With knowledge of the structures and function of the muscles, joints, and connective tissue and the dynamic pathology that intrudes and impedes normal function, nurses can readily provide quality and often life-saving actions. An awareness of why symptoms appear leads to quicker reporting to physicians of changes in the patient's condition. Nurses can also perform immediate interventions based on standing orders and recognition of what needs to be done in order to provide safe quality care. This knowledge changes what could be only technical care to professional care through the use of decision-making skills built upon the knowledge of pathophysiology.
- INTRODUCTION
- MUSCLES, JOINTS, AND CONNECTIVE TISSUES: STRUCTURAL AND FUNCTIONAL INTER-RELATIONSHIPS
- THE PROCESS OF SKELETAL MUSCLE CONTRACTION
- PATHOPHYSIOLOGIC INFLUENCES AND EFFECTS
- RELATED INFLUENCES AND EFFECTS
- NURSING ASSESSMENT: ESTABLISHING THE DATA BASE
- SPECIFIC DISORDERS OF CONNECTIVE TISSUE AND MUSCLES
- SPECIFIC DISORDERS OF THE JOINTS
- CASE STUDIES
- CONCLUSION
- Works Cited
- Evidence-Based Practice Recommendations Citations
This course is designed for nurses in all practice settings.
As health care becomes more complex, it is essential that the theoretical concepts of the basis of illness (pathophysiology) be well understood. The purpose of this course is to reinforce the scientific rationales for the interventions nurses perform and the decisions nurses make as patients move through the ever-changing struggle with their illness.
Upon completion of this course, you should be able to:
- Describe the structure and function of the muscles, joints, and connective tissues.
- Discuss the pathophysiologic influences that may affect the muscles, joints, and connective tissues.
- Outline the role of subjective data in completing a full nursing assessment of the muscles, joints, and connective tissues.
- Describe objective data compiled during a nursing assessment of the muscles, joints, and connective tissues.
- Identify imaging and diagnostic studies used in the identification and classification of muscles, joints, and connective tissues.
- Discuss genetic conditions manifesting in the muscles and connective tissues.
- Evaluate the presentation and differential diagnosis of inflammatory muscle and connective tissue disorders.
- Describe the clinical presentation and treatment of immunologic disorders of the muscles and connective tissues.
- Review the assessment and treatment of traumatic conditions of the muscles and connective tissue.
- Discuss disorders of the joints with multifactorial origin.
- Analyze the manifestations and therapeutic approaches for degenerative joint diseases.
- Outline the presentation, treatment, and nursing considerations for patients with immunologic joint conditions, such as rheumatoid arthritis.
- Compare and contrast the various joint diseases with an infectious origin.
- Describe cancers of the joints, muscle, and connective tissues.
- Evaluate the appropriate assessment and management of traumatic joint injuries.
Jane C. Norman, RN, MSN, CNE, PhD, received her undergraduate education at the University of Tennessee, Knoxville campus. There she completed a double major in Sociology and English. She completed an Associate of Science in Nursing at the University of Tennessee, Nashville campus and began her nursing career at Vanderbilt University Medical Center. Jane received her Masters in Medical-Surgical Nursing from Vanderbilt University. In 1978, she took her first faculty position and served as program director for an associate degree program. In 1982, she received her PhD in Higher Education Administration from Peabody College of Vanderbilt University. In 1988, Dr. Norman took a position at Tennessee State University. There she has achieved tenure and full professor status. She is a member of Sigma Theta Tau National Nursing Honors Society. In 2005, she began her current position as Director of the Masters of Science in Nursing Program.
Contributing faculty, Jane C. Norman, RN, MSN, CNE, PhD, has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Sharon Cannon, RN, EdD, ANEF
The division planner has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Sarah Campbell
The Director of Development and Academic Affairs has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
The purpose of NetCE is to provide challenging curricula to assist healthcare professionals to raise their levels of expertise while fulfilling their continuing education requirements, thereby improving the quality of healthcare.
Our contributing faculty members have taken care to ensure that the information and recommendations are accurate and compatible with the standards generally accepted at the time of publication. The publisher disclaims any liability, loss or damage incurred as a consequence, directly or indirectly, of the use and application of any of the contents. Participants are cautioned about the potential risk of using limited knowledge when integrating new techniques into practice.
It is the policy of NetCE not to accept commercial support. Furthermore, commercial interests are prohibited from distributing or providing access to this activity to learners.
Supported browsers for Windows include Microsoft Internet Explorer 9.0 and up, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Supported browsers for Macintosh include Safari, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Other operating systems and browsers that include complete implementations of ECMAScript edition 3 and CSS 2.0 may work, but are not supported. Supported browsers must utilize the TLS encryption protocol v1.1 or v1.2 in order to connect to pages that require a secured HTTPS connection. TLS v1.0 is not supported.
The role of implicit biases on healthcare outcomes has become a concern, as there is some evidence that implicit biases contribute to health disparities, professionals' attitudes toward and interactions with patients, quality of care, diagnoses, and treatment decisions. This may produce differences in help-seeking, diagnoses, and ultimately treatments and interventions. Implicit biases may also unwittingly produce professional behaviors, attitudes, and interactions that reduce patients' trust and comfort with their provider, leading to earlier termination of visits and/or reduced adherence and follow-up. Disadvantaged groups are marginalized in the healthcare system and vulnerable on multiple levels; health professionals' implicit biases can further exacerbate these existing disadvantages.
Interventions or strategies designed to reduce implicit bias may be categorized as change-based or control-based. Change-based interventions focus on reducing or changing cognitive associations underlying implicit biases. These interventions might include challenging stereotypes. Conversely, control-based interventions involve reducing the effects of the implicit bias on the individual's behaviors. These strategies include increasing awareness of biased thoughts and responses. The two types of interventions are not mutually exclusive and may be used synergistically.
#38950: Pathophysiology: Muscles, Joints, and Connective Tissues
Along with the bones, muscles, ligaments, tendons, cartilage, and the joints provide the body with a supportive framework that allows flexibility of movement and protects the internal organs. These tissues also give shape to the body and act partially as a storage and supply area for minerals. When the tissues are unable to perform their usual functions because of trauma or rheumatic, inflammatory, or degenerative conditions, a person's physical support, protection, mobility, and ability to carry out activities of daily living are affected.
The musculoskeletal system is composed of many anatomical structures that work together to produce movement, support, and protection of the body and its parts. These structures include the bones and joints of the skeletal system; the skeletal muscles; and the tendons, ligaments, and other elements that connect these tissues. This course will focus on the components of the system excluding the bones.
Contraction of skeletal muscle is its primary function, with the intent of moving the bones of the skeleton. Bone serves as a lever, the joint serves as a fulcrum upon which the bone pivots, and the muscle provides the force that moves the lever. A second function of skeletal muscles is maintenance of body posture. A residual amount of contraction in the muscles, known as muscle tone, serves to keep the body erect. A third function is heat production. To combat hypothermia, small, rapid contractions of skeletal muscle (shivering) produce body heat [1].
A typical skeletal muscle is anchored at each end to bone by a tendon. The muscle often stretches across a joint. The muscle's attachment to the less movable bone is called its origin, and its attachment to the more movable bone is called its insertion. When the muscle contracts, one bone remains more or less stationary, forcing the other bone to move [2].
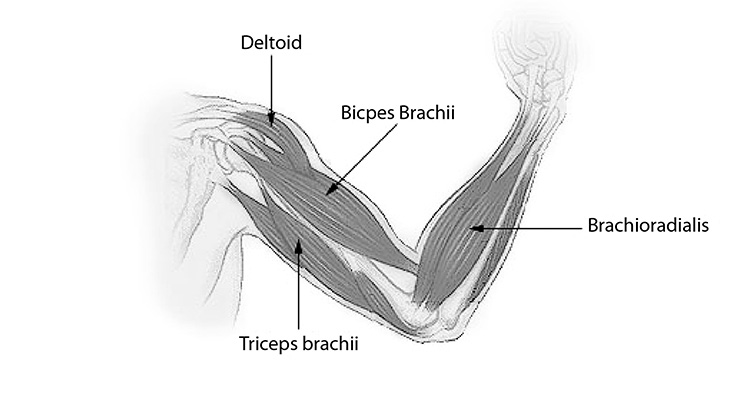
Most skeletal muscles work in groups. The prime mover is the muscle that contracts to produce the movement. Synergists are muscles that work with prime movers to assist in performing the movement. Antagonists are muscles that work opposite prime movers by relaxing during their contraction or by producing an opposite effect. For example, the arm is flexed by contracting the biceps brachia, which acts as the prime mover; at the same time, the triceps brachii on the opposite side of the humerus relaxes, acting as the antagonist (Figure 1). When the arm is extended, the roles of the biceps and triceps are reversed. An isotonic contraction occurs when a muscle shortens during contraction. An isometric contraction occurs when a muscle becomes tense while remaining the same length [2].
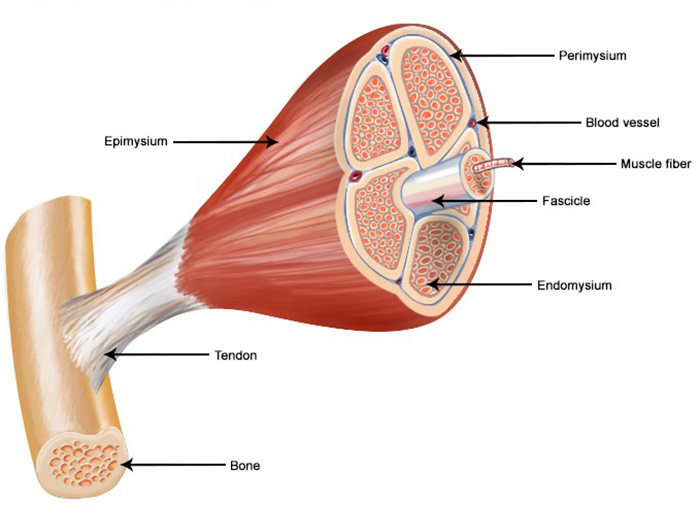
Muscle—skeletal, smooth, and cardiac—is made up of elongated cells called fibers (Figure 2). The fibers contain bands of contractile protein called myofibrils that extend the length of the cell. At the neuromuscular junction, the chemical acetylcholine creates the stimulus for muscle-nerve conduction of movement. Skeletal muscle fibers are multinucleated, and their myofibrils have striations: light and dark bands perpendicular to the long axis of the cell. The dark bands (anisotropic or A bands) are composed of the protein myosin, and the light bands (isotropic or I bands) contain the protein actin. A sense fibrous line called the Z line crosses the center of each I band and divides the myofibrils into a series of repeating units called sarcomeres. The bands are visible to the unaided eye and give skeletal muscle its alternate name: striated muscle. Smooth and cardiac muscles are made up of uninucleated cells. They further differ from skeletal muscle in that smooth muscle has tapered fibers with no striations and cardiac muscle has branched fibers [2].
Muscle fibers are bound together by connective tissue into small bundles called fascicles, visible to the unaided eye. Fascicles are bound into larger bundles, which collectively form the muscle. The entire muscle is enclosed by a connective tissue covering called the epimysium, which is continuous with the connective tissue surrounding the fascicles and fibers. The epimysium is also continuous with the tendon or other connective tissue at attachment of muscle to bone. Thus, there is a continuous network of connective tissue extending from individual muscle fibers to the tendon. Blood vessels and nerves penetrate the connective tissue of the muscle, so muscle has sufficient blood supply to furnish nutrients and oxygen and to remove the waste products of muscular activity [2,3].
Tendons are cords of connective tissue that attach muscles to the periosteum of the bones. During muscle contraction, the muscle pulls the tendon, which pulls the bone to which it is attached, producing movement. Flexion, extension, adduction, and abduction are normal movements of muscles and bones.
Ligaments, made of fibrous connective tissue, connect bones to one another. They have the ability to stretch while providing stability. The knee joint, for example, is stabilized by ligaments, such as the anterior and posterior cruciate ligaments, which bind the femur to the tibia within the joint capsule, and by the medial and lateral collateral ligaments outside the joint capsule [4].
A bursa is a fluid-filled sac that facilitates motion of structures that move against each other. It can be found between skin and bone, muscle and bone, tendons and bone, ligaments and bone, and between muscles. The bursae function as padding between structures to reduce friction caused by moving parts [4].
Connective tissue, in the broad sense of the term, include all tissues made up of cells in a matrix, including bone, cartilage, blood, and lymph. However, the term is used in a more limited sense when discussing diseases of the connective tissues. In this sense, connective tissue means the binding and covering tissues of the body, inducing tendons, ligaments, muscle fascia, and the deep layers of the skin. This kind of connective tissue (sometimes called "connective tissue proper") is essential in holding together all the components of the musculoskeletal system. Also included are intervertebral discs (or intervertebral fibrocartilage), which serve as "shock absorbers" to cushion the spine and help it move [4,5].
Skeletal muscle contraction begins with the stimulus of a muscle fiber by a motor neuron. Every motor neuron ends in many fine branches, with each branch connecting with an individual muscle fiber. A group of muscle fibers activated by a single motor neuron is called a motor unit. Motor units range in size from a single muscle fiber in muscles controlling fine, skilled movements to over one hundred fibers in muscles involved in gross movements. All the fibers of a motor unit contract together when the neuron is stimulated [6]. There are two types of motor units in skeletal muscle, Type 1 and Type 2. Type 1 has a small cell diameter, with a high excitability and fast conduction velocity. It has an oxidative profile with moderate contraction velocity and low fatigability. There are few muscle fibers of this type. In contrast, Type 2 has a large cell diameter, with low excitability but a very fast conduction velocity. Type 2 fibers are numerous in quantity, with a glycolytic profile and high fatigability. The small motor units, with Type 1 (also known as "slow-twitch") fibers, are recruited first and are frequently active, while the large motor units, with Type 2 ("fast-twitch") fibers, are used infrequently, in forceful contractions. Maximal efforts, in which fast motor units are recruited, cannot be sustained because of the rapid depletion of glycogen.
When a nerve impulse reaches the end of a motor neuron, small vesicles in the ends of the nerve branches release acetylcholine, which increases the permeability of the muscle cell and causes an influx of calcium ions into the cell. The calcium ions cause structural changes in the myofilaments that allow them to slide past each other, causing contraction. The structural changes also allow breakdown of adenosine triphosphate (ATP) to adenosine diphosphate (ADP) to provide energy for the contraction. The muscle relaxes as a result of the action of the enzyme cholinesterase, which breaks down acetylcholine, allowing the muscle to return to its resting state [6].
At the beginning of muscle contraction, ATP is formed from creatine phosphate stored in the muscle. The supply of creatine phosphate is limited, however, and even with mild muscle activity, additional ATP must be formed from ADP. The energy for forming this additional ATP is supplied by respiration. The first step in respiration is glycolysis, or anaerobic respiration, which produces lactic acid and small amounts of ATP. Under normal conditions, the lactic acid is broken down further by aerobic respiration, which requires an oxygen supply. The final products of aerobic respiration are carbon dioxide, water, and large amounts of ATP [6].
During sustained strenuous exercise, the blood cannot supply enough oxygen to keep pace with glycolysis, and lactic acid accumulates in the muscle, causing an oxygen debt. Muscle contractions continue for a short time using the small amount of ATP produced by glycolysis, but soon the demand exceeds the supply and the muscle is fatigued. The contractions decease in strength and then stop. The pain of muscle fatigue is the result of accumulated lactic acid. Oxidation of excess lactic acid occurs after exercise, when the person breathes deeply to pay off the oxygen debt [6].
The effects of exercise on the body's cells are significant. Physical activity increases the size and number of mitochondria, increases muscle's ability to use fat as a source of energy, increases the size of muscle fibers, and increases the content of myoglobin in muscle fibers. Exercise also results in increased fat oxidation. All of these increases lead to hypertrophy of the muscle, which leads to an increase in strength of the muscle. The wasting of muscle due to lack of use is assessed as atrophy
The primary function of the musculature and connective tissues of the body is to provide body movement. When disease or trauma alters the system, the individual's ability to move and ambulate can be affected, which can profoundly affect a person's lifestyle. Movement is often still possible, but not without pain or difficulty [7].
