Among the male-specific conditions addressed in this course are prostate disease (prostatitis, benign prostatic hypertrophy, and cancer), testicular conditions (testicular torsion, epididymitis, varicocele), premature ejaculation, erectile dysfunction, late-onset hypogonadism, infertility, and sexually transmitted infections (STIs). Prostate cancer is discussed in considerable detail. Prostate screening is among the most controversial issues in health care today, and the issue is explored here, with recommendations for how to discuss screening with male patients. Also included are brief overviews of male breast cancer, a rare disease but one that is rising in prevalence, and health issues of specific concern for men who have sex with men (MSM), a growing population in the primary care setting. The psychosocial well-being of men is integral to overall health. The link between anger and stress and disease is mentioned, as is the major role substance abuse has in mortality and morbidity. Alcohol misuse and depression have both been underdiagnosed in men, especially older men, and strategies for screening are explored. The course closes with suggestions for fostering enhanced healthy behaviors among men, with recommendations for reaching out to men, ensuring appropriate health screening, and encouraging healthy behaviors.
- INTRODUCTION
- OVERVIEW OF MEN'S HEALTH ISSUES
- DISEASES AND CONDITIONS OF THE PROSTATE
- DISEASES AND CONDITIONS OF THE TESTES
- MALE BREAST CANCER
- MALE SEXUAL HEALTH
- HEALTH ISSUES FOR MEN WHO HAVE SEX WITH MEN
- HEALTH ISSUES FOR TRANSMEN
- PSYCHOSOCIAL WELL-BEING OF MEN
- FOSTERING ENHANCED HEALTH BEHAVIORS IN MEN
- CONCLUSION
- Works Cited
- Evidence-Based Practice Recommendations Citations
This intermediate course is designed for psychologists involved in treating physical and mental conditions in men.
Continuing Education (CE) credits for psychologists are provided through the co-sponsorship of the American Psychological Association (APA) Office of Continuing Education in Psychology (CEP). The APA CEP Office maintains responsibility for the content of the programs.
The purpose of this course is to provide psychologists with necessary information regarding conditions and health issues that affect men in order to facilitate more effective diagnosis, treatment, and care. As male-specific factors influence the provision and compliance to therapy, tools to ensure effective patient education for men are provided to increase the likelihood of positive outcomes.
Upon completion of this course, you should be able to:
- Identify diseases that are more prevalent among men than among women.
- Describe the health implications of male gender identity and identify strategies to improve communication with male patients.
- Explain the diagnosis and treatment of benign prostate conditions and prostate cancer.
- Apply guideline recommendations for prostate cancer screening.
- Describe treatment options and assist patients in selecting a management strategy for localized prostate cancer.
- Distinguish among benign testicular conditions.
- Discuss the diagnosis and treatment options for testicular cancer.
- Discuss the differences between male and female breast cancer.
- Discuss diagnosis and treatment options, and assist patients in selecting a treatment strategy for sexual dysfunction (premature ejaculation and erectile dysfunction).
- Devise a strategy for diagnostic testing and treatment of late-onset hypogonadism.
- List factors affecting male infertility.
- Promote patient education and disease prevention, implement effective screening, and select guideline-appropriate treatment of sexually transmitted infections.
- Identify issues of particular concern for men who have sex with men.
- Discuss the effects of substance misuse, depression, and stress/anger on the physical and psychosocial well-being of men.
- Discuss the importance of educating men about the need for screening, routine health maintenance, and healthy lifestyle.
Lori L. Alexander, MTPW, ELS, MWC, is President of Editorial Rx, Inc., which provides medical writing and editing services on a wide variety of clinical topics and in a range of media. A medical writer and editor for more than 30 years, Ms. Alexander has written for both professional and lay audiences, with a focus on continuing education materials, medical meeting coverage, and educational resources for patients. She is the Editor Emeritus of the American Medical Writers Association (AMWA) Journal, the peer-review journal representing the largest association of medical communicators in the United States. Ms. Alexander earned a Master’s degree in technical and professional writing, with a concentration in medical writing, at Northeastern University, Boston. She has also earned certification as a life sciences editor and as a medical writer.
John M. Leonard, MD, Professor of Medicine Emeritus, Vanderbilt University School of Medicine, completed his post-graduate clinical training at the Yale and Vanderbilt University Medical Centers before joining the Vanderbilt faculty in 1974. He is a clinician-educator and for many years served as director of residency training and student educational programs for the Vanderbilt University Department of Medicine. Over a career span of 40 years, Dr. Leonard conducted an active practice of general internal medicine and an inpatient consulting practice of infectious diseases.
Contributing faculty, Lori L. Alexander, MTPW, ELS, MWC, has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Contributing faculty, John M. Leonard, MD, has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
James Trent, PhD
The division planner has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Sarah Campbell
The Director of Development and Academic Affairs has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
The purpose of NetCE is to provide challenging curricula to assist healthcare professionals to raise their levels of expertise while fulfilling their continuing education requirements, thereby improving the quality of healthcare.
Our contributing faculty members have taken care to ensure that the information and recommendations are accurate and compatible with the standards generally accepted at the time of publication. The publisher disclaims any liability, loss or damage incurred as a consequence, directly or indirectly, of the use and application of any of the contents. Participants are cautioned about the potential risk of using limited knowledge when integrating new techniques into practice.
It is the policy of NetCE not to accept commercial support. Furthermore, commercial interests are prohibited from distributing or providing access to this activity to learners.
Supported browsers for Windows include Microsoft Internet Explorer 9.0 and up, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Supported browsers for Macintosh include Safari, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Other operating systems and browsers that include complete implementations of ECMAScript edition 3 and CSS 2.0 may work, but are not supported. Supported browsers must utilize the TLS encryption protocol v1.1 or v1.2 in order to connect to pages that require a secured HTTPS connection. TLS v1.0 is not supported.
The role of implicit biases on healthcare outcomes has become a concern, as there is some evidence that implicit biases contribute to health disparities, professionals' attitudes toward and interactions with patients, quality of care, diagnoses, and treatment decisions. This may produce differences in help-seeking, diagnoses, and ultimately treatments and interventions. Implicit biases may also unwittingly produce professional behaviors, attitudes, and interactions that reduce patients' trust and comfort with their provider, leading to earlier termination of visits and/or reduced adherence and follow-up. Disadvantaged groups are marginalized in the healthcare system and vulnerable on multiple levels; health professionals' implicit biases can further exacerbate these existing disadvantages.
Interventions or strategies designed to reduce implicit bias may be categorized as change-based or control-based. Change-based interventions focus on reducing or changing cognitive associations underlying implicit biases. These interventions might include challenging stereotypes. Conversely, control-based interventions involve reducing the effects of the implicit bias on the individual's behaviors. These strategies include increasing awareness of biased thoughts and responses. The two types of interventions are not mutually exclusive and may be used synergistically.
#63764: Men's Health Issues
There are many reasons to be concerned about health issues that are unique to or more common in men. In 1900, women outlived men by an average of two years; that gap widened to seven years in 1970 through 1990 [1]. Advances in diagnosis and treatment, as well as heightened awareness of disparities in men's and women's health, led to a narrowing of the gap to slightly less than five years in 2014 [1]. Still of concern, however, is the high number of men's deaths that are potentially avoidable. Many factors contribute to the disparity in mortality and morbidity between men and women, but the factor thought to have the most significant impact on the health of men relates to male gender identity, including a tendency for risky behavior [2,3,4,5].
The concept of men's health was established to focus on the high rates of morbidity and mortality. Thus, men's health encompasses both male-specific conditions, such as those related to the prostate, as well as diseases that affect men at a higher rate compared with women. A discussion of all diseases that affect men is beyond the scope of this course. However, the leading causes of death among men are presented and discussed in the context of how they compare with the causes of death in women.
Among the male-specific conditions addressed are prostate disease (e.g., prostatitis, benign prostatic hypertrophy [BPH], cancer), testicular conditions (e.g., testicular torsion, epididymitis, varicocele, cancer), premature ejaculation, erectile dysfunction, late-onset hypogonadism, infertility, and sexually transmitted infections (STIs). Prostate cancer is discussed in considerable detail. Prostate screening and treatment have been controversial issues in health care, and the most recent recommendations for how to discuss screening and treatment options are included. Also provided are brief overviews of male breast cancer, a rare disease but one that is rising in prevalence, and health issues of specific concern for men who have sex with men (MSM), a growing population seen in the primary care setting.
The psychosocial well-being of men is integral to overall health. The link between anger and stress and disease is mentioned, as is the major role of substance misuse in mortality and morbidity. Alcohol misuse and depression have both been underdiagnosed in men, especially older men, and strategies for screening are explored.
The course closes with suggestions for fostering enhanced healthy behaviors among men, with recommendations for reaching out to men, ensuring appropriate health screening, and encouraging healthy behaviors.
The concept of men's health emerged in response to the documented trends in greater mortality rates for men compared with women. Over the past decade, attention to the causes of death and disease in men has increased, and a growing body of scientific literature has begun to elucidate gender differences in physiologic, psychologic, and sociologic aspects of disease. These differences have a strong influence on the health of men as well as on the response to treatment and health behaviors.
Men's health lacks the same type of clinical focus as women's health; that is, men's health does not have the equivalent of a specialist (gynecologist) to provide care for the reproductive tract. Care of the male reproductive tract is assumed by primary care physicians, urologists, endocrinologists, reproductive specialists, and possibly, oncologists. The discipline of andrology is in its early stages, and some have proposed that this discipline should be expanded beyond the reproductive tract to include all men's health issues, with a goal of developing appropriate training programs and establishing a distinct specialty [6]. Men's health programs at large academic centers as well as free-standing centers in large cities are providing multidisciplinary diagnostic and management services targeted to men.
As defined by most organizations around the world, the field of men's health encompasses a broad range of health issues, including diseases that are more prevalent among men than women or that differ with regard to risk factors, diagnosis, and treatment. Men's health also addresses the psychologic and social influences on men and acknowledges the need to model healthier attitudes beginning in boyhood.
Several initiatives have helped to promote awareness of men's health among the public, policy arena, and scientific community, including establishment of the Men's Health Network, a nonprofit organization based in Washington, DC, and targeted peer-review journals such as the Journal of Men's Health and the American Journal of Men's Health.
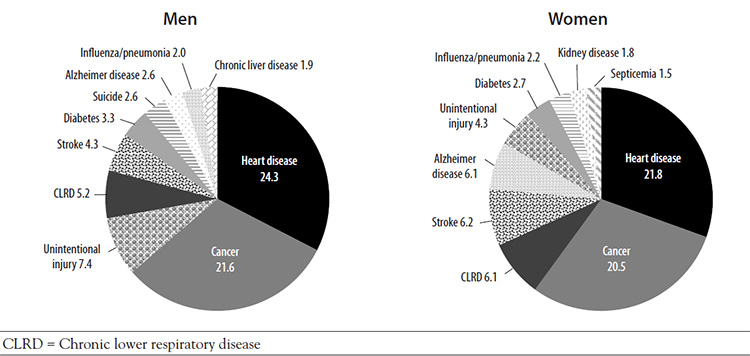
In general, the leading causes of death among men and women are the same; what differs are the age at the time of death, the number of deaths caused by each disease, and the ranking of the causes (Figure 1) [7,8]. The overall death rate in 2019 was higher for male than female individuals (all ages) (846.7 vs. 602.7 per 100,000) [9,10]. Cardiovascular disease and cancer are the two leading causes of death for both men and women, but a greater percentage of men die of each cause [9,10]. Deaths related to cardiovascular disease and cancer account for approximately 46% of the total number of deaths among all men [7]. In 2019, the death rate from Alzheimer disease was 30% lower among men than women; the death rates from cerebrovascular diseases, influenza/pneumonia, and chronic lower respiratory diseases were approximately the same for each biologic sex [7,8]. The causes of death differ within the male population according to age and race/ethnicity, highlighting disparities related to socioeconomic status, cultural differences, access to care, and possibly, genetic predisposition for specific diseases (Table 1) [11].
TEN LEADING CAUSES OF DEATH FOR MEN ACCORDING TO RACE/ETHNICITY, 2018
| Leading Causes of Death | Mortality Rate and Rank | |||||
|---|---|---|---|---|---|---|
| All Men | White | Black/African American | Hispanic/Latino | Asian/Pacific Islander | American Indian/Alaskan Native | |
| Cardiovascular diseases | 24.4% (1) | 24.8% (1) | 24.1% (1) | 20.2% (1) | 23.1% (2) | 18.9% (1) |
| Cancer | 22.2% (2) | 22.2% (2) | 19.7% (2) | 19.4% (2) | 24.7% (1) | 15.9% (2) |
| Unintentional injuries | 6.8% (3) | 6.9% (3) | — | 11.3% (3) | 5.3% (4) | 13.7% (3) |
| Chronic lower respiratory diseases | 5.3% (4) | 5.8% (4) | 3.2% (7) | 3.3% (6) | 3.2% (6) | 3.6% (7) |
| Stroke | 4.2% (5) | 4.1% (5) | 5.0% (4) | 4.7% (4) | 6.7% (3) | 2.9% (8) |
| Diabetes mellitus | 3.1% (6) | 2.9% (6) | 4.4% (6) | 4.2% (5) | 4.2% (5) | 5.7% (5) |
| Suicide | 2.5% (7) | 2.7% (8) | — | 3.1% (8) | 2.6% (8) | 4.2% (6) |
| Alzheimer disease | 2.5% (8) | 2.9% (7) | 7.9% (3) | 2.3% (9) | 2.3% (9) | — |
| Influenza and pneumonia | 2.0% (9) | 2.0% (9) | 1.7% (10) | 3.2% (7) | 3.2% (7) | 2.2% (10) |
| Chronic liver disease | 1.9% (10) | 1.7% (10) | — | 4.1% (6) | — | 6.1% (4) |
| Assault (homicide) | — | — | 4.5% (5) | 2.2% (10) | — | 2.3% (9) |
| Kidney disease | — | — | 2.7% (8) | — | 2.0% (10) | — |
| Septicemia | — | — | 1.7% (9) | — | — | — |
Review of the leading causes of death demonstrates that many men's deaths are potentially avoidable. Most notable is the third leading cause of death for all men: unintentional injuries [11]. Unintentional injuries cause substantially more deaths among men than women, for whom it is the sixth leading cause of death [12]. Suicide is the eighth leading cause of death among all men; this cause of death is not included in the top 10 causes for women. In addition, homicide is among the ten leading causes of death for Black, Hispanic/Latino, and American Indian/Alaska Native men [13,14,15]. Several of the other leading causes of death among men are associated with chronic diseases, for which modification of risk factors and early detection can improve outcomes.
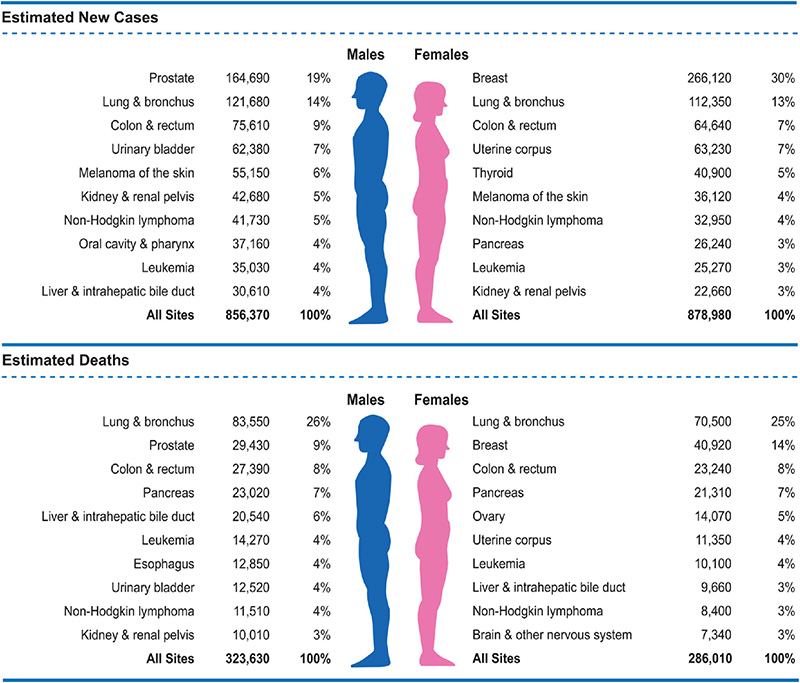
Gender differences exist in the prevalence of specific cancers and in deaths related to cancers [16]. The lifetime probability of being diagnosed with invasive cancer is higher for men than women (Table 2) [16]. The rate of deaths associated with cancer of the colon/rectum, urinary bladder, esophagus, and liver and intrahepatic bile duct are higher among men than among women (Figure 2) [16]. Although prostate cancer is the most prevalent cancer in men and receives widespread attention, lung cancer is responsible for a greater percentage of cancer-related deaths among men (23% vs. 11%) [16].
An increasing amount of research is supporting a relationship between men's risk for disease and death and male gender identity, and the traditional male role has been shown to conflict with the fostering of healthy behaviors [4,17]. Male gender identity is related to a tendency to take risks, and the predilection for risky behavior begins in boyhood [17,18,19]. In addition, boys are taught that they should be self-reliant and independent and should control their emotions, and societal norms for both boys and men dictate that they maintain a strong image by denying pain and weakness [4,18,19].
Issues related to male gender identity have several important implications for health. First, risky behavior is associated with increased morbidity and mortality. Second, the concept of masculinity leads to inadequate help- and information-seeking behavior and a reduced likelihood to engage in behavior to promote health [4,18,19]. These behaviors appear to be rooted in a decreased likelihood for men to perceive themselves as being ill or at risk for illness, injury, or death [4]. Third, male gender identity, coupled with lower rates of health literacy, creates special challenges for effectively communicating health messages to men [5,20,21]. Gender differences in health-related behaviors are consistent across racial/ethnic populations, although specific behaviors vary according to race/ethnicity [17].
Risky behavior affects health and well-being beginning at a young age. The overall rate of fatal injuries is approximately two times higher among boys than girls (0 to 19 years of age) [22]. Motor vehicle accidents are the leading cause of death for both genders, especially in the age category of teenage drivers (15 to 19 years of age). Although not all of these injuries and deaths are related to risky behavior, Youth Risk Behavior Surveillance (YRBS) data indicate that many of them are related; other risky behaviors identified in this survey are related to morbidity and mortality in adolescence and are also contributors to habits that affect health in adulthood. The 2019 YRBS showed that the rate of risky behaviors is predominantly higher among male respondents (Table 3) [23]. The rates of many of these behaviors continued to be higher among male adults (Table 4), which plays a role in premature deaths among men [1,24].
COMPARISON OF RISKY BEHAVIORS IN YOUTH (9th THROUGH 12th GRADES)
| Behavior | Male Respondents | Female Respondents |
|---|---|---|
| Did not always wear a seat belt | 43.3% | 42.7% |
| Rode with a driver who had been drinking alcohol | 15.6% | 17.5% |
| Texted or e-mailed while driving | 39.6% | 38.4% |
| Drove after drinking alcohol | 7.0% | 3.6% |
| Carried a weapon (gun, knife, or club) | 19.5% | 6.7% |
| Was in a physical fight in the previous 12 months | 28.3% | 15.3% |
| Currently smoke cigarettes daily | 6.9% | 4.9% |
| Currently use smokeless tobacco | 5.8% | 1.6% |
| Currently use electronic vapor product (e-cigarettes, e-cigars, e-pipes, vape pipes, vaping pens, e-hookahs, hookah pens) | 32.0% | 33.5% |
| Had >5 drinks of alcohol within a couple of hours on >1 of the previous 30 days | 12.7% | 14.6% |
| Ever used marijuana | 37.0% | 36.5% |
| Drove after using marijuana | 14.6% | 11.3% |
| Ever misused prescription opioids | 12.4% | 16.1% |
| Ever used cocaine | 4.9% | 2.7% |
| Ever used heroin | 2.3% | 1.0% |
| Ever used methamphetamines | 2.7% | 2.7% |
RISKY BEHAVIOR AMONG ADULTS
| Behaviorsa | Men | Women |
|---|---|---|
| Non-seat belt use | 11.6% | 7.2% |
| "Heavy" drinking (five or more drinks on the same occasion on at least five days of the last month) | 8.2% | 4.0% |
| Five drinks or more in a day at least one day within the previous month | 28.5% | 20.7% |
| Current smoking | 15.6% | 12.0% |
| Use of illicit drugsa | ||
| Any illicit drug (past month) | 14.0% | 9.5% |
| Cannabis (past month) | 12.3% | 8.0% |
| Psychotherapeutic drug (nonmedical use in past month) | 2.1% | 1.9% |
| aData for behaviors are based on individuals 18 years of age and older; the data on use of illicit drugs are based on individuals who were 12 years of age and older. | ||
Men's predilection for risky behavior is reflected in the high rate of unintentional injury, which accounts for 7.4% of deaths among men (compared with 4.3% for women) [7,8]. There is wide variation in this rate across race/ethnicity, with much higher rates among American Indian/Alaska Native men (13.7%) and Hispanic/Latino men (11.3%) [11]. The trend of more fatal unintentional injuries among men is evident in countries around the world; an analysis of accidental deaths among men and women in 36 countries showed higher rates for men [2]. Across all age-groups, the rates were higher in the United States than the median rate for all countries. Accidental deaths are related primarily to motor vehicle injuries, violence, and occupation, and the rates in all categories are higher for men than for women. The rate of death related to motor vehicle injuries for men is slightly higher than for women (16.0 vs. 6.3 per 100,000), and the percentage of fatal unintentional firearm-related injuries deaths occur overwhelmingly more often among men (82.7%) than women (17.3%) [25]. Similarly, fatal occupational injuries occur predominantly in men (57% vs. 6%) [26].
Substance misuse plays a significant role in both risky behavior and the development of chronic diseases. As demonstrated by the YRBS data, the use of tobacco, alcohol, and illicit drugs begins in the teenage years, with more boys than girls engaging in such behavior [23]. One exception appears to be prescription opioids, which are more likely to be misused by female adolescents than male adolescents. Among adults, substance misuse continues to be more prevalent among men than women [27]. Misuse of tobacco, alcohol, and drugs are associated with high rates of unintentional injuries, violence, STIs, and masking of depression [25,28,29,30].
The rate of tobacco use among men has declined over the past decade, but the rate continues to be higher than that among women [31]. The Centers for Disease Control and Prevention (CDC) estimates that men who smoke increase their risk of death from lung cancer by 25 times, with tobacco being the cause of approximately 90% of all lung cancer deaths in men [32]. In addition, smoking is a significant risk factor for many cancers, especially those that are more prevalent among men, and is linked to a two to four times greater likelihood of cardiovascular disease or stroke [32].
Excessive alcohol use is the third leading lifestyle-related cause of death for both men and women, and long-term use of alcohol is a well-recognized contributor to several chronic diseases [33]. Even consumption that is considered to be less than "hazardous" (three to five drinks per day) has been associated with increased morbidity and mortality [34].
Help- and information-seeking behavior related to male gender identity is another factor that affects men's health. In general, men are reluctant to seek health care or talk about their health because they see such help-seeking as a sign of weakness or vulnerability and a threat to their masculinity [4,35,36]. These reports are substantiated by data on utilization of healthcare resources, which indicate that men have fewer office visits to doctors or other health care professional than women; in 2018, 23.9% of men had no office visits, compared with 12.5% of women [37]. In addition, men are more likely to lack a usual source of health care (18.6% vs. 10.7%) [37]. Men have reported several reasons for not having a usual source of care, and the reasons vary among racial/ethnic populations [39]. The reason given most often is that they are seldom or never sick, and this may be related to men's perceptions of invulnerability [39,40]. Other reasons given include not finding time and not being able to take time away from work [38]. Cultural values, such as machismo, lead many Hispanic men to avoid health care until there is no other choice [40]. This may contribute to the low rate of healthcare use among Hispanic men, which is the lowest across racial/ethnic populations [40]. Other reasons for the low use of healthcare services among Hispanic men are lack of health insurance, low understanding of the healthcare system, fear of poor functional outcomes, and a low perception of the quality of the patient-clinician interaction [40]. In the Black population, men have reported to avoid healthcare services because of fears and concerns about their negative health behaviors and history [41].
Lower rates of healthcare use among men have a negative impact on preventive care, and rates of routine health assessments and recommended vaccinations and screening procedures have been lower among men than among women [42]. Several factors contribute to the avoidance of screening tests, including men's belief that they are healthy; their focus on their present, rather than future, health; the need for more information about the screening procedure; and other issues related to masculinity [42]. For example, Black men have reported avoiding screening for prostate and colorectal cancer because they see these procedures as "violating their manhood" [41,43].
Among men who do have physician office visits, many are not forthcoming about symptoms or information they seek [44]. Because of their traditional discomfort with expressing feelings and emotions, they are less likely to seek help for psychosocial problems or emotional symptoms [17,45]. Men tend to be more motivated to seek health care for male-oriented conditions, such as erectile dysfunction or sports-related injuries, or when their health or symptoms interfere with their routine activities [45].
Effective communication is essential in the healthcare setting but can be challenged by several factors. Specific challenges in communicating with men are related to male gender identity as well as to low health literacy and language and cultural barriers.
Male Gender Identity
Men's beliefs about masculinity and traditional male roles affect health communication, and healthcare practitioners should consider male-specific beliefs and perceptions when communicating with male patients. For example, because men tend to focus on present rather than future health, concepts of fear, wellness, and longevity often do not work well in health messages [40]. Instead, healthcare practitioners should focus more on "masculine" concepts, such as strength, safety, and performance, all of which tie into men's perceptions of their roles as providers and protectors. To address men's reluctance to admit pain, practitioners should avoid asking questions such as "Do you have pain?" and instead use phrases such as "Most men I see with this condition say they have quite a bit of pain—what about you?" Using numbers/statistics and metaphors relating the body to a machine may also help to communicate effectively by addressing male gender identity. In addition, practitioners should be nonjudgmental about their male patients' health and risk behaviors and develop open lines of communication to encourage them to express their health concerns.
Health Literacy, Language, and Culture
According to the National Assessment of Health Literacy, 14% of individuals in the United States have "below basic" health literacy, which means they lack the ability to understand health information and make informed health decisions [21,46]. The findings of the assessment demonstrated that the rate of "below basic" literacy was higher among men than women (16% vs. 12%) [21]. Although the rate of "basic" health literacy was similar for men and women, rates of "intermediate" and "proficient" health literacy were lower for men [21]. Similar rates of health literacy have been found in subsequent studies, with rates of adequate health literacy consistently lower among men and even lower among non-White men [47,48]. In one study, the rate of adequate health literacy was 48% among White men (compared with 63% among White women) and 23% among non-White men (compared with 30% among non-White women) [48].
Recognition of the importance of adequate health literacy to good health outcomes has led to assessment of health literacy being deemed "the newest vital sign," with development of an assessment tool by that name [48,49]. The Newest Vital Sign (NVS) tool has been shown to demonstrate the health literacy status in fewer than three minutes, with results that are comparable to those of more extensive literacy tests [48]. Clinicians are encouraged to use this tool to assess the literacy of their patients, especially those of racial/ethnic minorities, and to adapt discussions to literacy levels and provide low-literacy educational resources. Compounding health literacy are language and cultural barriers, which have the potential for far-reaching effects, given the growing percentages of racial/ethnic populations. According to U.S. Census Bureau data from 2020, 21.5% of the American population speak a language other than English, and of those, 8.2% speak English less than "very well" [50]. Clinicians should ask their patients what language they prefer for their medical care information, as some individuals prefer their native language even though they have said they can understand and discuss symptoms in English [51]. Translation services should be provided for patients who do not understand the clinician's language. "Ad hoc" interpreters (family members, friends, bilingual staff members) are often used instead of professional interpreters for a variety of reasons, including convenience and cost. However, clinicians should check with their state's health officials about the use of ad hoc interpreters, as several states have laws about who can interpret medical information for a patient [52]. Even when allowed by law, the use of a patient's family member or friend as an interpreter should be avoided, as the patient may not be as forthcoming with information and the family member or friend may not remain objective [52]. Children should especially be avoided as interpreters, as their understanding of medical language is limited and they may filter information to protect their parents or other adult family members [52]. Individuals with limited English language skills have actually indicated a preference for professional interpreters rather than family members [53].
