The emergence of Zaire ebolavirus in West Africa and the scope of the 2014–2016 Ebola virus disease (EVD) epidemic have come as a surprise in a region not previously known to harbor Ebola virus. However, sporadic, limited outbreaks of EVD have been documented in rural areas of central Africa for decades. This course will provide recommendations for patient care in the hospital setting that emphasize the importance of strict adherence to patient isolation and barrier precautions, including the proper use of personal protective equipment and environmental infection control measures applicable to any healthcare setting.
- INTRODUCTION
- THE EBOLA VIRUS
- TRANSMISSION
- EBOLA VIRUS DISEASE
- THE 2014–2016 EBOLA EPIDEMIC IN WEST AFRICA
- CLINICAL CASE MANAGEMENT: ADVANCED PLANNING
- EXPOSURE EVALUATION PROTOCOL
- DIAGNOSTIC SPECIMEN COLLECTION AND HANDLING
- ENVIRONMENTAL INFECTION CONTROL PROCEDURES
- HANDLING OF HUMAN REMAINS
- MONITORING, MANAGEMENT, AND TRAINING OF VISITORS
- PREVENTION
- SUMMARY
- RESOURCES
- Works Cited
- Evidence-Based Practice Recommendations Citations
This course is designed for physicians, physician assistants, nurses, and allied healthcare professionals involved in the treatment and care of patients with suspected or confirmed Ebola virus disease.
The purpose of this course is to provide healthcare professionals, interprofessional teams, and those working in allied health disciplines, an overview of the clinical features, modes of transmission, epidemic potential, and important public health measures required for control of Ebola virus disease outbreaks.
Upon completion of this course, you should be able to:
- Outline the characteristics and transmission of Ebola viruses.
- Describe the pathogenesis and clinical manifestations of Ebola virus disease (EVD).
- Identify a potential case of EVD based on clinical and epidemiologic considerations.
- Develop a patient management plan that includes supportive care, critical care and/or transfer to a referral hospital designated for this purpose.
- Describe the African Ebola epidemic and its potential impact on global public health.
- Discuss the implications for foreign travel and potential for introduction of Ebola into the United States.
- In the event of a known or suspect case, design a strategy for supportive care, patient contact isolation, and protection of healthcare workers, utilizing contact precautions appropriate for the nature and severity of illness.
- Discuss the steps to be taken if a breach in isolation protocol results in exposure of a healthcare worker to Ebola.
- Evaluate and discuss the importance of proper specimen collection, environmental hygiene, handling of human remains, and management of visitors in the control of EVD.
Carol Shenold, RN, ICP, graduated from St. Paul’s Nursing School, Dallas, Texas, achieving her diploma in nursing. Over the past thirty years she has worked in hospital nursing in various states in the areas of obstetrics, orthopedics, intensive care, surgery and general medicine.
Mrs. Shenold served as the Continuum of Care Manager for Vencor Oklahoma City, coordinating quality review, utilization review, Case Management, Infection Control, and Quality Management. During that time, the hospital achieved Accreditation with Commendation with the Joint Commission, with a score of 100.
Mrs. Shenold was previously the Infection Control Nurse for Deaconess Hospital, a 300-bed acute care facility in Oklahoma City. She is an active member of the Association for Professionals in Infection Control and Epidemiology (APIC). She worked for the Oklahoma Foundation for Medical Quality for six years.
John M. Leonard, MD, Professor of Medicine Emeritus, Vanderbilt University School of Medicine, completed his post-graduate clinical training at the Yale and Vanderbilt University Medical Centers before joining the Vanderbilt faculty in 1974. He is a clinician-educator and for many years served as director of residency training and student educational programs for the Vanderbilt University Department of Medicine. Over a career span of 40 years, Dr. Leonard conducted an active practice of general internal medicine and an inpatient consulting practice of infectious diseases.
Contributing faculty, Carol Shenold, RN, ICP, has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Contributing faculty, John M. Leonard, MD, has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Ronald Runciman, MD
Mary Franks, MSN, APRN, FNP-C
The division planners have disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Sarah Campbell
The Director of Development and Academic Affairs has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
The purpose of NetCE is to provide challenging curricula to assist healthcare professionals to raise their levels of expertise while fulfilling their continuing education requirements, thereby improving the quality of healthcare.
Our contributing faculty members have taken care to ensure that the information and recommendations are accurate and compatible with the standards generally accepted at the time of publication. The publisher disclaims any liability, loss or damage incurred as a consequence, directly or indirectly, of the use and application of any of the contents. Participants are cautioned about the potential risk of using limited knowledge when integrating new techniques into practice.
It is the policy of NetCE not to accept commercial support. Furthermore, commercial interests are prohibited from distributing or providing access to this activity to learners.
Supported browsers for Windows include Microsoft Internet Explorer 9.0 and up, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Supported browsers for Macintosh include Safari, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Other operating systems and browsers that include complete implementations of ECMAScript edition 3 and CSS 2.0 may work, but are not supported. Supported browsers must utilize the TLS encryption protocol v1.1 or v1.2 in order to connect to pages that require a secured HTTPS connection. TLS v1.0 is not supported.
The role of implicit biases on healthcare outcomes has become a concern, as there is some evidence that implicit biases contribute to health disparities, professionals' attitudes toward and interactions with patients, quality of care, diagnoses, and treatment decisions. This may produce differences in help-seeking, diagnoses, and ultimately treatments and interventions. Implicit biases may also unwittingly produce professional behaviors, attitudes, and interactions that reduce patients' trust and comfort with their provider, leading to earlier termination of visits and/or reduced adherence and follow-up. Disadvantaged groups are marginalized in the healthcare system and vulnerable on multiple levels; health professionals' implicit biases can further exacerbate these existing disadvantages.
Interventions or strategies designed to reduce implicit bias may be categorized as change-based or control-based. Change-based interventions focus on reducing or changing cognitive associations underlying implicit biases. These interventions might include challenging stereotypes. Conversely, control-based interventions involve reducing the effects of the implicit bias on the individual's behaviors. These strategies include increasing awareness of biased thoughts and responses. The two types of interventions are not mutually exclusive and may be used synergistically.
#94083: Ebola Virus Disease
Ebola virus is one of several human pathogenic filoviruses epizootic in regions close to the African equator[7,33,34]. Fruit bats have been identified as reservoirs for Ebola virus in nature, while small animals and subhuman primates serve as intermediate hosts. Sporadic zoonotic human infection is acquired from contact with infected animals, following which human-to-human transmission often leads to secondary spread of Ebola virus within the family and local community. Ebola virus is a highly virulent human pathogen; symptomatic, debilitating illness is the rule, outbreaks spread rapidly, and mortality rates are high.
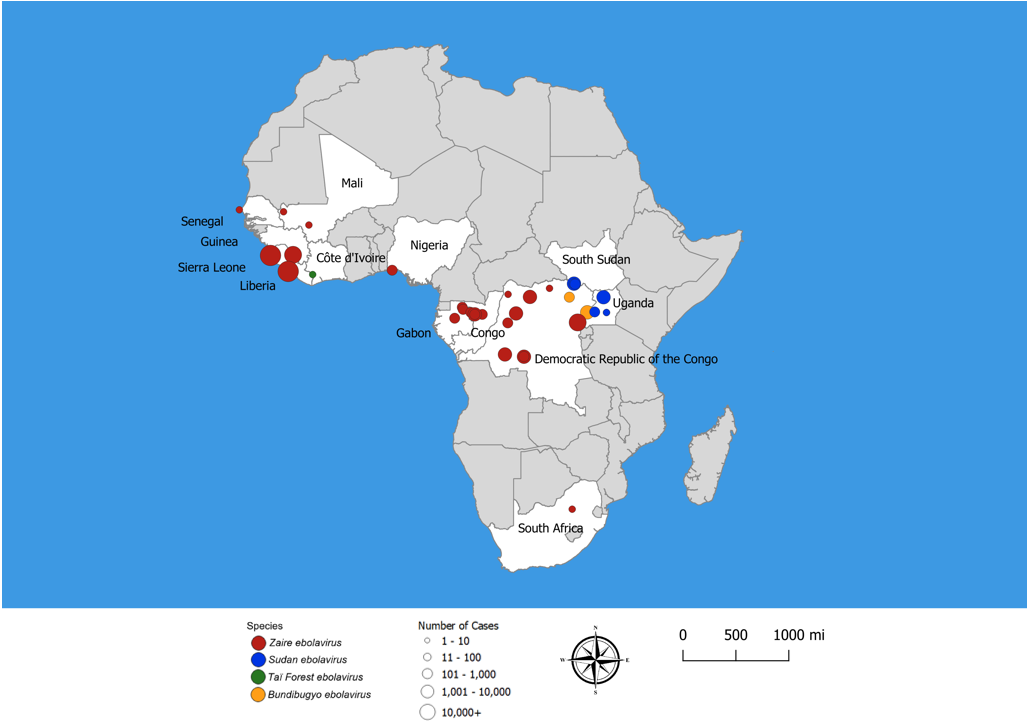
Ebola virus disease (EVD), formally known as Ebola hemorrhagic fever, is a severe multi-systemic illness having a high mortality risk. Periodic outbreaks of EVD with case fatality rates 40% to 60% have occurred in equatorial Africa, primarily in remote villages along tropical rainforests of Central Africa (Figure 1) [1,2,41]. Four species of Ebola virus have been identified in connection with symptomatic human infection. Zaire ebolavirus, Bundibugyo ebolavirus, and Sudan ebolavirus are the three species responsible for large outbreaks in Africa. Species Zaire ebolavirus is the most common and most lethal, with case fatality rates of 50% to 90%. Zaire ebolavirus is responsible for 18 of the 28 (64%) recorded EVD outbreaks since 1976, including the two largest EVD outbreaks in history: the 2014–2016 West Africa outbreak and the 2018 outbreak in eastern Democratic Republic of the Congo [22]. Sudan ebolavirus, with a fatality rate of 50%, has caused several outbreaks in Uganda and along the border between South Sudan and the Democratic Republic of the Congo (DRC). Bundibugyo ebolavirus, discovered in 2007, was associated with two outbreaks, one in DRC and the other on the border of DRC and Uganda. Taï Forest ebolavirus was the cause of one case identified in Côte d'Ivoire.