Inflammation may occur in muscle or connective structures as a result of excessive or repeated strain or pathogenic invasion. Restricted motion and pain usually result. One such example is rotator cuff injury, when the patient is unable to abduct the arm because of pain and muscle spasms. Other connective tissues of the body may be affected by inflammation, resulting in changes in other organs as well as the musculoskeletal system. Many of these connective tissue disorders are believed to be associated with immune processes [7].
The joint is the musculoskeletal structure most frequently influenced by degenerative disease. Changes are most often associated with aging, excess weight, trauma, and inflammatory conditions. In the presence of these factors, articular cartilage softens, thins, and ulcerates, and the joint surfaces become rough. There may be a narrowing of the joint space and swelling of adjacent soft tissue. The normal smooth-gliding joint action is diminished, and the periosteum becomes irritated by friction, stimulating the growth of bone spurs at the joint margins. The effects of this destruction include joint pain, stiffness, and joint deformity, which can result in slight to moderate limitation of movement. Crunching or grating sounds, called crepitus, may be heard upon movement [8,9].
The intervertebral discs can also be affected by degeneration. The water content of the discs decreases with age, causing them to become thinner. The surrounding ligaments also change with age, so the disc becomes unstable. These changes along with increased bone resorption cause decreased height and painless restriction of spinal movement in the elderly. In some cases, the condition becomes more severe, with pressure on nerves causing pain and neurologic deficits [8,9].
Somewhat akin to degeneration is the process of atrophy. Muscle can atrophy as a result of disuse. As noted, the normal strain on muscles contributes to their development and to the maintenance of their size, shape, strength, and composition. Through disuse, muscle cells become reduced in size and weakened, and the muscle mass becomes more fibrous. Inactivity can also lead to joint contracture; the muscle fibers become shortened and fixed, and the joint's range of motion becomes limited. These conditions are reversible with the resumption of activity. However, contractures can progress to an irreversible state without treatment [8,9].
Musculoskeletal structures, such as joints and bursae, can be infected by pathogens entering from penetrating wounds or via the circulation. Pain and restricted motion are common in these cases [10].
Malignant neoplasms of the bone, muscle, and cartilage are called sarcomas. Cancer affecting the muscle is called rhabdomyosarcoma; chondrosarcoma originates in the bones but can extend to the cartilage [11]. Depending on the specific cancer and location, patients may experience a temporary limitation in mobility (e.g., following surgery for tumor removal) or permanent limitation due to extensive surgical intervention, such as amputation [11].
Skeletal muscle can be injured by trauma. Fortunately, skeletal muscle fibers can regenerate, but when the damage is extensive, the fibers are replaced by scar tissue. Trauma to the musculoskeletal structures supporting the joints is common. Muscle fibers may be injured due to overuse, overstretching, forcible twisting and other abnormal movement. The fibers may be torn, or stretched too far, and joint surfaces may dislocate, that is, separate partially or completely. Associated blood vessels and nerves may be damaged in the process. Pain and limited motion are the result [12].
Direct muscle trauma, overuse, or exposure to high temperatures can induce rhabdomyolysis. Rhabdomyolysis is a complex syndrome involving the rapid dissolution of damaged skeletal muscle, resulting in the leakage of intracellular contents to such an extent that it results in organ (particularly kidney) damage.
Neurologic and vascular problems can cause or contribute to connective tissue and muscle disorders. Because muscle functioning is the result of the combined effect of muscle fibers and motor nerves, neurologic damage or interference can impair muscle functioning, causing atrophy and paralysis. Likewise, disruption of the vascular supply to these tissues can limit the nutrient and oxygen supply to cells and interfere with removal of cellular waste products. Prolonged interruption of circulation leads to necrosis [13,14].
Connective tissue disorders can also give rise to neurologic or vascular problems, which may in turn cause further musculoskeletal damage. Pressure from bandages, traction devices, tumor growth, and poor positioning are a few problems that can hinder nerve and blood vessel functioning. Trauma to muscles causes edema and hemorrhage in soft tissues, increasing the pressure within a confined space. Pressure on nerves and blood vessels in the area can become so great as to produce irreversible necrosis of the muscle tissue. A permanently disabling contracture of the limb may occur, as well as loss of motor and sensory functioning [13,14].
A person's occupation and lifestyle can contribute to alterations in the muscular and connective tissues. Interest in physical fitness has prompted many people to become active in athletic endeavors. Highly athletic activities, including weightlifting, distance running, and more intense sports, are associated with an increased risk for injury, particularly with improper conditioning and training [15].
Sport injuries can generally be categorized as acute or overuse. Acute injuries occur most often in contact sports and include strains, sprains, and dislocations. Overuse injuries are usually a result of repetitive motions or excessive intensity or duration of exercise. Acute injuries are typically traumatic (e.g., ligament tears), while the most common overuse injuries are tendinosis and osteoarthritis [15].
With muscle and connective tissue disorders, patients may be unable to continue their usual recreational activities. Further, roles within the family may change to accommodate impaired ability to conduct usual activities of daily living. Occasionally, it may be necessary to use assistive devices or to modify the environment, which requires a period of adjustment [15].
The nursing assessment of patients with muscle, joint, and/or connective tissue disorders requires special emphasis on the musculoskeletal, neurologic, and vascular systems [16].
As part of any nurse assessment, patients should provide important information about what they are experiencing as a result of their conditions.
Pain, in some cases severe, is a common manifestation of joint, muscle, and connective tissue problems. Patients should be asked to describe their pain thoroughly, including location, intensity, quality, duration, radiation, precipitating factors, and successful relief measures. Some patients ache all over and should indicate each of the areas involved. Knowing the quality of pain may help pinpoint a specific problem, but the patient may require help in describing the pain. All these data are helpful in reaching a diagnosis [16].
Some patients experience pain so severe they cannot tolerate moving or being touched. Others have learned to live with chronic pain. It is important to pay attention to descriptions of pain that seem unusual or excessive for the patient's condition; such complaints warrant a thorough assessment. Changes in pain status may indicate a new or undiagnosed condition [16].
Some patients with musculoskeletal conditions will experience paresthesia, such as tingling, numbness, and/or and diminished or absent sensation. The affected area should be defined as precisely as possible. Paresthesia is an indication of a neurologic problem and requires an in-depth assessment [16].
Nurses can obtain additional subjective data by asking the patient how the problem affects activities of daily living and mobility. Changes in normal activities may be from pain alone or from other effects of their illness, including fatigue, weakness, stiffness, or decreased mobility of a particular body part. Some patients may have abandoned activities or made adjustments to maintain independence. Patients should be encouraged to discuss their view of the situation to bring insights and misconceptions to the surface [16].
Patients should be asked about any assistive devices used to help maintain independence, including aids for walking, eating, dressing, bathing, or toileting. These may not be devices designed specifically for the tasks; some are creative and adaptive in finding new ways to meet their daily needs [16].
Objective data include the results of physical assessment and of laboratory and other diagnostic tests. When assessing patients with musculoskeletal disorders, vital signs, posture, muscle strength and tone, ability to ambulate, and neurologic status should all be included in the patient assessment [16].
Assessment of vital signs is of particular importance in cases of musculoskeletal trauma. Hyperthermia may accompany inflammation and is common with an infection. Observing respiration is essential when injury occurs to the face, neck, or chest. Patients with spinal or chest changes may also have abnormal respirations [16].
Inflammation is an immune response to infection, physical trauma, or autoimmune reaction. Swelling occurs as inflammatory exudate forms to defend the tissues from the injury. Edema may also be present. Inspection and palpation are used when assessing patients for swelling and inflammation and comparing one extremity to the other for size, warmth, and erythema. A joint will appear swollen when there is an increase in synovial fluid or when blood or purulent material is present in the joint capsule. This swelling is known as effusion. Effusion in the knee is detected by displacing the fluid with an upward stroke along the medial side of the knee and then pressing on the lateral side. The fluid will return and form a bulge (the bulge sign).
It is important to be gentle when assessing inflamed areas because they are usually tender. It is best to start palpating at the distance from the obvious tender area and work toward it, letting patients know when and where they will be touched and reassuring them that the touch will be gentle [16].
Injury or disease processes may cause changes in the skin. Discoloration results when trauma to soft tissues causes ecchymosis (bruising). The skin may be broken or torn as result of injury. Describe any lesions completely: include the occasion, length, depth, and appearance of the involved tissue. If there is any drainage, describe the amount, color, type, and odor [17,18].
Rashes are common in connective tissue disorders. Look for changes in the skin such as discoloration, dryness, scaliness, and lesions. With some types of arthritis, the hair, skin, and nails may show signs of changes. Discoloration, usually redness, may occur in the palms, over joints, and at the distal ends of the toes and fingers. Normal pigmentation may also be altered. Characteristic nodules may be noted when palpating and observing the skin [17,18].
Joints may be assessed for changes by observation and palpation. Heberden nodes may be noted on the distal interphalangeal joint of patients with osteoarthritis. Likewise, rheumatoid nodules may be noted near the joints of patients with rheumatoid arthritis, even in the absence of other signs. Joints may be compared bilaterally to assess symmetry, position, and changes in alignment [17,19].
The curvature of the spine should be assessed to identify the presence of scoliosis (lateral curve), kyphosis (convex curve of the thoracic spine), or lordosis (concave curve of the lumbar spine). Patients with skeletal changes may shift another body part in the opposite direction to compensate for the imbalance; for example, the pelvis may tilt to compensate when one leg is shorter than the other [17,18].
Range of motion can be measured with an instrument called a goniometer. Placing the arms of the goniometer parallel to the axis of the bones that form the joint, the examiner measures the angle for the typical positions of the joint. The elbow's normal flexion, for example, is 160o, whereas its normal extension is 0o. To determine what is normal for a patient, compare a joint with an apparently impaired range of motion to the corresponding joint in the other extremity, if possible. Patients can have differences in range of motion for a variety of reasons, particularly as they age, so it is vital to assess typical range of motion on an individualized basis. Dexterity is usually assessed by asking the patient to pick up an object from a flat surface [17,18].
If a patient is unable to move an extremity, range of motion may be determined through passive movement. Joints should not be moved beyond the point of comfort, and if possible, assessments should not include acutely inflamed joints, which may be tender [17,18].
During the assessment of range of motion, note joint stiffness, instability, and changes. Boney crepitation may be heard or felt during movement when there is a rough surface of the articular cartilage or when broken bone ends rub together. A limitation of motion may be due to a contracture. Early detection of signs movement limitation can allow for the implementation of measures to improve range of motion and prevent further limitations [17,18].
Observe the patient's standing posture for abnormalities. Posture can be affected by structural changes or differences, muscle weakness, or trauma. In addition, patients may hold themselves in positions that relieve or decrease pain. Patients should be observed for symmetry. Posture is also an indication of energy and muscle tone. Normally, posture is erect but not rigid [16].
Assessment of muscle strength, size, and tone can support the diagnostic process, but it can also provide information about the amount of assistance necessary for ambulation and participating in activities. Muscle strength is assessed by asking the patient to resist movements or to move against resistance [16].
Muscles should be observed and palpated bilaterally to check their size and asymmetry. If there seems to be a significant discrepancy in size, the limb circumferences should be measured [16].
Muscle tone is assessed by moving the extremities passively. While the patient is relaxed the examiner moves the extremity through the ranges of motion, noting resistance to movement. A muscle with diminished tone is described as flaccid. When the muscle is tight and tense from involuntary contraction, it is said to be spastic [16]. Function of the muscles depends on proper function of the nervous system. Muscle abnormalities noted during the assessment may be due to disorders of the nervous system rather than musculoskeletal disorders.
To assess the ability to ambulate, the patient should be asked to get up to walk across the room, turn around, and come back. Any difficulties rising to standing, starting or stopping walking, or turning should be noted. In a typical gait, the feet are 2–4 inches apart, and the body shifts from side to side about 1 inch. The posture is erect, with toes pointed straight ahead and shoulders in a straight line; the arms swing back and forth at the person's sides, and movement is smooth with good balance [16].
There can be a variety of irregularities in gait, with an equally diverse underlying etiology. A limp can occur from differences of leg length, joint motion, muscle strength, or other causes. The gait may appear stiff, unsteady, or wide-based; the feet may drag, or the steps may be very short. The body may lurch to the side as the individual shifts weight from one leg to the other. An irregular gait can cause fatigue because of the extra energy needed for walking. Ambulation may also be affected by pain, fear of falling, and loss of balance and coordination. As adults age, walking speed and balance may decrease. Steps may be short and shuffling, without the confidence and poise of youth [16].
Diagnostic studies provide information useful in diagnosing and following the course of the disease process.
Blood tests performed to detect presence of muscle disease measure levels of enzymes released when muscle tissues are destroyed or injured. These enzymes are creatine phosphokinase or creatine kinase, lactic dehydrogenase (LDH), and serum glutamic-oxaloacetic transaminase (SGOT), also known as aspartate amino-transaminase (AST). The same tests indicate cardiac muscle destruction in the patient with a myocardial infarction [20].
The antinuclear antibody (ANA) test is the most specific and sensitive test for lupus and is therefore the most commonly used autoantibody test. Ninety-seven percent of patients with lupus have a positive ANA blood test. The titer and patterns of the blood sample are reported. A titer greater than 1:80 is usually considered positive [21]. It is important to note that a positive ANA test is found in 97% of patients with lupus, but alone, it does not indicate a conclusive diagnosis of lupus [21]. A positive ANA test, although not always found, satisfies one of the four typical clinical characterizations required for a definitive diagnosis of lupus. ANA tests may also be positive in patients with other connective tissue diseases, chronic infectious diseases, and autoimmune diseases [21].
The 2010 American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) joint working group recommends several laboratory tests for the diagnosis of rheumatoid arthritis, including rheumatoid factor, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and anti-cyclic citrullinated peptide (anti-CCP) antibody [22]. A positive rheumatoid factor is the most specific and sensitive laboratory marker of rheumatoid arthritis, as it is seen in about 70% to 80% of patients [23,24,27]. It is also present in many healthy individuals, patients with other rheumatic diseases, and individuals with chronic infections [26]. The anti-CCP antibody test is a specific blood test available for diagnosing rheumatoid arthritis and distinguishing it from other types of arthritis [24,27]. The anti-CCP antibody test is a marker of anti-citrullinated protein antibody (ACPA) and is positive in about 80% to 90% of patients; it can also be present in other diseases, including active tuberculosis, and is especially useful in early synovitis. While rheumatoid arthritis differs from person to person, individuals with rheumatoid factor, the anti-CCP antibody, or subcutaneous nodules tend to have more severe forms of the disease [24,26,27]. However, biomarkers for the initial tissue processes that cause joint damage in rheumatoid arthritis lack prognostic accuracy and are therefore inadequate as stand-alone tests. As such, they are typically used to help rule out other causes of arthritis when a patient has clinical features of rheumatoid arthritis [28].
The presence of human lymphocyte antigen B27 (HLA-B27) is used to help diagnose or rule out ankylosing spondylitis and reactive arthritis. This antigen is present in 90% of those with these conditions, but it can also be found in those without pathology, so it is not diagnostic [20].
Serum uric acid is elevated during an acute episode of gout but may be normal during remission. Serum uric acid level is also used to assess kidney function [20].
The ESR is a test in which the settling of red blood cells in uncoagulated blood is timed. This is a nonspecific test, and elevations in ESR are indicative of generalized inflammation. Changes in the ESR give an indication of improvement or worsening of the condition [20].
CRP is also associated with disease activity, and the CRP value over time correlates with radiographic progression in patients with rheumatoid arthritis [24,26,29]. ESR is typically ≥30 mm/hour, and CRP level is typically ≥0.7 pg/mL.