Most important, perhaps, is the fact that clinical consequences are more likely with ad hoc interpreters than with professional interpreters [54]. A systematic review of the literature showed that the use of professional interpreters facilitates a broader understanding and leads to better clinical care than the use of ad hoc interpreters, and many studies have demonstrated that the lack of an interpreter for patients with limited English proficiency compromises the quality of care and that the use of professional interpreters improves communication (errors and comprehension), utilization, clinical outcomes, and patient satisfaction with care [55,56].
Clinicians should use plain language in their discussions with their patients who have low literacy or limited English proficiency. They should ask them to repeat pertinent information in their own words to confirm understanding, and reinforcement with the use of low-literacy or translated educational materials may be helpful.
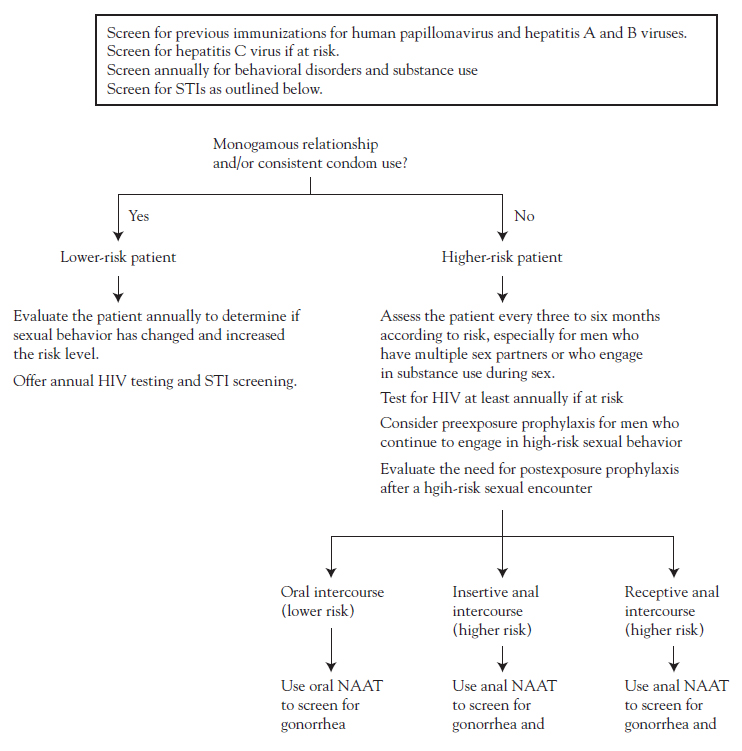
Among male-specific disorders, prostatic conditions are perhaps of most concern to men and have raised the most questions in the healthcare community about diagnosis, screening, and treatment. Sexual health issues, such as premature ejaculation and erectile dysfunction, are also of substantial concern to men, and treatments for these conditions gained increased attention beginning in the late 1990s. The prevalence of many STIs is on the rise, especially among younger men, posing a significant public health problem [57]. Infertility is an issue for many younger men, and interest in late-onset hypogonadism has increased, primarily because of the debate about the use of testosterone replacement therapy. Much attention has also been focused on the unique healthcare needs of a minority population—MSM. (This term has become preferred as a more accurate description because of the variation in how such men identify themselves sexually [58].) Another minority population is that of men with breast cancer, a disease that has become more prevalent since the 1980s. The diseases and conditions noted here by no means represent all of those related to the health care of men. Topics were chosen on the basis of their impact on the overall health of men and the implications for care.
Primary care and family medicine physicians and other general healthcare providers are at the forefront of managing all of these male-specific conditions. Consultation with and referral to specialists, such as urologists, endocrinologists, reproductive specialists, and oncologists, should be carried out as appropriate, and follow-up should be continued with the primary healthcare provider.
Prostate tissue undergoes changes as men age, and as such, prostatic conditions predominantly occur in older men. The three primary problems related to the prostate are prostatitis, BPH, and prostate cancer. These conditions can be challenging to diagnose because lower urinary tract symptoms, such as frequency, urgency, and dysuria, can be associated with all three conditions. Furthermore, the most serious of the prostate conditions—prostate cancer—usually produces no symptoms in the early stage of the disease. In addition to the diagnostic challenge created by similar, or no, symptoms, the interpretation of prostate-specific antigen (PSA) levels is difficult, and decisions regarding who and when to screen for prostate cancer are not easy.
Inflammation of the prostate is classified into four categories according to a system developed by the National Institutes of Health (NIH) International Prostatitis Collaborative Network [59]. These categories are:
Acute bacterial prostatitis
Chronic bacterial prostatitis
Chronic prostatitis (nonbacterial)/chronic pelvic pain syndrome (subcategorized as A [inflammatory] and B [noninflammatory])
Asymptomatic inflammatory prostatitis
Both acute and chronic bacterial prostatitis occur in approximately 5% to 10% of men with symptoms related to prostatitis. Chronic nonbacterial prostatitis/chronic pelvic pain syndrome is the most common type, occurring in approximately 90% of symptomatic men [60]. These three types of prostatitis are addressed here; asymptomatic inflammatory prostatitis is an incidental finding during evaluation of another genitourinary condition such as prostate cancer or infertility [61].
It has been estimated that prostatitis accounts for approximately 2 million outpatient visits per year in the United States, with a direct cost of care of nearly $4,000 per patient per year [61]. The condition can have a substantial impact on the quality of life, causing pain and sexual dysfunction, as well as decreased libido and erectile and ejaculatory dysfunction [62,63].
Chronic prostatitis/chronic pelvic pain syndrome has the greatest impact on the quality of life of all types of prostatitis. Studies have found that the effect of chronic pelvic pain syndrome on the quality of life is similar to that of angina, congestive heart failure, diabetes mellitus, and Crohn disease [61]. Symptoms fluctuate over time; one study showed that 43% of men had symptoms within 11 months of follow-up, and another showed that 31% of men had moderate or marked improvement during two years of follow-up [64,65]. Chronic prostatitis/chronic pelvic pain syndrome also causes patient anxiety at the initial visit. Most men with symptoms worry that they have an infection (71%) or cancer (68%), and concerns at one-year follow-up have included worsening symptoms without treatment, cancer, infection, and need for surgery [65]. These concerns have led to an increased number of physician visits [65].
The prevalence of prostatitis has been reported to be approximately 8%, ranging from about 2% to 10% [66]. In patients younger than 35 years of age, the most common variant of the syndrome is acute bacterial prostatitis. Among older patients, nonbacterial prostatitis (NIH types II and IV) is the most common [67]. The results of studies have suggested that the symptoms of prostatitis increase the risk for BPH, lower urinary tract symptoms, and prostate cancer [66].
The cause of acute and chronic bacterial prostatitis is usually lower urinary tract infection with gram-negative organisms, most notably Escherichia coli [60,61]. Most men with prostatitis, however, have no evidence of urinary tract infection [61]. Other causes may include a primary voiding dysfunction problem; presence of Chlamydia trachomatis, Ureaplasma species, or Trichomonas vaginalis; uncommon organisms (e.g., Mycobacterium tuberculosis); HIV; cytomegalovirus; and inflammatory conditions (e.g., sarcoidosis) [67].
The risk factors for prostatitis have not been clearly defined. In a study of 463 men with chronic prostatitis/chronic pelvic pain and 121 asymptomatic age-matched controls, the lifetime prevalence of several self-reported medical conditions were significantly greater among men with prostatitis, specifically neurologic disease (41% vs. 14%); hematopoietic, lymphatic, or infectious disease (41% vs. 20%); psychiatric conditions (29% vs. 11%); nonspecific urethritis (12% vs. 4%); and cardiovascular disease (11% vs. 2%) [68]. The authors of that study noted that more research is needed to determine if such conditions contribute to the pathogenesis of chronic prostatitis/chronic pelvic pain. A history of STIs has been noted to be associated with an increased risk for prostatitis symptoms [66].
Several other urogenital conditions should be considered in the differential diagnosis of prostatitis, including BPH, cystitis, erectile dysfunction, prostate cancer, STI, and urolithiasis [69,70,353]. Of the four types of prostatitis, acute bacterial prostatitis is the easiest to diagnose and treat. Patients with acute prostatitis present with irritative symptoms (dysuria, urinary frequency, and urgency), and obstructive voiding symptoms (hesitancy, incomplete voiding, straining to urinate); the syndrome may also include signs of systemic infection, such as chills and fever [70,353]. Pain most commonly occurs in the prostate/perineum and scrotum and/or testes; pain referred to the penis or lower back also occurs [70]. Urine samples should be cultured to determine the causative micro-organism.
Chronic bacterial prostatitis is distinguished from acute disease by time, being defined by persistence of symptoms for at least three months, and systemic symptoms are usually absent [58,70]. The condition should be suspected when the patient's history includes recurrent urinary tract infections, usually with the same bacterial strain [61]. The patient should complete an NIH Chronic Prostatitis Symptom Index to obtain a baseline score for the severity of symptoms [59]. This index includes questions related to three domains—pain, urinary symptoms, and quality-of-life impact—and has been shown to be a valid, reliable tool for measuring prostatitis symptoms [70,71]. Computed tomography (CT) can determine if there are structural or functional abnormalities of the urinary tract [60,61].
The diagnostic evaluation for acute or chronic bacterial prostatitis includes a urinalysis and urine culture [61,70]. When acute prostatitis is suspected, digital rectal exam should be performed gently so as not to precipitate bacteremia and sepsis. The prostate will usually be enlarged, boggy, and tender, though absence of tenderness on initial examination does not exclude the diagnosis of prostatitis. There are no standardized criteria for the diagnosis of chronic prostatitis/chronic pelvic pain syndrome [61,69]. The Meares-Stamey four-glass test was developed in the late 1960s to screen for prostatitis; the test involves collecting urine samples before and after prostatic massage, as well as collecting prostatic fluid during the massage [72]. Cultures are done on the specimens, and the presence of micro-organisms in the prostatic fluid indicates chronic prostatitis [61,72]. The accuracy and reliability of the test has not been established, and studies have shown that the test is not used often, even by urologists [61,69]. There is also a two-glass version of the test that has correlated well with the four-glass version, but that, too, is not often used [61]. The Meares-Stamey test is not helpful for diagnosing chronic pelvic pain syndrome. Men who have substantial lower urinary tract symptoms and pelvic pain may be candidates for urodynamic evaluation, as voiding dysfunction is common in such cases [61].
No U.S.-based guidelines have been developed, to date, for the treatment of prostatitis, but the European Association of Urology included recommendations for the treatment of prostatitis in its 2008 guidelines on the management of urinary and male genital tract infections [70]. Most patients with bacterial prostatitis can be managed as outpatients with oral antibiotics (e.g., a fluoroquinolone or trimethoprim-sulfamethoxazole) and close follow-up. Hospitalization and broad-spectrum parenteral antibiotics (e.g., piperacillin/tazobactam or ceftriaxone plus ciprofloxacin) should be considered in patients who are systemically ill, are unable to urinate voluntarily, or have risk factors for antimicrobial resistance [70,353]. An aminoglycoside may be added to any of these antibiotics as initial therapy [70]. A fluoroquinolone is the preferred choice for oral therapy because of the spectrum of antibacterial activity and good penetration into prostatic tissue. Duration of antibiotic treatment should be individualized in relation to duration of symptoms and clinical response; 10 to 14 days will suffice for most acute cases of prostatitis, but 21 to 28 days may be required for those with a more subacute onset or slow resolution of symptoms.
For chronic bacterial prostatitis, the choice of antibiotic depends on the sensitivity of the micro-organism, and the antibiotic should be one that penetrates the prostate [61]. The typical first-line treatment is a four- to six-week course of a fluoroquinolone, and treatment is usually more effective if begun soon after symptoms begin [61,70,73,74]. Trimethoprim-sulfamethoxazole may also be considered [70].
Treatment for chronic prostatitis/chronic pelvic pain syndrome is complex; evidence on the effect of traditional treatment options has been conflicting, and treatment options are often not effective in managing symptoms. The most commonly studied pharmacologic options are antibiotics, alpha-blockers, anti-inflammatory agents, steroid inhibitors, and muscle relaxants, and often, a combination of these agents provides the most effective management [74]. Antibiotics, particularly fluoroquinolones, have improved symptoms, even in some patients in whom a bacterial cause has not been identified [74]. Studies have shown that an antibiotic and an alpha-blocker is more effective than an antibiotic alone [70]. A meta-analysis showed that alpha-blockers, antibiotics, and a combination of the two all significantly improve symptoms (according to scores on the NIH Chronic Prostatitis Symptom Index), with the combination providing the greatest benefit [75]. However, another meta-analysis showed that these same agents—alone and in combination—were not associated with a statistically or clinically significant decrease in symptom scores [76]. The combination of an alpha-blocker (doxazosin) with an anti-inflammatory agent (ibuprofen) and a muscle relaxant (thiocolchicoside) led to a statistically and clinically significant reduction in the total score on the NIH Chronic Prostatitis Symptom Index in one systematic review; according to the findings of another systematic review, the three-agent combination was not superior to monotherapy [74,76]. Researchers have cautioned that publication bias may cause overestimation of the beneficial effects of alpha-blockers and that the placebo effect has been significant in many studies [75,76]. Addressing a hypothesis that the pain related to chronic prostatitis may have a neuropathic origin, pregabalin has been evaluated as a management strategy, but a systematic review found that the drug did not improve symptoms and caused side effects in a large percentage of men [77].
Trigger point release/paradoxical relaxation training to release trigger points in the pelvic floor musculature was found to significantly improve symptoms in men who had chronic prostatitis/chronic pelvic pain syndrome [63]. Seventy percent of the men in the study had a significant decrease in the score on the NIH Chronic Prostatitis Symptom Index, with improvement in pelvic pain, urinary symptoms, libido, ejaculatory pain, and erectile and ejaculatory dysfunction [63].
Benign prostatic hyperplasia (BPH), also referred to as benign prostatic hypertrophy, is a histologic diagnosis that refers to the proliferation of smooth muscle and epithelial cells within the prostatic transition zone [78]. BPH is one of the most common conditions among aging men. The onset of lower urinary tract symptoms usually begins after 40 years of age, increasing in prevalence and severity with age [78]. Serious complications and mortality are rare, but the condition has an impact on the quality of life, with symptoms that interfere with normal daily activities and sleep [78]. Complete evaluation is necessary for an accurate diagnosis of BPH; the condition must be differentiated from prostate cancer, which is associated with similar early symptoms. In addition, early detection of BPH leads to early treatment, which can control progression of the disease, preventing such complications as urinary tract infection, acute urinary retention, and obstructive nephropathy [79].
The prevalence of BPH increases with age, from approximately 8% of men 31 to 40 years of age to approximately 90% of men in their 80s [80,81]. Risk factors identified in one study included increased age, prostatic volume, and peak urinary flow rate [82]. Other factors, including some that are modifiable, include obesity, diet, dyslipidemia, hypertension, alcohol use, and smoking [83]. The relative risk for BPH (and common comorbidities) may be higher for Black and Hispanic men than for White men and is thought to be related in part to genetic differences based on race/ethnicity; however, observational studies have produced variable results [81,84].
As previously noted, distinguishing BPH from other prostate-related diseases is often difficult, as lower urinary tract symptoms are similar for a variety of conditions. The American Urological Association (AUA) evidence-based guidelines for the management of BPH, updated in 2021, recommend the following tests [78]:
Medical history
Assessment of lower urinary tract symptoms
Determination of severity and bother of symptoms
Physical examination
Urinalysis
Determination of a serum PSA level is also recommended if the patient has a life expectancy of more than 10 years (and the diagnosis of prostate cancer will alter management), and a frequency-volume chart is recommended if substantial nocturia is a predominant symptom [78]. Routine measurement of a serum creatinine level is not recommended as part of the initial evaluation of men with lower urinary tract symptoms related to BPH [78].
In obtaining a history, clinicians should ask about urinary tract symptoms, sexual function, previous surgical procedures, and general health issues in an attempt to identify other causes of voiding dysfunction or comorbidities that may complicate treatment. Diabetes, cerebrovascular disease, and Parkinson disease can cause urinary symptoms secondary to neurogenic bladder, and STIs or trauma may cause urethral stricture [85]. It may be appropriate to have the patient keep a diary of voiding habits (frequency, volume, etc.) [78].
Assessment of symptoms is an integral aspect of the initial evaluation for BPH, as it helps to determine the severity of disease. The International Prostate Symptom Score (IPSS) (previously called the AUA Symptom Index) is a validated, self-administered symptom frequency and severity assessment questionnaire originally developed by the AUA Measurement Committee [78]. The IPSS is a widely available, seven-question assessment tool that has been validated for clarity, test/retest reliability, internal consistency, and criteria strength [78,86]. The IPSS addresses [86]:
Urinary frequency
Hesitancy
Nocturia
Incomplete emptying
Urgency
Weak urinary stream
Intermittence
Symptoms should be discussed with the patient and questions addressed as necessary [78].
The physical examination should include a digital rectal examination (DRE) to determine the size, consistency, and shape of the prostate [78]. A symmetrically firm and enlarged prostate by DRE is indicative of BPH [79]. The true size of the prostate is often underestimated by DRE compared with transrectal ultrasound [78]. Examination should also include neurologic evaluation to assess the patient's general mental status, ambulatory status, neuromuscular function of the lower extremities, and anal sphincter tone [78].
A urinalysis (dipstick test) to screen for hematuria, proteinuria, pyuria, and other abnormalities can help to rule out such conditions as bladder cancer, carcinoma in situ of the bladder, urinary tract infection, urethral strictures, distal urethral stones, and bladder stones, which are less likely if the results of urinalysis are normal [78].
Optional studies that may be used to confirm the diagnosis or evaluate the presence and severity of BPH include post-voiding residual urine measurement (PVR) and uroflowmetry studies [78]. A PVR is useful in determining a baseline ability of the bladder to empty and detecting severe urinary retention that may not be amenable to medical therapy. Uroflowmetry is a simple, office-based procedure, an adjunct to evaluation of lower urinary tract symptoms and probability of bladder outlet obstruction. Flow rates of <10 mL/second have shown a specificity of 70%, a positive predictive value of 70%, and a sensitivity of 47% for bladder outlet obstruction [78].
According to the AUA guideline, the benefits, risks, and costs of treatment options should be discussed with patients who have moderate-to-severe symptoms (IPSS score of 8 or more) who are bothered enough by the symptoms to consider therapy [78]. The treatment options for BPH include:
Watchful waiting
Medical therapy (minimally invasive procedures)
Surgical interventions
The AUA guideline recommends watchful waiting as the preferred approach for men who have mild symptoms (a score of less than 8 on the AUA Symptom Index) [78]. This approach may also be taken for men with moderate-to-severe symptoms (score of 8 or more) who are not bothered by the symptoms and have no complications [87]. Watchful waiting should include yearly evaluations similar to the initial one [78]. Lifestyle changes and behavioral interventions are considered reasonable first-line treatments for all patients. Symptoms may be reduced by avoiding decongestants and antihistamines, decreasing fluid intake (and avoiding caffeine and alcohol) prior to bedtime, and increasing physical activity and weight loss [78].
AUA guidelines recommend offering monotherapy with an alpha-blocker as initial preferred option for patients with bothersome -to-severe symptoms [78]. Clinicians should consider performing a PVR measurement or uroflowmetry prior to treatment intervention. Five alpha-blockers have FDA-approved indications for BPH (Table 5). Clinical studies show that all five of these drugs—alfuzosin, doxazosin, tamsulosin, terazosin, and silodosin—are equally effective in terms of symptom relief and expected range of improvement in symptom index (IPSS) score [78]. The choice of alpha-blocker should be based on the patient's age and comorbidities, and different adverse event profiles (e.g., ejaculatory dysfunction, changes in blood pressure).
PHARMACOLOGIC THERAPY FOR BENIGN PROSTATIC HYPERTROPHY
| Agent | Daily Dose |
|---|---|
| Alpha-blockers | |
| Alfuzosin ER (Uroxatral) | 10 mg |
| Doxazosin (Cardura) and doxazosin ER (Cardura XL) | 4–8 mg |
| Silodosin (Rapaflo) | 8 mg |
| Tamsulosin (Flomax) | 0.4–0.8 mg |
| Terazosin (Hytrin) | 1–2 mg |
| 5-alpha reductase inhibitors | |
| Dutasteride (Avodart) | 0.5 mg |
| Finasteride (Proscar)a | 5 mg |
| Combination (alpha-blocker and 5-alpha reductase inhibitor) | |
| Dutasteride/tamsulosin (Jalyn) | 1 capsule (0.5 mg dutasteride and 0.4 mg tamsulosin hydrochloride) |
| Phosphodiesterase 5 inhibitors | |
| Tadalafil (Cialis)a | 5 mg |
| aCombination finasteride/tadalafil (5 mg each) may also be used. | |
The adverse events associated with alpha-blockers are orthostatic hypotension, dizziness, fatigue (asthenia), and ejaculatory problems [78]. These drugs should not be used for men who are taking medication for erectile dysfunction, as the interaction between the two drugs can cause profound hypotension [79]. Alpha-blocker agent use also has been associated with the rare complication of intraoperative floppy iris syndrome; patients anticipating cataract surgery should be informed of the risks and advised to discuss these risks with their ophthalmologist [78].
Two 5-alpha reductase inhibitors, finasteride and dutasteride, are also approved for treatment of BPH-related symptoms and are recommended options in the AUA guideline [78]. This is less effective than therapy with alpha-adrenergic antagonists for relieving lower urinary tract symptoms, leading to an average improvement of 3 points on the AUA Symptom Index [78]. The advantage of 5-alpha reductase inhibitors is that they also act to prevent progression of disease and reduce the size of the prostate. As such, the AUA notes that these drugs should be used only for men who have evidence of prostatic enlargement [78]. Men should be made aware of the need for long-term therapy with either of these drugs, and clinicians should also discuss the possible adverse events, which include decreased libido, ejaculatory dysfunction, and erectile dysfunction. These effects usually resolve within one year [78,79].
In 2011, the FDA issued a safety announcement that the Warnings and Precautions section of the labels of 5-alpha reductase inhibitors was revised to include new safety information about the increased risk of a diagnosis of high-grade prostate cancer [92]. The revision came after FDA review of two prostate cancer prevention trials, in which finasteride and dutasteride reduced the incidence of lower risk forms of prostate cancer but were associated with an increased incidence of high-grade prostate cancer [92].
The AUA guideline also supports the use of combination therapy with an alpha-blocker and a 5-alpha reductase inhibitor for men with lower urinary tract symptoms and evidence of prostate enlargement, as demonstrated on volume measurement, PSA level as a proxy for volume, or on DRE [78]. A fixed-dose combination of dutasteride (0.5 mg) and tamsulosin (0.4 mg) is available, and the results at four years showed that, for men with a baseline prostate volume ≥40 mL and PSA level of ≥1.5 ng/mL, the combination led to greater reductions in the relative risk of clinical progression, acute urinary retention, or BPH-related surgery than either drug alone [93].
The AUA guideline also notes that anticholinergic agents are appropriate and effective options for managing BPH-related symptoms in men who do not have an elevated post-void residual and when symptoms are predominantly irritative [78].
Phosphodiesterase type-5 inhibitors have also been shown to be effective for reducing the symptoms associated with BPH [94]. This class of drugs also offers advantages over other drugs in its rapid onset of action, fewer adverse events, and enhanced sexual function [94]. Potential adverse events include back pain, dyspepsia, headache, and dizziness [95]. In 2011, the first phosphodiesterase type-5 inhibitor—tadalafil—was approved by the FDA for BPH-related symptoms, with indications for symptoms in men who have prostate enlargement, with or without erectile dysfunction [95]. Before prescribing tadalafil, clinicians should ensure that patients are not taking drugs that interact with tadalafil, such as nonselective alpha-blockers, nitrates, and cytochrome P450 inhibitors [95].
Saw palmetto, a commonly used alternative therapy for BPH, is not recommended for BPH-related symptoms, as the most recent data have shown no clinically meaningful effect on symptoms [78].
Minimally invasive therapies such as transurethral needle ablation and transurethral microwave thermotherapy are treatment options for men with bothersome moderate or severe symptoms [78]. However, the AUA guideline notes that, although these therapies improve symptoms, flow rate, and quality of life, the outcomes are not as good as those after transurethral resection of the prostate [78].
Surgical interventions are typically reserved for worsening disease and severe symptoms that do not respond to medical treatment. The AUA guideline recommends surgery for patients with renal insufficiency secondary to BPH, refractory urinary retention secondary to BPH, recurrent urinary tract infections, bladder stones, or gross hematuria due to BPH; or symptoms refractory to other therapies [78]. The most common procedure is transurethral resection of the prostate, which comprises 90% of all prostate surgeries done for BPH and is the benchmark for therapy [78,96]. Open prostatectomy; transurethral laser ablation or enucleation; laser resection; photoselective vaporization; and transurethral incision, vaporization, and resection are other surgical options, and the selection of intervention is based on the surgeon's experience, the patient's anatomy, and a discussion of the benefits and risk of complications [78].
Prostate cancer is the most commonly diagnosed cancer among men, accounting for 19% of all cancer diagnoses in men and the second leading cause of cancer-related deaths, responsible for 9% of cancer-related deaths in men [16]. The lifetime risk of a prostate cancer diagnosis is approximately 15% [16].
Prostate cancer is a complex issue for both men and their healthcare providers for many reasons, including variation in tumor biology, lack of specific symptoms, accuracy of levels of PSA and its several derivatives, questions about optimum treatment, and, most notably, controversy surrounding screening.
In 2022, the estimated projected number of new prostate cancer diagnoses was 268,490, with 34,500 prostate cancer-related deaths [16]. The majority of newly diagnosed prostate cancers have localized disease. The highest incidence is found among Black men (172.6 per 100,000), and the lowest is among Asian American and Pacific Islander men (55.0 per 100,000) [16]. The death rate related to prostate cancer is also highest for Black men, with a rate that is more than twice that for men of all other races/ethnicities (37.9 per 100,000 vs. 17.8 [White], 21.0 [American Indian and Alaska Native], 15.6 [Hispanic/Latino], and 8.6 [Asian American and Pacific Islander]) [16]. The mortality rate associated with prostate cancer decreased 4.1% per year between 2009 and 2019, in part, because of improvements in early detection and treatment [16].
The known risk factors for prostate cancer are advanced age, Black race, and a family history of the disease (especially when diagnosed at a younger age) [16,97]. The risk for prostate cancer may also be increased for men with symptoms of prostatitis [66].
Several studies have been undertaken to determine the efficacy of chemoprevention agents and dietary supplements to reduce the risk of prostate cancer. The chemoprevention agents evaluated belong to the class of 5-alpha reductase inhibitors, a class of drugs approved for the treatment of BPH. One drug in this class, finasteride, was evaluated in the first large-scale chemoprevention study, the Prostate Cancer Prevention Trial (PCPT), a seven-year study involving nearly 19,000 men 55 years of age or older. In that study, finasteride significantly reduced the prevalence of prostate cancer (18% vs. 24% for the placebo group) [98]. Dutasteride was shown to decrease the risk of prostate cancer in the REDUCE trial, and extended follow-up indicated a low rate of new prostate cancer diagnoses [99,100]. The initial results of the PCPT and REDUCE trials led the American Society of Clinical Oncology (ASCO) and the AUA to develop a joint guideline recommending finasteride and dutasteride for the prevention of prostate cancer [90]. However, reanalysis of the results of the trials showed that the risk for high-grade prostate cancer was increased and the reduction in prostate cancer risk was seen primarily for less fatal subtypes of prostate cancer that are often not treated [100,101]. In 2011, the FDA decided against approving the two drugs for the prevention of prostate cancer, noting that the risk-benefit profile is not favorable for chemoprevention [91,101,102]. As stated earlier, the FDA revised the labels of all 5-alpha reductase inhibitors to note the increased risk of higher-grade prostate cancer associated with the drugs [92]. The ASCO/AUA guideline was withdrawn, and experts have called for more research to determine whether 5-alpha reductase inhibitors have a role in the prevention of prostate cancer [101,102,103].
Dietary supplements have not been shown to substantially reduce the prevalence of prostate cancer. In the Selenium and Vitamin E Cancer Prevention Trial (SELECT), a randomized study of more than 35,000 men, neither of those two vitamins, alone or in combination, prevented prostate cancer in relatively healthy men [104]. A subsequent phase III trial showed that selenium supplementation had no effect on prostate cancer risk among men with high-grade prostatic intraepithelial neoplasia [105]. There is insufficient evidence for the routine recommendation of other dietary supplements, such as soy, milk thistle, omega fatty acids, lycopene, or green tea, to prevent prostate cancer [106,107,359].