The 2014---2016 Ebola outbreak in West Africa (Guinea, Liberia, and Sierra Leone) was the first to affect densely populated urban areas where transmission is rapid and the attack rate high, resulting in a prolonged, deadly epidemic with global public health concerns [3,4]. This EVD outbreak totaled 28,616 cases and caused11,310 deaths [22]. The 2018—2020 EVD outbreak in DRC resulted in 3,470 cases and 2,287 (66%) deaths. Control of this outbreak was complicated by armed conflict between rebel groups and government armed forces, which hindered timely medical and public health response activities.
Sporadic cases and small, confined outbreaks of EVD are reported almost yearly in locales across central Africa. In 2022, a single case in April and five cases in August of Zaire ebolavirus infection were reported from DRC; an outbreak of 164 cases with 55 (34%) deaths from Sudan ebolavirus was reported from Uganda in September, lasting until January 2023. This was the sixth such EVD outbreak in Uganda; Pre-planned rapid response teams were deployed to support outbreak public health activities, including surveillance, investigation of unexplained deaths, contact tracing, and communication with the public [45].
Importation of EVD to the United States by someone traveling from an epidemic region is a recognized risk with potential for spread to other people. During the 2014–2016 outbreak in West Africa, 11 persons were treated for EVD in the United States, and 2 of them died. Nine of these cases were imported and two were domestic healthcare workers who had cared for the first travel-associated EVD case [22]. Both healthcare workers recovered.
Prompt identification of Ebola within a community is difficult because early EVD, like other acute infections, presents as a febrile illness with non-specific symptoms and signs. This puts healthcare workers, travelers, and families at risk of inadvertently contracting and transmitting the virus before the true import is known. Consequently, in clinical settings where the possibility for EVD exists, it is imperative that healthcare workers practice stringent Standard Precautions at all times when caring for a febrile patient until the specific diagnosis is known. This requires adherence to basic hand hygiene, isolation precautions, and the use of personal protective equipment (PPE) designed to prevent contact with body fluids and other potentially contaminated materials, such as clothing, bed covers, and burial garments [5,6].
The Centers for Disease Control and Prevention (CDC) website (https://www.cdc.gov/vhf/ebola) provides updated general information and addresses important issues of public health concern, including clinical guidance and recommendations, diagnosis, laboratory specimen transport, and protection of healthcare workers. Selected material from the CDC website that covers practical issues of case evaluation, infection control, and healthcare worker safety are reproduced in the latter portion of this course.
Ebola virus is a negative stranded RNA virus in the Filoviridae family. Zaire ebolavirus was discovered in 1976, as the cause of an outbreak of hemorrhagic fever located near the Ebola River in the DRC (formerly Zaire). Sporadic cases and small outbreaks of EVD have been reported subsequently in sparsely populated regions of Central Africa. The 2014–2016 outbreak began in the West Africa country of Guinea and spread rapidly to the neighboring countries of Sierra Leone and Liberia [3,7].
The taxonomy, or phylogenetic classification of Ebola, has been revised in recent years, and can be a source of confusion in usage of terms [2,33]. The family Filoviridae includes two genera that are pathogenic for humans: Ebolavirus and Marburgvirus. The family name is derived from the Latin term filum meaning "thread" and refers to the unique elongated (filamentous) morphology of filoviruses. The genus Ebolavirus includes five distinct species named for the locale in which each was first identified (Table 1) [33]. The Zaire, Sudan, and Bundibugyo species have caused periodic large outbreaks in Africa, whereas the Reston and Taï Forest species have not [7]. The pathogenicity of the Reston species, identified in pigs from the Philippines and China, is uncertain; it has been isolated from asymptomatic persons but no human illness or death from this species has been reported to date.
EBOLA VIRUSES
| Species | Virus | Endemic Countries |
|---|---|---|
| Bundibugyo ebolavirus | Bundibugyo virus (BDBV) | Uganda |
| Zaire ebolavirus | Ebola virus (EBOV) | Democratic Republic of Congo |
| Reston ebolavirus | Reston virus (RESTV) | Philippines, China |
| Sudan ebolavirus | Sudan virus (SUDV) | South Sudan |
| Taï Forest ebolavirus | Taï Forest virus (TAFV) | Ivory Coast |
Both Marburgvirus and Ebolavirus cause sporadic outbreaks of hemorrhagic fever with high case fatality rates in the range of 60% to 90%. A variant strain of Zaire ebolavirus, EBOV-Makona, is the apparent cause of the 2014–2016 epidemic in West Africa [7,33].
The natural reservoir of Ebola virus and the ecologic perturbations by which the virus emerges to infect humans are poorly understood [34]. EVD is considered a true zoonosis in that a reservoir of Ebola virus exists in the wild, combined with low-level infection in small mammals and nonhuman primates. Humans are an accidental host, increasingly so because of deforestation, hunting, and dietary practices that lead to increased contact with small animals. The 2014–2016 West Africa outbreak has been linked to consumption of fruit bats, which are sometimes used for dietary purposes [7]. Although filoviruses have been identified in bats, and EVD outbreaks have been temporally associated with bat migrations and die-offs, neither bats nor any other mammal has yet been accepted as a definitive Ebola virus reservoir [34].
Ebola virus is transmitted through direct contact with body fluids (e.g., saliva, vomitus, urine, feces, blood) of infected persons or contact with surfaces and materials, such as bedding and clothing, contaminated with these fluids. There is no evidence of respiratory transmission among humans, nor is Ebola virus transmitted by mosquitoes, ticks, or other insect vectors. Burial ceremonies, which bring mourners into direct contact with the body or garments of the deceased, have played an important role in the transmission of Ebola in West Africa. Sexual transmission may also play a role, as Ebola virus has been isolated from semen of male survivors up to months after onset of illness [13. 19]. There is no evidence that ebolaviruses can spread through sex or other contact with vaginal fluids from a woman who has had EVD [46].
Environmental studies show that Ebola virus can be recovered hours after contamination of inanimate surfaces by infected body fluids. Unless thoroughly disinfected, contaminated surfaces such as clothing, flooring, and furniture may be sources of transmission, as for example if one touches the mouth, nose, or eyes after manually handling contaminated material or objects [6].
Individuals who do not follow correct isolation precautions when caring for patients with EVD are at highest risk for infection [3]. Common risk exposures include unprotected close contact with patients, handling of soiled clothing or bedding, and improper environmental decontamination, hence the importance of providing healthcare workers with effective protective equipment and observing strict Contact Precautions. Ebolaviruses pose little risk to travelers or the public who have not cared for or been in contact with someone sick with EVD [46].
Ebola virus is a nonsegmented negative-strand RNA virus that varies greatly in size and form, with the filamentous form being the most characteristic [34]. The Ebola virus core structure consists of the RNA genome wrapped in nucleoprotein (the nucleocapsid) and other proteins having distinct functions in the replication cycle. The particle is enveloped within a lipid bilayer (the viral envelope) derived from the host cell. Spikes on the surface of the particle are formed by a transmembrane glycoprotein that mediates viral entry into the host cell.
Ebola virus is thought to gain entry to the human host through mucous membranes or skin abrasions, after which it binds to receptors on the surface of macrophages, monocytes, and dendritic cells [2]. Infected macrophages and monocytes migrate to regional lymph nodes, where continued viral replication and cellular propagation of infection occurs. From lymph nodes, infected monocytes, macrophages, and free virions spread through the lymphatic system to the blood stream, liver, spleen, and adrenal glands. Amplification of infection at these distant sites leads to focal areas of tissue necrosis and the release of ever-increasing numbers of virions into the general circulation [2]. The trophism of Ebola virus extends to multiple cell types, including endothelial cells, fibroblasts, hepatocytes, and adrenocortical cells.
The rapid pace of virus replication and dissemination results not only from the direct effect of virus on host cells, but also from mechanisms Ebola virus employs to evade host immune responses. [2,34]. These mechanisms include antagonism of the type I interferon response, inhibition of antigen presentation to CD8 and CD4 T cells, and suppression of T-cell activation, impeding the linkage between innate and adaptive immune responses [34]. Although Ebola virus does not invade lymphocytes, widespread and massive lymphocyte apoptosis develops early in the course of EVD [2].
Infected macrophages elaborate a variety of inflammatory mediators (cytokines and chemokines) that act in various ways to produce severe multisystem injury. These include cell-surface expression of tissue factors that activate the coagulation cascade and cytokine-mediated vascular endothelial injury, which together lead to disseminated intravascular coagulation (DIC), hypotension, and multi-organ failure [2]. This syndrome resembles the septic shock caused by gram-negative bacillary endotoxemia.
As noted, EVD is an acute, severe, multisystem viral infection that is highly contagious and often fatal [1,3,8,9,33]. The onset of symptoms is usually 5 to 7 days after exposure, though the observed incubation period varies from 2 to 21 days. Illness begins abruptly with fever, chills, and malaise, followed soon after by the rapid onset of weakness, myalgia, headache, vomiting, diarrhea, and abdominal pain. An erythematous maculopapular rash, appearing first on the buttocks and trunk, then more generalized, is often seen between the 5th and 7th day of illness [8]. Other findings include pharyngitis, lymph node enlargement, hepatomegaly, and abdominal tenderness.