In certain instances, clinicians may perform an arthrocentesis in order to differentiate rheumatoid arthritis from other arthropathies [30]. Findings from synovial fluid aspiration that support a diagnosis of rheumatoid arthritis include straw-colored fluid with a significant number of fibrin flecks, synovial fluid ability to clot at room temperature, and 5,000–25,000 white blood cells/mm3 (5–25 × 109/L) with 85% polymorphonuclear leukocytes [23,24]. In addition, bacterial cultures are negative, no crystals are present, and the synovial fluid glucose level is low [23,24].
Examination by x-ray helps diagnose joint problems; it also allows following of the progress of a condition and its response to treatment. X-rays are able to show joint changes, such as erosion of joint margins, joint space narrowing, bone spurs, loose bodies, and dislocation. Specific injuries to soft tissues such as tendons and ligaments do not show on x-rays, but soft tissue swelling may be obvious [20].
Magnetic resonance imaging (MRI) can produce a detailed and highly useful image of the joints and soft tissues. As such, it is usually the best option when evaluating major joints, the spine and the muscles, tendons, and ligaments of the extremities. MRI has a role in the diagnosis of a variety of musculoskeletal disorders, including osteoarthritis, back pain, tears in the connective tissues of the joints, congenital disorders of the joints, and occupational/sports-related injuries [31].
Because it is readily available and avoids the use of radiation, ultrasonography is often a good option in the assessment of musculoskeletal disorders and injuries. Ultrasound allows for the visualization of joints, tendons, muscles, bursae, ligaments, cartilage, nerves, fascia, and related soft tissue and can have a role in diagnosis and/or evaluation of disease progression for a variety of conditions. The American Academy of Physical Medicine and Rehabilitation indicates that ultrasound is an essential component in the diagnosis of tendinopathies/tendon tears, nerve entrapments (e.g., carpal tunnel syndrome), and acute or chronic muscle injury [32]. It may also be involved in the evaluation of ligamentous injury and joint instability syndromes, subluxations/dislocations, and fascia injury or inflammation. When joint aspiration is necessary, it may be guided by ultrasound, as may therapeutic injections.
The electromyogram is a test to measure the electric currents produced by muscles, at rest and during contraction. Small needle electrodes are inserted into the muscle being tested and then connected by wires to an electromyography machine. Changes in muscle electrical activity may be helpful in diagnosing neuromuscular disease, and the test is particularly useful in differentiating muscular disease from neurologic disease [20].
Various biopsies may be performed on the musculoskeletal system. Skin samples, obtained by a punch biopsy, may be examined to diagnose certain connective tissue disorders. Muscle biopsies are usually operative procedures done to evaluate muscle disease. The synovial membrane can be biopsied, and analysis can be useful in diagnosing different types of arthritis. Buccal mucosa may be biopsied to help diagnose Sjögren syndrome, and the temporal artery may be biopsied to diagnose temporal arteritis [20].
Injury to connective tissue and muscle may arise from congenital or acquired disease or from trauma. Diagnosis and treatment/management of these conditions are specific to the disorder.
Genetic disorders of connective tissue are structural connective tissue changes present at birth. Most of these disorders are transmitted by a single autosomal dominant gene. Although there are many congenital connective tissue disorders, most are rare; two more widely known conditions are Marfan syndrome and Ehlers-Danlos syndrome. The obvious manifestations of these disorders may not appear until the second decade of life or later [33].
Both syndromes are serious and require collaborative assessment and treatment by the entire healthcare team. Healthcare providers should gather careful family histories detailing the patterns of disease transmission so families can see the degree of risk [33].
Marfan syndrome is one of the most common inherited (autosomal dominant) disorders of connective tissue, occurring in 1 in every 10,000 to 20,000 individuals [34]. It is the result of mutations in the FBN1 gene. FBN1 mutations are associated with a broad continuum of physical features ranging from isolated features of Marfan syndrome to a severe and rapidly progressive form in newborns.
Clinical Manifestations
There is wide variability in clinical symptoms in Marfan syndrome, with the most notable occurring in eye, skeleton, connective tissue, and cardiovascular systems. The most common symptom is myopia. Ocular problems are a result of defective supporting tissue of the lens, which can cause bilateral subluxation or total dislocation of the lens. The dislocation is usually upward, but slit-lamp examination is done to detect more subtle variations. Complications such as reduced visual acuity, uveitis, glaucoma, cataracts, and retinal detachment may also occur [33].
Cardiovascular complications of Marfan syndrome are potentially life-threatening and commonly involve the aorta. Marfan syndrome causes degeneration of the elastic fibers of the aortic media, which can lead to dissecting aneurysm. Aortic regurgitating may occur, producing a diastolic murmur. Mitral valve prolapse, thickening of the coronary arteries, conduction system abnormalities, and aortic coarctation have also been associated with this condition [33].
Echocardiogram is useful in following aortic and mitral valve abnormalities. Patients with valve involvement are at risk for endocarditis. These patients should be prescribed antibiotic prophylaxis for any dental work causing bleeding or for any other invasive procedures, to prevent bacteremia [33].
The most obvious skeletal manifestations in patients with Marfan syndrome are extreme height and long extremities. These patients are usually much taller than other members of their families and have excessively long arms and legs in relation to their bodies. The measurement from fingertip to fingertip with the arms outstretched is typically greater than the body height. Arachnodactyly (extremely long fingers) is commonly noted. The sternum may bulge outward (pectus carinatum, or pigeon breast), or it may be depressed (pectus excavatum, or funnel breast). If the chest differences are extreme, the echocardiogram becomes unreliable [33].
Kyphoscoliosis may be quite severe because of the weakness of the ligaments and other supporting connective tissues. Other skeletal manifestations include a long and narrow skull, with a high, arched palate, and flat feet. Joints and ligaments are hyperextensive, leading to recurrent dislocations of the knees and hips [33].
Therapeutic Measures
Therapeutic approaches in Marfan syndrome are directed toward the specific manifestations. Corrective lenses are almost universally necessary, and yearly ophthalmologic examinations aid in early detection of retinal detachment and lens dislocation [33].
Because cardiovascular problems are the major cause of mortality, most diagnostic and treatment efforts are directed here. Echocardiograms are done yearly, unless the diameter of the aorta exceeds the upper limits by 50%, in which case echocardiogram is performed every six months [33]. Beta blockers are used to decrease the stress on the aorta at the time of diagnosis or when there is progressive aortic dilatation. There is some evidence that angiotensin receptor blockers may be used, and clinical trials are underway to evaluate this use. Surgery to repair the aorta is done when the aortic diameter is greater than 5 cm in adults and older children, when the aortic diameter increases by 1.0 cm per year, or when there is progressive aortic regurgitation [34].
Kyphoscoliosis is the most deforming and disabling skeletal manifestation of Marfan syndrome. Patients should be examined biannually, and therapy (e.g., bracing, physical therapy, spinal fusion) should be initiated as soon as possible to prevent or slow further changes [33]. In more severe cases, the thoracic cavity in patients with kyphoscoliosis can be so reduced that cardiac and respiratory function are compromised. These patients are particularly susceptible to upper respiratory infections and should be treated aggressively if an infection occurs [33].
Prepubertal girls are often given estrogen and prepubertal boys given androgens to decrease height and help prevent kyphoscoliosis. While these hormones induce early epiphyseal closure, they also trigger the physical and psychosocial changes of puberty, which can create additional psychosocial stresses.
While Marfan syndrome is not always inherited, it is always heritable. Approximately 75% of cases are inherited, and the offspring of patients with Marfan syndrome have a 50% chance of developing the syndrome. In addition, patients with Marfan syndrome who become pregnant are at risk for potentially dangerous aortic changes resulting from cardiovascular overload and increased intra-abdominal pressure [33].
Specific Nursing Measures
The health history is extremely important in patients with congenital disorders such as Marfan syndrome. Particular attention should be paid to the patient's coping abilities in terms of living with a chronic disease that involves numerous changes in body image [7,35].
At each visit, the patient should be thoroughly assessed, with particularly attention to the eyes, cardiovascular system, and musculoskeletal system [7,35]. When examining the patient's eyes, look for tremor of the iris as it is moved horizontally. This is an indication of subluxation of the lenses. These patients may also have myopia and blue sclera (due to the presence of thin sclera through which the vessel-rich choroid can be seen).
Patients may display early diastolic murmurs of aortic regurgitation. This consists of a high-pitched blowing sound, heard best with the stethoscope over the second right or third left intercostal space. Increased pulse pressure and collapsing (water-hammer) pulse may also be evident. Occasionally, a midsystolic click indicative of mitral valve prolapse may be auscultated.
Nursing interventions for these patients will focus on supportive symptomatic care and education needs. The nurse should be prepared to discuss the nature and course of the disease and the importance of genetic and pregnancy counseling. The patient should be urged to keep current with biannual exams. Patients should also be counseled to avoid trauma, including contact sports, and invasive surgical procedures (when possible) [7,35]. They are also advised to avoid medications and foods that can lead to chronic increases in blood pressure and stretch the connective tissue in the cardiovascular system.
Ehlers-Danlos syndromes are a group of rare genetic disorders of connective tissue that affect the skin, joint, and hematopoietic systems. It is usually transmitted by an autosomal dominate gene, but it may also be recessive or an X-linked recessive gene [33].
Clinical Manifestations
The major manifestations of Ehlers-Danlos syndromes are fragile and increased elasticity of skin, hyperextensible joints, and fragility of blood vessel walls [33]. In the 2017 classification system, 13 types of Ehlers-Danlos syndrome were identified, including rarer forms [36]. They are generally organized according to the dominant system(s) involved, severity, and mode of transmission.
The skin of most patients with an Ehlers-Danlos syndrome is very smooth and hyperextensible; it can be pulled away from the body but returns to its original shape. Fragility and bruising are often evident. Minor cuts cause gaping wounds with little bleeding. Even the slightest trauma may cause purpura or hematomas that calcify, particularly over pressure points such as knees and elbows [33].
An unusually large range of joint movement (hypermobility) occurs in most forms of Ehlers-Danlos syndrome, and it is a hallmark feature of the hypermobile type. Dislocations, effusion, and hemarthrosis of the hip, patella, and shoulders may occur. Kyphoscoliosis, flat feet, and hyperextensible knees are often present. Thoracic changes are not as common but do sometimes occur, as does a forward slipping of the lower lunar vertebrae (spondylolisthesis) [33].
The patient may have episodes of bleeding, including spontaneous epistaxis; bleeding into the joints (hemarthrosis); blood in the sputum (hemoptysis); dark, tarry stools (melena) indicating bleeding in the digestive tract; and bleeding gums. It is not known whether the abnormal bleeding is from weakness in blood vessel walls or abnormal interactions of platelets with collagen [33]. Patients with Ehlers-Danlos syndrome who become pregnant are at risk for uterine rupture.
Abnormalities of the heart and blood vessels occur in patients with the cardiac-valvular type. These include mitral valve prolapse, right bundle branch block, and other conduction abnormalities. Patients with this type of Ehlers-Danlos syndrome have friable arteries, increasing the risk for adverse events during invasive angiography [33].
Other manifestations of Ehlers-Danlos syndrome can include spontaneous bowel rupture, pneumothorax, and diaphragmatic hernias or diverticula. In rare instances, a patient may have glaucoma, retinal detachment, or corneal abnormalities [33].
Specific Nursing Measures
Care for patients with an Ehlers-Danlos syndrome is limited to symptomatic treatment and support; there is no curative treatment. The main concern is to protect the patient's skin and joints from cuts, bruises, and dislocations. At each visit, the patient should be assessed for bleeding gums, melena, hemoptysis, and nosebleeds. Inadequate wound healing or wound dehiscence after a surgical procedure should be noted. Assessment of the lungs for pneumothorax, particularly following surgery, is important [35].
As with any chronic condition, the nurse needs to teach patients and their families about the nature and course of the disease. The patient should also be referred to a genetic counselor, as there are varying modes of heritability. A patient with Ehlers-Danlos syndrome who becomes pregnant is at risk for abortion, preterm birth, exacerbation of joint problems, increased bruisablity, abdominal hernia, and varicosities. Serious complications may arise with cesarean deliveries, because sutures do not hold well and wound dehiscence may result [7].
Many pathologic conditions involve inflammation of connective tissue. In this section, most of the inflammatory conditions are related to alterations in the immune system [37].
Bursitis is an inflammation of the synovial membrane lining a bursa; tendinitis is an inflammation of a tendon. These inflammations may result from trauma, or they may be secondary to disease. Although both conditions are usually acute, they can become chronic and disabling with repeated injury or inadequate care [37]. Note that tendinitis is distinct from tendinosis, which is the result of a noninflammatory condition characterized by degeneration of the tendon in response to chronic overuse.
Bursitis and tendinosis develop from prolonged overuse of a particular muscle group that can eventually damage a bursa or tendon. Overuse may be due to repetitive work movements or to a sports activity. Because the vascular supply of tendons is poor, their healing is limited and inflammation can become chronic, resulting in tissue damage and persistent pain. Often, the patient becomes unable to continue performing the movements that led to the condition, potentially impairing their ability to continue working.
Calcium deposits in tendons or bursae may also be the cause of inflammation. Tendon sheaths may become inflamed secondarily to systemic disease, such as gout, rheumatoid arthritis, or scleroderma [37].
Clinical Manifestations
The major symptom of bursitis/tendinitis/tendinosis is pain, often so severe that the patient is unwilling to move the affected part. Swelling may be present, and this alone may keep the patient from moving the joint. Any of the body's many bursae and tendons can become inflamed, but some joint areas are more commonly affected than others. Differential diagnosis of acute pain and erythema in joint areas should include infection, gout, and rheumatoid arthritis [37].
Bursitis and tendinitis/tendinosis of the shoulder involve the subacromial and subdeltoid bursa (different sections of the same large bursa) and the tendon of the supraspinatus muscle. The onset of bursitis or tendinitis in the shoulder usually follows activities involving repetitive movements of the whole arm, such as sanding, painting, sawing, throwing, or repeated lifting. Pain in the deltoid area increases when the patient lies on the shoulder or actively abducts the arm. A classic sign of bursitis/tendinitis/tendinosis of the shoulder is the "painful arc" between 80o and 120o of active arm abduction. The patient is often unable to support the weight of the arm at these angles. Further abduction causes no pain, and the examiner can perform assisted range of motion. If passive range of motion causes pain, capsulitis, rather than a periarticular disorder, is suspected [37].
Inflammation of the elbow region most often involves the olecranon bursa and the medial and lateral epicondyles. "Tennis elbow" is generally lateral epicondylitis, and "pitcher's elbow" is medial epicondylitis. These conditions cause pain that radiates from the elbow down to the forearm. The patient may drop heavy objects because of a feeling of decreased strength, although there is no real loss of strength or range of motion. Palpation of the involved epicondyle causes pain. Activities involving lower arm movement, such as tennis or hammering, may precipitate an attack. Olecranon bursitis usually is caused by leaning or falling on the elbow. There may not be severe pain, but swelling is often extensive [37].
Tenosynovitis involves inflammation of the tendon and tendon sheath and is also known as de Quervain tenosynovitis of the wrist [38]. When the tendons at the base of the thumb become irritated or inflamed this causes the tunnel around the tendon to swell and results in pain and difficulty grasping and holding objects. Overuse is the most common cause [38]. New repetitive activity, hormonal fluctuations associated with pregnancy and breastfeeding, and wrist fractures also are possible causes of de Quervain tenosynovitis [39].
Stenosing tenosynovitis, also referred to as "trigger finger," occurs when the pulley/tendon relationship between the hand and fingers is restricted by thickening or swelling at the base of the fingers. This creates pain and a distinctive catching, popping, or locking action in the finger or thumb. A cycle of triggering, inflammation, and swelling is common. Like carpal tunnel syndrome, stenosing tenosynovitis has been associated with other health conditions, such as gout, diabetes, and rheumatoid arthritis. In many cases, the actual cause is not clear [40].