There is no question that available screening methods and enhanced awareness has led to an increased number of men in whom prostate cancer is diagnosed at an earlier stage. The primary benefit of screening is a lower stage and grade of cancer at the time of diagnosis, and the high rate of localized disease at the time of diagnosis (92% to 96%) reflects, in part, the increased number of cancers that are detected earlier through screening [102,108,109]. Despite this benefit, an effect of screening on mortality has not been clearly demonstrated. After 13 years of follow-up in the National Cancer Institute's Prostate, Lung, Colon, and Ovary (PLCO) trial, there was no benefit of annual screening on mortality [110]. A meta-analysis (five randomized controlled trials) similarly demonstrated no effect of screening on prostate cancer-specific or overall mortality [111]. However, data from the European Randomized Study of Screening for Prostate Cancer demonstrated that screening reduced the risk for prostate cancer death by 7% to 9% per year [112].
In addition to a lack of effect on mortality, screening is associated with high rates of false-positive results, overdiagnosis and subsequent overtreatment, and complications. Among men who had four PSA tests, the cumulative risk for at least one false-positive result was 12.9% [102]. Rates of overdiagnosis have been estimated at 17% to 50%, and 23% to 42% of all screen-detected prostate cancers are overtreated [102,113]. Furthermore, treatment is associated with complication rates of 20% to 50% [102,114]. These findings led several expert panels to update their screening recommendations (Table 6) [97,102,108,114,115,116,117]. Overall, experts recommend against routine screening for most men and emphasize the need to consider life expectancy and the patient's age and risk factors for the disease. The age to start a discussion about screening varies slightly among the guidelines. The AUA guideline notes that decisions about screening should be individualized for men younger than 55 years who are at high risk for the disease (positive family history or Black race) [114]. The guideline also states that the greatest benefit of screening appears to be for men 55 to 69 years of age and strongly recommends shared decision making for men in this age-group. The ACS guideline notes that screening should be discussed beginning at 50 years of age for men at average risk and before 50 years of age for men at higher risk [108]. The NCCN guideline suggests that clinicians talk to patients about the risks and benefits of a baseline DRE and PSA beginning at 40 years of age [97]. The American College of Physicians (ACP) recommends that clinicians inform their male patients, 50 to 69 years of age, about the limited potential benefits and substantial harms of screening [115].
RECOMMENDATIONS FOR PROSTATE CANCER SCREENING
| Organization | Year of Publication | Screening Recommendation | Notes | ||
|---|---|---|---|---|---|
| American Cancer Society | 2010 | — | Discuss the potential benefits, risks, and uncertainties associated with prostate cancer screening with men ≥50 years | ||
| American Society of Clinical Oncology | 2012 | Discourage general screening for men with a life expectancy of ≤10 years, as the harms outweigh the benefits | Discuss the individual appropriateness of screening with men who have a life expectancy >10 years | ||
| American Urological Association | 2013, reconfirmed 2018 | No routine screening in men 40 to 54 years of age at average risk |
| ||
| American College of Physicians | 2013 | No routine screening with PSA for average-risk men younger than 50, men older than 69, or men with a life expectancy of less than 10 to 15 years | Clinicians should inform men 50 to 69 years of age about limited potential benefits and substantial harms of screening and should individualize decision based on patient's general health, life expectancy, and preferences. | ||
| U.S. Preventive Services Task Force | 2018 | No routine screening for men 70 years of age and older. For men 55 to 69 years of age, the decision should individualized. | Clinicians should discuss the potential benefits and harms of screening. | ||
| National Comprehensive Cancer Network | 2022 | — |
|
Researchers continue to investigate ways to make screening more effective. Using a higher PSA threshold for biopsy for older men and less frequent screening for men with low PSA levels are strategies that may reduce the risk of overdiagnosis as well as prostate cancer-related mortality [118].
Informed decision making is integral in selecting approaches to screening, with every guideline emphasizing the need to discuss the potential benefits, harms, and limitations associated with screening with their male patients. The American Cancer Society notes that men should receive information about screening directly from their healthcare provider or be referred to reliable and "culturally appropriate" sources [108]. Decision aids can be especially useful in helping men and their healthcare providers weigh the benefits and risks of screening, and studies of decision aids have led to improved knowledge and have increased men's desire for an active role in decision making [108,114,119,120,121]. The NCCN guideline offers talking points for discussion, and ASCO provides a decision aid tool (https://www.asco.org/sites/new-www.asco.org/files/content-files/practice-and-guidelines/documents/2012-psa-pco-decision-aid.pdf).
Despite the continued emphasis on informed decision making, the percentage of men who report having had a discussion with their healthcare providers about screening has been suboptimal, with a rate of about 63% to 66% of the general male population [122,123]. Black men were most likely to have had a discussion, and men without a usual source of care were the least likely [123].
For men who choose to have screening for prostate cancer, the combination of DRE and PSA is the preferred method, providing better predictive value than either method alone [102]. The sensitivity of PSA testing is higher than that of DRE, especially for tumors that are more aggressive [109]. However, the PSA level can vary as a result of several factors.
PSA and Its Derivatives
In an effort to enhance the specificity of PSA testing, variations of the PSA test have been developed, including free PSA, PSA density, PSA velocity, and complexed PSA [97]. Each has its benefits and limitations, and the AUA notes that none increases the benefits-harms ratio of screening [114]. Levels of free PSA have been shown to be significantly lower in men with prostate cancer than in men without the disease [97]. The FDA has approved percent-free PSA for the early detection of prostate cancer in men with PSA levels between 4 ng/mL and 10 ng/mL [97].
PSA density is the result of dividing the PSA level by the volume of the prostate, as measured by transrectal ultrasonography, and a higher result suggests a greater likelihood of prostate cancer [97]. Greater PSA density has correlated with the presence of prostate cancer, as well as with the pathologic stage of the tumor and its aggressiveness and progression after treatment [124]. The use of PSA density has been limited by the lack of precision of total PSA, of measurement of prostate volume, and of the need to carry out transrectal ultrasonography [97]. In addition, PSA density does not offer much benefit compared with other PSA derivatives [97]. PSA velocity is the rate at which a PSA level increases over a period of time, and it has been most helpful for longitudinal monitoring of men younger than 50 years of age who have normal PSA levels and no prostate enlargement [97]. A high PSA velocity alone should not prompt biopsy but instead, aid in decision making [97]. The test is not useful for men with PSA values greater than 10 ng/mL [97]. The ratio of complexed PSA to total PSA provides information comparable to the ratio of free to total PSA, and the use of complexed PSA has been approved as a detection aid (in conjunction with DRE) for men 50 years of age or older; however, the test is not widely used in practice [97].
Threshold for Biopsy
Prostate cancer is found in about 25% of biopsy specimens, illustrating a problem regarding a well-defined threshold at which to obtain a biopsy specimen [125]. Although most cancer is detected with use of a PSA threshold of 4 ng/mL, some studies have shown that prostate cancer is subsequently found in men with levels in the range of 2.5–4.0 ng/mL [97]. The NCCN concluded that while these values have been used by many, a level of 3.0 ng/mL is supported by trials and would more robustly limit the risk of overdetection. However, there was not a consensus among NCCN panel members regarding limiting the option to biopsy to prespecified PSA thresholds [126]. The NCCN panel also concluded that DRE alone is not an absolute indication for biopsy in men with low PSA, as the positive predictive value of DRE in this population is poor. However, a very suspicious DRE, independent of PSA, could indicate high-grade cancer in men with normal PSA values, and therefore, biopsy should be considered in these men [126].
Men with early prostate cancer are usually asymptomatic. More advanced disease may be associated with changes in urinary habits, such as a slowing of the urinary stream, sense of incomplete voiding, nocturia, and frequency, as well as dysuria, hematuria, or pain in the lower back or pelvis. Because many of these symptoms are similar to those linked to benign prostate conditions, prostate cancer cannot be diagnosed on symptoms alone. The diagnostic methods are the same as those used for screening: PSA, DRE, and transrectal ultrasonography. In performing the DRE, the clinician should focus on the size, consistency, and abnormalities within or beyond the gland. Prostate cancers are characteristically hard, nodular, and irregular.
In its 2013 Best Practice Statement on PSA, the AUA emphasizes the importance of PSA in staging, noting that the PSA level predicts response of prostate cancer to local therapy [127]. Response is most likely in men with a PSA level <10 ng/mL [127].
Biopsy of the prostate with analysis of the tissue provides the most definitive diagnostic procedure. It also gives evidence of the aggressiveness of the tumor when cancer is detected. The pathologist quantifies the aggressiveness of the tumor with use of the Gleason score, assigning a number between 2 and 10 (with 10 representing the most aggressive). Pathologic review involves both staging according to the American Joint Committee on Cancer staging manual and classification of the tumor with the Gleason score [128]. Further staging with imaging (CT, MRI, bone scan) is done only for tumors that are confined to the prostate with a Gleason score of 8 or higher or a PSA level of greater than 20 ng/mL or for tumors that extend beyond the prostate or are symptomatic [97]. As part of the Choosing Wisely campaign, the AUA notes that a routine bone scan is not necessary for men with newly diagnosed prostate cancer with a PSA level <20.0 ng/mL and a Gleason score of ≤6 [127].
Recognizing that many prostate cancers have an indolent natural history, guidelines recommend utilization of a risk stratification classification for patients with newly diagnosed localized disease [358]. Stratification facilitates patient counseling and should be used with a shared decision-making approach in which treatment decisions are based on the patient's estimated life expectancy and the risk of biochemical recurrence [126]. Risk of biochemical recurrence has been classified by the NCCN into five categories (Table 7) [126].
CLASSIFICATIONS OF RISK OF BIOCHEMICAL RECURRENCE
| Risk Level | Tumor | Gleason Score | PSA Level (ng/mL) | Other | ||
|---|---|---|---|---|---|---|
| Very low | T1c | ≤6 | <10 |
| ||
| Low | T1–T2a | ≤6 | <10 | — | ||
| Intermediate | T2b–T2c | 7 (or PSA level as noted) | 10–20 ng/mL | — | ||
| High | T3a (or other criteria) | 8–10 (or other criteria) | >20 | — | ||
| Very high | T3b–T4 (locally advanced) | Primary Gleason pattern 5 (or other criteria) | — | Biopsy cores: >4 with Gleason score 8–10 | ||
| NCCN = National Comprehensive Cancer Network, PSA = prostate-specific antigen. | ||||||
A new prostate cancer grading system was developed during a 2014 consensus conference of the International Society of Urological Pathology (ISUP). The new system resulted in changes to the assignment of Gleason pattern based on pathology. This system assigns grade groups from 1 to 5, derived from the Gleason score. Many experts believe that the ISUP grade groups enable patients to better understand their true risk level and limit overtreatment. The NCCN has accepted the new grade group system. Patients remain divided into very-low-, low-, intermediate-, high-, and very-high-risk groups [126].
The primary options for localized prostate cancer are watchful waiting (also known as active surveillance), radiation therapy (either three-dimensional external-beam radiation or brachytherapy), and radical prostatectomy. Other options include androgen-deprivation therapy (ADT, also referred to as hormone therapy), chemotherapy, cryosurgery, and immunotherapy.
Each treatment option is associated with benefits and harms, and clinicians should discuss each option in detail and provide educational resources and decision aids [129,130,131]. To gain a true understanding of a patient's preferences, treatment options should be discussed only after the patient has described his preferences [132]. Clinicians should carefully assess their patients' understanding of treatment options; studies of underserved men have shown low comprehension of common terms used in prostate cancer treatment discussions [133,134]. Attention should also be paid to how to best communicate risk. A study has shown that such terms as "number needed to treat," "odds ratio," and "relative risk reduction" were confusing to men [135]. In that study, men best understood information when it was presented as an absolute risk reduction and in a positive context; men preferred that treatment options be discussed in terms of the probability of an increase in survival (rather than a decrease in mortality) and that the discussion include the impact of treatment on patient-centered quality-of-life outcomes [135].
Active Surveillance
Active surveillance has also been referred to as watchful waiting, but the terms have not always been defined the same way, and researchers are calling for a distinction between the two terms. Active surveillance denotes an approach in which men with localized, low-risk prostate cancer are followed up closely for clinical signs that prompt definitive treatment with curative intent should this become necessary [136,358]. Watchful waiting refers to the strategy recommended for asymptomatic patients with prostate cancer and limited life expectancy [358]. Some studies draw further distinction, defining watchful waiting as observation and provision of palliative care when prostate cancer becomes symptomatic, and active surveillance as close follow-up (with DRE, PSA levels, and biopsies) and provision of treatment at signs of disease progression [138]. Patients with a life expectancy of less than five years do not benefit from prostate cancer screening, diagnosis, or treatment as prostate cancer treatment does not improve survival within five years of follow-up [358].
For patients with favorable intermediate-risk prostate cancer, clinicians should discuss with patients the options of active surveillance, radiation therapy, or radical prostatectomy [358]. Choosing active surveillance rather than definitive treatment is difficult because of the myriad advantages and disadvantages to the approach (Table 8) [126]. Data on active surveillance have also conflicted. In a cohort of 450 men followed up for a median of nearly seven years, the rate of prostate cancer-specific mortality was low [139]. Two later systematic reviews indicated that the evidence was insufficient to determine whether active surveillance with curative intent was an appropriate option for men with localized prostate cancer [136,137]. Most recently, radical prostatectomy was compared with active surveillance, and the intervention did not significantly reduce all-cause or prostate cancer-specific mortality through at least 12 years of follow-up [140]. In addition, a cost-effectiveness analysis demonstrated that active surveillance was most effective and least expensive compared with several interventions (brachytherapy, intensity-modulated radiation therapy, or radical prostatectomy) [138].
ADVANTAGES AND DISADVANTAGES OF ACTIVE SURVEILLANCE FOR PROSTATE CANCER
| Advantages | Disadvantages | ||||
|---|---|---|---|---|---|
| Ensure that small indolent cancers are not treated unnecessarily | Lack of definitive prompt for treatment may lead to missed opportunity for cure | ||||
| Avoid side effects of treatment that may be unnecessary | Cancer may progress or metastasize before treatment | ||||
| Maintain quality of life and normal activities | Treatment of larger, more aggressive cancer may be more complex, with increased side effects | ||||
| Decrease initial costs |
|
The NCCN Panel recommends active surveillance for all men with very-low-risk prostate cancer and a life expectancy of less than 20 years and believes that surveillance should be considered for men with very-low-risk prostate cancer and a life expectancy of 20 years or more [126]. In addition, the Panel recommends active surveillance for all men with low- and favorable intermediate-risk prostate cancer and a life expectancy of less than 20 years and believes that it should be considered for men with low- and favorable intermediate-risk and a life expectancy of 10 years or more [126]. With active surveillance, recommended monitoring is measurement of a PSA level no more than every 6 months, unless clinically indicated, and physical exam with DRE every 12 months [126]. An increase in PSA should prompt re-testing as transient PSA elevations are common; serial PSA increases, new DRE abnormalities, or other concerns for clinical progression should prompt re-evaluation with prostate MRI and possible prostate biopsy [126,358].
Radiation Therapy
Radiation therapy is an option for men at various levels of risk for biochemical recurrence, except for men for whom active surveillance is recommended [126]. Radiation to pelvic lymph nodes may be considered for men with intermediate risk and should be done for men at high risk [126]. Radiation therapy offers progression-free survival similar to that of prostatectomy while avoiding the complications associated with surgery [126].
The advent of three-dimensional (3D) CRT, which integrates external-beam radiation with CT images, has allowed for the delivery of higher radiation doses but with a lower risk of side effects because of enhanced precision [126]. About half of men will have temporary bladder or bowel symptoms during treatment with external-beam radiation therapy [126]. The disadvantage to external-beam radiation therapy is the time needed for treatment, as the recommended duration of treatment is eight to nine weeks [126].
Intensity-modulated radiation therapy (IMRT), a second-generation 3D technique, has been used increasingly in clinical practice [141]. IMRT reduced the risk of gastrointestinal toxicities and rates of salvage therapy compared with 3D-CRT in some retrospective, population-based studies, but treatment cost was increased [142,143]. More recently, moderately hypofractionated image-guided IMRT regimens have been tested in randomized trials, but additional research is needed [126].
Brachytherapy has been used increasingly for men with early localized prostate cancer; however, increasing evidence suggests that technical advancements in brachytherapy may have a role in treatment of high-risk localized and locally advanced prostate cancer [126,144,145]. This approach is a recommended option as monotherapy for men at low risk and a life expectancy of at least 10 years and in combination with external-beam radiation therapy for men at intermediate risk, regardless of life expectancy [126,146]. Complications are increased when the two forms of radiation therapy are used together [126]. Brachytherapy alone yields control rates comparable to those of surgery (approximately 90%), and added advantages are short treatment duration, minimal risk of incontinence, and short-term preservation of erectile function; the seeds are implanted in one procedure, and men typically recover in one day [126]. Disadvantages include the need for general anesthesia and a risk of acute urinary retention [126].
Radical Prostatectomy
Radical prostatectomy is an option for men with a life expectancy of at least 10 years who have clinically localized disease that can be completely excised [126]. It also may be an option for men with high-risk disease and for select patients with very-high-risk disease, although several factors (e.g., PSA >10 ng/mL, stage T2b or higher, Gleason score 9 or 10, higher number of biopsy cores with high-grade cancer, more than 50% core involvement) predict unfavorable outcome in these patients [147]. Radical prostatectomy is a salvage option for patients experiencing biochemical recurrence after primary external beam radiation therapy, but morbidity remains significantly higher than when the treatment is used as initial therapy [148,149]. This treatment option has been most often associated with the highest survival rates but also with side effects that have been reported to have a significant impact on quality of life, such as impotence, incontinence, urethral stricture, and surgery-related morbidity [126,150,151]. Despite the potential side effects, the sense of being cancer free has led men who chose to have radical prostatectomy to be satisfied with their decision [152]. Laparoscopic and robot-assisted procedures have been found to yield results similar to those for open procedures, but rates of incontinence and erectile dysfunction may be higher [126]. The AUA notes that no conclusive benefit to pelvic lymph node dissection has been found [127]. Such dissection for clinically localized disease may not be necessary if the PSA is less than 10 ng/nL and the Gleason score ≤6 [127].
Androgen Deprivation Therapy (ADT)
ADT involves medical or surgical castration (with luteinizing hormone-releasing hormone [LHRH] agonists or orchiectomy, respectively). It is recommended as an adjunct to radiation therapy or prostatectomy for men with local or locally advanced disease and at high or intermediate risk for recurrence [126]. Meta-analyses have shown clinical benefit for adjuvant ADT after either radiation therapy or prostatectomy or neoadjuvant therapy before radiation therapy [153; 154].
Both NCCN and ASCO recommend ADT as initial treatment for metastatic prostate cancer [126,155]. Researchers have evaluated the timing of ADT—early (before symptoms occur) or delayed—and early therapy has provided no overall survival benefit and only a modest decrease in risk for prostate cancer-specific mortality; because of this, the ASCO guideline does not make a recommendation for early ADT [155]. Several studies have demonstrated that intermittent ADT is as effective as continuous ADT for metastatic or locally advanced disease, with better quality of life and fewer side effects [156,157,158].
Use of ADT as a primary therapy for men with localized prostate cancer has increased significantly among men at low and intermediate risk, but this approach should not be considered standard [126,146]. ADT is associated with several adverse events, including osteoporosis, increased risk for fracture, obesity, insulin resistance, and increased risk for cardiovascular disease and diabetes [126].
Chemotherapy
The use of chemotherapy is typically reserved for men with metastatic castration-resistant prostate cancer, and docetaxel-based regimens have been shown to confer survival benefit [159,160]. The duration of therapy is not well-defined, but 10 cycles were used in the phase III trials in which these regimens were evaluated.
Cryosurgery
Cryosurgery is a minimally invasive procedure that is an option for prostate cancer (of any grade) that is clinically confined to the prostate in men at low, intermediate, or high risk [161]. The five-year biochemical disease-free survival rates have ranged from 48% to 92%, depending on the risk of recurrence, but long-term data on prostate cancer-specific survival are not yet available and there are no clearly defined guidelines for patient selection for cryosurgery as a salvage procedure [161]. The authors of a meta-analysis published in 2007 and updated in 2018 concluded that it was difficult to determine the relative benefits of this treatment because of the poor quality of the available studies [162].
Options for Metastatic Castration-Resistant Prostate Cancer
Since 2010, three agents, an immunotherapy, and a radiopharmaceutical have been approved for metastatic castration-resistant prostate cancer. Cabazitaxel (Jevtana), enzalutamide (Xtandi), and abiraterone acetate (Zytiga) are indicated for treatment following docetaxel [126]. Sipuleucel-T (Provenge), an autologous cellular immunotherapy, is approved for men with metastatic castration-resistant prostate cancer who are asymptomatic or minimally symptomatic. Lastly, radium 223 dichloride (Xofigo) was approved in May 2013 for the treatment of metastatic castration-resistant prostate cancer with bone metastases (but not visceral involvement) [126].
Survival after treatment of prostate cancer is related to the extent of the tumor at the time of diagnosis, and the relative five-year survival rate is 100% for localized or regional prostate cancer [16]. The five-year survival rate is substantially lower (30%) when prostate cancer is metastatic at the time of diagnosis [16].
Primary care physicians, nurses, and other healthcare professionals who see patients on a regular basis play an important role in the follow-up evaluation for men who opt for active surveillance, as well as for those who have been treated by an oncologist. After treatment for prostate cancer, men should be followed up with an annual DRE and PSA testing every 6 to 12 months for five years and annually thereafter [163]. Primary care clinicians can also aid in the management of the side effects of treatment and screening for secondary cancers.
Patient A is an active man, 59 years of age, who missed his yearly DRE and PSA. The results of these tests had been within normal limits in all previous examinations. At his next examination, a firm prostate nodule, approximately 2 mm in diameter, is palpated, and the PSA level is 14 ng/mL. A needle biopsy of the prostate is performed within one week of the PSA measurement. The biopsy shows several sites containing cells indicative of adenocarcinoma of the prostate, with a Gleason score of between 8 and 9.
After carefully evaluating the treatment options for an aggressive tumor, Patient A chooses radical prostatectomy and seeks care at an institution where nerve-sparing surgery is performed with the assistance of a robotic, computer-controlled device, to help reduce the risk of adverse events. According to the pathologic evaluation, the tumor is an adenocarcinoma that has extended beyond the capsule of the gland but has not involved the seminal vesicles.
Staging studies, including an MRI of the pelvis and abdomen and a bone scan, confirm the extent of the tumor and demonstrate lack of lymph node involvement or distant metastasis (T3a, N0, M0). Because of the T3a finding, a course of external-beam radiation therapy to the local site is prescribed.
At the three-month follow-up visit, the PSA level has increased to 20 ng/mL, and a bone scan demonstrates multiple skeletal lesions, primarily in the ribs, pelvis, and skull, none of which had been seen on the previous scan. Due to the rapid progression of disease and the metastatic lesions, the patient's survival is estimated to be less than three years.
After a discussion with his surgeon, oncologist, and urologist, the patient decides to forego ADT, choosing instead to enroll in a clinical trial for treatment consisting of chemotherapy with docetaxel in combination with the angiogenesis inhibitor bevacizumab over a course of several months. The treatment causes some nausea, malaise, and hair loss, but the patient tolerates the effects well. The primary bothersome adverse effect is oral ulcers, which require topical treatment. The PSA level drops steadily during follow-up, reaching a level of 0.4 ng/mL after approximately six months of treatment.
Patient A continues to feel well after two years of follow-up, and the PSA level has remained at 0.2 ng/mL or less. Incontinence that was present after the surgery has ended, but erectile dysfunction remains, despite the use of medications.
Testicular conditions are fairly uncommon but are more prevalent among younger men than older men [164,165]. As with conditions of the prostate, testicular conditions may be associated with similar symptoms, creating a challenge for accurate diagnosis. When evaluating a man who has acute scrotal pain, a primary objective is to distinguish benign conditions from those requiring immediate intervention and from testicular cancer.
Testicular torsion occurs in approximately one in 4,000 male individuals younger than 25 years of age each year [164]. In 90% of cases, intravaginal torsion is caused by a congenital malformation of the processus vaginalis [164]. Predisposing factors include increased testicular volume, testicles with horizontal lie, history of cryptorchidism, and a spermatic cord with a long intrascrotal portion [166]. Surgery to repair the torsion is necessary to save the testicle; thus, early diagnosis is critical [164,165].
The most common misdiagnosis of testicular torsion is epididymitis [164,167]. The first step should be to determine the onset of pain, as testicular torsion is associated with pain of sudden onset; in contrast, the onset of pain is insidious in epididymitis and other conditions [164,165]. The physical examination also plays an important role in distinguishing testicular torsion from epididymitis. A key distinction is the absence of the cremasteric reflex in testicular torsion, which has been found to have a sensitivity of at least 99% in two studies of boys [167,168]. To elicit this reflex, the medial thigh is stroked or pinched, which causes contraction of the cremaster muscle and elevation of the testis. If the testicle moves at least 0.5 cm, the reflex is positive [164]. Other distinguishing features include the area of tenderness, appearance of the scrotum, and testicular lie (Table 9) [164,165,167,168].
DISTINGUISHING BETWEEN TESTICULAR TORSION AND EPIDIDYMITIS
| Sign/Symptom | Testicular Torsion | Epididymitis |
|---|---|---|
| Onset of pain | Sudden (<12 hours) | Insidious |
| Cremasteric reflex | Absent | Present |
| Tenderness | Diffuse; spermatic cord | Epididymal area |
| Appearance of scrotum | Usually normal | Edematous, "orange peel" appearance |
| Testicular lie | High | Normal |
If the diagnosis of testicular torsion is still in question after physical examination or if the onset of pain was 6 to 12 hours previously, color Doppler ultrasonography should be carried out [164,165]. This imaging study has been found to have a sensitivity of 88% and a specificity of 90% in detecting testicular torsion in boys [169]. Decreased or absent blood flow and rotation of the spermatic cord on the affected side are indicators of testicular torsion [164,166]. Scintigraphy with technetium 99m pertechnetate has a higher sensitivity, but this modality is not as readily available as ultrasonography in some institutions [164,170].
A diagnosis of testicular torsion, whether highly suspected or definitive, requires immediate surgical intervention, and a surgical consultation should be obtained [164,165]. The success rate for manual detorsion has been low (approximately 26%), so this procedure should be avoided as an alternative to surgical treatment [164,171].
Inflammation of the epididymis affects a small proportion of men. Few epidemiologic studies are available, but the prevalence has been estimated to be approximately 0.29% to 0.9% and is the same across racial/ethnic populations [172]. Acute epididymitis is usually caused by bacterial infection, and the source of the infection varies. For men who are younger than 35 years of age and sexually active, the source is most commonly an STI. The most frequently identified micro-organisms are C. trachomatis and Neisseria gonorrhoeae [57,173]. The diagnosis and treatment of epididymitis caused by STIs are discussed later in this course.
Among men who are older than 40 years of age, epididymitis is usually associated with bacterial infection of the urinary tract. Epididymitis has also been reported as a side effect of the drug amiodarone, used for ventricular arrhythmias [174]. A review of the literature indicated that the time to onset of the condition ranged from 4 to 71 months and developed at a daily dose of 200–800 mg [174,175]. In many cases, there is no known etiology [176]. When pain, swelling, and/or inflammation persist for more than three months, the condition is considered to be chronic.
Men with acute epididymitis usually present with unilateral pain and tenderness in the testicle [173]. Additional symptoms include dysuria, urinary frequency or urgency, and symptoms related to the source of infection (e.g., fever, chills, or pain). Urinalysis and urine culture should be done to determine the presence of infection [175,176].
Obtaining a careful history is an important first step in the diagnosis of epididymitis. The practitioner should ask about the sexual history; surgical history, especially in the scrotal area; the location, severity, and frequency of pain; and the presence and duration of symptoms [176]. When symptoms have been present for three months or longer, the Chronic Epididymitis Symptom Index can help determine the impact of symptoms on the quality of life [176].
As stated previously, several findings on physical examination can distinguish epididymitis from testicular torsion [164,165,167,168]. The physical examination should also include evaluation of the abdomen, especially to check for tenderness in the flank and bladder distention, and the inguinal regions [165]. Examination of the scrotum should be carried out bilaterally, assessing the degree of swelling, presence of erythema, and differences in size [165].