Early laboratory abnormalities include neutropenia, lymphocytopenia, thrombocytopenia, and mild-to-moderate elevations of hepatic aminotransferase enzymes. Hemoglobin and hematocrit values are elevated secondary to fluid losses and hemoconcentration. At peak illness, total white blood cell counts rebound, anemia develops, and a variety of metabolic abnormalities emerge, including hypoalbuminemia, hypoglycemia, and elevated blood urea nitrogen [34].
With disease progression, patients experience profound weakness, postural hypotension, prostration, confusion, and bleeding abnormalities. Hemorrhagic manifestations, seen in fewer than half of affected patients, include petechiae, ecchymoses, bleeding from the gums, and blood in vomitus and stool [1,8,33]. Hemorrhagic complications are evidence of ongoing thrombocytopenia and the development of DIC. Metabolic acidosis and electrolyte abnormalities, principally hypokalemia, are common.
The case fatality rate for EVD is in the range of 40% to 80% [8]. Adverse prognostic factors include age older than 45 years, presence of comorbidities, delay in diagnosis, and limited availability of clinical resources. Patients who survive beyond two weeks usually recover, though after a prolonged convalescence marked by weight loss, asthenia, easy fatigue, and hair loss [8]. In survivors, clinical stability or improvement begins at about day 10 of illness and coincides with the appearance of antibodies and a sharp decline in circulating viral load.
Death from EVD most often results from hemodynamic collapse, hemorrhage, renal failure, or other inter-current complications such as pneumonia, metabolic acidosis, and cardiorespiratory failure. Fatal illness is associated with persistence and increasing levels of viremia and the near-absence of virus-specific antibody. While there is no evidence that pregnancy affects susceptibility to Ebola virus infection, pregnant women with EVD are at increased risk for severe illness, pregnancy-associated bleeding, spontaneous abortion, and death [28].
Autopsy studies of fatal EVD reveal extensive atrophy of lymphoid tissue, hepatic necrosis, focal necrosis of other organs, and acute tubular necrosis of the kidney [1,8].
The diagnosis of acute EVD is difficult because early symptoms and signs are nonspecific and may suggest other, more common febrile illnesses such as malaria or typhoid fever. The clinical criteria for a presumed case of EVD have been established by the CDC and are as follows [20]:
Elevated body temperature or subjective fever and additional symptoms such as severe headache, muscle pain, vomiting, diarrhea, abdominal pain, or unexplained hemorrhage
Epidemiologic risk factors within the past three weeks before the onset of symptoms, such as any of the following:
Contact with blood or other body fluids of a patient known to have or suspected to have EVD
Residence in—or travel to—an area where Ebola virus transmission is active
Direct handling of bats, rodents, or primates from disease-endemic areas
Contact with semen from a man who has recovered from EVD
In the event of a confirmed or suspected case of EVD, the patient should be isolated, diagnostic specimens collected, and public health authorities notified.
The presentation of a febrile patient in an endemic area or after foreign travel raises suspicion for a variety of common acute infections, depending on geographic locale. In residents of or travelers from Central Africa, primary considerations are malaria, typhoid, shigellosis, leptospirosis, dengue fever, rickettsiosis, meningoccocal septicemia, relapsing fever, and hepatitis [1,10].
For clinical purposes, early confirmation of the diagnosis of EVD relies on detection of viral genomic material or demonstration of virus-specific antigen in a sample of blood (Table 2). Ebola virus is detectable in blood within one to three days after onset of initial symptoms by use of the reverse transcriptase polymerase chain reaction (RT-PCR). Virus detection in blood samples remains positive for 10 days or more after onset of illness. If the initial diagnostic evaluation occurs more than three days after onset of illness, a subsequent specimen for RT-PCR may be necessary to confidently exclude EVD.
LABORATORY TESTS USED IN DIAGNOSIS OF EVD
| Timeline of Infection | Diagnostic Tests Available | ||||
|---|---|---|---|---|---|
| Within a few days after symptoms begin |
| ||||
| Later in disease course or after recovery | IgM and IgG antibodies | ||||
| Retrospectively in deceased patients |
|
An enzyme-linked immunosorbent assay (ELISA), for detection of viral antigen, is also available. This assay is slightly less sensitive than RT-PCR, usually becoming positive three to five days after onset of illness.
In 2019, the U.S. Food and Drug Administration (FDA) allowed marketing of a new rapid diagnostic test for Ebola virus [25]. The OraQuick Ebola Rapid Antigen Test uses blood samples from symptomatic patients or cadaveric oral fluid to identify Ebola antigens; the results are available within 30 minutes. For living patients, the test is intended for use in patients suspected of and with signs or symptoms consistent with EVD and when the patient meets the CDC's Ebola virus epidemiologic criteria, such as history of residence in or travel to a geographic region with active EVD transmission at the time of travel [25]. Negative results do not rule out Ebola virus infection, and definitive identification of EVD requires additional testing and confirmation.
Specimen collection and transfer for diagnostic purposes is a serious biohazard risk. Workers have been exposed to the disease during this process, especially when strict infection control policies are not followed and enforced [3]. The CDC provides guidance for collection, transport, and submission of specimens for Ebola virus testing, last updated December 2022 [21]. If a diagnosis of EVD is considered, clinicians should coordinate with their clinical laboratory, local public health officials, and the CDC to ensure appropriate precautions are taken to prevent spread and coordinate care. The CDC's Viral Special Pathogens Branch (VSPB) is available at any time for consultations on EVD by calling (770) 488-7100.
The CDC recommends that Ebola testing be conducted only for persons who have both consistent signs or symptoms and risk factors as follows [21]:
Elevated body temperature or subjective fever or symptoms, including:
Severe headache
Fatigue
Muscle pain
Vomiting
Diarrhea
Abdominal pain
Unexplained hemorrhage
An epidemiologic risk factor (e.g., travel exposure, personal contact with a known or suspected case of EVD, laboratory work handling human specimens) within the 21 days preceding the onset of symptoms.
Patients with EVD should be admitted to a single-bed room separate from the usual patient care areas, preferably one with an adjacent anteroom for donning and removing PPE. Ideally, care should be provided by an interprofessional team (physician and nursing staff) designated for this purpose. The team members should have critical care experience or advanced knowledge and training in the care of severely ill and highly infectious patients. Adherence to strict isolation, standard contact precautions, proper utilization of PPE, and effective environmental decontamination practices are essential for limiting transmission and protecting healthcare workers [6,11].
Because survivors can produce infectious virions for prolonged periods, strict barrier isolation in a private room away from traffic patterns should be maintained throughout the illness. The patient's urine, stool, sputum, and blood, along with any objects that have come in contact with the patient or the patient's body fluids (such as laboratory equipment), should be disinfected with a 0.5% sodium hypochlorite solution [6].
There is no satisfactory antiviral agent for treatment of EVD. Care is supportive and initial management goals include fluid resuscitation, prevention of intravascular volume depletion, and correction of electrolyte and other metabolic abnormalities [9,11]. Severe hypokalemia and varying degrees of lactic acidosis are common. Vigilance is required for fluid resuscitation that matches losses from vomiting and diarrhea, preventing complications of shock (e.g., metabolic acidosis, acute renal failure, acute lung injury).
Efforts to develop specific treatments for Ebola virus have accelerated in response to the public health challenges of EVD. Promising therapies initially tested in animal models have been followed by clinical trials conducted during recent outbreaks. These novel approaches include transfusion of convalescent plasma, monoclonal antibody infusions, and antiviral drugs developed for treatment of other RNA viruses. In addition, new strategies have been applied to vaccine development and application for therapeutic and preventive purposes.
ZMapp is a combination of three monoclonal antibodies having neutralizing activity against Ebola virus. In a primate treatment trial, rhesus macaques were given three doses of ZMapp, beginning at varying times up to five days after exposure. All 18 macaques survived [18]. During the 2014–2016 Ebola outbreak, two infected American healthcare workers who survived received ZMapp as part of their therapy.
A randomized controlled trial to assess the benefit of ZMapp combined with the current standard of care for EVD was conducted in West Africa in 2015 [29]. Of 71 evaluable patients enrolled, 31 died—an overall case fatality rate of 30%. Of the group who received the current standard of care alone, 13 of 35 (37%) patients died, compared with 8 of 36 (22%) patients in the group who received standard care plus ZMapp. Although the addition of ZMapp appeared to be beneficial, the result fell just short of statistical criteria for proven efficacy [29].
As of March 2024, there are two therapeutic agents approved by the FDA to treat EVD caused by Zaire ebolavirus [22]. In 2020, the FDA approved a monoclonal antibody combination atoltivimab, maftivimab, and odesivimab-ebgn (Inmazeb) for treatment of Zaire ebolavirus (Ebola virus) infection in adult and pediatric patients [35]. Inmazeb targets the surface glycoprotein that mediates virus-host membrane fusion, thereby preventing entry of virus into host cells and interrupting viral replication. Safty and efficacy were evaluated in a randomized clinical trial involving 154 patients who received one intravenous infusion and 168 patients who received an investigational control. The 28-day mortality in the treatment group was 33.8%, compared with 51% in the control group. Common symptoms reported during and after infusion were fever, chills, tachycardia, tachypnea, and vomiting; however, these are also common symptoms of EVD. A single monoclonal antibody preparation (Ebanga), which also binds to virus surface glycoprotein, interrupting cell entry, has also received FDA approval for treatment of EVD. The efficacy of Inmazeb and Ebanga was further evaluated in randomized controlled trials conducted during the 2018—2020 Ebola virus outbreak in the DRC. Overall survival was significantly higher for patients who received either of the two treatments [22]. Efficacy of Inmazeb and Ebanga has not been established for viruses other than Ebola virus (species Zaire ebolavirus).