The most common inflammatory problem of the hip is trochanteric bursitis. Pain, which is distributed over the lateral aspect of the hip and thigh, may inhibit ambulation. An increase in pain is seen with abduction and internal rotation against resistance. The patient feels tenderness with palpation over the greater trochanter. Patients who have leg length discrepancy may develop this inflammation in the hip of the longer leg [37].
Four bursa in the knee can cause significant discomfort for the patient when inflamed [37]:
Prepatellar bursa
Superficial infrapatellar bursa
Deep infrapatellar bursa
Pes anserine bursa
Prepatellar bursitis ("housemaid's knee") results from the combined action of excessive kneeling and leaning forward, as when gardening. Superficial infrapatellar bursitis ("clergymen's knee") can result from excessive kneeling. Deep infrapatellar bursitis and pes anserine bursitis are secondary to excessive weight bearing or unusually strenuous exercise [37].
Achilles tendinitis is a painful inflammation of the tendon of the ankle with or without swelling. This injury often results from a single episode of overuse. It can also occur in runners who wear shoes with rigid soles. Recurrent episodes of Achilles tendinitis, when a patient resumes activity before complete healing has occurred, can result in progressive scar formation, which may require surgical repair [37].
Therapeutic Measures
The measures employed for relief of bursitis and tendinitis vary according to the patient's age and the location, cause, and severity of the injuries. Recommendations usually include [37]:
Short-term immobilization, particularly during differential diagnosis
Ice packs applied to the affected area
Physical therapy and structured exercise after the initial period of rest
Anti-inflammatory medication
Occasionally, local corticosteroid injections are administered to the inflamed bursa or tendon area. While this approach is relatively widespread, it is not supported by well-designed systematic reviews [41].
Physical therapy and increasing return to activities is the best practice for these patients. Physical therapy consists of a four-step approach [42]:
Pain reduction and load management (isometric loading and avoiding positions of compression)
Isotonic loading (heavy-slow resistance through concentric-eccentric phases)
Energy-storage loading (plyometric loading)
Return to activity/sport
Exercise is crucial in the rehabilitation process, and active movement is started early. For example, in bursitis of any bursa of the knee, quadriceps-setting exercise is begun as soon as pain allows. When pain and tenderness have completely subsided, range of motion and full quadriceps activity are initiated. Physical therapists are often involved in designing and implementing exercises for patients, according to their individual needs. Occupational therapists may also participate if the nature of the problem involves a modification or change in job [15].
In some cases, fluid may be aspirated from the bursal space to relieve the symptoms. Any fluid obtained should be cultured and inspected. X-rays of joints are usually normal, but in some instances, calcium deposits can be identified as the precipitating factor. Arthrography is indicated in specific types of shoulder trauma to rule out any disruption of the joint capsule. Surgery is rarely used for bursitis or tendinitis unless rupture of the tendon occurs [37].
Specific Nursing Measures
Goals of nursing care are to relieve the patient's pain, maintain maximum mobility, and prevent joint contracture. Assessment of pain and range of motion is important both initially and after treatment to measure improvements. Reassurance and support can contribute to the relief of pain, so it is helpful to assure the patient that the pain of bursitis or tendinitis/tendinosis is usually of short duration [35].
Instrumental to the success of treatment is comprehensive patient education. Patients should receive instruction on physical therapy exercises (including frequency), pain management techniques, and return to activities; written instruction should also be provided. If pain is relieved with pharmacotherapy, the patient may be tempted to use the affected area too soon. It is important to caution patients to refrain from early resumption of activity to avoid reinjury and/or the creation of scar tissue [35].
Polymyalgia rheumatica is an immune-mediated inflammatory disorder characterized by muscle stiffness, pain, and weakness around the neck, shoulders, and hip. While this is an inflammatory disorder, the cause of trigger is unclear; genetic, infectious (e.g., Epstein-Barr virus, parvovirus), and gut health-related etiologies have all been suggested, with varying levels of evidence [43]. The incidence increases with age, with the greatest incidence in White patients older than 50 years of age; the average age at diagnosis is 70 years.
Clinical Manifestations
As noted, the characteristic symptoms of polymyalgia rheumatica are pain and stiffness in the shoulders, neck, upper arms, and hip area. The pain and stiffness are usually worse upon waking in the morning or after resting, and usually last an hour or more. Patient may experience difficulty performing normal activities, including rising from bed or a chair, dressing, and brushing hair. Many patients will have difficulty raising their arms above the shoulders [44]. Less common signs and symptoms include flu-like symptoms (e.g., low-grade fever, weakness, loss of appetite, weight loss) and swelling of the wrists or joints in the hands. Onset of symptoms is typically over the duration of a few days but may be as short as overnight.
Diagnosis is typically based on the presence of elevated inflammatory markers, particularly ESR and immunoglobulin G (IgG). In addition, these patients will display a decreased number of circulating B cells compared with healthy adults [43].
A significant portion of patients with polymyalgia rheumatica are also diagnosed with giant cell arteritis, and research indicates the co-occurrence of these conditions is common even without the presence of symptoms [43].
Therapeutic Measures
The EULAR and the ACR have issued a joint guideline for the management of polymyalgia rheumatica [45]. The cornerstone of treatment is at least 12 months of glucocorticoid therapy. This typically consists of 12.5–25 mg prednisone, although a lower dose may be preferred in patients at risk for glucocorticoid-related adverse events (e.g., those with osteoporosis, glaucoma, diabetes). Drug therapy should be tapered up to effective dose and tapered down when discontinued.
Care of patients with polymyalgia rheumatica includes monitoring for and preventing (when possible) the adverse effects of long-term steroid therapy. This can include vitamin D and calcium supplementation as well as bisphosphonate prophylaxis for those at increased risk for fracture [43]. Because close monitoring is necessary, patient education should include the necessity for keeping all follow-up appointments.
The disorders in this section are believed to have an autoimmune etiology. As with many autoimmune disorders, there are a variety of potential initiating factors, including viral infections, genetic predisposition, and exposure to toxins [46].
Autoimmune disorders may be generally classified as organ-specific or generalized. Autoimmune connective tissue diseases are generalized, usually involving a progressive degradation of collagen in connective tissue throughout the body. Rheumatoid arthritis is among autoimmune disorders but will be discussed later in this course, because joint involvement is the major problem [46]. Some autoimmune disorders result in musculoskeletal manifestations but have an etiology in another body system. For example, fibromyalgia is characterized by widespread musculoskeletal pain and fatigue, but it is believed to be the result of nervous system dysfunction.
Autoimmune connective tissue disorders can be associated with significant morbidity mortality. However, early diagnosis and treatment have improved prognosis, though they remain chronic (incurable) conditions. Successful therapy for patients with autoimmune disease requires an interprofessional team approach in order to ensure the best outcomes for patients [46].
Familiarity with each disorder will prepare the nurse to be alert for manifestations, exacerbations, and patient education needs. Because patients are often prescribed several medications to help manage the disorder, nursing management often includes medication management. Comfort measures are another important aspect of nursing care during acute phases or exacerbations. Proper positioning, use of splints, and small comfort measures (e.g., backrubs, smoothing wrinkled sheets, creating a calm environment) all contribute to the patient's well-being [46].
The nurse will explain to patients how they can try to prevent exacerbations of specific manifestations of their disease and how to cope with them when they do occur. Prevention measures may include avoiding stress, cold, sun, or certain drugs [46].
Four different forms of lupus have been identified: cutaneous lupus erythematosus, drug-induced lupus, neonatal lupus, and systemic lupus erythematosus (SLE) [47]. Cutaneous lupus mainly affects the skin. It is associated with chronic skin eruptions that, if left untreated, can lead to scarring and permanent disfigurement. Drug-induced lupus is associated with ingestion of various drugs that result in lupus-like symptoms. Neonatal lupus is a rare, non-systemic condition affecting infants of women with lupus. SLE, which affects multiple organ systems as well as the skin, is considered the most common of the four forms.
SLE, often referred to simply as lupus, is a chronic inflammatory autoimmune disorder of the connective tissue, primarily affecting the skin, joints, blood, and kidneys [47,48,49]. In this autoimmune disorder, antibodies are formed within the body that target healthy body systems, causing inflammation and structural changes. The word lupus means "wolf" in Latin, while erythematosus means "redness." The disease is named for the characteristic red rash that appears on the face and is thought to resemble a wolf's face [47,49]. The term "lupus erythematosus" was coined in 1851 by Pierre Cazenave, a French dermatologist, but writings describing lupus date to ancient Greece [49,50].
Lupus has been characterized as a multidimensional, unique, complex, challenging, unpredictable, and often elusive disease [47]. It is a non-organ-specific systemic disease with a varying prognosis that can be mild, serious, life-threatening, or even fatal. The disease is characterized by recurring remissions and exacerbations, often called flares, that occur most commonly in the spring and summer [48,51]. Periods of remission vary considerably among those diagnosed with lupus [47].
The number of reported cases of lupus varies based on different sources; it is believed that there are at least 1.5 million affected individuals in the United States [52,53]. More than 90% of SLE cases occur in women, with most women developing symptoms in their childbearing years (15 to 45 years of age) [54]. New diagnoses of lupus in women older than 45 years of age are uncommon [49]. SLE is most common among African Americans, with African American women having three times the incidence of White American women [54]. The incidence of lupus is also greater in Hispanic, Asian, and Native American women when compared to White women [55]. Statistics show that Black and Hispanic women tend to develop the disease at a younger age, are more likely to develop more serious complications (particularly cardiovascular complications and kidney disease), and tend to have a higher mortality rate from the disease as compared to White women [54].
The exact cause of lupus remains a mystery, but researchers believe that it results from multiple factors [49,56]. Possible causes may be interrelated and include immunologic dysfunction, genetic factors, hormones, and environmental influences [50,51].
Immune dysregulation, in the form of autoimmunity, is thought to be the prime cause of lupus. In patients with lupus, the body produces an accelerated inflammatory response, resulting in the production of autoantibodies, causing immune complexes (antigens combined with antibodies) [49,56]. These autoantibodies and complexes assault the body's own healthy cells and tissues [47,49,50,51]. Symptoms of SLE are the result of the damage to the body's tissues secondary to the immunologic response. One of the hallmark indicators of lupus is the formation of autoantibodies, and the presence of autoantibodies in the blood is a key factor to the diagnosis of lupus [47,49,51].
The strong hereditary component of lupus is supported by the fact that first- and second-degree relatives of patients with lupus are at a greater risk for developing lupus [57]. Estimates indicate that 5% to 13% of relatives will develop lupus, but only 5% of children whose mothers had lupus will develop the disease [57]. For those with a genetic predisposition, environmental factors may trigger lupus [47]. Environmental factors that may precipitate or exacerbate lupus include physical or emotional stress, streptococcal or viral infections, exposure to sunlight, immunizations (live vaccines), surgery, smoking, chemical agents (drugs, metals, or toxins), certain foods or supplements, and other environmental irritants [47,50,58]. Further, female sex hormones are believed to have a potential role, as women in their reproductive years are most susceptible to lupus.
Diagnosis
The diagnosis of lupus may be a challenge for the healthcare provider as well as the patient. In 2019, the EULAR and the ACR published updated classification criteria for lupus (Table 1) [59]. The EULAR/ACR criteria classifies a person as having lupus if they meet entry criterion of an ANA titer of >1:80, followed by additive weighted criteria (seven clinical and three immunologic) in which the patient must meet one clinical criterion and ≥10 points between the clinical criteria and immunologic criteria [59].
CLASSIFICATION CRITERIA FOR THE DIAGNOSIS OF SYSTEMIC LUPUS ERYTHEMATOSUS
| Domain | Criteria | Weight | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Entry Criterion | |||||||||||
| Positive antinuclear antibody (ANA) titer | ANA titer of >1.80 on Hep-2 cells or an equivalent positive test (ever) | Must be positive to continue to additive criteria | |||||||||
| Additive Criteria, Clinical | |||||||||||
| Constitutional | Fever | 2 | |||||||||
| Hematologic |
|
| |||||||||
| Neuropsychiatric |
|
| |||||||||
| Mucocuteanous |
|
| |||||||||
| Serosal |
|
| |||||||||
| Musculoskeletal | Joint involvement | 6 | |||||||||
| Renal |
|
| |||||||||
| Additive Criteria, Immunology | |||||||||||
| Antiphospholipid antibodies |
| 2 | |||||||||
| Complement proteins |
|
| |||||||||
| SLE-specific antibodies |
| 6 | |||||||||
Clinical Manifestations
No two people with lupus will experience identical symptoms. The onset of lupus may be acute or insidious, vague, or even nonspecific. On average, individuals with lupus have symptoms of the disease for two to three years before a diagnosis is made [49]. Symptoms are the result of the inflammatory and immune response of the individual's body to the disease process [49]. Repetitive cycles of exacerbations and remissions of symptoms are a hallmark of the lupus disease process.
Common symptoms of lupus include fever, weight loss, malaise, fatigue, skin rashes, polyarthralgia, vasculitis, Raynaud syndrome (discussed in detail later in this course), patchy alopecia (hair loss), and painless ulcers of the mucous membranes [51]. Fatigue is probably the most universal symptom, described as a persistent complaint of a paralyzing fatigue that normal rest may not relieve [47]. Vague symptoms of lupus include aching, fatigue, low-grade or spiking fever, chills, and malaise. Episodic fever is reported by more than 80% of all patients with lupus, with a low-grade fever most often noted [47]. Infection is certainly a major concern and is a potential symptom for patients with lupus. Those with lupus are more susceptible to opportunistic infections due to alterations in their hematologic system, especially in white blood cells. Women with lupus may also experience irregular periods or amenorrhea due to the disease process [47,49].
Skin rashes are very common among patients with lupus; approximately 80% of patients report skin involvement [47]. A red, raised rash over the nose and cheeks characterizes the classic "butterfly rash" of lupus. The butterfly rash is reported by 55% to 85% of all patients with lupus at some point during their disease process [47]. Discoid lupus lesions may also be seen. Ultraviolet light often aggravates skin eruptions, and approximately one-third of all patients with lupus are found to be photosensitive [47,60]. Oral, nasal, and vaginal ulcers may occur. Conditions such as alopecia, pruritus, alteration in wound healing, and bruising are other common dermatologic symptoms.
Polyarthralgia (pain in multiple joints) occurs in more than 90% of lupus cases [47]. The joint pain associated with lupus is similar to that experienced by rheumatoid arthritis patients and is often called lupus arthritis. Most patients complain of morning joint stiffness and pain. The pain is typically symmetrical, and joints may become tender, warm to the touch, and swollen. The dominant extremities are usually more inflamed. Joints commonly affected include the toes, ankles, fingers, wrists, elbows, and knees [61]. Joint pain is often one of the first and most common complaints of those with lupus and is often what initially brings them to a healthcare provider [50]. Additional musculoskeletal symptoms that may occur include subcutaneous nodules, tendonitis, tendon rupture, and carpal tunnel syndrome [47].
Anemia and cardiopulmonary abnormalities are relatively common among patients with SLE, affecting 50% of patients [47,49,62]. The most common cardiac complication of lupus is pericarditis, while pleurisy is the most common respiratory complication [47,49].
Nervous system involvement secondary to lupus is common and can range from mild to severe. Central nervous system involvement may result in cognitive disorders, including confusion, fatigue, memory impairment, and difficulty in articulating thoughts [49]. Cognitive dysfunction is estimated to occur in up to 90% of patients with lupus and is not associated with lupus disease activity [63].
Renal damage is one of the most serious complications of lupus, often causing such symptoms as hematuria, proteinuria, urine sediment, cellular casts, urinary tract infections, and fluid/electrolyte imbalance. Renal involvement has the potential to cause renal failure, affecting up to 50% of patients [47]. Renal disease is a leading cause of death in patients with lupus [47].
Ophthalmic disease affects approximately 20% of patients with lupus [47]. Ophthalmic symptoms associated with lupus may include a lupus rash on the eyelids, conjunctivitis, dry eyes, glaucoma, and cataracts [47]. In severe cases, retinal exudates or blindness may occur.