Acute infectious epididymitis is treated by addressing the underlying infection, and antibiotics should be chosen according to the causal micro-organism. Symptomatic relief for both infectious and noninfectious epididymitis can be achieved with bed rest, scrotal support and elevation, ice packs, and anti-inflammatory agents or analgesics. If tenderness or swelling persists after treatment with antibiotics or if a mass becomes palpable, further evaluation should be carried out to rule out testicular cancer [173,177]. Watchful waiting is suggested for chronic epididymitis [176].
Consultation with a urologist may be appropriate for men with complications or with chronic epididymitis [173]. Scrotal exploration may be necessary if abscess, testicular infarction, or pyocele develops. Epididymectomy has been used to treat chronic epididymitis, but the outcomes have varied widely [176].
A varicocele is a dilated, tortuous inflammation of the veins of the spermatic cord above the testicle. A prevailing thought has been that the superior mesenteric artery compresses the left renal vein over the aorta, also known as the "nutcracker effect" [178]. This theory has been confirmed by studies that have shown that varicoceles are less common in obese men [178,179]. It has also been suggested that the condition is caused by damage to the contractile mechanism of the smooth muscle organization of spermatic veins [180]. As a result of anatomic differences, the condition is more common in the left testicle, but advances in imaging have led to reports of high rates of bilaterality [181]. Varicocele can cause discomfort in the scrotal area, but usually the condition is asymptomatic [165].
The frequency of varicocele among adolescents and young adults is approximately 15% to 20%, and the rate is higher among men who have some level of infertility, with reports of 77% and 81% in some studies [181,182]. A study of older men (mean age: 60.7 years) demonstrated a prevalence of 42% [183].
Varicoceles vary in size, and large ones can be identified through physical examination alone. Varicoceles can have an adverse effect on spermatogenesis, and infertility has been associated with varicoceles that can be palpated [182]. The most significant finding is a feeling of a "bag of worms" when the scrotum is palpated [165,182]. The varicocele may disappear or be substantially reduced when the patient is recumbent [182]. Smaller varicoceles can be detected by asking the patient to perform the Valsalva maneuver in the standing position [182]. In older men (at least 60 years of age), varicoceles have been associated with significantly smaller and soft testes [183]. Color Doppler ultrasonography is the diagnostic procedure of choice when the findings of the clinical examination are inconclusive [182].
The treatment of varicocele depends on several factors, including the age of the patient, the size of the varicocele, the results of semen analyses, and the patient's desire for fertility [182]. Varicoceles in adolescents and young adults have been associated with significant loss of testicular volume and growth arrest of the testes, the risk of which increases with the size of the varicocele [184,185]. These individuals should be monitored with physical examination and semen analyses to detect changes in testicular function, as earlier treatment will increase the likelihood of recovering normal spermatogenetic function [182,186]. Advances in minimally invasive procedures and surgeries have led to significant strides in the management of symptomatic varicoceles [187]. Many experts agree that indications for surgical intervention in adolescents are pain, large varicoceles, hypotrophy of the involved testicle, bilateral varicocele, intratesticular varicocele, and abnormal semen parameters on serial evaluation. The ideal method for treating adolescent varicocele has not been clearly established, but the main task is to decrease the number of recurrences and complications while retaining optimum testicular function. Because of this, many surgeons respect the attitude "catch-up growth" [188]. Treatment approaches and outcomes of therapy are discussed more fully in the section on infertility.
Testicular cancers are primarily germ cell tumors and are classified as seminomas and nonseminomas, the latter type being more clinically aggressive [177]. Testicular cancer is rare, accounting for 0.5% of all malignant tumors [177,190]. However, the worldwide incidence of this type of cancer has been increasing in the past six decades [177]. As with other testicular conditions, this cancer is most common among male individuals 20 to 34 years of age [177,189]. Early detection results in a cure rate of approximately 95% [177].
In 2019, there were an estimated 283,792 men living with testicular cancer in the United States [190]. In 2022, there will be an estimated 9,910 new cases of testicular cancer and 460 deaths. According to 2000–2019 SEER data, the incidence is highest among non-Hispanic White men (7.3 per 100,000), followed by American Indian/Alaska Native (10.6 per 100,000) and Hispanic men (5.9 per 100,000), Asian/Pacific Islander men (2.4 per 100,000), and Black men (1.5 per 100,000) [191].
Among the several risk factors for testicular cancer, the primary one is cryptorchidism, which can increase the risk 11-fold [177]. Other risk factors include a family history of the disease, testicular dysgenesis, and Klinefelter syndrome [177]. A history of cancer in one testicle confers an increased risk (2% to 5%) of cancer in the contralateral testicle over the 25 years following diagnosis [192].
The USPSTF does not recommend routine screening for testicular cancer—by either clinician examination or self-examination—for asymptomatic adolescent and adult male individuals, as there is no evidence that screening reduces mortality [193]. The USPSTF notes that instead of screening, men should be advised to report testicular problems promptly, as cure rates are high for any stage of testicular cancer [193].
Testicular cancer usually presents as discomfort or swelling in the testicles that is suggestive of epididymitis or orchitis [177]. Physical examination will demonstrate a palpable mass [177]. Occasionally, the patient may note tender or swollen breasts or loss of sex drive.
According to the NCCN guideline for the treatment of testicular cancer, testicular ultrasonography is optional if a diagnosis is obvious from the physical examination, but the guideline notes that this diagnostic test is usually done to define the lesion [177]. Both the NCCN and ASCO recommend measuring serum levels of alpha-fetoprotein (AFP), human chorionic gonadotropin (beta-hCG), and lactate dehydrogenase (LDH) to help determine if the testicular mass is a germ cell tumor and, if so, whether it is a seminoma or a nonseminoma [177,194]. A nonseminoma is associated with an elevated AFP level; in contrast, an elevated level of beta-hCG, with a normal AFP level, usually indicates a seminoma [177]. Additional evaluation should include a chest x-ray and CT of the abdomen and pelvis to determine if lymph nodes are involved [177]. If metastatic disease is suspected, further imaging studies, such as bone scan, magnetic resonance imaging, or positron emission tomography, may be necessary. Open biopsy is not usually performed [177].
Men with suspected testicular cancer should be referred to an oncologist who will discuss treatment options, which include orchiectomy and radiation therapy or chemotherapy, depending on the type of tumor and the stage of disease. Lymph node dissection may also be necessary for metastatic disease. The possibility of sperm banking should be discussed before any type of treatment is initiated [177].
Treatment options for early-stage seminoma (stage I, confined to the testicle and epididymis) are active surveillance (preferred), single-agent carboplatin (one or two cycles), or radiation therapy [177].
Radiation therapy is recommended for stage II seminoma (involvement of nearby lymph nodes), with the treated area extended to include the ipsilateral iliac lymph nodes [177]. If radiation is contraindicated, chemotherapy with three cycles of bleomycin, etoposide, and cisplatin (BEP) or four cycles of etoposide and cisplatin (EP) is recommended. If chemotherapy is given, both regimens are recommended [177]. Chemotherapy with EP or BEP is recommended for stage III seminoma (involvement of distant lymph nodes and/or viscera) [177].
Treatment options for nonseminoma include surveillance, chemotherapy, and retroperitoneal lymph node dissection [177]. Selecting the appropriate therapy involves consideration of many factors, including the extent of disease in the lymph nodes, the levels of serum tumor markers before and during treatment, radiographic findings, and the commitment of the patient to adhere to surveillance protocols that involve frequent blood work and CT [177]. Chemotherapy involves either EP or BEP [177].
The cure rates for testicular cancer are high, even when cancer is at an advanced stage at the time of diagnosis [177]. The overall five-year survival for testicular cancer (all stages) is 95.2% [190].
Men who have been treated for testicular cancer should be followed up at regular intervals to monitor for signs of recurrence. Follow-up visits typically include a history and physical examination and serum tumor markers. The ASCO guideline on the serum tumor markers for male individuals with germ cell tumors notes that there is insufficient evidence to determine whether monitoring tumor markers improves survival or health outcomes but nonetheless recommends measuring AFP and beta-hCG levels during each surveillance visit, and the NCCN also recommends an LDH as part of surveillance [194]. Evidence is also lacking regarding optimal surveillance intervals, and the intervals vary according to diagnosis (seminoma or nonseminoma) and stage of disease [177]. In general, the recommended intervals are every two months in the first year, every three months in the second year, every six months in the third and fourth years, and annually thereafter [177]. It is recommended that surveillance continue for at least 10 years [177,194]. Chest x-ray and computed tomography of the abdomen and pelvis are recommended at greater intervals [177].
The follow-up evaluation plays an important role in assessing for the long-term effects of treatment. The primary effect of chemotherapy is oligospermia, but sperm production can be recovered [195,196]. A population-based study found that 70% of testicular cancer survivors fathered children [197]. Secondary acute leukemias have been reported to develop after chemotherapy and radiation therapy, and other consequences of platinum-based chemotherapy include hearing deficits and impaired renal function [198,199]. Melanomas and cancers at many sites have been associated with radiation therapy, occurring 10 years or more after treatment [198]. Lastly, the risk of cardiac events has been increased for testicular cancer survivors who had been treated with radiation therapy and/or chemotherapy [200].
Breast cancer in men is rare; an estimated 2,710 new cases will be diagnosed in the United States in 2022, and an estimated 530 men will die of the disease [16]. These figures represent less than 1% of all breast cancer diagnosed in this country. Although the numbers are low, the prevalence has increased 26% since the early 1980s, prompting increased attention and highlighting the need to emphasize to men—and their healthcare providers—that breast cancer is not confined to women [201]. The lack of awareness of the disease has led to a longer time between the development of symptoms and diagnosis and to a later age (mean age: 67 years) and stage of disease at the time of diagnosis compared with women [201,202].
Male breast cancer has not been extensively studied, and research is difficult because of the small numbers of men with the disease. Reviews of the literature have been helpful in identifying risk factors, clinical and pathologic characteristics, and the role of genetics [201,202,203]. Studies have shown that male breast cancer differs from female breast cancer in many ways. For example, some risk factors unique to men include the following [203]:
Undescended testes
Orchiectomy
Infertility
Gynecomastia
Mastitis
Breast trauma
Infertility
Klinefelter syndrome
Radiation to the chest wall
BRCA2 mutation is found in approximately 4% to 16% of men with breast cancer [203].
A painless subareolar lump or swelling is the most common presenting symptom, occurring in approximately 85% of men with breast cancer [201,204]. Other common symptoms are nipple retraction, localized pain, or nipple ulceration, bleeding, or discharge. About 1% to 2% of men will have no symptoms [201,204]. In diagnosing male breast cancer, the primary consideration is to distinguish cancer from gynecomastia, which is present in about 30% of healthy men [202].
The approach to the diagnostic evaluation of male breast cancer is the same as for female breast cancer. A history and physical examination will help determine potential risk factors and identify the clinical features. Mammography has good sensitivity and specificity, and ultrasonography may be useful, especially for detecting involvement of the lymph nodes [202]. Biopsy is essential for elucidating the pathologic characteristics. In male breast cancers, the overexpression of estrogen receptor and progesterone receptors is likely [203,205].
As noted, data on male breast cancer are limited, and recommendations for treatment have been extrapolated from the literature on female breast cancer and from small series of men with the disease. Modified radical mastectomy is used most often, with lumpectomy rarely performed [203]. Sentinel node biopsy has also been effective in men [206,207]. Adjuvant radiation therapy has been associated with a lower local recurrence rate and a higher survival rate [202,203]. Adjuvant chemotherapy has been carried out according to guidelines for women at high risk for recurrence. Adjuvant hormone therapy has a clear role in the treatment of men with hormone receptor-positive cancer, with reductions in recurrence and death [204,208]. In addition, tamoxifen has led to a 50% response rate for metastatic breast cancer [202].
Five-year survival rates for men with breast cancer have been reported to be between 40% and 65% [201,202]. In one retrospective study, the median survival was 87 months (83 months for men with invasive disease) [203]. Older age, higher stage of disease, and increasing tumor size have been associated with shorter survival [203]. The risk of second cancers (breast and nonbreast) appears to be high [209].
Sexual dysfunction affects more than a quarter of men, yet attention to sexual health is low because of the lack of validated evidence-based guidelines for diagnosis and treatment as well as men's hesitancy to discuss sexual health issues with their primary healthcare providers [210,211]. Clinicians should include questions about sexual function in routine health evaluations and foster an environment of trust and open dialogue to help elicit information on sexual health from their male patients.
Issues related to sexual health change over the course of a man's lifetime. Early ejaculation is of concern to men across the ages, erectile dysfunction and late-onset hypogonadism are of special concern to older men, and infertility and STIs are more common issues among younger men.
The AUA definition of premature ejaculation is "poor ejaculatory control, associated bother, and ejaculation within about two minutes of initiation of penetrative sex that has been present since sexual debut" [354]. This definition and others have not been evidence based, however, and the International Society of Sexual Medicine charged a panel of experts with developing an evidence-based definition. According to this definition, premature ejaculation is "a male sexual dysfunction characterized by ejaculation which always or nearly always occurs prior to or within about one minute of vaginal penetration, and the inability to delay ejaculation on all or nearly all vaginal penetrations, and negative personal consequences, such as distress, bother, frustration, and/or the avoidance of sexual intimacy" [213]. The definition is limited to men with lifelong premature ejaculation and those for whom the condition is not caused by another physical, mental, or psychological health condition. Some have called for the condition to be called "early" ejaculation as a more accurate description of the condition [214].
Premature ejaculation is thought to be the most common sexual disorder among men, and the condition is associated with a high rate of psychosocial distress and has a substantial impact on men's relationships with their partners [215,216].
The reported prevalence of premature ejaculation in the United States has varied widely, ranging from 5% to 40%, depending primarily on the definition [210,212]. The highest prevalence is found among men who are 60 years of age or older [214].
There are no established criteria for the diagnosis of premature ejaculation; clinicians should assess medical, relationship, and sexual history and perform a focused physical examination to make the diagnosis [354]. Laboratory studies or physiologic testing is needed only if the history or physical examination suggests a complex cause [212,354]. Among the details to be elicited from the history are [212]:
Frequency and duration of premature ejaculation
Relationship of premature ejaculation to specific partners
Degree of stimulus resulting in premature ejaculation
Nature and frequency of sexual activity (foreplay, masturbation, intercourse, use of visual cues)
Impact of premature ejaculation on sexual activity
Types and quality of personal relationships and quality of life
Aggravating or alleviating factors
Relationship to drug use or misuse
The patient's partner may be helpful in providing a description of the problem, and care should be taken to distinguish premature ejaculation from erectile dysfunction [212]. The AUA recommends that, for men with concomitant premature ejaculation and erectile dysfunction, erectile dysfunction should be treated first [212].
The treatment approaches for premature ejaculation include psychologic, behavioral, and pharmacologic therapies, and the risks and benefits of all options should be discussed with the patient and, when possible, his partner [212,354]. Behavioral therapy was once considered to be the standard therapy, but studies have shown that the best approach may involve a combination of therapies to address the limitations of each approach as well as the multimodal causes of premature ejaculation [210,217,218]. The 2022 AUA/Sexual Medicine Society of North America guideline recommends that, in addition to pharmacologic treatment, providers consider referring men with premature ejaculation to a mental health professional with expertise in sexual health [354].
No medication has been approved for the treatment of premature ejaculation, leaving the pharmacologic treatment to involve the off-label use of serotonin reuptake inhibitors or topical anesthetics that act by prolonging the latency of ejaculation [210,212,218,219,354]. The recommended first-line pharmacotherapeutic options are "on demand" clomipramine; a nonselective serotonin reuptake inhibitor; daily selective serotonin reuptake inhibitor (e.g., fluoxetine, paroxetine, sertraline); and topical penile anesthetics [212,354]. The doses studied have varied, and dosing is prescribed as either continuous (daily regimen) or situational (taken only before sexual activity); the optimal duration of therapy has not been determined (Table 10) [212,354]. The side effects of these drugs have not been evaluated outside the depression setting, but the effects appear to be similar for men who are not using the drug for depression, with the most common effects being nausea, dry mouth, and drowsiness [212].
AUA RECOMMENDED PHARMACOLOGIC THERAPY OPTIONS FOR PREMATURE EJACULATION
| Agent | Daily Dosea | Pre-Intercourse Dose (On Demand) |
|---|---|---|
| Nonselective serotonin reuptake inhibitor | ||
| Clomipramine (Anafranil) | 12.5–50 mg | 25–50 mg (4 to 24 hours prior to sexual activity) |
| Selective serotonin reuptake inhibitors | ||
| Fluoxetine (Prozac) | 5–20 mg | — |
| Paroxetine (Paxil) | 10 mg, 20 mg, or 40 mg | 20 mg (3 to 4 hours prior to sexual activity) |
| Sertraline (Zoloft) | 25–200 mg | 50 mg (4 to 8 hours prior to sexual activity) |
| Topical agent | ||
| Lidocaine/prilocaine cream (EMLA cream) | — | Lidocaine 2.5%/prilocaine 2.5% (20 to 30 minutes prior to sexual activity) |
| aThe lowest dose should be used when beginning therapy, with upward titration based on response. | ||
Treatment with topical lidocaine/prilocaine has also been shown to be effective in increasing the latency of ejaculation and is another option recommended by the AUA [212,220,221]. The drug is typically applied 20 to 30 minutes before sexual activity; earlier application (30 to 45 minutes prior to sexual activity) has led to numbness of the penis and loss of erection in a substantial number of men [221]. Topical treatment avoids adverse events associated with systemic therapy [222]. In 2016, the European Union approved a topical eutectic lidocaine/prilocaine metered-dose spray (Fortacin) for use in the treatment of primary premature ejaculation [223,224]. The spray has not been approved for this use in the United States [225].
One drug, dapoxetine, a short-acting selective serotonin reuptake inhibitor, is the first drug developed specifically for premature ejaculation, and it has been approved for use in several European countries, but not in the United States or Canada [222]. Several studies and systematic reviews have shown dapoxetine to substantially improve (compared with placebo) intravaginal ejaculatory latency time, perceived control, and patient-reported global impression of change and decrease related personal distress and difficulty [222,226,227,228]. However, the agent is characterized by discontinuation rates of up to 90%, primarily due to side effects, cost issues, efficacy below expectations, and the need for scheduling sexual intercourse [224]. The most common side effects have been nausea, dizziness, diarrhea, insomnia, and headache.
Psychological and behavioral therapies are valuable components of treatment [210,217,218]. Relationship counseling and sex therapy can help facilitate communication between the patient and his partner and ease tension surrounding sexual activity. Psychologic and behavioral therapies should focus on gaining confidence, learning control techniques, lessening performance anxiety, overcoming barriers to intimacy, achieving pleasure, and gaining satisfaction [210,217].
Erectile dysfunction can be conceptualized as an impairment in the arousal phase of sexual response and is defined by the AUA as "the consistent or recurrent inability to attain or maintain penile erection sufficient for sexual satisfaction, including satisfactory sexual performance" [355]. Erectile dysfunction is primarily a vascular disorder, but hormonal, neurologic, and psychologic factors are also involved. Approximately 70% of cases are organic and not of psychologic origin [229]. The term erectile dysfunction has come to replace "impotence" to more accurately describe a condition that is not associated with a loss of sexual desire or problems with ejaculation or orgasm [230].
Erectile dysfunction is estimated to affect 50 million men in the United States and more than 150 million men worldwide [231]. The prevalence has ranged from 10% to 30% among men 40 to 49 years of age and from 25% to 76% among men older than 70 years of age [232,233,234]. Ethnicity has also been a factor, with a higher rate for Black men and a lower rate for Hispanic men compared with White men [232]. However, another study showed that Hispanic men were more likely to report erectile dysfunction [234].
Erectile dysfunction has been reported to be more common among men with comorbidities; independent risk factors include age, diabetes, metabolic syndrome, cardiovascular disease, obesity, and sedentary lifestyle [214,234,235]. Among men with no known cardiovascular disease, erectile dysfunction has preceded coronary artery disease, stroke, and peripheral artery disease by an average of three years (range: two to five years) [236]. In addition, a meta-analysis (14 cohort studies; 92,757 men) showed that erectile dysfunction was an independent risk factor for cardiovascular and cerebrovascular events [237]. Other risk factors for erectile dysfunction include hormone disorders, neurologic conditions, psychologic disorders, history of surgery or radiation in the pelvic region, use of illicit drugs, and some prescription drugs (most notably, antihypertension agents) [238]. Encouraging men with these risk factors to modify their lifestyle and/or treating comorbidities may help reduce the risk of erectile dysfunction [239].
A detailed medical history is integral to diagnosing erectile dysfunction, as the history may elucidate an underlying cause. It is important to also document a psychosocial and sexual history to evaluate the potential of other related or contributing factors [230]. The physical examination should involve assessment of the abdomen, genitals, and pulses in the lower extremity [230]. Validated questionnaires are recommended to assess the severity of erectile dysfunction, to measure treatment effectiveness, and to guide future management [355]. A morning serum total testosterone should be measured routinely; selected laboratory studies to consider are fasting glucose and serum lipid profile, hemoglobin A1c, and thyroid function tests [355].
Erectile dysfunction is best managed with a combination approach [235]. Because of the strong relationship between erectile dysfunction and modifiable risk factors, lifestyle changes should be a first-line approach to managing the condition. The importance of achieving or maintaining a healthy body mass index, increasing exercise, and smoking cessation should be emphasized, especially given the relationship between erectile dysfunction and cardiovascular disease.
After treatment of erectile dysfunction is initiated, referral to a mental health professional should be considered to promote treatment adherence, reduce performance anxiety, and integrate therapies into a sexual relationship [355]. Both the AUA and the ACP recommend oral phosphodiesterase-5 inhibitors as first-line pharmacotherapy for erectile dysfunction in men for whom this class of drugs is not contraindicated [230,231,355]. Four drugs in the class have been approved for use in the treatment of erectile dysfunction: sildenafil (Viagra), tadalafil (Cialis), vardenafil (Levitra), and avanafil (Stendra, Spedra). Sildenafil and vardenafil differ from tadalafil with respect to the time to maximum serum level (1 hour vs. 2 hours) and serum half-life (4 hours vs. 18 hours) [230]. Furthermore, the duration of action is longest for tadalafil (up to 36 hours) [240]. The inhibitory effect of these drugs causes vascular smooth muscle relaxation in the corpus cavernosum, resulting in increased erection hardness and prolonged duration in men with erectile dysfunction who have sufficient intact vasculature [355].
Data from multiple trials and systematic reviews have demonstrated similar efficacy for phosphodiesterase-5 inhibitors in treating erectile dysfunction, particularly for sildenafil, tadalafil, and vardenafil [355]. Each of these drugs substantially improves erectile function and successful sexual intercourse compared with placebo [231]. Relative efficacy is less clear for avanafil because published comparative studies are limited. The ACP notes that there is insufficient evidence for recommending one drug over another and suggests that the choice be made according to the preferences of an individual patient with respect to ease of use, cost, and the adverse effects profile [231]. One systematic review and meta-analysis found evidence that tadalafil is the most effective agent, followed by vardenafil, with no major differences in the safety profile of any of the phosphodiesterase-5 inhibitors [241].
The side effects of all four drugs are similar, with headache, dyspepsia, facial flushing, nasal congestion, and visual disturbances being the most common events [230,240,242]. The FDA has issued two mandates to revise labeling of these agents. In 2005, the agency required labels for sildenafil, tadalafil, and vardenafil to reflect the possibility of sudden vision loss after taking the drugs for a period of time [243]. The alert was associated with several case reports suggesting a temporal association between use of one of the drugs and nonarteritic anterior ischemic optical neuropathy (NAION), a rare disorder characterized by sudden loss of vision in one eye [243,355]. However, subsequent studies showed that the risk of NAION was similar among men who were and were not taking a phosphodiesterase-5 inhibitor [244,245]. Risk factors for spontaneous NAION include older age, White race, small optic discs with low cup-to-disc ratio, and vascular disease, leading some investigators to suggest an examination of the fundus be performed on men who may be at higher risk for NAION before a phosphodiesterase-5 inhibitor is prescribed [243].
In 2007, the FDA mandated changes to the labels of phosphodiasterase-5 inhibitors to more prominently display warnings about the potential for sudden hearing loss [246]. A cross-sectional population-based study of more than 11,000 men subsequently demonstrated a higher likelihood of self-reported hearing loss associated with use of any phosphodiasterase-5 inhibitor (odds ratio: 2.23), but the association was significant only for sildenafil [247].
Use of a phosphodiesterase-5 inhibitor is contraindicated in several situations. They should not be taken by men who take organic nitrates (nitroglycerin) or nitrites (amyl nitrite) [248,249]. Vardenafil should not be used for men with a history of prolonged QT interval (or who take medication to prolong the QT interval) [230]. The use of a phosphodiesterase-5 inhibitor concomitantly with an alpha-blocker for lower urinary tract symptoms may lead to increased systemic vasodilation and hypotension [230].
Men who are being treated with a phosphodiesterase-5 inhibitor should be followed up closely to monitor efficacy and side effects. Attention to changes in health status and other medications is essential to avoid drug interactions. Clinicians should emphasize the importance of men providing information about treatment with a phosphodiesterase-5 inhibitor in case of a cardiovascular emergency [230].
Although the initial treatment option preferred by most men with erectile dysfunction is a phosphodiesterase-5 inhibiter, the AUA Panel notes that it is valid for men to begin with any type of established treatment, and recommends that patients be informed of all treatment options that are not medically contraindicated. The AUA guideline provides data on success rates, patient and partner satisfaction rates, and potential adverse effects for the following treatment options [355]:
Vacuum erection device: An effective, low-cost option with high rates of patient and partner satisfaction. May have a role as "rescue device" or adjunct to pharmacologic therapy.
Intraurethral alprostadil: Involves insertion of a delivery catheter into the urethral meatus and depositing an alprostadil tablet in the urethra; requires an in-office trial to insure effectiveness and safety. Variable rates of success (30% to 78%).
Intracavernosal injection: Administered by injecting medication (i.e. alprostadil) into the corpus cavernosa of the penis to produce an erection; an in-office injection test should be performed. Reported success rates range from 58% to 100%.
Penile prosthesis implantation: Surgical procedure that requires thorough patient and partner counselling. Available devices include malleable (non-inflatable) models as well as inflatable prostheses. Satisfaction rates vary across models, ranging from 66% to 88%.
Intracavernosal injection of a vasoactive drug is associated with the highest potential for priapism, and clinicians should ensure that men understand the correct technique and the importance of seeking medical intervention for a prolonged erection [230]. Only vacuum erection devices with a limiter (a feature that limits the amount of vacuum pressure and reduces potential for penile injury) should be recommended, whether purchased over the counter or procured by prescription [230,355]. The AUA advises that for men with erectile dysfunction, low-intensity extracorporeal shock wave therapy and intracavernosal stem cell therapy are considered investigational treatment options [355]. The risks associated with penile prostheses include mechanical failure, erosion, and infection [230]. The AUA guideline does not recommend the use of trazodone, testosterone therapy (for men with normal serum levels), or yohimbine and other herbal therapies [230].
Psychosocial therapy is an important component of treatment for erectile dysfunction. A meta-analysis showed that group psychotherapy in combination with sildenafil significantly improved erectile function and successful sexual intercourse compared with sildenafil alone [250].
In both men and women, levels of sex hormones decline with age. However, the ways in which these levels change and the symptoms associated with the decline differ greatly between men and women. There is no well-defined equivalent of menopause in men, although the phrase "andropause" is used frequently to refer to decreased testosterone and resulting symptoms. Other phrases, most notably androgen deficiency syndrome and late-onset hypogonadism, may be more accurate descriptors of the process. By any name, the condition is a complex of symptoms that includes loss of sexual satisfaction and overall well-being [251]. The condition is related to lower testosterone levels, which begin to decrease 1% to 2% each year beginning at 30 years of age [252].
Late-onset hypogonadism is distinct from hypogonadism in younger male individuals. For boys and young men, hypogonadism is related to testicular failure and is usually associated with a congenital abnormality, most often Klinefelter syndrome [251]. In older men with hypogonadism, testosterone levels are rarely as low as the levels in young men with primary hypogonadism [251].
Several important questions about late-onset hypogonadism remain unanswered [252,253]:
It is unclear whether the symptoms are caused by a reduction in testosterone or are a result of the normal physiologic process of aging.
There is no consistent level of testosterone to define hypogonadism, and there is confusion about what testosterone levels should be measured.
There is ongoing debate about the risk-benefit ratio of testosterone therapy for older men.