Anti-RNA virus drugs under development for treatment of other infections have also been tested for activity against Ebola virus. Favipiravir, also designated T-705, is one such drug, an antiviral agent currently in development for treatment of influenza. This drug acts as a nucleotide analog, selectively inhibiting viral RNA-dependent RNA polymerase. Favipiravir suppresses Ebola virus replication in cell culture. In a mouse model of (lethal) Ebola infection, administration of T-705 on day 6 of infection resulted in prompt clearance of viremia and 100% survival. Recovery was associated with virus-specific antibody production and directed T-cell function [12].
Recovery from EVD is dependent on good supportive care and effectiveness of a patient's immune response. Relapse during convalescence from EVD is rare; however, recovery is often prolonged and complicated by residual functional impairment. Complications include suppurative parotitis, pericarditis, orchitis, uveitis, loss of vision, hearing impairment, memory loss, and post-traumatic stress disorder [30]. A variety of persistent symptoms and functional deficits have been reported in survivors of EVD, referred to as the "post-Ebola syndrome" [33,36]. These include musculoskeletal pains, headache, persistent fatigue, ocular problems, and neurologic disturbances (e.g., memory loss, cognitive difficulty, depression). In a case-control study of patients with EVD in the 2014–2016 Ebola outbreak, major limitations in vision, mobility, cognition, and affect were observed in 21 of 27 (77.8%) survivors one year following hospital discharge [36].
A 2020 review of EVD reported that up to 87% of survivors experience arthralgias affecting (in order of decreasing frequency) the knees, back, hips, fingers, wrists, neck, shoulders, ankles, and elbows—often severe enough to impede functional status [50]. Ocular symptoms and signs (e.g., retro-orbital pain, blurry vision, light sensitivity, conjunctival injection) were also found to complicate EVD recovery in a large proportion of adult (14% to 60%) and pediatric (32%) survivors. The ocular complications were most frequently attributed to uveitis, usually with onset within 12 weeks following recovery from acute manifestations of EVD. Clinical reports indicate involvement of all anatomic locations of the uveal tract including anterior uveitis (anterior chamber, iris, or ciliary body) in 46% to 62% of cases, posterior uveitis (choroid or retina) in 26%, and pan-uveitis in 21% to 25% of those examined [50]. Timely diagnosis and early appropriate anti-inflammatory treatment for uveitis is important to avoid long-term visual disability.
Ebola virus may persist for months in some convalescing patients, sequestered in organs relatively impervious to the immune system (e.g., testes, eye, central nervous system). Late recovery of infectious Ebola virus from survivors has been reported for semen (82 days), ocular fluid (98 days), urine (26 days), breast milk (15 days), and cerebrospinal fluid (10 months) [30]. Among a group of patients who provided semen specimens at various times after discharge from an EVD treatment unit, Ebola virus RNA was detected in the semen of all 7 patients tested within 3 months, in 26 of 42 men (62%) tested at 4 to 6 months, in 15 of 60 (25%) at 7 to 9 months, in 4 of 26 (15%) at 10 to 12 months, and in 1 of 40 men tested 16 to 18 months after discharge [37]. The long-term presence of Ebola virus in semen, albeit with declining prevalence, suggests the possibility of sexual transmission. The risk of infectivity from persons with residual foci of Ebola virus is unknown but considered to be low and to decrease over time [30].
Following infection, survivors of EVD develop protective antibodies against Zaire ebolavirus known to last 10 years. The duration of immunity and residual susceptibility to other Ebola species are not known.
In 2019, the FDA approved the first vaccine for the prevention of EVD [23]. The Ebola vaccine rVSV-ZEBOV (Ervebo) is a live, attenuated, vectored vaccine based on a recombinant vesicular stomatitis virus expressing the Zaire ebolavirus glycoprotein [33]. This vaccine is administered as a single injection for protection against Ebola virus species Zaire ebolavirus only, which has caused the largest, most deadly Ebola outbreaks to date [39]. The use of Ervebo vaccine was initially restricted to individuals 18 years of age and older. In September 2023, after randomized trials had established safety and immunogenicity, FDA approval extension of the indication for use of Ervebo to include individuals 12 months of age and older [47].
The approval of Ervebo is supported by a study conducted in Guinea during the 2014–2016 outbreak in individuals 18 years of age and older [27]. The study was a randomized cluster (ring) vaccination study in which 3,537 contacts and contacts of contacts of individuals with laboratory-confirmed EVD received either "immediate" or 21-day "delayed" vaccination with Ervebo. This noteworthy design was intended to capture a social network of individuals and locations that might include dwellings or workplaces where a patient spent time while symptomatic, or the households of individuals who had contact with the patient during that person's illness or death. In a comparison of cases of EVD among 2,108 individuals in the "immediate" vaccination arm and 1,429 individuals in the "delayed" vaccination arm, Ervebo was determined to be 100% effective in preventing Ebola cases with symptom onset greater than 10 days after vaccination. No cases of EVD with symptom onset greater than 10 days after vaccination were observed in the "immediate" cluster group, compared with 10 cases of EVD in the 21-day "delayed" cluster group.
Pre-exposure prophylaxis Ervebo vaccination is recommended for healthcare personnel at potential occupational risk of exposure to Zaire ebolavirus. This recommendation includes workers who are responding to an EVD outbreak, those involved in the care and transport of patients with suspect or confirmed EVD, and laboratorians and support staff at facilities handling specimens that may contain Ebola virus [39,44].
Ebola first appeared in 1976 in simultaneous outbreaks in Nzara, Sudan, and in Yambuku, Democratic Republic of Congo. The latter was in a village situated near the Ebola River, from which the disease takes its name. Since that time, there have been sporadic outbreaks of EVD in rural villages, effectively contained by public health measures and quarantine. [1,2]. Before 2013, these outbreaks were small and occurred in remote regions with limited opportunity for spread. In contrast, the 2014–2016 EVD epidemic in West Africa dispersed rapidly over large geographic areas and major urban centers in three countries, reaching a total of 28,646 cases that overwhelmed the public health infrastructure [38]. This reflects the population growth, urbanization, and technologic connectedness of peoples across current-day Central Africa.
The propensity for nosocomial transmission of Ebola was quickly recognized during the 1976 outbreak, after numerous severely ill patients were admitted to an inpatient clinical unit. At that time, in the 120-bed Yambuku Mission Hospital, secondary cases developed promptly in other patients and staff, in part related to the use of non-sterile syringes and needles. The hospital closed, and many infected people and their contacts fled to their home villages out of fear and suspicion of the Western medicine and began seeking treatment from traditional healers [1].
The 2014–2016 outbreak has been traced to an index case in Guinea involving a child hospitalized in December 2013. In March 2014, public health officials in Guinea reported more than 40 additional cases, the first formal indication of a regional outbreak [7]. It is thought that during the initial stages of the outbreak severely ill patients were taken to provincial hospitals, where unsuspecting staff and visitors came into direct contact with patients. This in turn led to secondary foci of infection, further expanding the outbreak and establishing new chains of transmission.
Contiguous regions in Liberia, Sierra Leone, and Guinea in West Africa constitute the major locus of the epidemic. A small number of cases appeared transiently in Nigeria, where control measures appear to have been effective in limiting spread. In an analysis of 4,507 cases reported for the first nine months of the outbreak, the World Health Organization (WHO) found that the majority of cases occurred in persons 15 to 44 years of age, and the mean incubation period was 11 days, with a range of 2 to 21 days [3]. Lower attack rates in young children and older adults and higher rates in women presumably reflects differences in exposures associated with caregiving for ill persons [34]. The estimated case fatality rate for the period reported was 71% overall, 64% among hospitalized patients, and 56% among healthcare workers.
The virulence of Ebolavirus, ease of transmission, and rapidity of spread within large population centers combined to overwhelm existing clinical care facilities and public health resources within these West African countries [9]. Success in achieving infection control was further confounded by the inadequacy of usual sanitation practices within the populace with respect to care of the sick and burial of the dead.
On August 8, 2014, the WHO declared the West Africa Ebola epidemic a Public Health Emergency of International Concern, thereby invoking the powers of the 2005 International Health Regulations (IHR). In response to a potential pandemic, the IHR requires all countries to develop a national preparedness program, conduct surveillance, exercise public health measures, and report any internationally significant event [4]. The director-general of the WHO further urged those countries with active Ebola transmission to declare a national emergency, activate disaster management plans, and establish emergency operation centers.
With international support, a coordinated effort emerged in West Africa with a strategy for clinical care and infection control aimed at curbing the spread of the epidemic. Public health countermeasures included isolation and quarantine, social distancing, public education, travel restrictions, and enhanced protection of healthcare workers [4]. These measures embrace the following recognized priorities for an effective infection control program:
Sufficient numbers of healthcare workers trained and properly equipped for rendering care and maintaining strict contact precautions
Rigorous identification and isolation of cases combined with surveillance of primary contacts
Rapid, safe, and culturally sensitive disposal of the dead
Education of the public and community leaders regarding the importance and the means for effective sanitation precautions and safe burial practices
By the end of April 2016, the West Africa Ebola epidemic had been contained and the outbreak rapidly diminished over the ensuing weeks. According to data at the CDC, there had been 28,616 cases (suspected, probable, and confirmed) and 11,310 deaths [13].