Therapeutic Measures
There is currently no cure for lupus, and long-term disease management is required. Due to the variability of lupus symptoms, treatment protocols differ for each individual. The range of treatments, however, are increasing in number and becoming more effective; thus, the disease can be controlled reasonably well in most people. The ultimate goal of treatment is to suppress immune system abnormalities, prevent disease flares, and reduce inflammation and other complications secondary to lupus [51].
Treatment is based on such factors as symptoms and severity, overall general health, activity level, school and/or family schedule, age, family and social situations, other medical conditions, and financial and insurance considerations [50].
Although there is no cure for lupus, there are several types of drugs available to aid in the treatment and management of secondary symptoms. Among these drug classes are nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, antimalarials, biologics, and immunosuppressives. In cases of severe lupus kidney disease not helped by pharmacologic intervention, dialysis or kidney transplant may be necessary.
Specific Nursing Measures
Nurses may see patients with SLE in both inpatient and ambulatory care settings. Discovering early symptoms and signs of exacerbations and complications is important in prolonging the life of patients with SLE. Carefully monitor all diagnostic study reports to remain well informed about the patient's progress [46].
Individuals diagnosed with lupus are encouraged to do all of the following [47,49,50,51]:
Get plenty of physical and emotional rest.
Maintain a healthy diet.
Establish an exercise regimen.
Avoid sunlight.
Seek prompt treatment of infection.
Limit stress.
Set realistic goals and priorities.
Maintain effective communication with their healthcare providers.
Develop a support system, including family, friends, healthcare professionals, community organizations, and organized support groups.
Avoid triggering or aggravating factors.
Seek regular health care.
Eight to 10 hours of sleep per night along with naps are recommended for patients with lupus. In addition, individuals with lupus should minimize stress to reduce emotional distress, as well as avoid direct prolonged sunlight, especially during the hours between 10 a.m. and 4 p.m. The use of a sunscreen with a sun protective factor (SPF) of 15 or greater that protects against both ultraviolet A and B rays is recommended along with protective clothing such as long sleeves and a hat [47]. Routine exercise is important to reduce fatigue and maintain joint mobility.
Social support can have a positive impact on individuals diagnosed with lupus. However, seeking and gaining social support can be difficult when one is experiencing a chronic illness such as lupus, because tremendous energy is necessary to maintain social networks [64]. Lupus symptoms, as well as treatment side effects, can present a challenge for individuals in maintenance of their pre-illness social relationships and activities. Furthermore, to gain necessary support, individuals with lupus should understand and then communicate to others what they need to assist them in managing their disease.
Keller noted similar findings in her research on social support and psychologic distress in women with lupus. She concluded that "younger women with lupus were more psychologically distressed than older women with lupus and that women with shorter duration since diagnosis were more distressed" [65]. Keller also found that the perception of having social support and being satisfied with the social support were more important than the number of social supports [65]. Thus, perception of and satisfaction with social support has been found to reduce psychologic distress.
One important potential source of assistance can be support groups. It has been noted that "participating in a support group can provide emotional assistance, boost self-esteem and morale, and help to develop or improve coping skills" [51]. Successful support groups can assist patients to gain insights into how to live with their lupus [66]. Darner found that women with lupus who had been diagnosed for longer periods of time had a healthier psychosocial adjustment [67]. Therefore, those newly diagnosed with lupus may require more support and interventions to aid in psychosocial adjustment.
Systemic scleroderma, also called sclerosis, is an autoimmune connective tissue disorder that causes fibrous changes in the skin, synovium, and small arteries of the digits, as well as in various internal organs, most notably the esophagus, intestines, heart, lungs, kidneys, and thyroid. The disease occurs in various forms, ranging from a primarily skin condition (localized scleroderma) to the CREST (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) syndrome, which is thought to be more benign, to involvement of visceral organs (systemic scleroderma). Some patients with mild-to-moderate types of scleroderma can progress to the visceral and more extensive cutaneous lesions associated with systemic scleroderma [46].
In all forms of the disease, there is vascular injury at the level of small arteries and capillaries, and the resulting decrease in circulation is the cause of the tissue changes. The precipitating factor for the onset of systemic scleroderma is not clear, although there is some evidence that genetic and environmental factors play a role. Silica and certain organic solvents are recognized as risk factors of occurrence of systemic scleroderma. In addition, the prevalence of the disease is 13 times higher in first-degree relatives of patients than in the general population [68]. The result is an activation of the immune system, causing blood vessel damage and injury to tissues that result in scar tissue formation and the accumulation of excess collagen.
There are no definitive tests to diagnose systemic scleroderma, and diagnosis is primarily based on clinical evaluation. Autoantibodies occur in this disorder, and the ESR may be elevated. As part of the diagnostic workup, the following tests may be performed [68]:
Nailfold capillaroscopy
Screening for antinuclear antibodies (mainly anti-centromere and anti-scl70/anti-topoisomerase antibodies)
Transthoracic echocardiography
High-resolution computed tomography (CT) of the chest
Diffusing capacity of the lung for carbon monoxide and spirometry
Hand x-ray
Esophageal manometry
Clinical Manifestations
The most frequent presentation in systemic scleroderma is the clinical triad of skin changes, Raynaud phenomenon, and esophageal hypomotility. However, manifestations are often present in other organ systems, requiring continual monitoring [46].
The most typical changes in all types of scleroderma occur in the skin. Typically, skin changes begin with swelling of the hands and gradual thickening, tightening, and hardening of the skin of the fingers (sclerodactyly). The fingers become tapered and in severe cases claw-like, with impaired mobility. Ulcers may develop on fingertips and over knuckles as the skin becomes taught. Skin changes can progress proximally at a slow rate, eventually affecting the face. In these cases, the skin of the face becomes tight and shiny, with a loss of normal wrinkles and skin folds. The nose may become beaked, and sometimes radial furrowing is seen around the mouth. Patients may experience an impaired ability to fully open their mouths. In extreme cases, the face becomes expressionless [46].
Most patients with systemic scleroderma have Raynaud syndrome, and this is often the first symptom to appear. With Raynaud syndrome, there is diminished blood flow to the digits secondary to vasoconstriction of the digital arteries, typically triggered by cold, vasoconstriction drugs, or emotional states. The initial sign is digital pallor, which progresses to cyanosis, then to erythema on rewarming [46].
The patient may have pain and stiffness in both small and large peripheral joints. Occasionally, patients develop arthritis and synovial effusion. Contracture and atrophy of the fingers may eventually occur [46].
Hypomotility of the esophagus occurs in most patients with systemic scleroderma. This typically presents as gastroesophageal reflux, with resulting heartburn and stricture, and potentially difficulty swallowing. In some cases, patients require esophageal dilation. Gastrointestinal involvement can progress to the intestine and colon, with development of hypomotility of the small intestine and wide-mouth diverticula [46]. In patients with gastrointestinal involvement, impaired nutrition is common.
Systemic scleroderma can also cause cardiopulmonary problems. Dyspnea may develop as a result of pulmonary hypertension and interstitial fibrosis. The examiner may hear fine dry rales or crackles at the bases of the lungs, and spirometry is often abnormal. Manifestations involving the heart are primarily the result of lung complications, but dysrhythmias, conduction disturbances, pericarditis, and pericardial effusions uncommonly occur [46].
In some patients, the kidneys can be seriously affected, with malignant hypertension rapidly producing renal failure, the leading cause of death for these patients. High renin levels and proteinuria are signs of kidney involvement [46].
Hematologic problems, in addition to a mild normochromic, normocytic anemia, include vitamin B12/folic acid deficiency anemia, which may occur secondary to bacterial overgrowth in an atonic small intestine. There is also a risk for gastrointestinal bleeding and resultant iron-deficiency anemia [46]. Other manifestations include thyroid disease, biliary cirrhosis, trigeminal sensory neuropathy, and Sjögren syndrome [46].
Therapeutic Measures
Treatment of systemic scleroderma is symptomatic and driven by the stage and organ involvement of the disease. In its 2017 guideline for the treatment of systemic scleroderma, the EULAR has established guidelines for the management of manifestations, organized by affected body system [69]. For patients with systemic scleroderma-associated Raynaud phenomenon, evidence supports nifedipine to reduce the frequency and severity of attacks. As such, oral nifedipine should be considered as first-line therapy. Phosphodiesterase-5 (PDE-5) inhibitors should also be considered [69]. For patients with severe disease who do not improve on oral therapy, intravenous iloprost is the recommended approach.
Intravenous iloprost is also recommended for patients with systemic scleroderma who experience digital ulcers [69]. PDE-5 inhibitors have been proven to expedite healing and prevent the development of digital ulcers and should be considered for these patients. Patients who do not respond to calcium channel blockers, PDE-5 inhibitors, or iloprost therapy, may be prescribed bosentan, which has been shown to reduce the number of new digital ulcers in patients with systemic scleroderma. Physical therapy for the hands is important to prevent contractures. For patients with Raynaud phenomenon, biofeedback is sometimes useful for controlling temperature in the hands and feet [46].
For patients whose systemic scleroderma is characterized by pulmonary arterial hypertension, EULAR recommends treatment with endothelin receptor antagonists (e.g., ambrisentan, bosentan, macitentan), PDE-5 inhibitors (e.g., sildenafil, tadalafil), or riociguat [69]. In cases of severe disease, intravenous epoprostenol is the first-line option. In cases of malabsorption by the small intestine, absorption often improves with the use of tetracycline, which destroys the bacterial overgrowth that occurs with hypomotility [46].
Hypertension is treated aggressively with angiotensin-converting enzyme (ACE) inhibitors to prevent irreversible renal damage [46]. The risk for scleroderma renal crisis in increased in patients taking glucocorticoids, and these patients should be closely monitored [69].
Arthritis responds to NSAIDs, and the dry eyes (sicca syndrome) of Sjögren syndrome are helped by artificial tears. A patient with dry mouth (xerostomia) should have frequent dental exams, because this condition predisposes patients to severe dental caries [46].
Specific Nursing Measures
Patients with known or suspected systemic scleroderma should be thoroughly assessed, including the skin, joints, and cardiovascular, pulmonary, and gastrointestinal status. The eyes and mouth should be evaluated for adequate lacrimal and salivary gland secretions. It is important to closely monitor blood pressure and review laboratory results. Venipuncture in the antecubital area may be difficult because of skin changes; further, finger sticks should be avoided. If only a small amount of blood must be drawn, the earlobe may be the best site [35].
Patient education should include a clear explanation of the nature and course of systemic scleroderma, including signs of more serious involvement. For some patients, demonstration of range-of-motion exercises to prevent joint contracture may be warranted. Patients should be encouraged to use moisturizing lotions to decrease dryness [35].
Patients with Raynaud phenomenon are advised to avoid cold, ergotamine, and amphetamines. They should be cautioned to take precautions against cold weather, including the use of warm gloves and socks. The use of nicotine should be avoided, as it is associated with pronounced peripheral vasoconstriction, which markedly aggravates Raynaud syndrome [35].
Patients with esophageal dysmotility should be advised to eat small, frequent meals and to chew their food thoroughly; meals should be followed with water. Proton pump inhibitors (PPIs) and antacids after meals and at bedtime can help to help to relieve gastroesophageal reflux disease. Resting and sleeping with the head of the bed elevated may also help to relieve symptoms [35].
The face and hands often undergo considerable changes in scleroderma, which alters the patient's appearance and manual dexterity. The facial skin becomes taut, the nose may become beaked, and telangiectasias may appear on the face. Tapering of the fingers, with tightness of the overlying skin, occurs as flexion contractures may be present [35]. These physical changes may cause varying levels of disability, but they can also have a negative effect on the patient's self-esteem and self-worth. Referral to mental health care and participation in support groups can be helpful.
Traumatic injuries to the soft tissues surrounding joints—muscles, ligaments, and tendons—are called sprains and strains; chronic injury is joint instability. The acute injury may arise from blunt trauma to the muscle or joint; excessive exercise; or twisting, stretching, or forcible extension of a joint (e.g., "twisting" the ankle). Surgery is seldom needed unless complete rupture occurs, but the pain of such an injury can be severely limiting [70].
A sprain is an injury to a ligament caused by forcing a joint beyond its normal range of motion. The ligament may be stretched or actually torn. Sprains usually occur following a blunt blow during sports activities or falls. A strain is an injury to a muscle and/or tendon at any location from origin to insertion.
Strains are associated with excessive stretching of a muscle or muscle unit; they usually do not occur because of a blow or direct trauma. Poor conditioning, improper warm-up before activity, muscle fatigue or weakness, and strength imbalance can all contribute to muscle or tendon strain. Both strains and sprains have a high incidence of recurrence [70].
Clinical Manifestations
A sprain causes pain, swelling, local hemorrhage, spasm of the muscle that moves that joint, and disability. Pain occurs with passive movement of the joint, and there is intense pain over the involved ligament itself. Sprains are graded according to damage to the ligaments and the resultant joint instability [70]. A Grade I sprain is characterized by slight stretching and microscopic tearing of the ligament fiber, mild tenderness, and swelling around the joint. A Grade II sprain is identified by partial tearing of the ligament, moderate tenderness and swelling, and an abnormal looseness in the joint. The most severe is a Grade III sprain, which consists of a complete tear of the ligament, significant swelling and tenderness, and substantial instability.
The most common sprains affect the ankle and occur when inversion of the foot tears a ligament, usually the anterior talofibular ligament. Knee sprains cause swelling, hemarthrosis, significant decrease in range of motion, and joint laxity. Often the person hears a "pop" when the injury occurs and later describes the knee as feeling as it is going to "give way." The medial collateral ligament is most commonly involved [70]. Following the acute injury, patients are usually able to bear weight.
Strains cause pain, swelling, muscle spasm, and hemorrhage into the muscle. Discoloration and weakness may also be present. Pain increases with active flexion or passive stretching, which helps in differentiating strains from sprains. Strains are graded according to loss of muscle strength [70,71]:
Grade 1: A mild injury with no appreciable tissue tearing and no substantial (less than 5%) loss of function or strength
Grade 2: A moderate injury with nearly half of muscle fibers torn, reduced strength, and some residual function
Grade 3: A severe injury resulting from the complete rupture of the muscle, severe swelling and pain, and complete loss of function
Therapeutic and Specific Nursing Measures
Approaches to the treatment of strains and sprains are similar. Before initiating treatment, a thorough assessment and history to determine the nature and cause of the injury as well as any significant health problems that may influence the treatment. When a suspected strain or sprain occurs, the first-line treatment consists of five components known by the acronym PRICE:
Protection: The affected joint or muscle should be covered to minimize the risk of additional traumatization.
Rest: The patient should take steps to avoid use of the joint, tendon, or muscle to allow time for repair and healing.
Ice: The application of cold will reduce pain and swelling (by causing vasoconstriction), and patients should be instructed to apply cold compresses up to several times per day, but to limit duration to 20 minutes or less.
Compression: In order to reduce diapedesis and promote lymphatic drainage, the area may be bandaged. Patients should be instructed that wrappings should not be so tight as to restrict circulation.
Elevation: The affected limb should be elevated to the level of the heart (or as close as possible) to promote venous return and reduce inflammation.
The PRICE regimen is usually continued for one week after injury, though there is some controversy about whether cold or heat is used after the first 24 hours. Cold is usually recommended for five to seven days because of its anti-inflammatory and analgesic effect. Then, wet heat may be used to aid in muscle relaxation and promote blood flow to the area [35].
With a second- or third-degree sprain, an x-ray should be taken to rule out fracture. Patients with sprains are usually immobilized for one week. When all pain on motion has ceased, patients can begin active range-of-motion and muscle-strengthening exercises. NSAIDs are the treatment of choice [35].
The PRICE regimen and NSAIDs are also appropriate for management of a strain. Emphasis is placed on prevention of recurrence through the use of muscle-strengthening and stretching exercises. Patients should be advised to engage in warm-up exercises before engaging in strenuous activity. For example, for patients with chronic ankle sprain/instability, slow stretching of the Achilles tendon daily can effectively reduce the incidence of recurrent sprain. Surgical intervention is recommended only in cases of complete muscle rupture [35].