There is a wide range in the reported prevalence of late-onset hypogonadism. In a population-based observational study, symptomatic androgen deficiency was found in nearly 6% of men 30 to 79 years of age, whereas in the Hypogonadism in Males (HIM) study, the prevalence was nearly 39% among men 45 years of age and older visiting primary care practices [254,255]. The prevalence increases substantially with age and is similar across racial/ethnic populations [254,255].
A diagnosis of late-onset hypogonadism requires both documentation of relevant symptoms and measurement of testosterone levels. The condition is associated with a variety of physiologic, psychologic, cognitive, and sexual symptoms; some signs and symptoms are more specific than others, and no combination of symptoms is typical (Table 11) [252,255].
SYMPTOMS AND SIGNS SUGGESTIVE OF TESTOSTERONE DEFICIENCY IN MEN
| Specific |
| |||||||
| Suggestive |
| |||||||
| Nonspecific |
|
Diagnosing late-onset hypogonadism (testosterone deficiency) is challenging because many signs and symptoms are associated with the normal process of aging or can be attributed to coexisting conditions. Two questionnaires that can help to identify late-onset hypogonadism are the Androgen Deficiency in Aging Males (ADAM) questionnaire and the Aging Males' Symptoms (AMS) scale [256,257,258,259,260]. The ADAM questionnaire consists of 10 questions, and the condition is defined by a positive response to two specific questions: "Do you have a decrease in libido (sex drive)?" and "Are your erections less strong?" or to any three of the other questions [256]. The AMS scale asks men to provide a score of 1 to 5 to each of 17 somatic, psychologic, and sexual symptoms. The ADAM questionnaire has been validated against testosterone levels, whereas the AMS scale was designed to evaluate the quality of life and has not been correlated to testosterone levels [261]. Both have excellent specificity but poor sensitivity [251].
In its updated practice guidelines on the treatment of androgen deficiency, the Endocrine Society recommends making a diagnosis of hypogonadism "in men with symptoms and signs of testosterone deficiency and unequivocally and consistently low serum testosterone and/or free testosterone concentrations (when indicated)" [252]. Serum testosterone level fluctuates in relation to time of day and food intake; peak concentrations occur during the morning hours. Therefore, clinicians should measure total testosterone concentrations on two separate mornings while the patient is fasting [252]. Measured levels should be interpreted with caution as not all laboratories use total testosterone assays harmonized to the national standard [355]. Intercurrent acute illness, nutritional deficiency, and certain medications (e.g., opioids, glucocorticoids) can alter the expected serum testosterone concentration. In general, a total testosterone concentration of 300 ng/dL is the cut-off level below which testosterone replacement therapy is considered for most men with suspected late-onset hypogonadism.
The increase in treatment with testosterone has been tremendous. Although there are benefits of testosterone therapy, there are also many potential risks (Table 12), and the risk-benefit ratio for men with late-onset hypogonadism has not been clearly defined [255,256,261]. Because of questions about the benefits and harms of testosterone, the Endocrine Society is specific in its recommendations for testosterone therapy (Table 13) and recommends against a general policy of offering testosterone therapy to all older men with low testosterone levels [252].
POTENTIAL BENEFITS AND RISKS OF TESTOSTERONE THERAPY
| Benefits | Potential Risks | ||||
|---|---|---|---|---|---|
| Improvement in sexual desire and function | Stimulation of growth of prostate cancer | ||||
| Increase in bone mineral density | Worsening of symptoms related to benign prostatic hypertrophy | ||||
| Improvements in mood, energy, and quality of life | Liver toxicity and liver tumor | ||||
| Change in body composition and improvement in muscle mass and strength | Gynecomastia | ||||
| Improvement in cognitive function |
|
RECOMMENDATIONS OF THE ENDOCRINE SOCIETY REGARDING TESTOSTERONE THERAPY FOR ADULT MEN WITH HYPOGONADISM
| Diagnosis and evaluation |
| ||||||||||
| Treatment |
| ||||||||||
| Monitoring |
| ||||||||||
| Screening |
|
Testosterone replacement is available in several forms, including oral agents, injectable formulations, transdermal gels and patches, and buccal tablets [252,263]. In general, a decision on the type of therapy should be made according to the patient's preference, with consideration of several factors, including pharmacokinetics, cost, ease of use, and side effect profile [252,263].
Close follow-up is essential for men being treated with testosterone replacement. The clinical response and side effects should be monitored at intervals of three to six months [252]. The treatment target should be a testosterone level in the middle of the normal range [252]. Follow-up should include evaluation of the prostate, through determination of PSA levels and DRE at three to six months for men 40 years of age and older who have a baseline PSA greater than 0.6 ng/mL. In addition, a hematocrit level should be determined at three to six months and then annually; treatment should be discontinued if the hematocrit is greater than 54%.
Infertility is clinically defined as the inability to conceive after one year of unprotected intercourse [264]. Approximately 15% of couples are unable to conceive after one year of unprotected intercourse. A male factor is the only cause in approximately 20% of infertile couples and is a contributing factor in another 20% to 40% [264]. Fertility declines with age, and research has shown that men older than 35 years of age are twice as likely to be infertile as men younger than 25 years of age [265,266]. Approximately 15% of infertile men have azoospermia, the complete absence of sperm in the ejaculate [267].
More than half of male infertility or subfertility is potentially correctable; often, the cause is unknown. The causes, both correctable and uncorrectable, include [264,268]:
Varicocele
Obstruction of a duct (epididymal, vasal, or ejaculatory)
Ejaculatory dysfunction
Testicular atrophy
Hypogonadotropic hypogonadism
Infection
Side effects of medication
Environmental toxins
Bilateral cryptorchidism
Genetic abnormality (Y chromosome microdeletion)
Congenital absence of vas deferens
According to the AUA guidelines, evaluation of suspected male infertility should include a complete medical and reproductive history, physical examination, and one or more semen analyses [264,356]. Men with one or more abnormal semen parameters or presumed male infertility should be evaluated by a male reproductive expert. It is important not to rely solely on semen analysis, as an underlying medical or genetic cause of infertility may be missed [268]. Other tests may be necessary, depending on the findings of this initial evaluation. Clinicians should obtain hormonal evaluation including follicle-stimulating hormone (FSH) and serum testosterone for infertile men with any of the following: impaired libido, erectile dysfunction, oligozoospermia or azoospermia, atrophic testes, or evidence of hormonal abnormality on physical examination [356].
The medical history can help to detect an underlying cause of infertility. Factors that can affect fertility include [268]:
Kallmann, Young, or Kartagener syndrome
Pituitary disease
Previous testicular disorders
History of inguinal, scrotal, or retroperitoneal surgery
Anticancer chemotherapy
The reproductive history should address the following issues: frequency and timing of intercourse, duration of fertility effort, use of lubricants, and sexual history (including STIs) [264,267,268].
Physical examination may identify a varicocele, the most common cause of male infertility [165,182]. Other findings on physical examination that may suggest a cause of infertility include small testes (less than 4 cm in greatest dimension or less than 20 cm3), signs of ductal obstruction (induration or engorgement of the vas deferens or epididymis), and abnormal distribution of hair and fat, which may indicate endocrinopathy [268].
As noted, the semen analysis should be carried out on at least two specimens, obtained at least one month apart [264]. The specimens should be collected after two to three days of abstinence. The World Health Organization (WHO) first established reference values for semen analysis in 1987 and published its sixth update in 2021 [269]. The 2020 AUA guideline references the 2010 WHO semen parameters and lower reference limit criteria for male infertility [356]:
Semen volume: 1.5 mL
Total sperm number: 39 million/ejaculate
Sperm concentration: 15 million/mL
Vitality: 58% live
Total motility (progressive + nonprogressive): 40%
Morphologically normal forms: 4.0%
Initially, the updated criteria met with controversy, with some noting that the new reference values would lead to fewer men being classified as infertile based on semen analysis alone [271,272,356]. No single abnormality among sperm parameters is diagnostic of infertility; the odds ratio for infertility increases with the number of abnormal semen parameters, rising sharply with two or more abnormal parameters [356].
Treatment options are available for correctable causes of infertility. Varicoceles can be repaired through open or laparoscopic surgery or by percutaneous embolization [182]. Surgical treatment leads to elimination of the varicocele in 90% of men, with improvement in the semen quality, production of testosterone, and rates of subsequent pregnancy [182,273]. For men with infertility related to obstruction, microsurgical reconstruction of the obstructed duct has led to the appearance of sperm in the ejaculate and higher rates of subsequent pregnancy [267]. Several techniques for retrieving sperm are also available. Options for reproductive assistance and adoption should be explored for men who have uncorrectable infertility. Genetic counseling should be offered to men with nonobstructive azoospermia due to primary testicular failure [267].
STIs are a serious public health concern. There are an estimated 26 million new infections annually and 68 million total STIs in the United States, of which youth 15 to 24 years of age account for about half [357]. In addition to the substantial morbidity associated with STIs, the financial cost is tremendous; nearly $16 billion in direct medical costs annually are associated with the eight major STIs (chlamydia, gonorrhea, hepatitis B virus, HIV, human papillomavirus [HPV], herpes simplex virus type 2 [HSV-2], trichomoniasis, and syphilis) [275]. The large majority of costs are attributable to HIV ($13.7 billion), followed by chlamydia ($691 million), gonorrhea ($271 million), and HSV-2 ($91 million) [57].
The discussion here is confined to STIs having the greatest impact on men: chlamydia, gonorrhea, syphilis, HSV-2, and HPV [57]. Although HSV-2 and HPV infections are more common among women than men, the infections have serious implications for men. For example, nearly one-third of the 22,000 HPV-associated cancers that occur each year in the United States develop in men [276]. Infection with HSV-2 increases the risk for HIV, which is particularly important for Black men, who are at greater risk for both HSV-2 and HIV [277].
Despite the availability of comprehensive guidelines for the testing and treatment of STIs, studies have shown poor compliance; in one study, fewer than one-third of individuals with an STI seen in an emergency department received recommended antibiotic treatment, and compliance with history-taking, diagnostic testing, and counseling ranged from 14% to 79% [278]. In addition, improvements in rates of HPV vaccination are needed [279].
The prevalence of STIs according to gender vary with infection; chlamydia, HSV-2, and HPV occur more often among female than male individuals; gonorrhea occurs at similar rates among female and male individuals; and syphilis occurs more often among male than female individuals [57,277,280]. Overall, almost two-thirds of all STIs occur in individuals 15 to 24 years of age [57]. Among men, most STIs are far more prevalent in the non-Hispanic Black population than in other ethnic/racial populations and are least prevalent in the Asian population (Table 14) [57,277,281].
RATE OF COMMON SEXUALLY TRANSMITTED INFECTIONS (STIs) AMONG MEN ACCORDING TO RACE/ETHNICITY, 2020
| STI | Prevalence (per 100,000) | ||||||
|---|---|---|---|---|---|---|---|
| All Men | Black (Non-Hispanic) | American Indian/Alaskan Native | Hispanic | White (Non-Hispanic) | Asian | Native Hawaiian/Other Pacific Islander | |
| Chlamydia | 336.7 | 883.7 | 315.8 | 198.0 | 113.2 | 72.0 | 300.6 |
| Gonorrhea | 236.3 | 819.5 | 272.3 | 144.8 | 77.4 | 46.6 | 195.8 |
| Syphilis (primary and secondary) | 20.7 | 57.7 | 32.6 | 23.4 | 11.0 | 8.9 | 30.7 |
Chlamydia
More than 1.5 million cases of chlamydia were reported to the CDC in 2020 [57]. The 2020 rate of chlamydia infection (481.3 cases per 100,000) represents a decrease of 13% over the rate in 2019. During 2019–2020, rates of reported chlamydia decreased among both men and women. Chlamydial infection occurs more than twice as commonly in women than men, and rates are highest among adolescents and young adults.
Gonorrhea
In 2020, a total of 677,769 cases of gonorrhea were reported to the CDC, making it the second most commonly reported notifiable sexually transmitted disease in the United States [57]. Rates of gonorrhea have increased 111% since the historic low of 98.1 cases per 100,000 in 2009. In 2020, the rate of gonorrhea among men was 236.3 cases per 100,000, compared with 150 cases per 100,000 among women [57].
Syphilis
In 2000–2001, the rate of syphilis (primary and secondary) was 2.1 cases per 100,000; however, the rate has increased almost every year since that time, increasing 6.8% between 2019 and 2020 [57]. In 2020, 133,945 cases of syphilis were reported, including 41,655 cases of primary and secondary syphilis, the most infectious stages of the disease. Rates of syphilis have increased in most racial/ethnic groups, with greatest increases among non-Hispanic American Indian/Alaska Native persons and non-Hispanic persons of multiple races [57]. Young men who have sex with men are disproportionally impacted, accounting for a majority (53%) of all male syphilis cases in 2020 [57].
HSV-2
Genital herpes is a chronic, lifelong viral infection; the prevalence is unknown as the majority of persons infected have not had the condition diagnosed. Many individuals with HSV-2 have mild symptoms or unrecognized infection but shed the virus intermittently in the urogenital area. Consequently, most genital infections are transmitted by persons unaware that they have the infection. Most cases of recurrent genital herpes are caused by HSV-2, and 11.9% of persons 14 to 49 years of age in the United States are estimated to have acquired this infection [173]. In 2020, the CDC estimated the prevalence of HSV-2 at 18.6 million persons, though the actual number is likely to be considerably higher [57,173]. The seroprevalence of HSV-2 is more than twice as high among female individuals (about 34%) than among male individuals (about 15%) [57]. As with other STIs, HSV-2 infection is more common among non-Hispanic Black men than other racial/ethnic populations [57].
HPV
Data on HPV infection in men are limited. According to a data brief published by the National Center for Health Statistics (NCHS), during 2011–2014, the seroprevalence of any HPV was 7.3% among adults 18 to 69 years of age, with 11.5% among men and 3.3% among women [282]. In the HIM study, an ongoing prospective cohort study of the natural history of HPV in men (from the United States, Mexico, and Brazil), the overall prevalence of HPV infection was 65.2%, with the highest rates among White and Black men (71.5% and 66.2%, respectively) and the lowest, among Asian/Pacific Islander men (42.2%) [281,283]. An estimated 34,800 new HPV-attributable cancers occurred every year during 2012–2016; before introduction of HPV vaccines, approximately 355,000 new cases of anogenital warts occurred every year [173].
Prevention and control are keys to lowering the prevalence of STIs, and the primary preventive strategies are: risk assessment, education, and counseling; limiting the number of sexual partners; abstinence or the use of condoms and barriers; and, in the case of HPV, with vaccination [173,276]. The importance of abstaining from sexual activity should be emphasized to individuals with a confirmed STI [173].
Control of STIs involves the identification of asymptomatic individuals and of symptomatic individuals who may not seek health care; effective diagnosis and treatment; and the evaluation, treatment, and counseling of sex partners of infected individuals [173]. The CDC encourages clinicians to promote prevention with patient-centered education that focuses on risk reduction measures directed at an individual patient's personal risk [173]. Obtaining a thorough sexual history is an essential component of prevention, and the CDC suggests asking questions related to [173]:
Partners (gender and number)
Protection (from STIs)
Practices (types of sexual activity)
Past history of STIs (patient and partners)
Prevention (of pregnancy)
Use of injected drugs (patient and partners)
Exchange of money for sex (patient and partners)
Other sexual practices
Practical strategies for risk assessment and counseling are provided in the CDC treatment guidelines document [173]. Healthcare providers should use simple, direct language when asking these questions, taking care to exhibit respect, compassion, and a nonjudgmental attitude [173]. Organizations such as the National Network of STI/HIV Prevention Training Centers, a CDC-funded group, can help providers enhance skills in counseling individuals about prevention. Resources can be found on the organization's website, available at https://www.cdc.gov/std/treatment/resources.htm.
Recommendations for screening vary according to risk and the type of STI (Table 15) [284]. The USPSTF also recommends high-intensity behavioral counseling for all sexually active adolescents and for adults at increased risk for STIs and HIV [284]. The USPSTF has not issued recommendations for screening for HPV, but beginning in 2011, the Advisory Committee on Immunization Practices (ACIP) recommended HPV vaccination for male individuals [276]. The ACIP recommends routine use of quadrivalent HPV vaccine for boys 11 or 12 years of age and for male individuals 13 to 26 years of age who have not initiated or completed the three-dose series [276,286]. The ACIP also notes that men 27 to 45 years of age may also be vaccinated if at high risk, as determined through shared decision-making [276,285,286]. In addition, hepatitis B vaccination is recommended for any patient who is being evaluated for an STI [173].
U.S. PREVENTIVE SERVICES TASK FORCE RECOMMENDATIONS FOR SCREENING FOR SEXUALLY TRANSMITTED INFECTIONS (STIs) IN MALE INDIVIDUALS
| STI | Recommendation |
|---|---|
| Chlamydia and gonorrhea | Insufficient evidence to recommend for or against screening in men |
| Syphilis | Strongly recommend screening for individuals at increased risk |
| Genital herpes | No screening for asymptomatic adults and adolescents |
The symptoms of STIs vary and are often similar to symptoms associated with other conditions of the urogenital tract, and some infected individuals may be asymptomatic.
Infection with chlamydia is often asymptomatic [173]. Diagnosis can be made by testing of a urethral or rectal swab or a urine specimen [173]. Nucleic acid amplification tests are the most sensitive tests and can be used for urine specimens [173].
Primary syphilis usually presents as a solitary chancre that develops at the site of infection approximately three weeks after exposure to the spirochete Treponema pallidum [287]. The chancre is typically painless and must be distinguished from other genital lesions, such as genital herpes, venereal warts, chancroid, and lymphogranuloma venereum (caused by C. trachomatis) [287].
Dark-field microscopy to detect T. pallidum is the optimum method of diagnosing syphilis. Although no such detection tests are commercially available, some laboratories provide locally developed and validated polymerized chain reaction (PCR) tests for the detection of T. pallidum [173]. A presumptive diagnosis of syphilis can be made with two types of serologic tests: nontreponemal tests (Venereal Disease Research Laboratory [VDRL] and rapid plasma regain [RPR] tests) and treponemal tests (such as fluorescent treponemal antibody absorbed [FTA-ABS] tests or the T. pallidum passive particle agglutination [TP-PA] assay) [173]. The CDC notes that using only one type of serologic test is insufficient for a diagnosis [173].
Gonococcal infection, which is caused by Neisseria gonorrhoeae (a gram-negative diplococcus), can lead to either urethritis or epididymitis [288]. Urethritis is accompanied by such symptoms as purulent discharge from the penis, dysuria, or erythema at the meatus [288]. Epididymitis caused by gonococcal infection is usually associated with unilateral testicular pain and no other symptoms [288]. Disseminated infection is rare (1% to 3%) [289]. A diagnosis of gonorrhea is confirmed by Gram stain and culture of urethral discharge or swab specimen for N. gonorrhoeae, or by nucleic acid amplification testing done on a urine sample [173,288]. Both techniques have similar sensitivity and specificity [173].
The CDC recommends that all individuals who are evaluated for gonorrhea should also be evaluated for chlamydia, syphilis, and HIV infection [173]. In one study of more than 3,800 men and women, approximately 10% to 30% of individuals with gonorrhea had concomitant infection with chlamydia [290]. The typical lesions of genital HSV-2 in men appear on or around the penis and are first noted as either a single or multiple erythematosus macular lesion(s). However, these lesions are absent in many infected individuals [173]. Viral culture is the preferred test for the diagnosis of HSV-2, but it requires two to seven days for results. The sensitivity of viral culture depends on the quality of the sample and the time at which the sample is obtained; sensitivity declines as the lesion begins to heal. A PCR test is available and is suggested by the CDC for analysis of cerebrospinal fluid when central nervous system disease is suspected [173]. Type-specific serologic tests are available as laboratory assays and point-of-care tests [173]. These tests have varying degrees of sensitivity for the detection of the HSV-2 antibody (80% to 90%) and specificity of at least 96% [173].
The treatment of STIs has four main goals [173]:
Eradicate infection
Alleviate symptoms and signs
Decrease complications (infertility, chronic pain, dissemination of disease)
Prevent transmission
The CDC has developed comprehensive guidelines for the treatment of STIs, last updated in 2021 (Table 16 and Table 17) [173]. For chlamydia, gonorrhea, or syphilis, single-dose regimens generally offer an advantage for the treatment of individuals with poor healthcare-seeking or compliance behaviors [173]. The CDC notes that for the treatment of syphilis, neither combinations of benzathine penicillin and procaine penicillin nor oral penicillin preparations are considered appropriate and emphasizes the importance of distinguishing the standard benzathine penicillin product widely used in the United States (Bicillin L-A) from the combination benzathine-procaine penicillin (Bicillin C-R); the latter is not appropriate for the treatment of syphilis [173].
TREATMENT OF CHLAMYDIA, SYPHILIS, AND GONORRHEA AS RECOMMENDED BY THE CENTERS FOR DISEASE CONTROL AND PREVENTION
| Infection | Recommended Treatment | Notes | |||||
|---|---|---|---|---|---|---|---|
| Chlamydia |
| A meta-analysis showed treatment failure among men was higher for azithromycin than for doxycycline. | |||||
| Gonorrhea |
|
| |||||
| Primary and secondary syphilis | Benzathine penicillin G 2.4 million units IM (single dose) |
|
TREATMENT OF HSV-2 AS RECOMMENDED BY THE CENTERS FOR DISEASE CONTROL AND PREVENTION
| Drug | Treatment Dosage | ||
|---|---|---|---|
| Initial Infection | Episodic Recurrent Infection | Long-Term Suppression | |
| Acyclovir | 400 mg three times daily for 7 to 10 days OR 200 mg, five times daily for 7 to 10 days | 800 mg two times daily for 5 days OR 800 mg three times daily for 2 days | 400 mg twice daily |
| Famciclovir | 250 mg, three times daily for 7 to 10 days | 125 mg two times daily for 5 days OR 1.0 g two times (single day) | 250 mg twice daily |
| Valacyclovir | 1 g two times daily for 7 to 10 days | 500 mg two times daily for 3 days OR 1.0 g once daily for 5 days | 500–1,000 mg once daily |
In addition to antibiotic treatment, bed rest, scrotal elevation, and analgesics can help to alleviate symptoms such as fever and local inflammation, which are primarily associated with gonorrhea. Beginning treatment as early as possible decreases the likelihood of complications and spread of infection, especially in the case of syphilis [173]. To prevent the transmission of infection, a patient with a confirmed or suspected STI should be told to avoid sexual contact until therapy is completed and he (and/or his partner) no longer has symptoms [173]. The need for sexual partners to be evaluated for treatment should also be emphasized. State and local health departments may provide assistance in arranging for the evaluation and treatment of sex partners of infected men.
HSV-2
The antiviral medications used to treat HSV-2 can only partially control the signs and symptoms of infection; they cannot eradicate the virus or reduce the risk, frequency, or severity of recurrence after the treatment course has been completed [173]. Men with HSV-2 infection should be given medication for episodic treatment of recurrent infection; treatment should begin within one day after the onset of a lesion [173]. If recurrences are frequent (six or more within a year), long-term suppression therapy may be appropriate; such therapy has been shown to reduce the frequency of recurrence by 70% to 80% [173].
Peterman et al. found a 14.7% rate of reinfection among men during the first year after treatment for an STI [291]. An unexpected finding in the study was the high percentage (66%) of asymptomatic infections. The authors suggested that treated individuals be rescreened at three months. The CDC recommends follow-up with clinical examination and serologic evaluation at 6 and 12 months after treatment [173].
All states require that cases of chlamydia, gonorrhea, syphilis, HIV, and acquired immune deficiency syndrome (AIDS) be reported to local health authorities [173]. Clinicians should seek advice from state or local health departments if reporting requirements are unclear [173].
It is difficult to determine an accurate percentage of MSM in the overall population because of the under-reporting of sexual behavior, but surveys indicate that this group of men represents at least 4% and up to approximately 16% of the population seen by any given healthcare professional [58,292,293]. The population that includes MSM (made up of gay, bisexual, and transgender individuals) has been identified as one of the six most underserved groups in the United States, yet medical training and standard resources for healthcare providers lack information on addressing the routine health concerns of this population [292,294]. MSM have specific healthcare needs that clinicians must understand in order to provide appropriate, comprehensive care.
Perhaps the most important health risk for MSM is their avoidance of routine health care [293]. MSM do not seek routine health care for a variety of reasons. They may have difficulty coming to terms with their sexual identity, fear being judged by healthcare professionals, or be embarrassed to discuss their sexual behavior. In addition, many MSM do not recognize their health risks or their need for screening and preventive health care [58,294]. Health risks also may not be recognized by MSM who do seek health care, and they may not be forthcoming about sexual behavior [294,295]. A study has indicated that less than 20% of MSM had discussed their risk of HIV infection with their healthcare provider [296].
Creating a welcoming clinical environment is the first step in fostering an open dialogue between healthcare providers and MSM [240,295]. Among the factors that contribute to such an environment are educational materials about specific healthcare needs for gay and lesbian individuals, a posted statement of nondiscriminatory care, and forms that contain more inclusive choices and gender-neutral language [240,295]. In addition, healthcare professionals and office personnel should maintain a nonhomophobic attitude, communicate clearly and sensitively using gender-neutral terms, and recognize how their own attitudes affect clinical judgments [293,297]. Confidentiality is an important issue for MSM, and healthcare personnel should assure the patient that some information could be kept out of the medical record [240].
Comprehensive health care for MSM must focus on the population's disproportionate risks for several conditions, including STIs, anal and other types of cancer, substance misuse, eating disorders, suicide, and victimization [294]. Thus, it is essential for clinicians to address several issues with MSM [58,173,292,298]:
Use of safe sexual practices
Screening and immunization for hepatitis A and B viruses
Testing and consideration of pre-exposure prophylaxis for HIV infection
Routine screening for STIs
Routine screening for anal HPV-related neoplasia
Potential risk for specific cancers (testicular, Hodgkin lymphoma, Kaposi sarcoma)
Assessment of substance misuse (tobacco, alcohol, cocaine, methamphetamine)
Nutrition and exercise
Evaluation of psychologic well-being and mental health
Screening for violence
Health risks should be addressed at the patient's first visit and each subsequent visit [58]. An algorithm has been developed to help guide recommended screening for MSM (Figure 3) [58]. In addition, because of an increased risk of HPV-related cancer, the ACIP now recommends HPV vaccination for MSM up to 26 years of age if they did not receive the vaccine when they were younger [276].
Sensitivity should be used in obtaining the medical and sexual history, and the sexual history should be placed in context by emphasizing that an understanding of sexual behaviors is essential to evaluating risks and providing optimal care. It should also be noted that a sexual history is an important component in the care of all patients, regardless of their sexual orientation or behaviors. Because of the various stages a man may be in with respect to his sexual identity, care should be taken to distinguish sexual behavior from sexual identity [295,297].
It is also vital to have resources readily available to provide to MSM as needed. Such resources include information on STI clinics, substance misuse facilities, services for victims of abuse, and referrals for counseling. The Gay and Lesbian Medical Association (GLMA) has developed resources to help clinicians provide appropriate care to gay, lesbian, bisexual, and transgender individuals. The GLMA also has a guideline for the care of this population, and the brochure (available at http://www.glma.org) includes a variety of additional resources [295].
It is likely that most healthcare providers will encounter transgender individuals in the course of their professional careers, and all healthcare agencies and providers should be prepared to provide competent and compassionate care for gender-variant individuals. Based on data from 2008, the prevalence of female-to-male (FTM) transsexualism (transmen) is 1 in 30,400–200,000 [362]. A transman is a transgender individual who, assigned female at birth, currently identifies as a man. It is important to note that these patients are men and do not require additional description unless medically necessary.
Caring for transgender individuals is complex and requires some preparation and forethought, taking into account knowledge of anatomical reassignments, the effects of therapy, and cultural sensitivity. Very little has been published regarding the unique ongoing healthcare needs of patients who have undergone gender confirmation. In general, health care should be based on the treatments the patient has received and at what stage he may be in the gender transition. Health promotion awareness and health screening will vary somewhat, but generally the patient will have the same needs as most adult patients in a primary care setting; the patient's gender confirmation process will have little effect on many aspects of health care [363]. Basic preventive services, like sexually transmitted infection testing and cancer screening, can be provided without specific expertise in transgender care [364]. Keep in mind that in some cases, older transmen may not disclose their transgender history to their healthcare providers, as they initially sought treatment at a time when it was common for providers to use very strict guidelines to determine who could and could not receive treatment [365].