Apart from the horrific burden of illness and death, the socioeconomic impact of the Ebola epidemic was enormous. It is estimated that during 2015, the countries of Guinea, Liberia, and Sierra Leone sustained a combined loss of $2.2 billion in gross domestic product. In addition, the epidemic resulted in lower investment, a decline in private sector growth, and reduced cross-border trade in goods and services [31].
The impact of the Ebola outbreak on the healthcare system was especially troubling. Healthcare workers caring for patients with EVD were among those at highest risk for contracting the infection. From the start of the outbreak through November 2015, a total of 881 confirmed cases of EVD in health workers were reported in Guinea, Liberia, and Sierra Leone, and there were 513 reported deaths [31]. Liberia lost 8% of its doctors, nurses, and midwives to Ebola; Sierra Leone and Guinea lost 7% and 1% of their healthcare workers, respectively [31]. The reduction in the healthcare workforce, combined with reduced access to services and setbacks in care for other indigenous infection, is estimated to have resulted in an additional 10,600 deaths from human immunodeficiency virus, tuberculosis, and malaria.
Children suffered not only from the disease but also because of the impact of the epidemic on the family and the educational process [31]. Approximately 20% of all EVD cases occurred in children younger than 15 years of age. Recovery plan data from the three countries most affected estimate that more than 17,000 children were orphaned because of Ebola. All schools closed for a period of 33 to 39 weeks, and by the time they reopened, an estimated 1,848 hours of instruction had been lost [31].
The cost of the international response to the West Africa Ebola epidemic reached $3.611 billion (in U.S. dollars) by December 2015. The U.S. government allocated approximately $2.369 billion, including $798 million to the CDC, $632 million to the U.S. Department of Defense, and $939 million to the Agency for International Development [31].
The risk of the Ebola virus transmission during air travel is low because, unlike infections such as influenza or tuberculosis, this virus is not spread by inhalation of aerosolized particles from an infected person. Moreover, a person only becomes infectious after having developed symptoms of illness, and transmission of the virus requires direct physical contact with bodily fluids in a manner likely to be extraordinarily rare for travelers on a commercial flight.
There is the possibility that a newly infected traveler from an epidemic zone could enter the United States during the (asymptomatic) incubation period, only to become ill (and thus infectious) some days later. Such a case occurred in October 2014, when a man became ill and was hospitalized in Dallas, Texas, several days after arriving from Liberia. Shortly before his departure from Guinea he had provided bodily assistance to a severely ill woman who subsequently was diagnosed with EVD. This was the first case of EVD to be diagnosed in the United States and was followed shortly by two secondary cases among the hospital nurses who had cared for the patient. This series of events spawned a national discourse on the potential risks from Ebola and on the measures required for effective protection of healthcare workers and control of transmission in the event of an index case [14]. This in turn lead to enhanced assistance to West Africa and to the implementation of a program for identification and surveillance of potentially infected travelers entering this country [15].
During the West Africa outbreak, the CDC provided personnel to assist with active screening and education efforts on the ground in West Africa, aimed at preventing sick travelers from boarding a plane. In addition, airports in Liberia, Sierra Leone, and Guinea screened all outbound passengers by means of a health questionnaire, symptom assessment, and temperature monitoring.
In October 2014, the CDC implemented an enhanced screening program at five U.S. airports that receive greater than 90% of air passengers whose travel originated from Sierra Leone, Liberia, or Guinea [15]. Travelers from these three countries were taken to a designated area, where each was asked about health status and exposure history, observed for symptoms, and screened for fever. Those with exposure history, symptoms or fever were evaluated further by CDC officers and referred to local and state health authorities. The remaining travelers were given health information and asked to participate in a self-monitoring, post-arrival, surveillance program [15].
Active post-arrival surveillance included travelers without fever or other symptoms consistent with Ebola, who were monitored daily by state and local health departments for 21 days from the date of their departure from West Africa. Six states (New York, Pennsylvania, Maryland, Virginia, New Jersey, and Georgia), accounting for the large majority of such travelers, implemented active post-arrival monitoring in late October 2014. The CDC also provided post-arrival surveillance assistance to state and local health departments, including information on travelers arriving in their states, and upon request, technical support, consultation, and funding.
In response to a small outbreak in 2021, the CDC implemented entry screening procedures for travelers arriving from Guinea and the Democratic Republic of the Congo [42].
If an ill passenger entered the United States, CDC protocols were in place to protect against further disease spread. These include:
Notification to CDC of ill passengers on a plane before arrival
Investigation of ill travelers
Isolation if necessary
The CDC has also provided guidelines to airlines for managing ill passengers and crew and for disinfecting aircraft. The CDC issued a Health Alert Notice reminding U.S. healthcare workers of the importance of taking steps to prevent the spread of Ebola virus, including how to test and isolate suspected patients and how to protect themselves from infection [5].
There are well-established CDC protocols in place to ensure safe transport and care of patients with infectious diseases back to the United States. These procedures cover each step in the process: transport from bedside to the airport; boarding a non-commercial airplane equipped with a special transport isolation unit; and arrival at a medical facility in the United States that is appropriately equipped and staffed to minimize risk for spread of infection and to ensure that the American public is protected. The protocol is similar to that used for evacuation of patients during the severe acute respiratory syndrome (SARS) outbreak.
On October 6, 2014, the CDC issued a Health Alert Notice Advisory to remind healthcare personnel and health officials to [16]:
Increase vigilance in inquiring about a history of travel to West Africa in the 21 days before illness onset for any patient presenting with fever or other symptoms consistent with Ebola.
Isolate patients who report a travel history to an Ebola-affected country (i.e., Liberia, Sierra Leone, and Guinea) and who are exhibiting Ebola symptoms in a private room with a private bathroom and implement Standard, Contact, and Droplet Precautions (including gowns, facemask, eye protection, and gloves).
Immediately notify the local/state health department.
Early recognition is critical to controlling the spread of Ebola virus. Healthcare personnel should examine the patient's travel history and consider the possibility of Ebola in patients who present with fever, myalgia, headache, abdominal pain, vomiting, diarrhea, or unexplained bleeding or bruising. If a patient reports a history of recent travel to one of the affected West African countries and is symptomatic, immediate action should be taken [16].
An active early warning approach, under the framework of a surveillance response system, for veterinary and human populations is a key element for prevention in all countries. The institution of electronic-based reporting systems based on advances in information and communication technologies is also an important tool for surveillance [17].
Another crucial component during an outbreak is to understand cultural practices and customs that can affect behavioral attitudes toward Ebola outbreaks. Lack of understanding about EVD and misinformation concerning hospitalization, treatment, and transmission frustrated attempts to curtail the epidemic in West Africa. Myth, rumor, and fear frequently led to avoidance of healthcare centers, refusal of hospitalization, concealing of sick family members at home, and reliance upon local custom and traditional healers. These factors, taken together, contributed to a heightened mortality risk and served to perpetuate spread of the infection [17,26].
Events surrounding the 2014–2016 Ebola epidemic have led to the recommendation that all inpatient facilities have a plan in place to prevent transmission of EVD in healthcare settings. This plan should address where these patients will be cared for, who will render that care (designated physician and nursing staff), and detailed protocols for PPE utilization and environmental decontamination procedures. The CDC has published clinical care guidelines for hospitals and any healthcare facility staff who may encounter patients with suspected and confirmed EVD or other viral hemorrhagic fevers [32].
Most U.S. hospitals are capable of admitting a patient to a private room (with bathroom) and observing standard isolation and Contact Precautions. With advanced planning in conformity with CDC infection control recommendations and with the availability of critical care specialists, most should be able to safely and effectively care for a patient with EVD [6].
Patient Placement
Patients should be placed in a single patient room (containing a private bathroom) with the door closed. Facilities should maintain a log of all persons entering the patient's room. Consider posting personnel at the patient's door to ensure appropriate and consistent use of PPE by all persons entering the patient room.
Personal Protective Equipment
All persons entering the patient room should wear at least:
Gloves
Gown (fluid resistant or impermeable)
Eye protection (goggles or face shield)
Facemask
Additional PPE might be required in certain situations (e.g., copious amounts of blood, other body fluids, vomit, or feces present in the environment), including but not limited to:
Double gloving
Disposable shoe covers
Leg coverings
Healthcare personnel should don PPE before entry into patient rooms or care areas. Upon exit from the patient room or care area PPE should be carefully removed without contaminating one's eyes, mucous membranes, or clothing with potentially infectious materials and either discarded or, for re-useable PPE, cleaned and disinfected according to the manufacturer's reprocessing instructions and hospital policies. Hand hygiene should be performed frequently, including before and after patient contact, contact with potentially infectious material, and upon removal of PPE, including gloves.
Patient Care Equipment
Dedicated medical equipment (preferably disposable, when possible) should be used for the provision of patient care. All non-dedicated, non-disposable medical equipment used for patient care should be cleaned and disinfected according to manufacturer's instructions and hospital policies.
Patient Care Considerations
Limit the use of needles and other sharps as much as possible when caring for patients with known or suspected EVD. Phlebotomy, invasive procedures, and laboratory testing should be limited to the minimum necessary for essential diagnostic evaluation and medical care. All needles and sharps should be handled with extreme care and disposed in puncture-proof, sealed containers.
Aerosol-Generating Procedures
All aerosol-generating procedures (AGPs) should be avoided for patients with EVD if at all possible. If performing AGPs, use a combination of measures to reduce exposures. This includes prohibiting visitors during these procedures and limiting the number of healthcare personnel present to only those essential for patient care and support. Conduct the procedures in a private room, ideally in an airborne infection isolation room (AIIR) when feasible. Room doors should be kept closed during the procedure except when entering or leaving the room, and entry and exit should be minimized during and shortly after the procedure.