Rhabdomyolysis is a condition that develops as a result of the rapid dissolution of damaged or injured skeletal muscle [72]. Though not strictly a traumatic disorder, the most common cause of rhabdomyolysis is direct trauma to the skeletal muscle. However, any trigger of muscle destruction can theoretically result in rhabdomyolysis, and additional causes include infection, drugs/toxins, electrolyte disorders, endocrine disorders, extremes of body temperature, and excessive exertion [73].
As discussed, the function of skeletal muscle relies on ATP metabolism, electrolyte exchange, and intact myocytes. When these factors break down, the intracellular components of the muscle (e.g., electrolytes, creatine kinase, lactate dehydrogenase, myoglobin) are released into the body and enter the bloodstream. In more severe cases, this can lead to acute kidney injury, electrolyte imbalances, renal failure, and even death.
Clinical Presentation
The presentation of rhabdomyolysis is typically believed to consist of muscle pain, weakness, and discolored (reddish-brown) urine. Though this is considered the "classic" triad of symptoms, less than 10% of patients will present with all of these symptoms [73]. More than half of patients present only with myoglobinuria.
Diagnosis of rhabdomyolysis depends on detection of plasma creatine kinase. A diagnostic level has not been definitively identified, but most experts use a concentration five times the upper limit of the normal reference range (1,000 IU/L) [72].
Therapeutic Measures
Treatment of rhabdomyolysis focuses mainly on prevention of kidney damage and acute renal failure. Therefore, fluid therapy to increase urine output (and dilute urine) is the standard of care. The American Society of Nephrology has identified an ideal fluid regimen for these patients consisting of half isotonic saline (0.45%, or 77 mmol/L sodium), to which 75 mmol/L sodium bicarbonate is added [74]. At least 3–6 L should be administered per 24 hours; however, up to 10 L (or more) may be given if continuous supervision is possible. If necessary, 10 mL/hour of mannitol 15% may be added to further increase urine output. In cases that have already progressed to overt renal failure, extracorporeal blood purification is warranted [74].
In addition, supportive treatment of resultant hypovolemia and electrolyte imbalances (e.g., hyperkalemia, hypocalcemia) is necessary. Measures to help stabilize temperature are often necessary. Patients' input and output should be monitored and documented. Pain management is often necessary, and patients should be assessed for severity and quality of ongoing pain.
Because of their location and constant use, joints are particularly susceptible to stress, injury, and inflammation. In addition, many autoimmune disorders manifest in the joints.
A wide variety of joint conditions are multifactorial in origin, including ankylosing spondylitis, reactive arthritis, psoriatic arthritis, gout and pseudogout, low back pain, scoliosis, Charcot arthropathy, and carpal tunnel syndrome [37,75].
Joint disorders of multifactorial origin can disrupt normal life activities, and families and job security can be negatively affected unless patients seek proper medical attention and counseling. Among the disorders discussed in this section, only gout can be cured, but the other disorders can be controlled to varying degrees so that in most instances the patient can maintain a fairly normal lifestyle [37,75].
Psoriasis is often associated with inflammatory arthritis and a negative rheumatoid factor. Psoriatic skin lesions usually precede the development of arthritis, and in most cases, there is correlation between joint flares and skin flares. However, some patients with psoriatic arthritis have very mild or no psoriatic skin lesions. Heredity is the most specific risk factor, but environmental factors also play a role; the exact etiology is unknown [76].
Clinical Manifestations
The manifestations of psoriatic arthritis vary from patient to patient. Some have distal joint involvement, while others have widespread deformity, ankyloses, and joint destruction. The disease can be symmetrical or asymmetrical, and some patients have spondylitis, sacroiliitis, eye problems, or a combination. Nodules are not present with psoriatic arthritis [76].
In patients with psoriasis, silver-white scaly patches develop on the elbows, legs, scalp, and back. Nails are often pitted (20 pits or more per nail), and arthritis is more common with nail changes than with skin lesions. Onycholysis is common [76].
Joint symptoms usually begin with the acute onset of pain and swelling of distal interphalangeal (DIP) joints. A gout-like symptom in the great toe often gives a "sausage" appearance to the joint, but the disability is usually not as great as with rheumatoid arthritis. Spondylitis is often found in families with a strong background of psoriatic arthritis. Upon x-ray examination, some people show marked articular destruction with resorption of bone. A shortening of the middle phalanx of the DIP joints of fingers and toes has a characteristic cuplike appearance, and in some cases, an entire phalanx is destroyed. Extra-articular symptoms include conjunctivitis, episcleritis, or uveitis [76].
Laboratory studies of patients with psoriatic arthritis reveal mild anemia, an elevated ESR, negative rheumatoid factor, positive ANA, and an elevated uric acid level. Clinical diagnosis is made by considering nails, peripheral arthritis, and spinal involvement. Nail and skin changes in psoriatic arthritis may be hard to differentiate from those in reactive arthritis [76].
Therapeutic Measures
Therapeutic measures for psoriatic arthritis are aimed at both the arthritis and the psoriasis [76]. Nonpharmacologic approaches include physical and occupational therapy, exercise, smoking cessation, weight loss, and massage therapy. Symptoms may be controlled with NSAIDs and/or glucocorticoids (oral or injection). In treatment-naive patients with active psoriatic arthritis, a tumor necrosis factor (TNF) inhibitor is recommended over oral, small-molecule drugs (OSMs) as a first-line option. However, OSMs may be used instead of a TNF inhibitor in patients without severe disease, particularly if they prefer an oral treatment option [77].
Gout is a metabolic disorder associated with elevated urate levels in the body and is the most common cause of inflammatory arthritis in the United States. Gouty arthritis is characterized by recurring episodes of acute, usually monoarticular, arthritis that tend to remit over several days to weeks; however, undiagnosed, untreated patients are at risk for developing a chronic deforming arthritis. An estimated 9.2 million adults in the United States are affected [78]. Gout is rarely encountered in persons younger than 30 years of age, with the predominant age range being 30 to 60 years. However, onset may occur in men in their early 20s who have a genetic predisposition and lifestyle risk factors. The peak age of onset in women is the sixth to eighth decade of life [78]. The estimated prevalence of gout is 5.9% in men and 2.0% in women [78]. The prevalence and incidence of gout has increased over the past several decades [78,79].
Gout develops in persons with hereditary or acquired chronic hyperuricemia or in those with marked perturbations in serum urate associated with such factors as alcohol consumption, drug use, eating foods high in purines, overweight/obesity, and myeloproliferative disorders [78,80]. The normal serum urate is generally considered to be ≤6.8 mg/dL. The majority of patients at the time of an acute flare have demonstrable hyperuricemia (in excess of 7 mg/dL); however, about 20% do not. The presence of hyperuricemia in the absence of symptoms is not diagnostic of gout [78]. In all cases, the hyperuricemia is caused by some dysregulation in the balance between production and excretion of urate. An estimated 80% to 90% of gout cases are due to urate underexcretion and not overproduction [78]. Hyperuricemia can occur without precipitating gout, and in the absence of symptoms, it may not warrant intervention [78,81].
Uric acid is a final metabolic product of purine nucleotides found in many foods and in human tissue. Intermediary processes of purine metabolism include the initial breakdown of purines to inosine and then to hypoxanthine. Hypoxanthine is metabolized to xanthine, and xanthine to uric acid, with both stages catalyzed by the enzyme xanthine oxidase (the primary site for pharmacologic intervention by allopurinol) [82].
The human body is limited in its capacity to excrete a heavy urate load. In the setting of persistent hyperuricemia, often combined with stress to weight-bearing joints such as the great toe, monosodium urate crystals precipitate within joint synovial fluid, producing an intense inflammatory reaction. With chronicity, adjacent tissues may become saturated with urate, leading to deposits within articular, periarticular, bursal, bone, auricular, and cutaneous sites. These deposits, termed tophi, are detectable on physical exam or by radiographs and are a cardinal pathognomonic feature of gout. The presence of crystals, within joint fluid or in tissue, activates monocytes and macrophages to clear the crystals through phagocytosis. The release of proinflammatory cytokines and chemokines into the immediate area triggers an acute inflammatory reaction and influx of neutrophils into the joint space [83,84,85].
Clinical Manifestations
The clinical presentation of gout is typically one of arthritis and intense pain, and patients may exhibit inflammation and edema in the afflicted joint. Although the great toe is the most common site, other joints and their surrounding tissue can be affected, including the insteps, ankles, heels, knees, wrists, fingers, and elbows [78]. Gout may be confused with other causes of arthritis as all forms share the cardinal signs of inflammation: pain, redness, warmth, tenderness, and swelling [80,86]. While gout initially manifests in severe, discrete episodes of pain, the condition may progress to more frequent attacks with shorter asymptomatic periods between attacks [78,86]. Synovial fluid analysis is the gold standard for diagnosing gout, confirmed by the presence of monosodium urate.
Therapeutic and Nursing Measures
Gout is perhaps the most easily treated, and preventable, form of arthritis. This is due to widespread understanding of its underlying mechanisms and the availability of effective treatment [80]. It is managed by controlling the current acute attack and preventing future attacks. Medications addressing the underlying pathophysiology include the xanthine oxidase inhibitors (XOIs) allopurinol and febuxostat and the uricosuric agents probenecid, fenofibrate, and losartan [80,87]. (Note: The use of fenofibrate and losartan for the treatment of gout is off label.)
The initial steps include patient education, testing to rule out other causes of hyperuricemia, and evaluation of the disease burden to determine appropriate treatment. All patients with hyperuricemia and established gout should be advised to begin dietary modification. This involves avoiding organ meat high in purine content, high-fructose corn syrup, and excessive alcohol use. Portions of high purine-content seafood, sugar, and salt should be limited. The ideal diet will include low- or non-fat dairy products and vegetables. Other lifestyle modifications can also assist in managing gout, including weight loss in overweight patients, regular exercise, smoking cessation, and adequate hydration [83,87,88].
The acute pain of gout may be treated with NSAIDs, a cyclooxygenase-2 (COX-2) inhibitor, systemic corticosteroids, or oral colchicine monotherapy in mild-to-moderate disease (≤6 on a 10-point pain scale). Combination therapy (i.e., colchicine and NSAIDs, oral corticosteroids and colchicine, or intra-articular steroids with each of the other options) may be used in cases of severe disease with intense pain and polyarticular presentation. Intramuscular triamcinolone acetonide is recommended in patients unable to take oral medication or likely to be poorly adherent to the multidose oral regimen [87].
An inadequate response to therapy after escalation (<20% pain reduction within 24 hours or <50% pain reduction after ≥24 hours) should prompt reconsideration of the diagnosis. If gout is confirmed, switching to another form of monotherapy or adding a second agent may prove effective [83,87].
Urate-lowering therapy should be initiated in all patients with tophaceous gout, radiographic damage due to gout, or frequent gout flares [88]. Therapy should be started within 24 to 36 hours of the onset of an acute gout attack unless otherwise contraindicated. Urate-lowering therapy is not recommended for patients experiencing their first flare, or for patients with asymptomatic hyperuricemia (serum urate >6.8 mg/dL) with no prior gout flares or subcutaneous tophi [88]. Allopurinol (≤100 mg/day) is the preferred first-line agent. Febuxostat (≤40 mg/day) is an acceptable alternative [88]. Probenecid may be used as an alternative to allopurinol or febuxostat if there is contraindication or intolerance to these preferred agents. However, probenecid should be avoided in patients with a history of urolithiasis [83,87,88].
Clinicians may also consider screening for the HLA-B*5801 allele, which is associated with high risk of severe allopurinol hypersensitivity reaction. High-risk persons include Koreans with an estimated glomerular filtration rate <60 mL/min/1.73 m2 or those with Han Chinese or Thai ancestry [89].
Anti-inflammatory prophylaxis (against precipitating an acute flare) is recommended when initiating urate-lowering therapy in asymptomatic patients [88]. Colchicine was once the treatment of choice but is now less commonly used than NSAIDs because of its narrow therapeutic window and risk of toxicity [90]. To be effective, colchicine therapy is ideally initiated within 36 hours of onset of the acute attack [78]. In the case of colchicine intolerance or contraindication, prednisolone may be used [88]. Prophylaxis should continue after achieving target serum urate level for three months in patients without tophi, for six months in patients with resolved tophi, and with any remaining signs of gout activity in all patients [88].
Patients with intermittent symptoms or chronic synovitis with tophi (chronic tophaceous gouty arthritis) should be treated with a single-agent XOI, such as allopurinol, at a dose to achieve and maintain the serum urate level within normal range [83,87]. If the serum urate target is not achieved or disease activity persists, a uricosuric agent may be added to the XOI. Pegloticase therapy should be considered if the serum uric target is not achieved, disease activity persists, more than seven attacks occur per year and no tophi, two or more attacks per year and tophi, or chronic tophaceous gouty arthritis is present [88,89].
Calcium pyrophosphate deposition disease, or "pseudogout," is a similar crystalline arthritis that occurs in patients with underlying osteoarthritis and is identified by the presence in synovial fluid of calcium pyrophosphate dehydrate crystals [78,80,85]. X-ray findings of articular cartilage calcification usually accompany it. Many patients with pseudogout have other disorders, such as diabetes, hypothyroidism, and gout [37].
Clinical Manifestations
In pseudogout, arthritis occurs in a large joint, which is erythematous, swollen, warm, and painful. Like gout, pseudogout is usually monoarticular, but involvement of other joints can follow in succession. Attacks are often precipitated by trauma, surgery, or medical illness. Onset of symptoms is rapid, with a peak in 12 to 36 hours. Episodes are intermittent, usually involve the same joint, and typically last about one to two weeks. Joints are normal between attacks [37].
Therapeutic and Nursing Measures
The deposition of calcium pyrophosphate dihydrate crystals cannot be reversed. Acute attacks of pseudogout are treated with NSAIDs, colchicine, and/or oral corticosteroids; in more severe cases, drainage of the affected joint may be helpful [37].
Nursing measures for pseudogout include careful joint assessment, thermo- or cryotherapy, and monitoring for symptoms and signs of systemic illness and side effects of medications [18]. Patient education for home care includes instruction in the safe application of heat or ice, range of motion exercises, and the nature and side effects of prescribed medications. A weight-reduction diet can be helpful promote the long-term health of weight-bearing joints [18].
When it occurs, back pain is most often localized to the lower back, and chronic back pain is almost always chronic low back pain. Although acute-onset low back pain is a common problem that usually resolves within four to six weeks, many patients develop a persistent, disabling pain syndrome with a diminishing prognosis for return to normal function. When low back pain continues beyond 12 weeks, the prospect for subsequent remission is poor and progression to chronic low back pain is likely. Chronic low back pain imposes a great burden: for patients, pain and disability; for society and the healthcare system, an enormous expense in direct and indirect costs.
Risk factors for developing low back pain can be generally categorized as nonmodifiable, such as old age, female sex, poverty, and lower education level, and modifiable, including higher body mass index (BMI), smoking, lower perceived general health status, physical activity (e.g., bending, lifting, twisting), repetitive tasks, job dissatisfaction, and depression. The greatest contributors to low back pain episodes are single-event or repetitive exposures to mechanical stress and age-related degenerative spinal changes. With chronic low back pain, mechanical and biophysiologic factors play a minimal secondary role to the primary contribution from psychosocial factors [91].
Clinical Manifestations
The onset of low back pain is described as discomfort in the vicinity of the low back ranging from a dull ache to a sudden, sharp, shooting or stabbing pain and may include limited flexibility and/or range of motion or inability to stand straight [92]. Although the symptoms of back pain can originate anywhere from the thoracic spine to the sacrum and coccyx, most cases originate in the lumbar spine, as this is the site of support for upper body weight [92].
With low back pain, the clinical presentation varies according to etiology. In general, radicular pain suggests nerve root involvement, while axial pain suggests disk degeneration, facet arthropathy, sacroiliac (SI) joint arthropathy, or myofascial pathology of the spine.