For the FTM patient, any residual female organs will require lifelong modified physical exams and risk screenings. These patients may require occasional modified pelvic exams and/or mammograms, and both the provider and the patient may have difficulty finding a comfortable clinical environment [366]. For FTM individuals, gynecologic examinations can heighten their emotional conflict between self-perception and physical anatomy. Respectful communication that maintains dignity, agency, and control is central to mitigating distress during pelvic exams [367]. The routine physical exam should include a breast exam, Pap test, and assessment of bone health and other possible effects of long-term testosterone supplementation.
Psychosocial well-being is important to men, and many conditions or situations can disrupt the sense of well-being. Among the more common factors that can have a negative effect on well-being for both sexes are everyday stressors (positive as well as negative), personal conflicts, traumatic events, and depression. In general, men lack the social support and interpersonal relationships that help women to cope with stresses [299]. Because of this, men differ in their ability to handle stress, with many men resorting to anger, violence, and substance misuse to deal with stress or depression [28,300]. As a result, stress/anger, substance misuse, and depression are among the psychosocial conditions with the most serious health implications for men. Most men will not seek help for psychosocial disorders and may not recognize the symptoms of depression [45,300,301]. Thus, it is important for healthcare providers to address psychosocial well-being and potential threats to well-being as part of routine health evaluations of men.
Stress and anger have long been associated with negative health consequences. Most of the earlier research focused on the effects of stress and hostility on coronary heart disease, and additional research has found a link between hostility and a more rapid decline in lung function in older men [302,303,304]. Appropriate expression of anger has been suggested as a way to improve health, and controlling anger has been shown to promote well-being in older individuals [305].
Safety is also of concern, as anger has been associated with an increased incidence of injuries and violence. In one study, higher levels of anger (at a given moment) were associated with an increased risk of injury, especially in men [306]. In that study, nearly 32% of individuals who had been injured reported having some degree of irritability before the injury. Men are the usual perpetrators of intimate partner violence causing injury, and these men tend to be younger (18 to 35 years of age), to be from a racial/ethnic minority population, and to have low socioeconomic status [307,308]. Substance misuse and unemployment are also associated with such violence [307]. However, identifying a perpetrator of intimate partner violence in a clinical setting is difficult [308]. It is important to remember that men can also be victims of intimate partner violence, and this is especially true for MSM [309].
Although the USPSTF found insufficient evidence for or against routine screening for intimate partner violence (including child abuse and elder abuse), a survey of patients within a private family practice network showed that 97% of respondents believed that physicians should ask patients about family stress and conflict [310,311]. The survey sample included women who had been physically hurt by intimate partner violence as well as men who had admitted perpetrating such injury. These findings support early studies that indicated patient preference for clinicians to ask questions about physical and sexual abuse [312]. The American Academy of Family Physicians (AAFP) notes that family physicians have the opportunity to provide early intervention in family violence through routine screening and identification of abuse; thus, physicians should be alert for the presence of family violence in virtually every patient encounter [313]. It seems reasonable and appropriate for clinicians to include within routine health assessments of men questions about feelings of anger and frustration and urges to strike family members [307,309]. Suggestions for strategies that focus on anger management and conflict resolution may be helpful, especially for adolescents and young men [309].
As noted, substance misuse is higher among men than among women in all age categories, and men are more likely to have psychosocial problems related to the misuse [28,307]. Although the rate of alcohol misuse is highest among younger men, men older than 65 years of age are of special concern because they are much more likely than women to be "problem" drinkers and to misuse a wide range of illicit as well as prescription drugs [307]. As the general population ages, the misuse of illicit drugs is expected to increase [314]. Adding to this problem is the low rate of screening for alcohol misuse in the older population and the secrecy of many men about drug use [314,315].
Additional concerns are the use of anabolic steroids among adolescents and young adult men and the use of methamphetamine among MSM. Use of anabolic steroids begins during the teenage years in approximately 25% of cases, and about 10% of all users are teenagers [316]. The prevalence of methamphetamine use among MSM is approximately 10% to 20%, a rate that is 10 times higher than that in the general population [317].
Several professional organizations, including the USPSTF, recommend screening and behavioral counseling intervention to reduce alcohol misuse [318]. However, reported rates of screening have been low [319]. Several screening instruments have been developed, and they vary in the number of questions, the populations for which they are best suited, and their usefulness in specific situations; no one tool is perfect [320,321,322,323]. The CAGE questionnaire, which includes four questions, is best for detecting alcohol dependency and is easy and quick to perform [320,321]. However, the test may not detect low, but risky, levels of drinking [307,324]. The Alcohol Use Disorders Identification Test (AUDIT) is the most accurate for detecting problem drinking [319,322].
Screening in the older population is especially important, as low levels of alcohol use can cause morbidity due to age-related physiologic changes, comorbidities, and the use of prescription medications [325]. Screening tools developed specifically for older individuals should be used, such as the geriatric version of the Michigan Alcohol Screening Test (MAST) or the Alcohol-Related Problems Survey (ARPS) [325,326,327]. Clinicians should also ask specific questions about drug use.
A medical history is also helpful, and a family history of alcoholism is a risk factor [319]. Clues to a problem with alcohol can be provided by such symptoms as amnesic episodes, mood swings, chronic fatigue, gastrointestinal symptoms, anxiety, and excessive sweating [319]. Several physical findings can suggest that a patient has a problem with alcohol or drugs, including [319,324]:
Mild tremor
Unsteady gait
Tachycardia
Odor of alcohol or marijuana
Enlarged, tender liver
Nasal irritation (cocaine use)
Conjunctival irritation (marijuana use)
Excessive use of aftershave or mouthwash
Signs of chronic obstructive pulmonary disease, hepatitis B or C, or HIV infection
Signs that should raise a "red flag" about substance misuse are frequent absences from work or school, history of frequent trauma or accidental injuries, depression or anxiety, other substance misuse, labile hypertension, sexual dysfunction, sleep disorders, poor nutrition, gastrointestinal symptoms, and interpersonal conflicts [307,319,324].
Clinicians should provide brief interventions, such as short counseling strategies, for men who are identified to have at-risk drinking. These interventions have been shown to be effective [284,319,324]. Alcoholism and drug addiction are best treated by an addiction medicine specialist or through an inpatient or outpatient program [324]. Primary care providers should have referrals for counseling and treatment readily available, as well as resources on support groups, such as Alcoholics Anonymous and Narcotics Anonymous.
To help healthcare professionals carry out the appropriate diagnosis and treatment of patients with alcohol problems, the National Institutes on Alcoholism and Alcohol Abuse (NIAAA) developed the publication Helping Patients Who Drink Too Much: A Clinician's Guide, which features an updated guideline on screening and brief intervention. The most recent edition is available on the NIAAA website at https://pubs.niaaa.nih.gov/publications/practitioner/cliniciansguide2005/guide.pdf.
Depression is often regarded as a "woman's disease" because it is diagnosed more frequently in women than men. However, researchers and the health community at large now realize that depression is of serious concern in men and is underdiagnosed [28,328]. According to data from 2020, the prevalence of major depressive episode was 6.2% among men and 10.5% among women [329].
Despite the lower rates of depression in men compared with women, the rate of completed suicide is nearly four times higher for men (25.8 vs. 7.1 per 100,000) [25]. Suicide is a leading cause of death for men in many age groups and across all racial/ethnic populations, except for the Black population [25].
The underdiagnosis of depression in men involves clinician-related and patient-related factors. Clinicians' lack of appropriate training and discomfort with dealing with depression contribute to a low rate of diagnosis, estimated to be about 50% [3,330]. In addition, no screening instrument for suicide risk has been shown to reliably detect suicide risk in primary care populations [331]. This is unfortunate, as primary care providers appear to be in a position to intervene. As many as 83% of people who died by suicide had contact with their primary care physician in the year before death, with approximately 20% seeing their physician one day before death [330,332]. In addition, 50% to 66% of individuals who committed suicide saw their primary care physician within one month of their death, with 10% to 40% committing suicide within one week of the visit [331]. Thus, better recognition of depression and suicide risk by primary care providers may help reduce suicide rates.
Many patient-related factors in the underdiagnosis of depression are primarily related to gender issues, including [28,300,328,330,333,334]:
Reluctance of men to seek help
Lack of men's recognition of the symptoms of depression
Hesitancy of men to express emotions
Tendency for men to see depression as a weakness
Men's misconceptions about mental illness and its treatment
Because men are less likely to express their emotions, they may recognize and discuss only the physical symptoms of depression, making diagnosis a challenge [300,301,333]. A carefully taken history can elicit information about risk factors, which include a family history of depression, the use of some medications (beta blockers, histamine H2-receptor antagonists, benzodiazepines, and methyldopa), chronic illness or other comorbidity, lack of social support, recent life stressor, and single marital status [307,335]. Substance misuse frequently occurs concomitantly with depression, more often in men than women, but the direction of the causal relationship is not clear [300,335].
Many of the symptoms of depression reported by women are the same for men: depressed mood, changes in appetite and sleep habits, problems with concentration, and an inability to find pleasure in once pleasurable activities [300]. It has been proposed that the symptoms of depression in men represent a male depressive syndrome, characterized by such symptoms as irritability, acting-out, aggression, low tolerance of stress, low impulse control, tendency to blame others, and a greater willingness to take risk [28,300,330,333]. Men with depression may thus present with a very different symptom profile [328].
Identification of suicide risk is an essential component of the evaluation of patients with depression. Many of the risk factors for suicide are similar to those for depression; when the circumstances surrounding completed suicides were reviewed, the following were found to be factors [25]:
Loss of a partner (through death or other means)
Loss of job
History of mental illness
Depressed mood
Previous suicide attempts
Physical health problems
Intimate partner problem
Preceding or impending crisis (within two weeks)
Financial problem
Clinicians should ask questions to determine the duration of symptoms and explore possible triggers of depression [328]. Because of their lack of experience with discussing emotions, many men may be uncomfortable with open-ended questions such as, "How do you feel?"; rather, discussing emotions in situational contexts can help men better express what they are feeling and why [333]. It may also be helpful to de-emphasize the negative connotation of depression and frame questions within the overall context of health and well-being [314].
The treatment approach will depend on the severity of symptoms and the patient's preference. In general, a combination of psychotherapy and pharmacologic management provides the best results for most men [328,335]. Potential psychotherapy approaches include cognitive behavior therapy and interpersonal psychotherapy [300,307,328]. First-line pharmacologic treatment involves the use of selective serotonin reuptake inhibitors, such as paroxetine, sertraline, and fluoxetine [307]. This treatment approach has efficacy rates of 30% to 70% [328]. Clinicians should emphasize the importance of taking the medication as prescribed, as it may be two to four weeks before a benefit is evident [328]. Depression that is associated with chronic illness is often seen as an inevitable consequence of the disease, but the depression should be treated. Frequently, the treatment improves the overall outcome [335].
The strong association between lifestyle choices and men's morbidity and mortality clearly demonstrates the need to foster healthier behaviors among men. Creating a better understanding of the importance of health care requires broad-scale campaigns to heighten awareness of the need for routine and preventive health care and to encourage men to schedule physician visits. Also needed are efforts at the community and practice levels to enhance health-seeking behavior and improve men's understanding of their health. The efficacy of all of these efforts depends on addressing the unique features of the masculine gender identity.
The Men's Health Network has established International Men's Health Week as the week leading up to Father's Day each June [336]. Highlights of the Week include health fairs, screening, and distribution of educational materials in workplaces and elsewhere in the community. Other Men's Health Network campaigns "speak" to men, with names such as "Men at Work" and "Time Out for Men's Health" maintenance schedule [336].
Some have suggested that large-scale campaigns that feature well-respected athletes and actors can increase appeal to men [45]. However, others have cautioned that, while celebrity endorsement of screening may have a positive effect on men, such campaigns may not target the right audience or address all the pertinent facts [337].
The optimal educational campaigns are those that target men and attempt to challenge men's perceptions of health and the need for preventive care. For example, to heighten awareness about depression in men, the National Institute of Mental Health launched the "Real Men, Real Depression" campaign and produced an accompanying booklet "Men and Depression" [335]. Both the campaign and the booklet feature quotations and vignettes from men who have been treated for depression.
Analysis of data about men who lack a usual source of care indicates that such men are more apt to be younger, Hispanic, single (never married or divorced), without insurance, and living in the southern or western parts of the United States or in urban areas [39]. Education about the importance of health care should be provided through public service announcements, media, schools, and workplaces as appropriate to target these groups of men [39]. Given men's propensity to see a physician only when they are sick or have symptoms, educational messages should emphasize the importance of preventive visits and discourage symptoms as a motivator for seeking health care [338]. Resources should also be culturally appropriate for diseases and conditions that disproportionately affect men of certain races and ethnicities.
As a result of men's reluctance to seek help, educational strategies that provide anonymity may be particularly well-suited for them [45,339]. Print resources should be distributed through a variety of venues that men frequent, such as the workplace, schools, religious organizations, sports arenas, men's organizations or clubs, pubs, supermarkets, car and motorbike dealerships, and barbershops [45,339,340]. In addition, digital media may be effective, especially for younger men. A study showed that 90-second educational video clips on men's health, sent by e-mail, were well-received [341].
Many community-based educational programs targeting men have been successful, especially among men in racial/ethnic minority populations. For example, a culturally tailored, language-concordant navigator program was successful at improving rates of colorectal cancer screening at a healthcare center serving a low-income, ethnically and linguistically diverse community [342]. The Black Barbershop Health Outreach Program (BBHOP) has been an effective program for promoting cardiovascular health, and the program can be used as a model for other health topics [343]. Another barbershop-based program involves training barbers to educate their clients about prostate cancer [344]. Focus groups of men from churches of a variety of denominations have indicated that church-based education may also be effective [35,345].
Men are more likely to use healthcare services that are quick and easy; consequently, making physician visits more convenient may increase the number of men who seek health care [339,346]. Evening office hours and walk-in appointments may be helpful in addressing this problem, and male-specific group appointments have been effective in enhancing men's education on health issues, with high satisfaction reported by participants [347]. In addition, nontraditional settings for healthcare services have been suggested, such as within workplaces and near sports venues, shopping centers, and men's organizations [45,339].
Men who are most likely to seek preventive care are those who live with a spouse or partner [348]. In addition, men have been shown to have strong feelings about women as the arbiters of health for the entire family and are likely to be influenced to seek health care by a member of the opposite sex; this is especially true for men in racial/ethnic minority populations [35,40,43,45]. Given these findings, healthcare providers should talk to their female patients to emphasize the importance of encouraging the men in their families to seek routine health care. Additionally, all interactions with male patients should be used to promote routine health assessments. Men who seek help for acute problems should be reminded of the need for screening and be counseled about risk factors [45,349]. A subsequent visit should be encouraged, and this message may be reinforced by providing a take-home reminder or by scheduling an appointment while the patient is in the office [45].
As noted earlier, fostering open communication in a nonjudgmental manner is essential. Clinicians should take care to raise health issues with their male patients and to overcome some masculine traits in communication, such as a reluctance to ask questions [240]. Asking open-ended questions may be helpful in some cases, and providing a questionnaire before the visit may foster discussion [45]. Assumptions about a man's willingness to share information should be avoided, as men have been more forthcoming when they receive cues that they are expected to provide valuable information [350]. Lastly, men often have a need to feel empowered, and shared decision making is important [351].
Decision aids are available in a variety of formats and literacy levels, and they may be useful in helping men make informed decisions about care [119,129,130,131]. Also, clinicians should review decision aids and educational resources carefully before using them to ensure that the information is comprehensive and accurate [129]. Resources should be available about the risks involved with not wearing a safety belt or motorcycle helmet, driving while intoxicated, speeding, handling firearms, stress/anger management, and safety issues in the home and at work.
Clinicians can help ensure that their patients receive reliable online information by posting the addresses of authoritative websites in their office, in print resources, and within the community (Table 18). Healthcare providers should be familiar with established guidelines for screening among men in various age categories and should emphasize the relative benefits and disadvantages of screening (Table 19). The Electronic Preventive Services Selector (ePSS) is an application for mobile devices that provides USPSTF information on screening and counseling, as well as preventive medication services. The AUA offers the Men's Health Checklist, a compact, downloadable reference for coordinating care of men; it is available at https://www.auanet.org/publications/mens-health-checklist.
ONLINE HEALTH RESOURCES FOR MEN
| General | ||||||||||
| ||||||||||
| Cancer | ||||||||||
| ||||||||||
| Smoking Cessation | ||||||||||
| ||||||||||
| Genitourinary Disorders | ||||||||||
| ||||||||||
| Depression | ||||||||||
| ||||||||||
| Alcohol and Drug Use | ||||||||||
| ||||||||||
| Sexually Transmitted Infections | ||||||||||
|
RECOMMENDATIONS AND SUGGESTIONS FOR HEALTH ASSESSMENTS, SCREENING, AND COUNSELING FOR MEN
| Intervention | Suggested Frequency | Relevant Ages (Years) | Recommending Body/Source | ||
|---|---|---|---|---|---|
| Routine physical examination (with determination of height, weight, and body mass index) | Every 3 to 5 years | 18 to 39 | — | ||
| Every 1 to 2 years | 40 to 49 | ||||
| Yearly | 50 and older | ||||
| Blood pressure screening | Every 1 to 2 years, depending on blood pressure | Beginning at 18 | USPSTF | ||
| Cholesterol level/lipid profile | At least every 5 years | 40 to 75 (earlier if at increased risk) | USPSTF | ||
| Diabetes (type 2) and prediabetes screening | Every 3 years | 35 to 70 in men with overweight or obesity | USPSTF | ||
| Cancer-related check-up (for cancer of the thyroid, testicles, lymph nodes, oral cavity, and skin) | At each routine examination | Beginning at 20 | ACS | ||
| Assessment, Counseling, and Behavioral Interventions as Appropriate | |||||
| Tobacco use | At each routine examination | All men | USPSTF | ||
| Alcohol use | At each routine examination | All men | USPSTF | ||
| Drug (illicit) use | At each routine examination | All men | ASAM | ||
| Depression | At each routine examination, when staff-assisted depression care supports are in place | All men | USPSTF | ||
| Counseling | |||||
| Healthy diet | At each routine examination | Men with risk factors for cardiovascular disease and diet-related chronic diseases | USPSTF | ||
| Exercise | At each routine examination | All men | AAFP, AMA, AHA, CDC | ||
| Sun avoidance and use of sunscreen | At each routine examination | All men | ACS, AAD, NIH Consensus Panel | ||
| Skin examination for melanoma | At each routine examination | All men | ACS | ||
| Avoidance of sexually transmitted infections | At each routine examination | All sexually active men at increased risk | CDC | ||
| Risk of HIV infection | At each routine examination | All men who have sex with men | AAFP | ||
| Risk for hepatitis A and B | At each routine examination | All men who have sex with men and others at high risk | AAFP | ||
| Sexual health | At each routine examination | All men | AAFP | ||
| Screening | |||||
| Colorectal cancer | Every 1 to 10 years, depending on risk and test used | 45 to 75 | USPSTF | ||
| Osteoporosis | At each routine examination | By 65 | ACP | ||
| HIV | Not established (encourage men to be tested) | 15 to 65 (younger and older men at increased risk) | USPSTF | ||
| Visual acuity (comprehensive eye examination) | Yearly | Beginning at 65 | AAO | ||
| Abdominal aortic aneurysm (ultrasonography) | Once | 65 to 75 (men who have ever smoked) | USPSTF | ||
| Immunizations | |||||
| Tetanus, diphtheria, pertussis (Td/Tdap) | Once (Tdap), with booster (Td or Tdap) every 10 years | All men | ACIP | ||
| Influenza vaccine | Yearly | All men | ACIP | ||
| Pneumococcal vaccine | Once | 65 and older (19 to 64 if risk) (one or two doses, depending on vaccine) | ACIP | ||
| Hepatitis A | Once | All men, if risk factors are present (2 or 3 doses, depending on vaccine) | ACIP | ||
| Hepatitis B | Once | 19 to 59, and 60 and older if risk factors are present (2, 3, or 4 doses, depending on vaccine or condition) | ACIP | ||
| Human papillomavirus (HPV) | Once |
| ACIP | ||
| Zoster (shingles) | Once | 50 and older or younger if risk factors present (2 doses) | ACIP | ||
| Haemophilus influenzae type b (Hib) | Once | All men, if risk factors present (1 or 3 doses depending on indication) | ACIP | ||
| Meningococcal A, C, W, Y | Once | All men, if risk factors present (1 or 2 doses depending on indication) | ACIP | ||
| Meningococcal B | Once | All men, if risk factors present (2 or 3 doses depending on vaccine and indication) | ACIP | ||
Routine health assessments should include screening and counseling about lifestyle factors that have an impact on health, such as substance misuse, diet, exercise, safe sex practices, and sun protection. Education about sun protection and self-examination for moles is especially important given the increase in the lifetime risk for melanoma among men [24]. At each routine visit, healthcare providers should assess each male patient's individual lifestyle, psychosocial, and occupational risks. The high rate of unintentional injury as a cause of death for men calls for increased attention to safety issues.
In response to high morbidity and mortality rates among men over the past decade, researchers have focused increased attention on men's health issues. Many factors contribute to health-related gender disparities, but male gender identity is thought to have the most significant impact. The characteristics of the traditional male role (self-reliance, independence, and maintenance of a strong image) cause men to seek health care much less often than women, especially for preventive care. As a result, disease in men may remain undiagnosed until more advanced stages. A tendency for risky behavior, another aspect of the traditional male role, also has a significant effect on men's mortality, as evidenced by unintentional injury being the third leading cause of death among all men. Such behaviors as substance misuse and non-use of protective devices (safety belts, helmets) begin in adolescence and continue into adulthood; across all age-groups, the rates of these behaviors are higher for male individuals than for female individuals. These behaviors are strongly associated with both chronic diseases and all-cause mortality in men.
Prostate cancer is a major concern for many men, and the issues of prostate cancer screening and treatment options are complex and confusing for patients as well as healthcare professionals. Informed decision making is also an important aspect of many benign conditions, such as prostatitis, BPH, premature ejaculation, erectile dysfunction, and late-onset hypogonadism. These conditions have a substantial effect on the quality of life for men, yet men are reluctant to initiate conversations on these topics because of embarrassment and a hesitancy to express feelings and symptoms. It is important to create an environment of open dialogue and ask questions to help men discuss these topics.
The psychosocial well-being of men is important for overall health. Alcohol misuse and depression have both been underdiagnosed in men, especially older men, and clinicians should remain diligent in screening for these disorders in their male patients.
Improvement of men's health relies on men gaining a greater understanding of their risk factors and becoming more involved in the health issues that affect them. Healthcare professionals have a critical role in helping to develop strategies to enhance men's utilization of healthcare resources and in encouraging their male patients to engage in screening and preventive care and to adopt healthy behaviors. Health assessments and screening should be carried out according to established guidelines, with consideration given to each individual patient's specific risks.
1. U.S. Department of Health and Human Services. Health, United States, 2019: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: National Center for Health Statistics; 2021.
2. White A, Holmes M. Patterns of mortality across 44 countries among men and women aged 15–44 years. J Mens Health Gender. 2006;3(2):139-151.
3. Rieker PP, Bird CE. Rethinking gender differences in health: why we need to integrate social and biological perspectives. J Gerontol B Psychol Sci Soc Sci. 2005;60(Special Issue II):S40-S47.
4. Addis M, Mahalik JR. Men, masculinity, and the contexts of help seeking. Am Psychol. 2003;58(1):5-14.
5. Galdas PM, Cheater F, Marshall P. Men and health help-seeking behaviour: literature review. J Adv Nurs. 2005;49(6):616-623.
6. Rankin T. Andrology as the medical specialty to focus medical training on men's health? J Mens Health Gender. 2005;2(1):45-48.
7. Centers for Disease Control and Prevention. Leading Causes of Death: Males, All Races and Origins—United States, 2018. Available at https://www.cdc.gov/healthequity/lcod/men/2018/all-races-origins/index.htm. Last accessed June 1, 2022.
8. Centers for Disease Control and Prevention. Leading Causes of Death in Females, United States. Available at https://www.cdc.gov/women/lcod/index.htm. Last accessed May 28, 2022.
9. Centers for Disease Control and Prevention. National Center for Health Statistics: Men's Health. Available at https://www.cdc.gov/nchs/fastats/mens-health.htm. Last accessed June 1, 2022.
10. Centers for Disease Control and Prevention. National Center for Health Statistics: Women's Health. Available at https://www.cdc.gov/nchs/fastats/womens-health.htm. Last accessed June 1, 2022.
11. Centers for Disease Control and Prevention. Health Equity: Leading Causes of Death, Males—United States, 2018. Available at: https://www.cdc.gov/healthequity/lcod/men/2018/index.html. Last accessed May 28,2022.
12. Centers for Disease Control and Prevention. Leading Causes of Death, Females—United States, 2018. Available at https://www.cdc.gov/women/lcod/2018/all-races-origins/index.htm. Last accessed June 1, 2022.
13. Centers for Disease Control and Prevention. Leading Causes of Death, Males—Non-Hispanic Black—United States, 2018. Available at https://www.cdc.gov/healthequity/lcod/men/2018/nonhispanic-black/index.htm. Last accessed June 1, 2022.
14. Centers for Disease Control and Prevention. Leading Causes of Death, Males—Hispanic—United States, 2018. Available at https://www.cdc.gov/healthequity/lcod/men/2018/hispanic/index.htm. Last accessed June 1, 2022.
15. Centers for Disease Control and Prevention. Leading Causes of Death, Males— American Indian/Alaska Native—United States, 2018. Available at https://www.cdc.gov/healthequity/lcod/men/2018/hispanic/index.htm. Last accessed June 1, 2022.
16. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33.
17. Courtenay WH. Key determinants of the health and well-being of men and boys. InternatJ Mens Health. 2003;2(1):1-30.
18. Mahalik JR, Lagan HD, Morrison JA. Health behaviors and masculinity in Kenyan and US male college students. Psychol Men Masculin. 2006;7(4):191-202.
19. Mahalik JR, Burns SM, Syzdek M. Masculinity and perceived normative health behaviors as predictors of men's health behaviors. Soc Sci Med. 2007;64(11):2201-2209.
20. Peerson A, Saunders M. Men's health literacy: advancing evidence and priorities. Crit Pub Health. 2009;19(3&4):441-456.
21. Kutner M, Greenberg E, Jin Y, Paulsen C. The Health Literacy of America's Adults: Results from the 2003 National Assessment of Adult Literacy. Washington, DC: National Center for Education Statistics; 2006.
22. Gilchrist J, Ballesteros MF. Vital signs: unintentional deaths among persons aged 0–19 years—United States, 2000–2009. MMWR. 2012;61(15):270-276.
23. Centers for Disease Control and Prevention. Youth Risk Behavior Surveillance—United States, 2019. MMWR Surveill Summ. 2020;69(Suppl 1):1-88.
24. U.S. Department of Transportation. Occupant Restraint Use in 2020: Results from the NOPUS Controlled Intersection Study. Washington, DC: National Highway Safety Administration; 2021.
25. Petrosky E, Ertl A, Sheats KJ, Wilson R, Betz CJ, Blair JM. Surveillance for violent deaths—National Violent Death Reporting System, 34 states, Four California Counties, the District of Columbia, and Puerto Rico, 2017. MMWR Surveill Summ. 2020;69(8):1-37.
26. U.S. Department of Labor, Bureau of Labor Statistics. Census of Fatal Occupational Injuries Summary, 2020. Available at https://www.bls.gov/news.release/cfoi.nr0.htm. Last accessed May 31, 2022.
27. Center for Behavioral Health Statistics and Quality. Results from the 2016 National Survey on Drug Use and Health: Detailed Tables. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2017.
28. Oliffe JL, Phillips MJ. Men, depression and masculinities: a review and recommendations. J Mens Health. 2008;5(3):194-202.
29. Substance Abuse and Mental Health Services Administration, Office of Applied Studies. The NSDUH Report: Sexually Transmitted Diseases and Substance Use. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2007.
30. Substance Abuse and Mental Health Services Administration. 2020 National Survey of Drug Use and Health (NSDUH) Releases. Available at https://www.samhsa.gov/data/release/2020-national-survey-drug-use-and-health-nsduh-releases. Last accessed June 1, 2022.
31. Higgins ST, Kurti AN, Redner R, et al. A literature review on prevalence of gender differences and intersections with other vulnerabilities to tobacco use in the United States, 2004–2014. Prev Med. 2015;80:89-100.
32. Centers for Disease Control and Prevention. Health Effects of Cigarette Smoking. Available at https://www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/effects_cig_smoking/. Last accessed May 31, 2022.