Healthcare personnel should wear gloves, a gown, disposable shoe covers, and either a face shield that fully covers the front and sides of the face or goggles and respiratory protection that is at least as protective as a National Institute for Occupational Safety and Health (NIOSH)-certified fit-tested N95 filtering face piece respirator or higher (e.g., powered air purifying respiratory or elastomeric respirator) during AGPs. Because of the potential risk to individuals reprocessing reusable respirators, disposable filtering face piece respirators are preferred.
It is important to conduct environmental surface cleaning following established procedures (discussed in detail later in this course). If re-usable equipment or PPE (e.g., powered air purifying respirator, elastomeric respirator) are used, they should be cleaned and disinfected according to manufacturer instructions and hospital policies.
Trained personnel, using PPE as described for routine patient care, should be in charge of collection and handling of soiled re-usable respirators. Although there are limited data available to definitively define AGPs, procedures that are usually included are bilevel positive airway pressure (BiPAP), bronchoscopy, sputum induction, intubation and extubation, and open suctioning of airways.
Facilities should develop policies for monitoring and management of potentially exposed healthcare personnel. Facilities should develop sick leave policies for healthcare personnel that are non-punitive, flexible, and consistent with public health guidance. All healthcare personnel, including staff who are not directly employed by the healthcare facility but provide essential daily services, should be aware of the sick leave policies. Persons with percutaneous or mucocutaneous exposures to blood, body fluids, secretions, or excretions from a patient with suspected EVD should stop working and immediately wash the affected skin surfaces with soap and water. Mucous membranes (e.g., conjunctiva) should be irrigated with copious amounts of water or eyewash solution. In addition, the individual should immediately contact an occupational health representative or supervisor for assessment and access to postexposure management services for all appropriate pathogens (e.g., human immunodeficiency virus, hepatitis C).
Healthcare personnel who develop sudden-onset fever, intense weakness or muscle pains, vomiting, diarrhea, or any signs of hemorrhage after an unprotected exposure (e.g., not wearing recommended PPE at the time of patient contact) to a patient with EVD should:
Not report to work or should immediately stop working
Notify their supervisor
Seek prompt medical evaluation and testing
Notify local and state health departments
Comply with work exclusion until they are deemed no longer infectious to others
Asymptomatic healthcare personnel who had an unprotected exposure to a patient with EVD should receive medical evaluation and follow-up care, including fever monitoring twice daily, for 21 days after the last known exposure. Hospitals should consider policies ensuring twice daily contact with exposed personnel to identify potential symptoms and document fever checks. Personnel may continue to work while receiving twice daily fever checks, based upon hospital policy and discussion with local, state, and federal public health officials. Infectious disease consultation is recommended for exposed persons who develop fever within 21 days of exposure.
The known or estimated level of risk exposure should guide testing of persons for possible Ebola virus infection. The CDC recommends testing for all persons with onset of fever within 21 days of having a high-risk exposure. A high-risk exposure includes any of the following:
Percutaneous or mucous membrane exposure or direct skin contact with body fluids of a person with a confirmed or suspected case of EVD without appropriate PPE
Laboratory processing of body fluids of suspected or confirmed EVD cases without appropriate PPE or standard biosafety precautions
Participation in funeral rites or other direct exposure to human remains in the geographic area where the outbreak is occurring without appropriate PPE
For persons with a high-risk exposure but without a fever, testing is recommended only if there are other compatible clinical symptoms present and blood work findings are abnormal (i.e., thrombocytopenia <150,000 cells/mcL and/or elevated transaminases) or unknown.
Persons considered to have a low-risk exposure include those who spent time in a healthcare facility where patients with EVD are being treated (including healthcare workers who used appropriate PPE, employees not involved in direct patient care, and other hospital patients who did not have EVD and their family caretakers) and household members of an Ebola patient without high-risk exposures (as defined). Persons who had direct unprotected contact with bats or primates from an endemic area would also be considered to have a low-risk exposure.
Testing is recommended for persons with a low-risk exposure who develop fever with other symptoms and have unknown or abnormal blood work findings. Persons with a low-risk exposure and with fever and abnormal blood work findings in the absence of other symptoms are also recommended for testing. Asymptomatic persons with high- or low-risk exposures should be monitored daily for fever and symptoms for 21 days from the last known exposure and evaluated medically at the first indication of illness.
Persons with no known exposures but who have fever and other symptoms or abnormal bloodwork within 21 days of visiting countries where Ebola virus transmission is active should be considered for testing. Consultation with local and state health departments is recommended in these cases.
If testing is indicated, the local or state health department should be immediately notified. Healthcare providers should collect serum, plasma, or whole blood. A minimum sample volume of 4 mL should be shipped refrigerated or on ice pack or dry ice (no glass tubes), in accordance with International Air Transport Association guidelines as a Category B diagnostic specimen [6,21].
Healthcare personnel should perform hand hygiene frequently, including before and after all patient contact, contact with potentially infectious material, and before putting on and taking off PPE, including gloves. Healthcare facilities should ensure that supplies for performing hand hygiene are available.
Acceptable hand hygiene in healthcare settings includes both washing with soap and water or using alcohol-based hand rubs. If hands are visibly soiled, use soap and water, not alcohol-based hand rubs.
The following sections are reprinted from the CDC's Guidance for Collection, Transport, and Submission of Specimens for Ebola Virus Testing, issued based on input from numerous hospital and laboratory directors, infectious disease physicians, CDC Ebola response teams, and state health officials [21].
The United States Occupational Safety and Health Administration (OSHA) Bloodborne Pathogens Standard (29 CFR 1910.1030) was developed to reduce the potential exposure of personnel to bloodborne pathogens. All U.S. laboratories handling patient specimens are required to comply with this standard at all times. Strict adherence is an initial step in providing protection to personnel.
Specimens should be obtained when a patient meets the criteria for person under investigation (PUI) including patients with clinical signs, symptoms, and epidemiologic risk factors for Ebola virus disease. If the first specimen is obtained one to three days after the onset of symptoms and tests negative and the patient remains symptomatic without another diagnosis, a later specimen is needed to rule out Ebola virus infection.
Staff who collect specimens from PUIs should wear appropriate PPE and should refer to Guidance on Personal Protective Equipment To Be Used by Healthcare Workers During Management of Patients with Ebola Virus in U.S. Hospitals, Including Procedures for Putting On (Donning) and Removing (Doffing) (online at https://www.cdc.gov/vhf/ebola/healthcare-us/ppe/guidance.html).
For adults, two samples of whole blood, each a minimum volume of 4 mL, is preferable. For pediatric patient samples, a minimum of 1 mL whole blood should be collected in pediatric-sized collection tubes. Blood must be collected in plastic collection tubes. Do not transport or ship specimens in glass containers or in heparinized tubes.
Whole blood preserved with ethylenediaminetetraacetic acid (EDTA) is preferred, but whole blood preserved with sodium polyanethol sulfonate, citrate or with clot activator is also acceptable. Do not separate and remove serum or plasma from the primary collection container.
Public health authorities will determine where ebolavirus testing will occur. Presumptive testing for Ebola virus and Sudan virus is available at select reference laboratories throughout the United States, Specimens should be packaged and transported at 2°–8°C with cold-packs to the final testing destination. Specimens other than blood may be submitted after consultation with CDC by calling the Emergency Operations Center at 770-488-7100.
If necessary, short-term storage of specimens before shipping should be at 2–8°C. Specimens sent to the CDC for testing must be sent on dry ice and must arrive in time to be tested within seven days of the date of specimen collection.
Real-time RT-PCR testing for Ebola virus is available at over 60 LRN laboratories located throughout the United States. LRN laboratories are currently using an FDA-approved Emergency Use Only (EUA) assay to detect the Ebola (Zaire species) virus. Samples that test positive using this assay are considered presumptive positive for Ebola Zaire RNA by real-time RT-PCR and should be submitted to CDC for additional evaluation.
PPE to be worn during transport within the facility should be determined by a site-specific risk assessment and may vary among facilities. Recommendations for PPE include disposable fluid-resistant closed lab coat, disposable gloves, covered legs and closed-toed shoes.
Before removing patient specimens from the site of care, it is advisable to plan the route of the sample from the patient area to the location where it will be packed for shipping to avoid high traffic areas.
Before removing patient specimens from the site of care, the outside of the specimen containers should be decontaminated with an approved disinfectant. Recommended disinfectants are those known to kill non-enveloped viruses and can be found in List L of the Environmental Protection Agency (EPA) Disinfectants for Use Against the Ebola Virus. This list of registered disinfectants meets the CDC's criteria for use against the Ebola virus on hard, non-porous surfaces.
In compliance with OSHA Bloodborne Pathogens Standard, specimens should be placed in a durable, leak-proof secondary container. After placing in a secondary container, specimens should be hand-carried to the laboratory or packing area. DO NOT use any pneumatic tube system (automated or vacuum specimen delivery system) for transporting specimens.
Samples from patients who are PUIs or confirmed to have Ebola virus infection should be packaged and shipped as Category A infectious substances in accordance with the Department of Transportation's (DOT's) Hazardous Materials Regulations. All persons packing and shipping infectious substances must be trained and certified in compliance with DOT or the International Air Transport Association (IATA) requirements every two years.
Specimens collected for Ebola virus testing should be packed and shipped without attempting to open collection tubes or aliquot specimens. Opening the tubes destroys the vacuum seal and thus increases the risk of leakage during transport. Specimens for shipment should be packaged following the basic triple packaging system, which consists of (1) a primary container (a sealable specimen container) wrapped with absorbent material, (2) a secondary container (watertight, leak-proof), and (3) an outer shipping package. For questions about (packaging) transportation regulations, contact the U.S. DOT Hazardous Materials Information Center at 1-800-467-4922.