Nonspecific Low Back Pain. Up to 85% of low back pain in patients presenting to the primary care setting is nonspecific, meaning that it lacks a clear origin and is not caused by specific local or systemic disease or spinal abnormality [93]. Nonspecific low back pain is a diagnosis of exclusion made after ruling out serious causes of the back pain. Although pain can originate from ligaments, facet joints, muscle, fascia, nerve roots, the vertebral periosteum, or outer portions of the disk, the effective management of nonspecific low back pain does not require a precise anatomic diagnosis [94]. The pain is usually unilateral and may radiate to the buttocks or posterior thigh but not past the knee. This can lead to incorrect diagnosis of radiculopathy or disk herniation. However, true radicular symptoms radiate below the knee in a dermatomal distribution and can involve sensory loss, weakness, or reflex changes. Painful spasm may be present, and pain may be worsened by movement, while lying flat decreases the pain. Complaints of numbness, weakness, or bowel or bladder dysfunction are absent [95]. Degenerative changes revealed by lumbar imaging should usually be considered nonspecific, because they poorly correlate with symptom severity [96].
Lumbosacral Radiculopathy. Lumbosacral radiculopathy is a clinical diagnosis of nerve root irritation and compression, resulting in a symptom distribution of the affected lumbar or sacral nerve root such as numbness, weakness, or paresthesia. Sciatica is the most common symptom of lumbar radiculopathy and refers to pain that radiates down the leg below the knee in the distribution of the sciatic nerve to indicate nerve root compromise from mechanical pressure or inflammation [96].
Causes of lumbar radiculopathy include disk herniation, arthritic degeneration, cord compression, spinal stenosis, tumor, and infection. With herniated disk, the pain is described as a deep, aching, axial midline pain concurrent with radicular pain. Discogenic pain results from a tear in the outer disk layer (annulus fibrosis) that causes the inner gelatinous material (nucleus pulposus) to prolapse, inflame, and compress a nerve root [95]. The resulting pain from pressure and nerve irritation improves with the resolution of local inflammation, and the disk protrusion may spontaneously remit with time. Although disk herniation and radiculopathy are often viewed as causally linked, herniation is often asymptomatic and only occasionally the cause of sciatica [95].
Lumbar Spinal Stenosis. Lumbar spinal stenosis refers to the frequently age-related narrowing of the spinal canal that may result in bony constriction of the cauda equina and the emerging nerve roots [96]. Spinal stenosis can produce pain in the low back that radiates down the back of both legs, often worsened with standing or walking. To make the pain more bearable, patients often walk a short distance with a hunched back, and then sit down for relief. The pain will then dissipate after several minutes. Congenital lumbar canal stenosis is a predisposing factor. Patients show less tenderness over the lumbar spine than those with acute lumbar disk herniation, and the straight leg-raising test may be normal [97].
Most persons 60 years of age and older exhibit varying degrees of spinal stenosis from disk herniation, osteophytes, or degenerative spondylolisthesis. Fortunately, clinical pain manifests in less than 30% and, just as with degenerative disk disease, there is poor correlation between symptom severity and extent of spinal canal stenosis revealed by MRI [95].
Myofascial Pain. Myofascial pain of the low back or neck is common, especially following trauma or repetitive motion injury. This is thought to result from strain or sprain to the muscles and ligaments. Myofascial pain is described as a deep, aching, poorly localized discomfort made worse by activity. It can be limited to discomfort in the paraspinal muscles or may extend to the buttocks and upper thigh areas [98].
Epidural Compression Syndrome. Epidural compression syndrome is an umbrella term that encompasses spinal cord compression, cauda equina syndrome, and conus medullaris syndrome. While these conditions differ in the level of neurologic deficit at presentation, they are otherwise similar in symptoms, evaluation, and management. Massive herniation of a midline disk, typically at the L4 to L5 disk level, is the most common cause of epidural compression syndrome. Tumor, epidural abscess, spinal canal hematoma, or lumbar spine spondylosis represent other causes [95].
In these patients, neurologic status at diagnosis is the greatest predictor of ultimate neurologic outcome and underscores the importance of early accurate diagnosis. The dominant symptom is back pain with accelerating pain severity. Pain from epidural spinal cord compression is made worse with recumbent positioning, and unilateral or bilateral radiculopathy may develop over time. For many patients, leg pain or neurologic symptoms are more dominant than back pain. Also common at diagnosis is symmetrical lower extremity weakness that may have progressed to gait disturbance or paralysis. Decreased lower extremity reflexes are associated with cauda equina syndrome [95].
Lumbar Facet Joint Syndrome. Lumbar facet joint syndrome is seen in as many as 35% of patients with low back pain and is frequently associated with arthritis or lumbar facet joint injury [97]. Dominant symptoms include unilateral low back pain that may radiate down the back or front of the thigh and morning stiffness with isolated facet arthropathy [99]. Tenderness is usually found over the lumbar paraspinal muscles and facet joints. Back pain is worsened with back extension and lateral rotation to the side of the pain, and the leg-raising test is negative. MRI and CT findings of facet joint arthropathy do not correlate with clinical findings [97].
Sacroiliac Joint Syndrome. SI joint syndrome typically manifests as localized pain in the lower back or upper buttock area that overlies the SI joint. Pain is intensified by attempts to walk up stairs, and while pain may be referred to the posterior thigh, extension below the knee is unusual [100]. Tenderness over the SI joint is often found in physical examination, and pain is aggravated by the Patrick test or single-leg standing [97]. The onset of SI joint pain is usually gradual (over months to years), and although etiology is often elusive, trauma, infection, and tumor represent infrequent yet known causes of SI joint pain [100].
Assessment and Diagnosis
Most patients with acute low back pain have ligamentous or muscle strain syndrome, follow a benign course, and show significant improvement within two to three weeks. The challenge for clinicians is to recognize early the possibility of serious disease, such as spinal metastatic cancer or vertebral and epidural space infection, and then to identify those with herniated disk, radiculopathy, or spinal stenosis.
The proper assessment of the patient with back pain requires vigilance and careful attention for factors and warning signs suggestive of serious or life-threatening disorders. A thorough history and physical examination should be performed on all patients, during which the patient is assessed for the presence of warning signs or ''red flags." Red flags represent alarm symptoms or signs that warrant prompt, specific diagnostic testing, urgent treatment, or referral to a specialist. Among these are weight loss, prior history of cancer, nocturnal or rest pain, age older than 50 years, recent trauma, fever and chills, history of injection drug use, chronic corticosteroid therapy, difficulty urinating, bowel or bladder incontinence, and neurologic deficits such as saddle anesthesia, perianal or perineal sensory loss, or motor weakness in the extremities [93,95,101]. As an example, there is a common association between spontaneous vertebral fracture and any combination of age older than 70 years, female gender, recent trauma, and prolonged corticosteroid use. There is also a moderate to highly significant predictive value for age older than 50 years, history of prior cancer, unexplained weight loss, and failure of conservative therapy in identifying spinal malignancy [101].
Patients should also be assessed for "yellow flags," or risk factors for poor prognosis and chronicity [94,101]. Areas to explore include maladaptive beliefs, attitudes, and behaviors regarding the back pain and recovery, such as passivity or reluctance to self-manage, dependency on the provider to "cure," fear avoidance beliefs, and beliefs that harm will come from activity and discomfort. Other areas include depression, anxiety, maladaptive coping response to stress, social withdrawal or isolation, and lack of social support. Adverse economic and work environment circumstances, such as job dissatisfaction, excessive and inflexible physical workplace demands, high levels of work-related stress, poor workplace social support, and adversarial or dysfunctional workplace relationships should also be noted [94]. Early detection and intervention (if indicated) for problematic motivational, emotional, or social dysfunction are important because these factors influence the selection and effectiveness of therapeutic interventions.
In the absence of red flags, use of imaging and diagnostic tests for acute low back pain is discouraged, as imaging findings rarely change clinical management. Overuse of lumbar imaging in low back pain correlates with, and likely contributes to, the two- to threefold increase in surgical rates for low back pain over the last 10 years [93]. Assigning significance to imaging anomalies requires skill at the specialist level to integrate historical, clinical, and imaging findings. Imaging abnormalities are essentially normative by 40 years of age; for instance, 80% of persons 60 years of age and older exhibit a protruding disk, which is symptomatic for only a fraction of patients. Incorrect communication of imaging findings to the patient may lead to patient fixation, contribute to fear-avoidance behaviors, and increase the risk of iatrogenic aggravation of chronic low back pain. Guidelines suggest that physicians without advanced training defer imaging tests to qualified specialists [93,102].
However, imaging and other testing should be performed in patients with new-onset or progressive neurologic deficits and those with suspicion of serious underlying conditions. In patients with persistent pain and symptoms consistent with radiculopathy or spinal stenosis, MRI should be performed only when such patients are candidates for surgery or epidural steroid injection. CT scanning is an alternative option to first-line MRI [93].
Therapeutic Measures
Although acute low back pain improves in most patients within three to six weeks using conservative therapy, up to 33% of patients with low back pain report pain of moderate or greater severity at one-year follow-up and 20% report ongoing pain severe enough to limit activity [96]. With chronicity, low back pain may become disabling and impose a severe emotional and functional burden. The management goals for chronic low back pain are to minimize pain and disability, improve functional status, and facilitate restoration of normal activity, while limiting the use of marginally effective or inappropriate medication [93].
Many pharmacologic therapies and minimally invasive or invasive procedures have been utilized in a strategy designed simply to relieve pain—with variable results. However, there is little evidence these focused pain approaches are comparable or superior to interventions that focus primarily on restoration of function instead of pain relief. This contradicts the biomedical model in medicine that emphasizes escalation of costly and invasive therapies to achieve "pain cure" in patients lacking response to lower-intensity approaches [102,103]. It is now recognized that treatment for conditions such as chronic low back pain persisting in the absence of a unique underlying pathologic lesion must address potential contributory factors such as affective disorders, maladaptive beliefs and coping skills, and interpersonal and occupational dysfunction. Dysregulated cortical, pre-frontal, and higher neural level mechanisms associated with chronic low back pain are being identified and may represent therapeutic targets in functional restoration-based approaches. As with other chronic pain syndromes, greater understanding of pain pathway alterations will better inform therapy selection.
Virtually universal among practice guidelines for chronic low back pain is the emphasis on a multidisciplinary, multi-modal approach that includes exercise and activity, cognitive restructuring of maladaptive attitudes and coping skills, a behavioral component addressing fear avoidance, physiotherapy and manual therapy, and analgesics as indicated [93,96,102,104,105,106,107]. This is often best accomplished by consultation or referral to an established pain treatment center. Multidisciplinary functional restoration programs, which are intensive (more than 100 hours) biopsychosocial interventions whereby physical rehabilitation is combined with cognitive-behavioral therapy and delivered by an interdisciplinary team, embody this recommendation. Moderate-to-strong evidence supports their efficacy in chronic low back pain. They have been found effective in reducing pain and improving physical function, work readiness, and return to work. Weaker outcomes are found in programs that are less intensive or lacking a behavioral component. Patients who do not improve with less intensive therapy options and have high levels of pain, distress, and disability should be considered for multidisciplinary functional restoration programs [94].
Scoliosis is a lateral curvature of the spine, most commonly in the thoracic area with convexity to the right and compensatory convex curve to the left in the cervical and lumbar areas. Scoliosis can be functional (a result of poor posture or leg-length discrepancy) or structural (a result of deformity of the vertebral bodies, paralysis, congenital malformations, or idiopathic causes). Idiopathic causes are the most common and appear with increased growth during adolescence. It disproportionately affects girls, who are 10 times more likely to be diagnosed than boys at 10 years of age or older [108].
Clinical Manifestations
Symptoms of backache, fatigue, and dyspnea occur only after scoliosis is well established. Untreated scoliosis can result in pulmonary insufficiency from decreased lung capacity, back pain, degenerative arthritis of the spine, intervertebral disease, and sciatica [108]. While screening for idiopathic scoliosis has typically occurred between 10 and 18 years of age, the current evidence is insufficient to assess the balance of benefits and harms [109].
Older patients may exhibit kyphosis, a postural curvature of the spine that is due to aging, disc degeneration, atrophy of spinal muscles, osteoporosis, or vertebral collapse. Adults with kyphosis have a rounded back and possible weakness and generalized fatigue. Kyphosis rarely produces local tenderness except in severe osteoporosis with compression fractures [108].
Therapeutic Measures
Early treatment of scoliosis consists of a combination of physical therapy, bracing, and/or surgery. If the condition is untreated in adolescence, problems that develop can only be treated symptomatically. Any upper respiratory tract infections are treated aggressively to prevent pneumonia and atelectasis [108].
Specific Nursing Measures
Patients with scoliosis often have body image issues and difficulty finding clothes that fit properly. When patients with scoliosis are hospitalized for any problem, careful attention to positioning is essential; improper positioning is not only extremely uncomfortable for the patient, but it can precipitate a vertebral fracture, especially in those with osteoporosis [108].
Carpal tunnel syndrome is generally associated with such umbrella terms as repetitive stress injuries, work-related upper extremity disorders, musculoskeletal disorders, entrapment neuropathies, and cumulative trauma disorders [110,111]. Specifically, carpal tunnel syndrome is a painful disorder of the wrist and hand that occurs when the median nerve (which runs from the hand to the forearm) becomes compressed [112,113].
The carpal tunnel is a narrow passageway on the palm side of the wrist. Surrounded by bones and ligaments, the carpal tunnel houses and protects the tendons of the hand and the median nerve, which controls sensations to the thumb and fingers. When the median nerve becomes pinched or compressed (due to swelling or irritation in adjacent tissues or tendons), the result can be pain, numbness, hand weakness, and in extreme cases, loss of hand function. Cases of bilateral carpal tunnel syndrome have been reported, but typically only one hand is affected [112,114,115]. Carpal tunnel syndrome is rare in children; it usually occurs only in adults [116].
Clinical Manifestations
The symptoms of carpal tunnel syndrome typically appear gradually and may include [114,116]:
Numbness, burning, or tingling in the fingers and palm of the hand
Pain in the wrist, palm, or forearm, especially during use
Decreased grip strength
Weakness in the thumb
Sensation of swollen fingers, whether or not swelling is apparent
Difficulty distinguishing between hot and cold
Symptoms may cause waking during the night with the urge to "shake out" the hand or wrist. Symptoms may occur with activities that require prolonged grasping and/or flexing of the wrist (e.g., driving, holding a book). Left untreated, carpal tunnel syndrome can progress to persistent numbness and permanent loss of hand function. In severe and chronic cases, irreversible muscle damage or atrophy may occur [112,116,117]. Complete sensory loss in the hand has also been reported.
Assessment and Diagnosis
Early diagnosis of carpal tunnel syndrome is important to prevent muscle atrophy or damage to the median nerve that cannot be reversed by treatment [112,116]. Early diagnosis, including a physical examination, medical history, routine laboratory tests, and imaging, can also help to identify or rule out other health conditions that may present with similar signs and symptoms and require specialized treatment [118,119]. The physical examination should include specific testing, such as Phalen's maneuver or Tinel's sign, that can produce the symptoms of carpal tunnel syndrome [114,116]. In elderly patients, particular attention should be given to the objective evidence of carpal tunnel syndrome rather than subjective complaints [120].
Therapeutic and Nursing Measures
Surgery, corticosteroids, NSAIDs, diuretics, wrist splints, exercise, ultrasound therapy, laser therapy, and yoga are among the methods that have been recommended for the treatment of carpal tunnel syndrome [121,122,123,124,125]. Although no single treatment method has been universally accepted, there is agreement that the treatment of carpal tunnel syndrome should begin as early as possible and should include attention to underlying causes, such as diabetes or rheumatoid arthritis. There is also agreement that successful treatment depends on patient compliance with the treatment program [116,126].
Corticosteroid injection has been found to improve patient satisfaction, symptoms, and function when measured at intervals of 2, 4, 8, and 12 weeks. As noted, it demonstrates a more significant overall improvement in the symptoms of carpal tunnel syndrome than oral corticosteroids but does not appear to provide a better long-term outcome (greater than six months) than splinting or NSAIDs. Two treatment injections do not appear to provide any added benefit when compared to one treatment injection [124,127].