33. Centers for Disease Control and Prevention. Alcohol and Public Health. Available at https://www.cdc.gov/alcohol. Last accessed June 1, 2022.
34. Reid M, Fiellin DA, O'Connor PG. Hazardous and harmful alcohol consumption in primary care. Arch Intern Med. 1999;159(15):1681-1689.
35. Gast J, Peak T. "It used to be that if it weren't broken and bleeding profusely, I would never go to the doctor:" men, masculinity, and health. Am J Mens Health. 2011;5(4):318-331.
36. Tannenbaum C, Frank B. Masculinity and health in late life men. Am J Mens Health. 2011;5(3):243-254.
37. Centers for Disease Control and Prevention. Summary Health Statistics Tables for U.S. Adults: National Health Interview Survey, 2018. Available at https://www.cdc.gov/nchs/nhis/shs/tables.htm. Last accessed May 31, 2022.
38. Kaiser Family Foundation. Gender Differences in Health Care, Status, and Use: Spotlight on Men's Health. Available at https://www.kff.org/womens-health-policy/fact-sheet/gender-differences-in-health-care-status-and-use-spotlight-on-mens-health/. Last accessed May 31, 2022.
39. Viera AJ, Pathman DE, Garrett JM. Adults' lack of a usual source of care: a matter of preference? Ann Fam Med. 2006;4(4): 359-365.
40. Peak T, Gast J, Ahlstrom D. A needs assessment of Latino men's health concerns. Am J Mens Health. 2010;4(1):22-32.
41. Sanders Thompson VL, Talley M, Caito N, Kreuter M. African American men's perceptions of factors influencing health-information seeking. Am J Mens Health. 2009;3(1):6-15.
42. Davis JL, Buchanan KL, Katz PV, Green BL. Gender differences in cancer screening beliefs, behaviors, and willingness to participate: implications for health promotion. Am J Mens Health. 2012;6(3):211-217.
43. Reynolds D. Prostate cancer screening in African American men: barriers and methods for improvement. Am J Mens Health. 2008;2(2):172-177.
44. Elder KT, Wiltshire JC, McRoy L, Campbell D, Gary LC, Safford M. Men and differences by racial/ethnic group in self-advocacy during the medical encounter. J Mens Health. 2010;7(2):135-144.
45. Sadovsky R, Levine L. Men's healthcare needs improvements: a recommendation for a midlife men's health assessment visit. J Mens Health Gender. 2005;2(3):375-381.
46. Committee on Health Literacy, Board on Neuroscience and Behavioral Health. Health Literacy: A Prescription to End Confusion. Washington, DC: The National Academies Press; 2004.
47. Jeppesen KM, Coyle JD, Miser WF. Screening questions to predict limited health literacy: a cross-sectional study of patients with diabetes mellitus. Ann Fam Med. 2009;7(1):24-31.
48. Shah LC, West P, Bremmeyr K, Savoy-Moore RT. Health literacy instrument in family medicine: the "newest vital sign" ease of use and correlates. J Am Board Fam Med. 2010;23(2):195-203.
49. Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the Newest Vital Sign. Ann Fam Med. 2005;3(6):514-522.
50. U.S. Census Bureau. Language Spoken at Home: 2-16-2020 American Community Survey (ACS). Available at https://www.census.gov/acs/www/about/why-we-ask-each-question/language. Last accessed May 28, 2022.
51. Karliner L, Napoles-Springer AM, Schillinger D, Bibbins-Domingo K, Pérez-Stable EJ. Identification of limited English proficient patients in clinical care. J Gen Intern Med. 2008;23(10):1555-1560.
52. Sevilla Matir J, Willis DR. Using bilingual staff members as interpreters. Fam Pract Manage. 2004;11(7):34-36.
53. Ngo-Metzger Q, Massagli MP, Clarridge BR, et al. Linguistic and cultural barriers to care: perspectives of Chinese and Vietnamese immigrants. J Gen Intern Med. 2003;18(1):44-52.
54. Flores G. Language barriers to health care in the United States. N Engl J Med. 2006;355(3):229-231.
55. Flores G. The impact of medical interpreter services on the quality of health care: a systematic review. Med Care Res Rev. 2005;62(3):255-299.
56. Karliner L, Jacobs EA, Chen AH, Mutha S. Do professional interpreters improve clinical care for patients with limited English proficiency? A systematic review of the literature. Health Serv Res. 2007;42(2):727-754.
57. Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance, 2020. Available at https://www.cdc.gov/std/statistics/2020/overview.htm. Last accessed May 28, 2022.
58. Knight D, Jarrett D. Preventive health care for men who have sex with men. Am Fam Phys. 2015;91(12):844-852.
59. Litwin M, McNaughton-Collins M, Fowler FJ Jr, et al. The National Institutes of Health Chronic Prostatitis Symptom Index: development and validation of a new outcome measure. Chronic Prostatitis Collaborative Research Network. J Urol. 1999;162(2):369-375.
60. Krieger JN, Nyberg L Jr, Nickel JC. NIH consensus definition and classification of prostatitis. JAMA. 1999;282(3):236-237.
61. Schaeffer AJ. Chronic prostatitis and the chronic pelvic pain syndrome. N Engl J Med. 2006;355(16):1690-1698.
62. Muller A, Mulhall JP. Sexual dysfunction in the patient with prostatitis. Curr Urol Rep. 2006;7(4):307-312.
63. Anderson R, Wise D, Sawyer T, Chan CA. Sexual dysfunction in men with chronic prostatitis/chronic pelvic pain syndrome: improvement after trigger point release and paradoxical relaxation training. J Urol. 2006;176(4 Pt 1):1534-1538, 1539.
64. Propert K, McNaughton-Collins M, Leiby BE, et al. A prospective study of symptoms and quality of life in men with chronic prostatitis/chronic pelvic pain syndrome: the National Institutes of Health Chronic Prostatitis Cohort study. J Urol. 2006;175(2):619-623.
65. Turner J, Ciol MA, Von Korff M, Berger R. Health concerns of patients with nonbacterial prostatitis/pelvic pain. Arch Intern Med. 2005;165(9):1054-1059.
66. Krieger JN, Lee SW, Jeon J, Cheah PY, Liong ML, Riley DE. Epidemiology of prostatitis. Int J Antimicrob Agents. 2008;31 (Suppl 1):S85-S90.
67. Turek PJ. Prostatitis. Available at https://emedicine.medscape.com/article/785418-overview. Last accessed May 6, 2019.
68. Pontari M, McNaughton-Collins M, O'Leary MP, et al. A case-control study of risk factors in men with chronic pelvic pain syndrome. BJU Int. 2005;96(4):559-565.
69. Watson RA. Chronic Pelvic Pain in Men. Available at https://emedicine.medscape.com/article/437745-overview. Last accessed June 1, 2022.
70. Grabe M, Bishop MC, Bjerklund-Johansen TE, et al. Guidelines on the Management of Urinary and Male Genital Tract Infections. Arnhem: European Association of Urology; 2008.
71. Turner J, Ciol MA, Von Korff M, Berger R. Validity and responsiveness of the National Institutes of Health Chronic Prostatitis Symptom Index. J Urol. 2003;169(2):580-583.
72. Meares E, Stamey TA. Bacteriologic localization patterns in bacterial prostatitis and urethritis. Invest Urol. 1968;5(5):492-518.
73. Murphy AB, Nadler RB. Pharmacotherapy strategies in chronic prostatitis/chronic pelvic pain syndrome management. Expert Opin Pharmacother. 2010;11(8):1255-1261.
74. Schiller DS, Parikh A. Identification, pharmacologic considerations, and management of prostatitis. Am J Geriatr Pharmacother. 2011;9(1):37-48.
75. Anothaisintawee T, Attia J, Nickel JC, et al. Management of chronic prostatitis/chronic pelvic pain syndrome: a systematic review and network meta-analysis. JAMA. 2011;305(1):78-86.
76. Cohen JM, Fagin AP, Hariton E, et al. Therapeutic intervention for chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS): a systematic review and meta-analysis. PLoS One. 2012;7(8):e41941.
77. Aboumarzouk OM, Nelson RL. Pregabalin for chronic prostatitis. Cochrane Database Syst Rev. 2012;8:CD009063.
78. Lerner LB, McVary, KT, Barry MJ et al: Management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline part I, initial work-up and medical management. J Urol. 2021;206:806-817.
79. Beckman TJ, Mynderse LA. Evaluation and medical management of benign prostatic hyperplasia. Mayo Clin Proc. 2005;80(10):1356-1362.
82. Wei JT, Calhoun E, Jacobsen SJ. Urologic Diseases in America Project: benign prostatic hyperplasia. J Urol. 2005;173(4):1256-1261.
83. Calogero AE, Burgio G, Condorelli RA, Cannarella R, La Vignera S. Epidemiology and risk factors of lower urinary tract symptoms/benign prostatic hyperplasia and erectile dysfunction. Aging Male. 2019;22(1):12-19.
84. Hoke GP, McWilliams GW. Epidemiology of benign prostatic hyperplasia and comorbidities in racial and ethnic minority populations. Am J Med. 2008;121(8 Suppl 2):S3-S10.
85. Lam JS, Cooper KL, Kaplan SA. Changing aspects in the evaluation and treatment of patients with benign prostatic hypertrophy. Med Clin North Am. 2004;88(2):281-308.
86. Barry MJ, Fowler FJ Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549-1557, 1564.
87. Deters LA. Benign Prostatic Hypertrophy Treatment and Management. Available at https://emedicine.medscape.com/article/437359-treatment. Last accessed June 1, 2022.
88. Marks LS, Gittelman MC, Hill LA, et al. Rapid efficacy of the highly selective α(1A)-adrenoceptor antagonist silodosin in men with signs and symptoms of benign prostatic hyperplasia: pooled results of 2 phase 3 studies. J Urol. 2013;189(1 Suppl):S122-S128.
89. U.S. Food and Drug Administration. FDA-Approved Drug Products. Available at https://www.accessdata.fda.gov/scripts/cder/daf. Last accessed June 1, 2022.
90. Kramer BS, Hagerty KL, Justman S, et al. Use of 5-α-reductase inhibitors for prostate cancer chemoprevention: American Society of Clinical Oncology/American Urological Association 2008 Clinical Practice Guideline. J Urol. 2009;181(4):1642-1657.
91. Wilt TJ, MacDonald R, Hagerty K, Schellhammer P, Kramer BS. Five-alpha-reductase inhibitors for prostate cancer prevention. Cochrane Database Syst Rev. 2008;(2):CD007091.
92. U.S. Food and Drug Administration. FDA Drug Safety Communication: 5-Alpha Reductase Inhibitors (5-ARIs) May Increase the Risk of a More Serious Form of Prostate Cancer. Available at https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-5-alpha-reductase-inhibitors-5-aris-may-increase-risk-more-serious. Last accessed June 1, 2022.
93. Roehrborn CG, Barkin J, Siami P, et al. Clinical outcomes after combined therapy with dutasteride plus tamsulosin or either monotherapy in men with benign prostatic hyperplasia (BPH) by baseline characteristics: 4-year results from the randomized, double-blind Combination of Avodart and Tamsulosin (CombAT) trial. BJU Int. 2011;107(6):946-954.
94. Miller MS. Role of phosphodiesterase type 5 inhibitors for lower urinary tract symptoms. Ann Pharmacother. 2013;47(2):278-283.
95. Cantrell MA, Baye J, Vouri SM. Tadalafil: a phosphodiesterase-5 inhibitor for benign prostatic hyperplasia. Pharmacotherapy. 2013;33(6):639-649.
96. National Kidney and Urologic Diseases Information Clearinghouse. Prostate Enlargement: Benign Prostatic Hyperplasia. Bethesda, MD: National Kidney and Urologic Diseases Information Clearinghouse; 2006.
97. National Comprehensive Cancer Network. NCCN Practice Guidelines in Oncology: Prostate Cancer Early Detection. Version 1.2022. Available at https://www.nccn.org/professionals/physician_gls/pdf/prostate_detection.pdf. Last accessed May 31, 2022.
98. Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349(3):215-224.
99. Andriole GL, Bostwick DG, Brawley OW, et al. Effect of dutasteride on the risk of prostate cancer. New Engl J Med. 2010;362(13):1192-1202.
100. Grubb RL, Andriole GL, Somerville MC, et al. The REDUCE Follow-Up Study: low rate of new prostate cancer diagnoses observed during a 2-year, observational, follow-up study of men who participated in the REDUCE trial. J Urol. 2013;189(3): 871-877.
101. Zimmerman MP, Mehr SR. Chemoprevention in prostate cancer: identifying patients at greatest risk may provide greatest value. Am J Manag Care. 2013;19(Spec No. 3):E8.
102. U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. JAMA. 2018;319(18):1901-1913.
103. American Urological Association. Prostate Cancer: Use of 5-α-Reductase Inhibitors for Prostate Cancer Chemoprevention: American Society of Clinical Oncology/American Urological Association. Available at https://www.auanet.org/Documents/education/Arc-Prostate-Cancer-Chemo.pdf. Last accessed June 1, 2022.
104. Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009;301(1):39-51.
105. Marshall JR, Tangen CM, Sakr WA, et al. Phase III trial of selenium to prevent prostate cancer in men with high-grade prostatic intraepithelial neoplasia: SWOG S9917. Cancer Prev Res (Phila). 2011;4(11):1761-1769.
106. Stephenson AJ, Abouassaly R, Klein EA. Chemoprevention of prostate cancer. Urol Clin North Am. 2010;37(1):11-21.
107. Ilic D, Forbes KM, Hassed C. Lycopene for the prevention of prostate cancer. Cochrane Database Syst Rev. 2011;(11):CD008007.
108. Wolf AWD, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60(2):70-98.
109. Mistry K, Cable G. Meta-analysis of prostate-specific antigen and digital rectal examination as screening tests for prostate carcinoma. J Am Board Fam Pract. 2003;16(2):95-101.
110. Andriole GL, Crawford ED, Grubb RL III, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104(2):125-132.
111. Ilic D, Neuberger MM, Djulbegovic M, Dahm P. Screening for prostate cancer. Cochrane Database Syst Rev. 2013:1:CD004720.
112. Tsodikov A, Gulati R, Heijnsdijk EAM, et al. Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO trials. Ann Intern Med. 2017;167(7):449-455.
113. Draisma G, Boer R, Otto SJ, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003;95(12):868-878.
114. Carter HB, Albertsen PC, Barry MJ, et al. Early Detection of Prostate Cancer (2018). Available at https://www.auanet.org/guidelines/guidelines/prostate-cancer-early-detection-guideline. Last accessed June 1, 2022.
115. Qaseem A, Barry MJ, Denberg TD, et al. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2013;158(10):761-769.
116. Basch E, Oliver TK, Vickers A, et al. Screening for prostate cancer with prostate-specific antigen testing: American Society of Clinical Oncology provisional clinical opinion. J Clin Oncol. 2012;30(24):3020-3025.
117. Smith RA, Andrews K, Brooks D, et al. Cancer screening in the United States, 2019: a review of current American Cancer society guidelines and current issues in cancer screening. CA Cancer J Clin. 2019;69(3):184-210.
118. Gulati R, Gore JL, Etzioni R. Comparative effectiveness of alternative prostate-specific antigen-based prostate cancer screening strategies: model estimates of potential benefits and harms. Ann Intern Med. 2013;158(3):143-153.
119. Allen JD, Mohllajee AP, Shelton RC, Drake BF, Mars DR. A computer-tailored intervention to promote informed decision making for prostate cancer screening among African American men. Am J Mens Health. 2009;3(4):340-351.
120. Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011;(10):CD001431.
121. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database SystRev. 2017:4:CD001431.
122. Ross LE, Powe BD, Taylor YJ, Howard DL. Physician-patient discussions with African American men about prostate cancer screening. Am J Mens Health. 2008;2(2):156-164.
123. McFall SL. U.S. men discussing prostate-specific antigen tests with a physician. Ann Fam Med. 2006;4(5):433-436.
124. Allan RW, Sanderson H, Epstein JI. Correlation of minute (0.5 mm or less) focus of prostate adenocarcinoma on needle biopsy with radical prostatectomy specimen: role of prostate specific antigen density. J Urol. 2003;170(2):370-372.
125. Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350(22):2239-2246.
126. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer. Version 4.2022. Available at https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Last accessed June 1, 2022.
127. Carroll P, Albertsen PC, Greene K, et al. PSA Testing for the Pretreatment and Posttreatment Management of Prostate Cancer, 2013 Revision of 2009 Best Practice Statement. Linthicum, MD: American Urological Association; 2013.
128. American Joint Committee on Cancer. Cancer Staging Manual. 8th ed. New York, NY: Springer; 2018.
129. Fagerlin A, Rovner D, Stableford S, Jentoft C, Wei JT, Holmes-Rovner M. Patient education materials about the treatment of early-stage prostate cancer: a critical review. Ann Intern Med. 2004;140(9):721-728.
130. Holmes-Rovner M, Stableford S, Fagerlin A, et al. Evidence-based patient choice: a prostate cancer decision aid in plain language. BMC Med Inform Decis Mak. 2005;5(1):16.
131. Kim SP, Knight SJ, Tomori C, et al. Health literacy and shared decision making for prostate cancer patients with low socioeconomic status. Cancer Invest. 2001;19(7):684-691.
132. Davison BJ, Parker PA, Goldenberg SL. Patients' preferences for communicating a prostate cancer diagnosis and participating in medical decision-making. BJU Int. 2004;93(1):47-51.
133. Kilbridge KL, Fraser G, Krahn M, et al. Lack of comprehension of common prostate cancer terms in an underserved population.J Clin Oncol. 2009;27(12):2015-2021.
134. Wang DS, Jani AB, Tai CG, et al. Severe lack of comprehension of common prostate health terms among low-income inner-city men. Cancer. 2013;119(17):3204-3211.
135. Ilic D, Murphy K, Green S. Risk communication and prostate cancer: identifying which summary statistics are best understood by men. Am J Mens Health. 2012;6(6):497-504.
136. Ip S, Dahabreh IJ, Chung M, et al. An evidence review of active surveillance in men with localized prostate cancer. Evid Rep Technol Assess (Full Rep). 2011;(304):1-341.
137. Dahabreh IJ, Chung M, Balk EM, et al. Active surveillance in men with localized prostate cancer: a systematic review. Ann Intern Med. 2012;156(8):582-589.
138. Hayes JH, Ollendorf DA, Pearson SD, et al. Observation versus initial treatment for men with localized, low-risk prostate cancer: a cost-effectiveness analysis. Ann Intern Med. 2013;158(12):853-860.
139. Klotz L, Zhang L, Lam A, et al. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28(1):126-131.
140. Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367(3):203-213.
141. Jacobs BL, Zhang Y, Schroeck FR, et al. Use of advanced treatment technologies among men at low risk of dying from prostate cancer. JAMA. 2013;309:2587-2595.
142. Jacobs BL, Zhang Y, Skolarus TA, et al. Comparative effectiveness of external-beam radiation approaches for prostate cancer. Eur Urol. 2014;65:162-168.
143. Goldin GH, Sheets NC, Meyer AM, et al. Comparative effectiveness of intensity-modulated radiotherapy and conventional conformal radiotherapy in the treatment of prostate cancer after radical prostatectomy. JAMA Intern Med. 2013;173:1136-1143.
144. Masson S, Persad R, Bahl A. HDR brachytherapy in the management of high-risk prostate cancer. Adv Urol. 2012;2012:980841.
145. Spratt DE, Soni PD, McLaughlin PW, et al. American Brachytherapy Society Task Group Report: combination of brachytherapy and external beam radiation for high-risk prostate cancer. Brachytherapy. 2017;16:1-12.
146. Thompson I, Thrasher JB, Aus G, et al. Guideline for the Management of Clinically Localized Prostate Cancer: 2007 Update. Linthicum, MD: American Urological Association; 2007.
147. Pierorazio PM, Ross AE, Lin BM, et al. Preoperative characteristics of high-Gleason disease predictive of favourable pathological and clinical outcomes at radical prostatectomy. BJU Int. 2012;110:1122-1128.
148. Chade DC, Eastham J, Graefen M, et al. Cancer control and functional outcomes of salvage radical prostatectomy for radiation-recurrent prostate cancer: a systematic review of the literature. Eur Urol. 2012;61:961-971.
149. Shekarriz B, Upadhyay J, Pontes JE. Salvage radical prostatectomy. Urol Clin North Am. 2001;28:545-553.
150. Bill-Axelson A, Holmberg L, Filen F, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian prostate cancer group-4 randomized trial. J Natl Cancer Inst. 2008;100(16):1144-1154.
151. Tomicich SF. Evaluation of quality of life for prostate cancer patients who have undergone radical prostatectomy surgery. Am J Mens Health. 2007;1(4):284-293.
152. Hoffman RM, Barry MJ, Stanford JL, Hamilton AS, Hunt WC, Collins MM. Health outcomes in older men with localized prostate cancer: results from the Prostate Cancer Outcomes Study. Am J Med. 2006;119(5):418-425.
153. Shelley MD, Kumar S, Coles B, et al. Adjuvant hormone therapy for localised and locally advanced prostate carcinoma: a systematic review and meta-analysis of randomised trials. Cancer Treat Rev. 2009;35(7):540-546.
154. Shelley MD, Kumar S, Wilt T, et al. A systematic review and meta-analysis of randomised trials of neo-adjuvant hormone therapy for localised and locally advanced prostate carcinoma. Cancer Treat Rev. 2009;35(1):9-17.
155. Loblaw DA, Virgo KS, Nam R, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2007 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25(12):1596-1605.
156. Klotz L, Toren P. Androgen deprivation therapy in advanced prostate cancer: is intermittent therapy the new standard of care?Curr Oncol. 2012;19(Suppl 3):S13-S21.
157. Mottet N, Van Damme J, Loulidi S, et al. Intermittent hormonal therapy in the treatment of metastatic prostate cancer: a randomized trial. BJU Int. 2012;110(9):1262-1269.
158. Niraula S, Le LW, Tannock IF. Treatment of prostate cancer with intermittent versus continuous androgen deprivation: a systematic review of randomized trials. J Clin Oncol. 2013;31(16):2029-2036.
159. Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513-1520.
160. Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer.N Engl J Med. 2004;351:1502-1512.
161. Babaian R, Donnelly B, Bahn D, et al. Best Practice Policy Statement on Cryosurgery for the Treatment of Localized Prostate Cancer. Linthicum, MD: American Urological Association; 2008.
162. Jung JH, Risk MC, Goldfarb R, Reddy B, Coles B, Dahm P. Primary cryotherapy for localized or locally advances prostate cancer. Cochrane Database Syst Rev. 2018;5:CD005010.
163. Skolarus TA, Wolf AMD, Erb NL, et al. American Cancer Society prostate cancer survivorship care guidelines. CA Cancer J Clin. 2014;64(4):225-249.
166. Arce JD, Cortes M, Vargas JC. Sonographic diagnosis of acute spermatic cord torsion. Rotation of the cord: a key to the diagnosis. Pediatr Radiol. 2002;32(7):485-491.
167. Kadish HA, Bolte RG. A retrospective review of pediatric patients with epididymitis, testicular torsion, and torsion of testicular appendages. Pediatrics. 1998;102(1):73-76.
168. Rabinowitz R. The importance of the cremasteric reflex in acute scrotal swelling in children. J Urol. 1984;132(1):89-90.
169. Kravchick S, Cytron S, Leibovici O, et al. Color Doppler sonography: its real role in the evaluation of children with highly suspected testicular torsion. Eur Radiol. 2001;11(6):1000-1005.
170. Wu HC, Sun SS, Kao A, Chuang FJ, Lin CC, Lee CC. Comparison of radionuclide imaging and ultrasonography in the differentiation of acute testicular torsion and inflammatory testicular disease. Clin Nucl Med. 2002;27(7):490-493.
171. Hawtrey CE. Assessment of acute scrotal symptoms and findings: a clinician's dilemma. Urol Clin North Am. 1998;25(4):715-723.
172. Nickel JC, Teichman JMH, Gregoire M, Clark J, Downey J. Prevalence, diagnosis, characterization, and treatment of prostatitis, interstitial cystitis, and epididymitis in outpatient urological practice: the Canadian PIE Study. Urology. 2005;66(5):935-940.
173. Centers for Disease Control and Prevention. Sexually Transmitted Infections Treatment Guidelines, 2021. Available at https://www.cdc.gov/std/treatment-guidelines/herpes.htm. Last accessed May 28, 2022.
174. Sadek I, Biron P, Kus T. Amiodarone-induced epididymitis: report of a new case and literature review of 12 cases. Can J Cardiol. 1993;9(9):833-836.
176. Nickel JC. Chronic epididymitis: a practical approach to understanding and managing a difficult urologic enigma. Rev Urol. 2003;5(4):209-215.
177. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Testicular Cancer. Version 2.2022. Available at: https://www.nccn.org/professionals/physician_gls/pdf/testicular.pdf. Last accessed May 28, 2022.
178. Nielsen ME, Zderic S, Freedland SJ, Jarow JP. Insight on pathogenesis of varicoceles: relationship of varicocele and body mass index. Urology. 2006;68(2):392-396.
179. Handel LN, Shetty R, Sigman M. The relationship between varicoceles and obesity. J Urol. 2006;176(5):2138-2140.
180. Tilki D, Kilic E, Tauber R, et al. The complex structure of the smooth muscle layer of spermatic veins and its potential role in the development of varicocele testis. Eur Urol. 2006;51(5):1402-1410.
181. Trussell JC, Haas GP, Wojtowycz A, Landas S, Blank W. High prevalence of bilateral varicoceles confirmed with ultrasonography. Int Urol Nephrol. 2003;35(1):115-118.
182. Sharlip ID, Jarow J, Belker AM, et al. Infertility: Report on Varicocele and Infertility: An AUA Best Practice Policy and ASRM Practice Committee Report. Linthicum, MD: American Urological Association; 2001.
183. Canales BK, Zapzalka DM, Ercole CJ, et al. Prevalence and effect of varicoceles in an elderly population. Urology. 2005;66(3):627-631.
184. Thomas JC, Elder JS. Testicular growth arrest and adolescent varicocele: does varicocele size make a difference? J Urol. 2002;168(4 Pt 2):1689-1691.
185. Kass EJ, Stork BR, Steinert BW. Varicocele in adolescence induces left and right testicular volume loss. BJU Int. 2001;87(6): 499-501.
186. Chan PT, Goldstein M. Medical backgrounder on varicocele. Drugs Today (Barc). 2002;38(1):59-67.
187. Raheem OA. Surgical management of adolescent varicocele: systematic review of the world literature. Urol Ann. 2013;5(3): 133-139.
190. Surveillance, Epidemiology and End Results Program. Cancer Stat Facts: Testicular Cancer. Available at https://seer.cancer.gov/statfacts/html/testis.html. Last accessed May 28, 2022.
191. Surveillance, Epidemiology and End Results Program. SEER Cancer Statistics Review 2000–2019. Browse the Tables and Figures: Testis: Rates and Trends by Race/Ethnicity. Available at: https://seer.cancer.gov/statistics-network/explorer/application.html. Last accessed May 28,2022.
192. Fossa SD, Chen J, Schonfeld SJ, et al. Risk of contralateral testicular cancer: a population-based study of 29,515 U.S. men. J Natl Cancer Inst. 2005;97(14):1056-1066.
193. U.S. Preventive Services Task Force. Screening for testicular cancer: U.S. Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2011;154(7):483-486.
194. Gilligan TD, Seidenfeld J, Basch EM, et al. American Society of Clinical Oncology clinical practice guideline on uses of serum tumor markers in adult males with germ cell tumors. J Clin Oncol. 2010;28(20):3388-3404.
195. Pont J, Albrecht W. Fertility after chemotherapy for testicular germ cell cancer. Fertil Steril. 1997;68(1):1-5.
196. Lampe H, Horwich A, Norman A, Nicholls J, Dearnaley DP. Fertility after chemotherapy for testicular germ cell cancers. J Clin Oncol. 1997;15:239-245.
197. Brydoy M, Fossa SD, Klepp O, et al. Paternity following treatment for testicular cancer. J Natl Cancer Inst. 2005;97(21):1580-1588.
198. Travis LB, Fossa SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors.J Natl Cancer Inst. 2005;97(18):1354-1365.
199. Osanto S, Bukman AW, Van Hoek F, Sterk PJ, De Laat JA, Hermans J. Long-term effects of chemotherapy in patients with testicular cancer. J Clin Oncol. 1992;10(4):574-579.