The CDC will not accept specimens without prior consultation and approval. The CDC's Viral Special Pathogens Branch (VSPB) is available 24/7 by calling CDC Emergency Operations Center at (770) 488-7100 and requesting VSPB's on-call epidemiologist. The following steps should be followed by persons certified to ship infectious substances.
Because guidelines may vary state to state, contact your state and/or local health department prior to shipping.
Email tracking number to spather@cdc.gov.
Do not ship for weekend delivery unless instructed to do so by the CDC.
Ship to:
Centers for Disease Control and Prevention ATTN STAT LAB: VSPB, UNIT #70 1600 Clifton Road NE Atlanta, GA 30333 Phone: 470-312-0094 Include the following information inside the package: your name, the patient's name, test(s) requested, date of collection, laboratory or accession number, and CDC Form 50.34 (available at https://www.cdc.gov/laboratory/specimen-submission/pdf/form-50-34.pdf) and Viral Special Pathogens Branch Specimen Submission Information (available at https://www.cdc.gov/ncezid/dhcpp/vspb/pdf/specimen-submission-508.pdf).
On the outside of the box, specify how the specimen should be stored: refrigerated.
Include documentation required by DOT or IATA.
For grossly soiled surfaces (e.g., vomitus, stool), use a 1:10 dilution of household bleach for cleaning and disinfection. In all other cases, a 1:100 dilution is sufficient. Soiled linens should be placed in clearly labeled, leak-proof bags at the site of use, transported directly to the laundry area, and laundered following routine healthcare laundry procedures.
Liquid medical waste, such as feces and vomitus, can be disposed of in the sanitary sewer following local sewage disposal requirements. Care should be taken to avoid splashing when disposing of these materials.
When discarding solid medical waste (e.g., needles, syringes, tubing) contaminated with blood or other body fluids from patients with EVD, contain the waste with minimal agitation during handling. Properly contained wastes should be managed according to existing local and state regulations for ensuring health and environmental safety during medical waste treatment and disposal. On-site treatment of the waste in an incinerator or a gravity-displacement autoclave for decontamination purposes will help to minimize the risks of handling contaminated waste. Alternatively, off-site medical waste treatment resources may be used.
If the patient dies, handling of the body should be minimized, and the remains should not be embalmed. Instead, remains should be wrapped in sealed, leak-proof material and cremated or buried promptly in a sealed casket. If an autopsy is necessary, the state health department and the CDC should be consulted regarding appropriate precautions.
Visitors (e.g., family and friends) who have been in contact with a patient with EVD before and during hospitalization are a possible source of Ebola infection for other patients, visitors, and staff. Visitor movement within the facility should be restricted to the patient care area and an immediately adjacent waiting area; avoid entry of visitors into the patient's room. Exceptions may be considered on a case-by-case basis for those who are essential for the patient's well-being. Establish procedures for monitoring, managing, and training visitors. For example, a visitor log should be kept of those who enter and exit the patient's room.
Visits should be scheduled and controlled to allow for:
Screening for active Ebola infection (e.g., fever and other symptoms) before entering or upon arrival to the hospital
Evaluating risk to the health of the visitor and ability to comply with precautions
Providing instruction before entry into the patient care area on hand hygiene, limiting surfaces touched, and use of PPE according to the current facility policy while in the patient's room
Community awareness of risk factors for Ebola infection and the protective measures individuals can take is an important way to reduce human transmission and interrupt the epidemic spread of EVD. During outbreaks in Africa, public health education efforts have focused on:
Reducing the risk of wildlife-to-human transmission from contact with infected animals such as fruit bats or monkeys/apes. Animals should be handled with gloves and other protective clothing. Animal products should be thoroughly cooked before consumption.
Reducing human-to-human transmission in the community arising from direct or close contact with infected patients, particularly with their bodily fluids. Gloves and appropriate PPE should be worn when taking care of ill patients at home. Regular hand washing is required after visiting patients in hospitals, as well as after taking care of patients at home.
Educating the public about the nature of the disease and about outbreak containment measures, including the prompt and safe burial of the dead.
The CDC has published guidance for personal protection and prevention of the spread of EVD for those living in or traveling to a region where Ebola virus is potentially present or to an area experiencing an outbreak of EVD [39]. These methods of protection emphasize that one should avoid general and sexual contact with all body fluids, clothing bedding, and other items that may have come in contact with the sick or persons known or suspected of having EVD. In addition, one should avoid contact with bats, forest antelopes, and nonhuman primates, including blood, fluids, or raw meat prepared from these or unknown animals (also known as bushmeat).
As noted, the Ebola virus vaccine is a replication-competent, live, attenuated recombinant vesicular stomatitis virus vaccine encoded for Zaire ebolavirus glycoprotein. It is known as rVSV/G-ZEBOV-GP Ebola vaccine (brand name Erbevo), manufactured by Merck. This vaccine is safe and effective against Zaire ebolavirus, the cause of the largest and most deadly Ebola outbreaks to date [40]. It is not possible to become infected with Ebola from vaccination, as the vaccine only contains a gene from the core particle, not the whole virus. Adverse effects following vaccination are typically mild and include headache, fatigue, myalgia, and fever. The duration of protection against Ebola virus is unknown. It is also not known whether it is effective when administered concurrently with antiviral medication, monoclonal antibody therapy, or plasma transfusion [40]. Erbevo is FDA-approved for the prevention of disease caused by Ebola virus (EBOV; species Zaire ebolavirus) in individuals 12 months of age and older as a single dose administration [47].
In February 2020, the Advisory Committee on Immunization Practices recommended pre-exposure vaccination with Ervebo for adults in the United States who are at potential risk of exposure to Zaire ebolavirus. Ervebo is not currently marketed in the United States but is stockpiled in the Strategic National Stockpile and is made available through the CDC for pre-exposure vaccination of individuals who fall into one of the three occupational categories [40]:
EVD responders to a Zaire ebolavirus outbreak.
Laboratorians or other staff working at biosafety-level 4 or Laboratory Response Network facilities in the United States that might handle specimens containing replication-competent EBOV
Healthcare personnel working at federally designated Ebola Treatment Centers involved in the care and transport of patients infected or suspected to be infected with EBOV in the United States
Continuing outbreaks of EVD, which come with high morbidity and mortality, are a tragedy for local communities, place an enormous burden on public health resources of affected countries, and pose a potential threat to global health stability. In response to this challenge, a consortium of agencies under the auspices of the WHO have sought to develop safe and effective Ebola virus vaccines applicable to adults and children. Such a vaccine, administered on a community-wide basis in response to an index case, would facilitate a ring strategy of containment designed to limit spread and shorten the outbreak. Two candidate vaccine regimens have received WHO prequalification status and were evaluated for this purpose during recent EVD outbreaks. One is rVSV-ZBOV vaccine (Ervebo) and the other is a combination of an adenovirus type-26—vectored vaccine encoding the ZEBOV glycoprotein followed by a booster dose of a modified vaccinia Ankara virus strain (MVA-BN-Filo) [48,49].
The WHO PREVAC Study Team has published results of two randomized, placebo-controlled trials evaluating three Ebola vaccine strategies in adults and children. The trials were conducted simultaneously in 2018 and enrolled 1,400 adults and 1,401 children (1- to 17 years of age) who were randomized to receive two injections of either Ebola vaccine or placebo in one of three regimens. Study participants were volunteers at sites in Guinea, Liberia, Sierra Leone, and Mali. All vaccine regimens were found to be safe and immunogenic in adults and children [49]. Serum IgG binding antibodies against the Ebola virus surface glycoprotein were evident by day 14 after vaccination and continued to be detectable, at varying levels depending on the regimen, in both age groups for one year. The trials were unable to assess protection from disease as there were no incident cases of EVD among vaccine or placebo group participants during the study period. However, the authors note that prior studies show that levels of glycoprotein-binding antibodies strongly correlate with neutralizing antibody titers in nonhuman and humans [49].
Sporadic, limited outbreaks of EVD have been documented in rural areas of Central Africa for decades. The emergence of Zaire ebolavirus in West Africa and the scope of the 2014–2016 Ebola epidemic came as a surprise in a region not previously known to harbor Ebola virus.
The outbreak of Zaire ebolavirus in West Africa was associated with a brief, focal cluster of cases in Dallas, Texas, demonstrating the clinical challenges of providing effective case management and control of transmission in hospital settings. The outbreak also exposed the limited preparedness of public health systems to respond to emerging, highly virulent communicable diseases. However, the medical and public health sectors here and abroad have moved to improve education, heighten vigilance, and put in place proven epidemiologic principles for limiting transmission and achieving control of epidemic spread.
The CDC website maintains a portfolio of information on EVD for hospitals and healthcare professionals, addressing all aspects of diagnosis, patient care, case management, and protection of healthcare workers. Recommendations emphasize the importance of strict adherence to patient isolation and barrier precautions, including proper use of PPE and environmental infection control measures applicable to any healthcare setting.
| Centers for Disease Control and Prevention |
| https://www.cdc.gov/vhf/ebola/clinicians/index.html |
| https://www.cdc.gov/vhf/ebola/laboratory-personnel/index.html |
| World Health Organization |
| https://www.who.int/health-topics/ebola |
| National Institute of Allergy and Infectious Disease |
| https://www.niaid.nih.gov/diseases-conditions/ebola-marburg |
2. Mahanty S, Bray M. Pathogenesis of filoviral haemorrhagic fevers. Lancet Infect Dis. 2004;4:487-498.
3. WHO Response Team. Ebola virus disease in West Africa: the first 9 months of the epidemic and forward projections. N Engl J Med. 2014;371:1481-1495.