200. van den Belt-Dusebout A, Nuver J, de Wit R, et al. Long-term risk of cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol. 2006;24(3):467-475.
201. Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: a population-based study. Cancer. 2004;101(1):51-57.
202. Giordano SH. A review of the diagnosis and management of male breast cancer. Oncologist. 2005;10(7):471-479.
203. Ravi A, Bang H, Karsif K, Nori D. Breast cancer in men: prognostic factors, treatment patterns, and outcome. Am J Mens Health. 2012;6:51-58.
204. Goss PE, Reid C, Pintilie M, Lim R, Miller N. Male breast carcinoma: a review of 229 patients who presented to the Princess Margaret Hospital during 40 years: 1955–1996. Cancer. 1999;85(3):629-639.
205. Korde LA, Zujewski JA, Kamin L, et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol. 2010;28:2114-2122.
206. Cimmino VM, Degnim AC, Sabel MS, et al. Efficacy of sentinel lymph node biopsy in male breast cancer. J Surg Oncol. 2004;86(2):74-77.
207. Goyal A, Horgan K, Kissin M, et al. Sentinel lymph node biopsy in male breast cancer patients. Eur J Surg Oncol. 2004;30(5):480-483.
208. Ribeiro G, Swindell R. Adjuvant tamoxifen for male breast cancer (MBC). Br J Cancer. 1992;65(2):252-254.
209. Wernberg JA, Yap J, Murekeyisoni C, et al. Multiple primary tumors in men with breast cancer diagnoses—a SEER database review. J Surg Oncol. 2009;99:16-19.
210. Althof SE. Prevalence, characteristics and implications of premature ejaculation/rapid ejaculation. J Urol. 2006;175(3 Pt 1):842-848.
211. Lue TF, Giuliano F, Montorsi F. Summary of the recommendations on sexual dysfunctions in men. J Sex Med. 2004;1(1):6-23.
212. Montague DK, Jarow J, Broderick GA, et al. Guideline on the Pharmacologic Management of Premature Ejaculation (Reviewed and Validity Confirmed 2010). Linthicum, MD: American Urological Association; 2004.
213. McMahon CG, Althof SE, Waldinger MD, et al. An evidence-based definition of lifelong premature ejaculation: report of the International Society for Sexual Medicine (ISSM) ad hoc committee for the definition of premature ejaculation.J Sex Med. 2008;5(7):1590-1606.
214. Hatzimouratidis K. Epidemiology of male sexual dysfunction. Am J Mens Health. 2007;1(2):103-125.
215. Revicki D, Howard K, Hanlon J, Mannix S, Greene A, Rothman M. Characterizing the burden of premature ejaculation from a patient and partner perspective: a multi-country qualitative analysis. Health Qual Life Outcomes. 2008;6:33.
216. Rosen RC, Althof S. Impact of premature ejaculation: the psychological, quality of life, and sexual relationship consequences.J Sex Med. 2008;5(6):1296-1307.
217. Althof S. Psychological approaches to the treatment of rapid ejaculation. J Mens Health Gender. 2006;3(2):180-186.
218. Perelman MA. A new combination treatment for premature ejaculation: a sex therapist's perspective. J Sex Med. 2006;3(6):1004-1012.
219. Assalian P. Guidelines for the pharmacotherapy of premature ejaculation. World J Urol. 2005;23(2):127-129.
220. Busato W, Galindo CC. Topical anaesthetic use for treating premature ejaculation: a double-blind, randomized, placebo-controlled study. BJU Int. 2004;93(7):1018-1021.
221. Atikeler MK, Gecit I, Senol FA. Optimum usage of prilocaine-lidocaine cream in premature ejaculation. Andrologia. 2002;34(6):356-359.
222. Morales A. Evolving therapeutic strategies for premature ejaculation: the search for on-demand treatment—topical versus systemic. Can Urol Assoc J. 2012;6(5):380-385.
223. Porst H, Burri A. Fortacin spray for the treatment of premature ejaculation. Urologia. 2007;84(2 Suppl):1-10.
224. Porst H, Burri A. Novel treatment for premature ejaculation in the light of currently used therapies: a review. Sex Med Rev. 2019;7(1):129-140.
225. Lexicomp Online. Available at https://online.lexi.com. Last accessed June 1, 2022.
226. Shabsigh R, Patrick DL, Rowland DL, et al. Perceived control over ejaculation is central to treatment benefit in men with premature ejaculation: results from phase III trials with dapoxetine. BJU Int. 2008;102(7):824-828.
227. Kaufman JM, Rosen RC, Mudumbi RV, et al. Treatment benefit of dapoxetine for premature ejaculation: results from a placebo-controlled phase III trial. BJU Int. 2009;103(5):651-658.
228. McMahon CG. Dapoxetine for premature ejaculation. Expert Opin Pharmacother. 2010;11(10):1741-1752.
229. Epperly T, Moore KE. Health issues in men: part I. Common genitourinary disorders. Am Fam Phys. 2000;61(12):657-3664.
230. Montague DK, Jarow JP, Broderick GA, et al. The Management of Erectile Dysfunction: An Update (Reviewed and Validity Confirmed 2011). Linthicum, MD: American Urological Association; 2005.
231. Qaseem A, Snow V, Denberg TD, et al. Hormonal testing and pharmacologic treatment of erectile dysfunction: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2009;151(9):639-649.
232. Laumann EO, West S, Glasser D, Carson C, Rosen R, Kang JH. Prevalence and correlates of erectile dysfunction by race and ethnicity among men aged 40 or older in the United States: from the Male Attitudes Regarding Sexual Health Survey.J Sex Med. 2007;4(1):57-65.
233. Rosen RC, Wing R, Schneider S, Gendrano N III. Epidemiology of erectile dysfunction: the role of medical comorbidities and lifestyle factors. Urol Clin North Am. 2005;32(4):403-417.
234. Saigal CS, Wessells H, Pace J, Schonlau M, Wilt TJ, for the Urologic Diseases in America Project. Predictors and prevalence of erectile dysfunction in a racially diverse population. Arch Intern Med. 2006;166(2):207-212.
235. Gooren L. How to optimise treatment of erectile dysfunction above and beyond the beneficial effects of a phosphodiesterase type 5 inhibitor. J Mens Health. 2008;5(2):163-170.
236. Jackson G, Boon N, Eardley I, et al. Erectile dysfunction and coronary artery disease prediction: evidence-based guidance and consensus. Int J Clin Pract. 2010;64(7):848-857.
237. Vlachopoulos C, Terentes-Printzios D, Ioakeimidis N, et al. Prediction of cardiovascular events and all-cause mortality with erectile dysfunction: a systematic review and meta-analysis of cohort studies. Circ Cardiovasc Qual Outcomes. 2013;6(1):99-109.
239. Esposito K, Giugliano F, Di Palo C, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA. 2004;291(24):2978-2984.
241. Yuan J, Zhang R, Yang Z, et al. Comparative effectiveness and safety of oral phosphodiesterase type 5 inhibitors for erectile dysfunction: a systematic review and network meta-analysis. Eur Urol. 2013;63(5):902-912.
242. Tsertsvadze A, Yazdi F, Fink HA, et al. Oral sildenafil citrate (Viagra) for erectile dysfunction: a systematic review and meta-analysis of harms. Urology. 2009;74(4):831-836.
243. Pomeranz HD. Can erectile dysfunction drug use lead to ischaemic optic neuropathy? Br J Ophthalmol. 2006;90(2):127-128.
244. Tsertsvadze A, Yazdi F, Fink HA, et al. Oral phosphodiesterase-5 inhibitors and hormonal treatments for erectile dysfunction: a systematic review and meta-analysis. Ann Intern Med. 2009;151(9):650-661.
245. McGwin G Jr, Vaphiades MS, Hall TA, Owsley C. Non-arteritic anterior ischaemic optic neuropathy and the treatment of erectile dysfunction. Br J Ophthalmol. 2006;90(2):154-157.
246. RxList. FDA Announces Revisions to Labels for Cialis, Levitra and Viagra. Available at https://www.rxlist.com/script/main/art.asp?articlekey=84652. Last accessed June 1, 2022.
247. McGwin G Jr. Phosphodiesterase type 5 inhibitor use and hearing impairment. Arch Otolaryngol Head Neck Surg. 2010;136(5): 488-492.
248. Kloner RA, Hutter AM, Emmick JT, et al. Time course of the interaction between tadalafil and nitrates. J Am Cardiol. 2003;42(10):1855-1860.
249. Kloner RA, Mullin SH, Shook T, et al. Erectile dysfunction in the cardiac patient: how common and should we treat? J Urol. 2003;170(2 Pt 2):S46-S50.
250. Melnik T, Soares B, Nasello AG. Psychosocial interventions for erectile dysfunction. Cochrane Database Syst Rev. 2007;(3):CD004825.
251. Haren MT, Kim MJ, Tariq SH, Wittert GA, Morley JE. Andropause: a quality-of-life issue in older males. Med Clin North Am. 2006;90(5):1005-1023.
252. Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2018;103(5):1715-1744.
253. AACE Hypogonadism Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients—2002 update. Endo Pract. 2002;8(6):439-456.
254. Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92(11):4241-4247.
255. Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60(7):762-769.
256. Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism. 2000;49(9):1239-1242.
257. Moore C, Huebler D, Zimmermann T, et al. The Aging Males Symptoms scale (AMS) as outcome measure for treatment of androgen deficiency. Eur Urol. 2004;46(1):80-87.
258. Heinemann LAJ, Zimmermann T, Vermeulen A, Thiel C. A new aging males symptoms rating scale. Aging Male. 1999;2(2):105-114.
259. Daig I, Heinemann LAJ, Kim S, et al. The Aging Males Symptoms (AMS) scale: review of its methodological characteristics. Health Qual Life Outcomes. 2003;1(1):77.
260. Heinemann LAJ, Saad F, Zimmermann T, et al. The Aging Males' Symptoms (AMS) scale: update and compilation of international versions. Health Qual Life Outcomes. 2003;1:15.
261. Liu PY, Swerdloff RS, Wang C. Relative testosterone deficiency in older men: clinical definition and presentation. Endocrinol Metab Clin North Am. 2005;34(4):957-972.
262. Bassil N, Alkaade S, Morley JE. The benefits and risks of testosterone replacement therapy: a review. Therapeutics Clin Risk Manage. 2009;5:427-448.
263. Brawer MK. Testosterone replacement in men with andropause: an overview. Rev Urol. 2004;6(suppl 6):S9-S15.
264. Jarow J, Sigman M, Kolettis PN, et al. The Optimal Evaluation of the Infertile Male: AUA Best Practice Statement. Linthicum, MD: American Urological Association; 2010.
265. Kidd SA, Eskenazi B, Wyrobek AJ. Effects of male age on semen quality and fertility: a review of the literature. Fertil Steril. 2001;75(2):237-248.
266. Ford WCL, North K, Taylor H, et al. Increasing paternal age is associated with delayed conception in a large population of fertile couples: evidence for declining fecundity in older men. Hum Reprod. 2000;15(8):1703-1708.
267. Jarow J, Sigman M, Kolettis PN, et al. The Evaluation of the Azoospermic Male: AUA Best Practice Statement. Linthicum, MD: American Urological Association; 2010.
269. World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 6th ed. Geneva: World Health Organization; 2021.
270. Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231-245.
271. Murray KS, James A, McGeady JB, et al. The effect of the new 2010 World Health Organization criteria for semen analyses on male infertility. Fertil Steril. 2012;98(6):1428-1431.
272. Esteves SC, Zini A, Azis N, et al. Critical appraisal of World Health Organization's new reference values for human semen characteristics and effect on diagnosis and treatment of subfertile men. Urology. 2012;79(1):16-22.
273. Li F, Yue H, Yamaguchi K, et al. Effect of surgical repair on testosterone production in infertile men with varicocele: a meta-analysis. Int J Urol. 2012;19:149-154.
274. Satterwhite CL, Torrone, Meites E, et al. Sexually transmitted infections among U.S. women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40(3):187-193.
275. Owusu-Edusei K, Jr, Chesson HW, Gift TL, et al. The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sex Transm Dis. 2013;40(3):197-201.
276. Dunne EF, Markowitz LE, Chesson H, et al. Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR. 2011;60(50):1705-1708.
277. Xu F, Sternberg MR, Gottlieb SL, et al. Seroprevalence of herpes simplex virus type 2 among persons aged 14–49 years—United States, 2005–2008. MMWR. 2010;59(15):456-459.
278. Kane BG, Degutis LC, Sayward HK, D'Onofrio G. Compliance with the Centers for Disease Control and Prevention recommendations for the diagnosis and treatment of sexually transmitted diseases. Acad Emerg Med. 2004;11(4):371-377.
279. Stupiansky NW, Alexander AB, Zimet GD. Human papillomavirus vaccine and men: what are the obstacles and challenges? Curr Opin Infect Dis. 2012;25(1):86-91.
280. Dunne EF, Nelson CM, Stone KM, et al. Prevalence of HPV infection among men: a systematic review of the literature. J Infect Dis. 2006;194(8):1044-1057.
281. Akogbe GO, Ajidahun A, Sirak B, et al. Race and prevalence of human papillomavirus infection among men residing in Brazil, Mexico and the United States. Int J Cancer. 2012;131(3):E282-E291.
282. Quillan G, Kruszon-Moran D, Markowitz LE, Unger ER, Paulose-Ram R. Prevalence of HPV in Adults Aged 18–United States, 2011–2014. NCHS Data Brief, No. 280. Hyattsville, MD: National Center for Health Statistics; 2017.
283. Giuliano AR, Anic G, Nyitray AG. Epidemiology and pathology of HPV disease in males. Gynecol Oncol. 2010;117(2Suppl): S15-S19.
284. U.S. Preventive Services Task Force. Screening for chlamydia and gonorrhea: U.S. Preventive Services Task Force recommendation statement. JAMA. 2021;326(10):949-956.
285. National Cancer Institute. Gardasil 9 Vaccine Protects Against Additional HPV Types. Available at https://www.cancer.gov/types/cervical/research/gardasil9-prevents-more-HPV-types. Last accessed June 1, 2022.
286. Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR. 2019;68(32):698-702.
287. Brown DL, Frank JE. Diagnosis and management of syphilis. Am Fam Physician. 2003;68(2):283-290, 297.
288. Miller KE. Diagnosis and treatment of Neisseria gonorrhoeae infections. Am Fam Physician. 2006;73(10):1779-1784, 1786.
289. Klausner JD. Disseminated Gonococcal Infection. Available at https://www.uptodate.com/contents/disseminated-gonococcal-infection. Last accessed June 1, 2022.
290. Lyss SB, Kamb ML, Peterman TA, et al. for the Project RESPECT Study Group. Chlamydia trachomatis among patients infected with and treated for Neisseria gonorrhoeae in sexually transmitted disease clinics in the United States. Ann Intern Med. 2003;139(3):178-185.
291. Peterman TA, Tian LH, Metcalf CA, et al. for the RESPECT-2 Study Group. High incidence of new sexually transmitted infections in the year following a sexually transmitted infection: a case for rescreening. Ann Intern Med. 2006;145(8):564-572.
292. Makadon HJ, Mayer KH, Garofalo R. Optimizing primary care for men who have sex with men. JAMA. 2006;296(19):2362-2365.
293. Bonvicini KA, Perlin MJ. The same but different: clinician-patient communication with gay and lesbian patients. Patient Educ Couns. 2003;51(2):115-122.
294. Dean L, Meyer IH, Robinson K, et al. Lesbian, gay, bisexual, and transgender health: findings and concerns. J Gay Lesbian Med Assoc. 2000;4(3):102-151.
295. Gay and Lesbian Medical Association. Guidelines for Care of Lesbian, Gay, Bisexual, and Transgender Patients. San Francisco, CA: Gay and Lesbian Medical Association; 2006.
296. Efford J, Bolding G, Maguire M, Sherr L. Do gay men discuss HIV risk reduction with their GP? AIDS Care. 2000;12(3):287-290.
297. Harrison AE, Silenzio VM. Comprehensive care of lesbian and gay patients and families. Prim Care. 1996;23(1):31-46.
298. Centers for Disease Control and Prevention. Sexually Transmitted Diseases Treatment Guidelines, 2021: Detection of STIs in Special Populations. Available at https://www.cdc.gov/std/treatment-guidelines/specialpops.htm. Last accessed June 1, 2022.
299. Plumb J, Brawer R. The social and behavioral foundations of men's health—a public health perspective. Prim Care. 2006;33(1): 17-34.
300. Winkler D, Pjrek E, Kasper S. Anger attacks in depression—evidence for a male depressive syndrome. Psychother Psychosom. 2005;74(5):303-307.
301. Chang T, Subramaniam PR. Asian and Pacific Islander American men's help-seeking: cultural values and beliefs, gender roles and racial stereotypes. Internat J Mens Health. 2008;7(2):121-136.
302. Bunker SJ, Colquhoun DM, Esler MD, et al. "Stress" and coronary heart disease: psychosocial risk factors. Med J Aust. 2003;178(6):272-276.
303. Knox SS, Siegmund KD, Weidner G, Ellison RC, Adelman A, Paton C. Hostility, social support, and coronary heart disease in the National Heart, Lung, and Blood Institute Family Heart Study. Am J Cardiol. 1998;82(10):1192-1196.
304. Kubzansky LD, Sparrow D, Jackson B, Cohen S, Weiss ST, Wright RJ. Angry breathing: a prospective study of hostility and lung function in the Normative Aging Study. Thorax. 2006;61(10):863-868.
305. Phillips LH, Henry JD, Hosie JA, Milne AB. Age, anger regulation and well-being. Aging Ment Health. 2006;10(3):250-256.
306. Vinson DC, Arelli V. State anger and the risk of injury: a case-control and case-crossover study. Ann Fam Med. 2006;4(1):63-68.
307. Epperly TD, Moore KE. Health issues in men: Part II. Common psychosocial disorders. Am Fam Physician. 2000;62(1):117-124.
310. U.S. Preventive Services Task Force. Intimate Partner Violence, Elder Abuse, and Abuse of Vulnerable Adults: Screening. Available at https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/intimate-partner-violence-and-abuse-of-elderly-and-vulnerable-adults-screening. Last accessed June 1, 2022.
311. Burge SK, Schneider FD, Ivy L, Catala S. Patients' advice to physicians about intervening in family conflict. Ann Fam Med. 2005;3(3):248-254.
312. Friedman LS, Samet JH, Roberts MS, Hudlin M, Hans P. Inquiry about victimization experiences: a survey of patient preferences and physician practices. Arch Intern Med. 1992;152(6):1186-1190.
313. American Academy of Family Practice. AAFP Policies: Intimate Partner Violence (Position Paper). Available at https://www.aafp.org/about/policies/all/intimate-partner-violence.html. Last accessed June 1, 2022.
314. Herman S, Sadovsky R. Psychosocial health screening and recognizing early signs of psychosocial distress. J Mens Health. 2010;7(1):73-82.
316. Laos C, Metzl JD. Performance-enhancing drug use in young athletes. Adolesc Med Clin. 2006;17(3):719-731.
317. Gay and Lesbian Medical Association. Breaking the Grip: Treating Crystal Methamphetamine Addiction Among Gay and Bisexual Men. San Francisco, CA: Gay and Lesbian Medical Association; 2006.
318. U.S. Preventive Services Task Force. Unhealthy Alcohol Use in Adolescents and Adults: Screening and Behavioral Counseling Interventions. Available at https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/unhealthy-alcohol-use-in-adolescents-and-adults-screening-and-behavioral-counseling-interventions. Last accessed June 1, 2022.
319. Enoch MA, Goldman D. Problem drinking and alcoholism: diagnosis and treatment. Am Fam Physician. 2002;65(3):441-450.
320. Mayfield D, McLeod G, Hall P. The CAGE questionnaire: validation of a new alcoholism screening instrument. Am J Psychiatry. 1974;131(10):1121-1123.
322. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction. 1993;88(6):791-804.
323. Selzer ML. The Michigan Alcoholism Screening Test: the quest for a new diagnostic instrument. Am J Psychiatry. 1971;127(12):1653-1658.
324. Mersy DJ. Recognition of alcohol and substance abuse. Am Fam Physician. 2003;67(7):1529-1532, 1535-1536.
325. Fink A, Morton SC, Beck JC, Hays RD, Spritzer K, Oishi S, Moore AA. The alcohol-related problems survey: identifying hazardous and harmful drinking in older primary care patients. J Am Geriatr Soc. 2002;50(10):1717-1722.
326. Culberson JW. Alcohol use in the elderly: beyond the CAGE. Part 2: screening instruments and treatment strategies. Geriatrics. 2006;61(11):20-26.
327. Moore AA, Beck JC, Babor TF, Hays RD, Reuben DB. Beyond alcoholism: identifying older, at-risk drinkers in primary care. J Stud Alcohol. 2002;63(3):316-324.
328. Robbins A. Biopsychosocial aspects in understanding and treating depression in men: a clinical perspective. J Mens Health Gender. 2006;3(1):10-18.
329. National Institute of Mental Health. Major Depression. Available at https://www.nimh.nih.gov/health/statistics/major-depression.shtml. Last accessed May 31, 2022.
330. Blashki G, Pirkis J, Morgan H, Ciechomski L. Managing depression and suicide risk in men presenting to primary care physicians. Prim Care. 2006;33(1):211-221.
331. U.S. Preventive Services Task Force. Screening for Suicide Risk in Adolescents, Adults, and Older Adults. Available at https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/suicide-risk-in-adolescents-adults-and-older-adults-screening. Last accessed June 1, 2022.
332. Pirkis J, Burgess P. Suicide and recency of health care contacts: a systematic review. Br J Psychiatry. 1998;173:462-474.
333. Kilmartin C. Depression in men: communication, diagnosis and therapy. J Mens Health Gender. 2005;2(1):95-99.
335. National Institute of Mental Health. Men and Depression. Bethesda, MD: National Institutes of Health; 2005.
336. Men's Health Network. Goals and Strategies. Available at https://www.menshealthnetwork.org/goals. Last accessed June 1, 2022.
337. Larson RJ, Woloshin S, Schwartz LM, Welch HG. Celebrity endorsements of cancer screening. J Natl Cancer Inst. 2005;97(9):693-695.
338. Ilic D, Risbridger GP, Green S. The informed man: attitudes and information needs on prostate cancer screening. J Mens Health Gender. 2005;2(4):414-420.
339. Wilkins D. "Getting it sorted:" identifying and implementing practical solutions to men's health. J Mens Health Gender. 2005;2(1):13-16.
340. Williams S, Bruno A. Worksite wellness programs—what is working. Am J Mens Health. 2007;1(2):154-156.
341. Campbell BB. A novel approach to educating men about preventative health in the digital age. J Mens Health. 2012;9(1):45-50.
342. Percac-Lima S, Grant RW, Green AR, et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial. J Gen Intern Med. 2009;24(2):211-217.
343. Releford BJ, Frencher SK Jr, Yancey AK, Norris K. Cardiovascular disease control through barbershops: design of a nationwide outreach program. J Natl Med Assoc. 2010;102(4):336-345.
344. Luque JS, Rivers BM, Kambon M, Brookins R, Green BL, Meade CD. Barbers against prostate cancer: a feasibility study for training barbers to deliver prostate cancer education in an urban African American community. J Cancer Educ. 2010;25(1):96-100.
345. Mount DL, Johnson DM, Rego MI, et al. Preliminary findings exploring the social determinants of black males' lay health perspectives. Am J Mens Health. 2012;6(1):71-79.
346. Xu KT, Borders TF. Gender, health, and physician visits among adults in the United States. Am J Public Health. 2003;93(7):1076-1079.
347. Campbell BB, Shah S, Gosselin D. Success with men's educational group appointments (MEGA): subjective improvements in patient education. Am J Mens Health. 2009;3(2):173-178.
348. Blumberg SJ, Vahratian A, Blumber JH. Marriage, cohabitation, and men's use of preventive health care services. NCHS Data Brief. 2014;154:1-7.
350. Oliffe J, Mroz L. Men interviewing men about health and illness: ten lessons learned. J Mens Health Gender. 2005;2(2):257-260.
351. McNutt RA. Shared medical decision making: problems, process, progress. JAMA. 2004;292(20):2516-2518.
352. Centers for Disease Control and Prevention. Recommended Adult Immunization Schedule for Ages 19 Years or Older, United States, 2022. Available at https://www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Last accessed June 1, 2022.
353. Coker TJ and Dierfeldt DM. Acute bacterial prostatitis: diagnosis and management. Am Fam Physician. 2016;93:114-120.
354. Shindel AW, Althof SE, Carrier S, et al. Disorders of ejaculation: an AUA/SMSNA Guideline. J Urol. 2022;207(3):504-512.
355. Burnett AL, Nehra A, Breau RH, et al. Erectile dysfunction: AUA guideline. J Urol. 2018;200:633.
356. Schlegel PN, Sigman M, Collura B, et al. Diagnosis and Treatment of Infertility in Men: AUA/ASRM Guideline, 2020. Available at https://www.auanet.org/guidelines/guidelines/male-infertility. Last accessed June 1, 2022.
357. Centers for Disease Control and Prevention. Sexually Transmitted Infections Prevalence, Incidence, and Cost Estimates in the United States. Available at https://www.cdc.gov/std/statistics/prevalence-incidence-cost-2020.htm. Last accessed June 1, 2022.
358. Eastham JA, Auffenberg GB, Barocas DA, et al. Clinically Localized Prostate Cancer: AUA/ASTEO Guideline (2022). Available at https://www.auanet.org/guidelines/guidelines/clinically-localized-prostate-cancer-aua/astro-guideline-2022. Last accessed June 1, 2022.
359. Zuniga KB, Chan JM, Ryan CJ, Kenfield SA. Diet and lifestyle considerations for patients with prostate cancer. Urologic Oncology. 2020;38(3):105-117.
360. U.S. Preventive Services Task Force. Serologic screening for genital herpes infection: U.S. Preventive Services Task Force recommendation statement. JAMA. 2016;316(23):2525-2530.
361. U.S. Preventive Services Task Force. Screening for syphilis infection in nonpregnant adults and adolescents: U.S. Preventive Services Task Force recommendation statement. JAMA. 2016;315(21):2321-2327.
362. Veale JF. Prevalence of transsexualism in New Zealand. Aust N Z J Psychiatry. 2008;42(10):887-889.
363. Sobralske M. Primary care needs of patients who have undergone gender reassignment. J Am Acad Nurse Pract. 2005;17(4):133-138.
364. Committee on Health Care for Underserved Women. Committee Opinion #512: health care for transgender individuals. Obstet Gynecol. 2011;118(6):1454-1458.
1. National Clinical Guideline Centre for Acute and Chronic Conditions. Lower Urinary Tract Symptoms in Men: Assessment and Management. London: National Institute for Health and Care Excellence; 2015. Available at https://www.nice.org.uk/guidance/cg97. Last accessed June 6, 2022.
2. American Urology Association, American Society for Reproductive Medicine. Diagnosis and Treatment of Infertility in Men: AUA/ASRM Guideline. Available at https://www.auanet.org/guidelines/guidelines/male-infertility. Last accessed June 6, 2022.
3. European Association of Urology. EAU Guidelines on Testicular Cancer. Available at https://uroweb.org/guidelines/testicular-cancer. Last accessed June 6, 2022.
4. Marcell AV, Male Training Center for Family Planning and Reproductive Health. Preventive Male Sexual and Reproductive Health Care: Recommendations for Clinical Practice. Philadelphia, PA: Male Training Center for Family Planning and Reproductive Health; 2014. Summary retrieved from Guideline Central at https://rhntc.org/sites/default/files/resources/mtc_male_prevrhc_2014.pdf. Last accessed June 6, 2022.
5. National Collaborating Centre for Women's and Children's Health. Fertility: Assessment and Treatment for People with Fertility Problems. London: National Institute for Health and Clinical Excellence; 2013. Available at https://www.nice.org.uk/guidance/cg156. Last accessed June 6, 2022.
6. U.S. Preventive Services Task Force. Behavioral counseling interventions to prevent sexually transmitted infections: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2020;324(7):674-681. Available at https://jamanetwork.com/journals/jama/fullarticle/2769474. Last accessed June 6, 2022.
7. Centers for Disease Control and Prevention. Preexposure Prophylaxis for the Prevention of HIV Infection in the United States, 2021 Update. Atlanta, GA: Centers for Disease Control and Prevention; 2021. Available at https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf. Last accessed June 6, 2022.
Mention of commercial products does not indicate endorsement.