5. Briand S, Bertherat E, Cox P, et al. The international Ebola emergency. N Engl J Med. 2014;371:1180-1183.
6. Centers for Disease Control and Prevention. Infection Prevention and Control Recommendations for Hospitalized Patients Under Investigation (PUIs) for Ebola Virus Disease (EVD) in U.S. Hospitals. Available at https://www.cdc.gov/vhf/ebola/clinicians/evd/infection-control.html. Last accessed March 8, 2024.
7. Baize S, Pannetier D, Oestereich L, et al. Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med. 2014;371:1418-1425.
8. Kortepeter MG, Bausch DG, Bray M. Basic clinical and laboratory features of filoviral hemorrhagic fever. J Infect Dis. 2011;204(Suppl 3):S810-S816.
9. Fowler RA, Fletcher T, Fischer WA 2nd, et al. Caring for critically ill patients with Ebola virus disease: perspectives from West Africa. Am J Respir Crit Care Med. 2014;190:733-737.
10. Wilson ME, Weld LH, Boggild A, et al. Fever in returned travelers: results from the GeoSentinal Surveillance Network. Clin Infect Dis. 2007;44:1560-1568.
11. Funk DJ, Kumar A. Ebola virus disease: an update for anesthesiologists and intensivists. Can J Anesth. 2015;62:80-91.
12. Oestereich L, Ludtke A, Wurr S, Reiger T, Munoz-Fontela C, Gunther S. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res. 2014;105:17-21.
13. Centers for Disease Control and Prevention. The 2014–2016 Ebola Outbreak in West Africa. Available at https://www.cdc.gov/vhf/ebola/history/2014-2016-outbreak/index.html. Last accessed March 8, 2024.
14. Edmond MB, Diekema DJ, Perencevich EN. Ebola virus disease and the need for new personal protective equipment. JAMA. 2014;312(23):2495-2496.
15. Gostin LO, Hodge JG, Burris S. Is the United States prepared for Ebola? JAMA. 2014;312(23):2497-2498.
16. Centers for Disease Control and Prevention. Ebola Virus Disease: Screening Patients. Available at https://www.cdc.gov/vhf/ebola/clinicians/evaluating-patients/index.html. Last accessed March 8, 2024.
17. Tambo E, Ugwu EC, Ngogang JY. Need of surveillance response systems to combat Ebola outbreaks and other emerging infectious diseases in African countries. Infect Dis Poverty. 2014;3:29.
18. Qiu X, Wong G, Audet J, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514:47-53.
19. Emond RT, Evans B, Bowen ET, Lloyd G. A case of Ebola virus infection. Br Med J. 1977;2(6086):541-544.
20. Centers for Disease Control and Prevention. Case Definition for Ebola Virus Disease (EVD). Available at https://www.cdc.gov/vhf/ebola/clinicians/evaluating-patients/index.html. Last accessed March 8, 2024.
21. Centers for Disorder Control and Prevention. Guidance for Collection, Transport and Submission of Specimens for Ebola Virus Testing. Available at https://www.cdc.gov/vhf/ebola/laboratory-personnel/specimens.html. Last accessed March 8, 2024.
22. Centers for Disease Control and Prevention. Ebola Virus Disease (EVD) Information for Clinicians in U. S. Healthcare Settings. Available at https://www.cdc.gov/vhf/ebola/clinicians/evd/clinicians.html. Last accessed March 8, 2024.
23. U.S. Food and Drug Administration. First FDA-Approved Vaccine for the Prevention of Ebola Virus Disease, Marking a Critical Milestone in Public Health Preparedness and Response. Available at https://www.fda.gov/news-events/press-announcements/first-fda-approved-vaccine-prevention-ebola-virus-disease-marking-critical-milestone-public-health. Last accessed March 8, 2024.
24. World Health Organization. Interim Infection Prevention and Control Guidance for Care of Patients with Suspected or Confirmed Filovirus Haemorrhagic Fever in Health-Care Settings, with Focus on Ebola. Available at https://www.who.int/publications/i/item/WHO-HIS-SDS-2014.4-Rev.1. Last accessed March 8, 2024.
25. U.S. Food and Drug Administration. FDA Allows Marketing of First Rapid Diagnostic Test for Detecting Ebola Virus Antigens. Available at https://www.fda.gov/news-events/press-announcements/fda-allows-marketing-first-rapid-diagnostic-test-detecting-ebola-virus-antigens. Last accessed March 8, 2024.
26. Shultz JM, Baingana F, Nuria Y. The 2014 Ebola outbreak and mental health: current status and recommended response. JAMA. 2015;313(6):567-568.
27. Henao-Restrepo A, Longini IM, Egger M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster randomised trial. Lancet. 2015;386(9996):857-866.
28. Jamieson DJ, Uyeki TM, Callaghan WM, et al. What obstetrician-gynecologists should know about Ebola: a perspective from the Centers for Disease Control and Prevention. Obstet Gynecol. 2014;124(5):1005.
29. PREVAIL II Writing Group, Multi-National PREVAIL II Study Team, Davey RT Jr, Dodd L, Proschan MA, et al. A randomized, controlled trial of ZMapp for Ebola virus infection. N Engl J Med. 2016;375(15):1448.
30. Centers for Disease Control and Prevention. Interim Guidance for Management of Survivors of Ebola Virus Disease in U.S. Hospital Settings. Available at https://www.cdc.gov/vhf/ebola/clinicians/evaluating-patients/guidance-for-management-of-survivors-ebola.html. Last accessed March 8, 2024.
31. Centers for Disease Control and Prevention. 2014–2016 Ebola Outbreak in West Africa, Cost of the Epidemic. Available at https://www.cdc.gov/vhf/ebola/history/2014-2016-outbreak/cost-of-ebola.html. Last accessed March 8, 2024.
32. Centers for Disease Control and Prevention. Infection Prevention and Control Recommendations for Hospitalized Patients with Known or Suspected Ebola Hemorrhagic Fever in U.S. Hospitals. Available at https://www.cdc.gov/vhf/ebola/clinicians/evd/infection-control.html. Last accessed March 8, 2024.
34. Baseler, L, Chertow DS, Johnson KM, et al. The pathogenesis of Ebola virus disease. Annu Rev Pathol Mech Dis. 2017;12:387-418.
35. U. S. Food and Drug Administration. FDA Approves First Treatment for Ebola Virus. Available at https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-ebola-virus. Last accessed March 8, 2024.
36. Jagadesh S, Sevalie S, Fatoma R, et al. Disability among Ebola survivors and their close contacts in Sierra Leone: a retrospective case-controlled cohort study. Clin Infect Dis. 2018;66:131-133.
37. Deen GF, Broutet N, Xu W, et al. Ebola RNA persistence in semen of Ebola Virus Disease survivors: final report. N Engl J Med. 2017;377:1428-1437.
38. Munster VJ, Bausch DG, de Witt E, et al. Outbreaks in rapidly changing Central Africa: lessons from Ebola. N Engl J Med. 2018;379:1198-1201.
39. Centers for Disease Control and Prevention. Ebola (Ebola Virus Disease) Prevention and Vaccine. Available at https://www.cdc.gov/vhf/ebola/prevention/index.html. Last accessed March 8, 2024.
40. Centers for Disease Control and Prevention. Ebola Vaccine: Information about Ervebo. Available at https://www.cdc.gov/vhf/ebola/clinicians/vaccine/index.html. Last accessed March 8, 2024.
41. Centers for Disease Control and Prevention. Ebola Virus Disease Distribution Map: Cases of Ebola Virus Disease in Africa Since 1976. Available at https://www.cdc.gov/vhf/ebola/history/distribution-map.html. Last accessed March 8, 2024.
42. Erickson B, Rangel C, O'Keefe E. U.S. to Implement Ebola Monitoring Program at Airports as New Cases Reported in Africa. Available at https://www.cbsnews.com/news/ebola-monitoring-program-u-s-considering-as-new-cases-reported-in-africa. Last accessed March 8, 2024.
43. Centers for Disease Control and Prevention. Guidance for Collection, Transport and Submission of Specimens for Ebola Virus Testing. Available at https://www.cdc.gov/vhf/ebola/laboratory-personnel/specimens.html. Last accessed March 8, 2024.
44. Malenfant JH, Joyce A, Choi MJ, et al. Use of Ebola vaccine: expansion of recommendations of the Advisory Committee on Immunization Practices to include two additional populations—United States, 2021. MMWR. 2022;71(8):290-292.
45. Centers for Disease Control and Prevention. Ebola Disease: History of Ebola Disease Outbreaks. Available at https://www.cdc.gov/vhf/ebola/history/chronology.html. Last accessed March 8, 2024.
46. Centers for Disease Control and Prevention. Ebola Disease: Transmission. Available at https://www.cdc.gov/vhf/ebola/transmission/index.html. Last accessed March 8, 2024.
47. U.S. Food and Drug Administration. Ebola Zaire Vaccine, Live (ERVEBO), Product Information. Available at https://www.fda.gov/vaccines-blood-biologics/ervebo. Last accessed March 8, 2024.
48. National Institute of Allergy and Infectious Diseases. News Releases: Ebola Vaccine Regimens Safe, Immunogenic in Adults and Children. NIAD Participated in International PREVAC Consortium, December 14, 2022. Available at https://www.niaid.nih.gov/news-events/ebola-vaccine-regimens-safe-immunogenic-adults-and-children. Last accessed March 8, 2024.
1. Lamontagne F, Fowler RA, Adhikari NK, et al. Evidence-based guidelines for supportive care of patients with Ebola virus disease. Lancet. 2018;391(10121):700-708. Available at https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(17)31795-6/fulltext. Last accessed March 21, 2024.
Mention of commercial products does not indicate endorsement.