Splinting has been found to improve patient satisfaction, symptoms, and function when measured at intervals of 2, 4, and 12 weeks. The American Academy of Orthopaedic Surgeons suggests that splinting be considered before surgery. This may be particularly helpful when weighing the risks of surgery versus the benefits. Splinting is not recommended for use after routine carpal tunnel release surgery. The benefit of splinting for postoperative rehabilitation is undetermined [126,127,128].
NSAIDs are used to treat a variety of acute and chronic pain conditions, including carpal tunnel syndrome, but opinion varies as to their effectiveness and safety for long-term use [129,130,131]. Specifically, NSAIDs have been associated with gastrointestinal and cardiovascular risks and toxicity with long-term use [132].
Diuretics and vitamin B6 (pyridoxine) may also help with temporary relief of symptomatic carpal tunnel syndrome, but their long-term benefits are unproven [127,131,133]. Acupuncture, yoga, exercise, laser therapy, activity modification, and ergonomic workplace modifications also have been mentioned as non-surgical treatment alternatives, but most experts agree that further research is needed to determine the viability and efficacy of these methods [116,124,126,127,131,134,135].
Carpal tunnel release is the preferred treatment for patients with chronic or severe carpal tunnel syndrome. It is achieved by either an open or endoscopic procedure [116,122,126,128]. Both types of surgery are generally performed on an outpatient basis under local anesthesia. Open release surgery involves making an incision of up to 2 inches at the base of the palm of the hand and cutting the transverse carpal ligament, which releases pressure on the median nerve [116,136]. Endoscopic surgery involves making a small, one-half inch incision at the wrist and introducing an arthroscope beneath the transverse carpal ligament. Using the scope as a guide, the ligament is cut, relieving pressure on the median nerve [116,134,136].
Osteoarthritis is the most common form of arthritis and is characterized by degeneration of cartilage and its underlying bone within a joint, with resultant bony overgrowth. This process of tissue breakdown eventually leads to pain and joint stiffness [137]. Osteoarthritis develops most frequently in the knee, hip, and hand. Although pain in the lower back and the neck are the most frequently occurring musculoskeletal conditions and are the leading cause of functional limitation and work absences, the etiology of back and neck pain is often unclear, with many cases involving muscles and ligaments rather than osteoarthritic changes [138,139,140].
Osteoarthritis is classified as primary or secondary. The cause of primary osteoarthritis is idiopathic; no abnormality is the cause of changes in the joint [141]. Secondary osteoarthritis is the result of a known cause, most often trauma/injury or systemic diseases. Secondary osteoarthritis is most often found in the shoulder, elbow, and ankle and is more likely to become clinically apparent at a younger age than primary osteoarthritis [141,142,143,144]. A population-based study showed that secondary osteoarthritis related to trauma accounts for approximately 12% of the overall prevalence of symptomatic osteoarthritis of the knee, hip, or ankle [145]. Injuries sustained in sports activities comprise a large portion of post-traumatic osteoarthritis [146]. A wide variety of systemic diseases have been identified as frequent causes of secondary osteoarthritis; these conditions include metabolic diseases, endocrine disorders, bone dysplasias, and crystal deposition diseases [141,147].
Clinical Manifestations
The diagnosis of osteoarthritis at most joints is made primarily on the basis of clinical findings, with imaging studies and laboratory tests more useful for ruling out other diagnoses rather than for confirming the diagnosis of osteoarthritis [148,149,150]. Although radiographic findings are considered to be diagnostic criteria for osteoarthritis, radiographs are not usually part of the initial diagnostic evaluation for several reasons. The primary reasons are the lack of evidence of early osteoarthritic changes on radiographs and the poor correlation between symptoms and radiographic evidence of osteoarthritis [148,151,152,153]. Thus, the absence of radiographic evidence of osteoarthritis in the presence of joint-related symptoms should not exclude the diagnosis of osteoarthritis.
When obtaining a history, questions should focus on the nature of joint-related symptoms, patients' self-reports of limitations in function or activities, and information related to established risk factors for osteoarthritis. The following questions can help elicit important information needed for a diagnosis:
Do you have any joints that hurt? If so, how long have they been bothering you?
When does the pain occur? After certain physical activities? At rest?
Do you have relief of pain if you rest?
Does the pain bother you at night? Does pain wake you up at night?
Are your joints stiff when you wake up in the morning? If so, how long does the stiffness last?
Do the joints that hurt ever lock up or give out on you?
Do you have a family history of osteoarthritis or rheumatoid arthritis?
What types of recreational activities or sports do you participate in? If you play sports, do you do so for leisure or competitively?
What is your occupation? Are there tasks or activities that are part of your job that bother any joints?
Have you ever had an injury to a joint?
Are there daily activities or other tasks that you cannot do because of pain or other symptoms in any joint?
The primary symptom of osteoarthritis of the knee is pain, especially with weight-bearing exercise or activity, that improves with rest. Stiffness in the joint occurs in the morning, lasting 30 minutes or less, and may occur after periods of inactivity [154]. The clinical presentation of hip osteoarthritis is similar to that of knee osteoarthritis, with pain being the most common symptom driving individuals to seek medical care [155]. Pain related to hip osteoarthritis is an ache—most often diffuse—that is usually felt during use of the joint and relieved by rest. Pain is typically gradual, variable, or intermittent; the joint may feel stiff after a period of inactivity [155]. The loss of function or mobility is usually related to the degree of pain.
Osteoarthritis of the hand is characterized by pain with use, which affects one or a few joints at any one time, and mild stiffness in the morning and/or after a period of inactivity [158]. The severity of osteoarthritis-related pain varies, and the pain may be intermittent. The joints most often affected are the distal and proximal interphalangeal joints and the base of the thumb [156,157,158]. Individuals who have evidence of osteoarthritis at several joints in the hand are at increased risk for generalized osteoarthritis, and clinicians should evaluate such patients as appropriate [158].
Pain related to osteoarthritis of the shoulder is typically progressive, related to activity, deep in the joint, and often localized posteriorly [142]. Pain is usually present at rest and interferes with sleep, with nocturnal pain becoming more common as the disease progresses. More advanced disease is also associated with stiffness that limits function.
Individuals with osteoarthritis of the elbow typically have pain, stiffness, and weakness in the joint [143]. Later stage disease is associated with pain when carrying a heavy object at the side of the body with the elbow in extension. The history is important when evaluating symptoms related to the elbow because of the strong relationship between trauma or occupation with osteoarthritis, especially in individuals who are younger than 40 years of age [159].
A history of ankle fracture or ligamentous injury is a hallmark feature of osteoarthritis of the ankle [144]. Diagnostic evaluation includes radiographs of the ankles made with the patient standing. MRI is also recommended, as it can provide evidence of osteonecrosis as well as indicate the amount of involvement, the extent of bone loss, and the size of subchondral cysts [144].
Therapeutic Measures
There is currently no curative therapy for osteoarthritis, and treatments to alter or arrest the disease process are few and mostly ineffective [151]. As clinicians on the frontline of care, primary care providers and nurses are typically the first to see individuals with symptoms indicative of osteoarthritis. Primary care providers can coordinate the management of osteoarthritis, and a multidisciplinary approach is best. The ACR and the Association of Rheumatology Health Professionals (a division of the ACR) support such an approach, noting that the healthcare team may include a rheumatologist, primary physician, nurse, nurse practitioner, physician assistant, physical therapist, occupational therapist, physiatrist, psychiatrist, psychologist, orthopedic surgeon, social worker, registered dietician, vocational counselor, and others [160].
The optimal management of osteoarthritis encompasses both nonpharmacologic and pharmacologic measures, beginning with basic modalities and following a so-called pyramid approach as the disease progresses or symptoms do not respond [161]. Several factors should be considered when selecting treatment modalities, including risk factors (e.g., age, comorbidity, overweight/obesity), the level of pain and functional limitations, signs of inflammation, and degree of structural damage [162].
Many treatment options are associated with benefits and risks, and the clinician should discuss the benefits and risks with patients and support their participation in the decision-making process [163,164]. Patient preferences are an important consideration when choosing treatment options and establishing treatment goals, and the ACR advocates care that addresses treatment goals that are meaningful to the individual patient [160]. Decision aids can help enhance patients' knowledge of treatment options, improve patients' participation in their care, and produce realistic expectations of outcomes [164]. Decision aids for osteoarthritis have been developed in a variety of media (e.g., print, online, video) and are available online (https://decisionaid.ohri.ca) [164].
The pain and disability associated with osteoarthritis often has a substantial psychologic and social effect. It is important to discuss these aspects with patients and to address psychologic issues, especially depression, in order for treatment measures to be effective [165].
Specific Nursing Measures
Education and self-management, through lifestyle modifications are universally recognized as the core of treatment in clinical guidelines [166]. This recommendation is based on research showing that education helps patients become more involved in their care, leading to improved outcomes [163]. The Agency for Healthcare Research and Quality notes that an effective partnership is the key to the effective management of osteoarthritis; the healthcare professional's role in this partnership is to [163]:
Encourage patients to change their behavior to improve symptoms or slow disease progression
Promote the proper use of medications
Instruct patients on how to interpret and report symptoms accurately
Support patients' efforts to maintain normal activities
Help patients adjust to new social and economic circumstances and cope with emotional consequences
Nurses should emphasize to patients that adhering to the management program will alleviate their symptoms, improve their function, and enhance their quality of life. Education should be tailored to address individual needs. For example, patients who participate in sports should be advised to avoid sports with direct contact and high impact and to wear protective equipment to prevent injury [167]. Similarly, for patients in occupations with high risk for osteoarthritis, clinicians should discuss the importance of avoiding high-risk tasks. It is also essential to encourage patients with osteoarthritis of the glenohumeral joint or the elbow to modify activities that led to the development of the disease [143,159]. Periodic contact during follow-up can help promote self-management [166].
Rheumatoid arthritis is defined as a chronic inflammatory disease characterized by uncontrolled proliferation of synovial tissue and a wide array of multisystem comorbidities [24]. In its most common presentation, rheumatoid arthritis affects the joints, causing inflammation of the synovium and cartilage and bone loss. The precise etiology of rheumatoid arthritis is presently unknown [26]. Most likely it has an autoimmune origin (whereby an individual's immune system confuses healthy synovial tissue for foreign substances, thereby attacking the synovial joint surfaces) given that autoantibodies (e.g., rheumatoid factor, ACPA) are present and often precede the clinical manifestation of rheumatoid arthritis by many years [22,25,168].
The course and severity of the illness can vary considerably, and infection, genetic factors, and hormones may contribute to the disease. Rheumatoid arthritis appears to require the complex interaction of genetic and environmental factors with the immune system and ultimately in the synovial tissues throughout the body. Triggers for rheumatoid arthritis have long been the target of active research. Purported triggers have included bacteria (Mycobacterium, Streptococcus, Mycoplasma, Escherichia coli, Helicobacter pylori), viruses (rubella, Epstein-Barr virus, parvovirus), and superantigens [25,26,27].
Although rheumatoid arthritis has a clear genetic component, only about 1 in 25 White individuals with the so-called shared epitope develop rheumatoid arthritis [27]. Even if one monozygotic twin has rheumatoid arthritis, there is only approximately a one in six chance that the other twin will develop the same disease. Thus, other factors in addition to genetics are active as precipitators or triggers of rheumatoid arthritis [27].
Clinical Manifestations
Findings on general physical examination are normal except for an occasional low-grade fever (38°C) and a slightly elevated pulse rate. The characteristic patient with rheumatoid arthritis initially presents with complaints of pain and stiffness in multiple joints. There is prominent and prolonged morning stiffness (lasting more than one hour) that usually begins gradually with fatigue, loss of appetite, widespread muscle aches, and weakness [23,25,27].
After this initial presentation, joint pain appears. When the joint is not used for some time, it can become warm, tender, and stiff. After inflammation of the joint, increased synovial fluid is produced and the joint becomes swollen. There is accompanying soft tissue swelling, and joint pain is often felt bilaterally, affecting the fingers, wrists, elbows, shoulders, hips, knees, ankles, toes, and neck [25]. Though the joints are tender, the small joints of the hands and feet are not usually painful when the patient is at rest. Palmar erythema and prominent veins on the dorsum of the hand and wrist indicate increased blood flow. Distal interphalangeal joints are rarely involved. The temperature over the involved joints (except the hip) can be elevated, but there is usually no accompanying erythema. There are limitations in the range of motion, muscle strength, and function around inflamed joints.
In addition, soft, poorly delineated subcutaneous nodules (rheumatoid nodules) are often found in the extensor surface of the forearm. Soft, small lymph nodes are found occasionally in epitrochlear, axillary, and cervical areas [24]. Other symptoms that may present include anemia due to deficits in bone marrow production; eye burning, itching, and discharge; or lung inflammation (pleurisy) [23,24,25,27]. Joint destruction may occur within one to two years after the appearance of the disease.
Rheumatoid arthritis is not solely a disease of joint destruction; it can involve almost all organs. Approximately 18% to 41% of patients with rheumatoid arthritis develop extra-articular manifestations [169,170]. Rheumatoid arthritis may cause inflammation of the outer cardiac lining (pericarditis) and cardiac muscle (myocarditis), leading to congestive heart failure. In a population-based cohort study, patients with rheumatoid arthritis had a significantly higher risk of cardiovascular disease than those without rheumatoid arthritis [171]. More than half of the patients 50 to 59 years of age and all of those older than 60 years of age with a new diagnosis of rheumatoid arthritis had a more than 10% increased risk of cardiovascular disease within 10 years of rheumatoid arthritis onset.
Pulmonary manifestations are also seen in patients with rheumatoid arthritis, occurring in approximately 30% to 40% of patients. In approximately 10% to 20% of these patients, involvement of the respiratory system is the first manifestation of rheumatoid arthritis [170]. There are several types of potential pulmonary manifestations of rheumatoid arthritis: pleural disease, interstitial pneumonitis, and fibrosis. Pleural effusions and pulmonary rheumatoid nodules are the most common manifestations, along with high rheumatoid factor titers [172,173,174]. Pleuritis is more often found in autopsies of patients with rheumatoid arthritis than in living patients. In about 20% of patients, pleuritis develops concurrently with rheumatoid arthritis onset [174]. Although pleuritic pain is not usually a major complaint, the effusions may be large enough to cause dyspnea. Pulmonary fibrosis can either be slowly progressive or result from pulmonary inflammatory disease; on physical exam of the lungs, they present with fine, diffuse, dry rales.
Ocular involvement is another major manifestation of rheumatoid arthritis, usually manifesting as scleritis, development of anterior uveitis, and peripheral ulcerative keratitis (corneal melt) [175,176]. These disorders are associated with inflammatory cytokines produced by ocular mononuclear cell infiltrates [176,177].
Osteopenia and osteoporosis are very common extra-articular complications in patients with rheumatoid arthritis [178]. The development of osteopenia in patients with rheumatoid arthritis appears to occur independent of corticosteroid use and is directly linked to elevated levels of the RANK ligand expressed by T cells, which promotes osteoclastic bone resorption [178,179,180].
Diagnosis
Rheumatoid arthritis is a clinical diagnosis [181]. As discussed, several laboratory tests are recommended for the diagnosis of rheumatoid arthritis, including rheumatoid factor, ESR, CRP, and anti-CCP antibody [22]. While the results of these tests are relatively sensitive and specific, false positives are possible. In 2010, a multi-biomarker disease activity test, Vectra DA, was introduced. This test uses a unique algorithm to derive a composite score (1 to 100) based on the results of 12 blood protein biomarkers, including vascular cell adhesion molecule-1, epidermal growth factor, vascular endothelial growth factor A, interleukin-6 (IL-6), TNF receptor type 1, matrix metalloproteinase-1 or collagenase-1, matrix metalloproteinase-3 or stromelysin-1, YKL-40, leptin, resistin, serum amyloid, and CRP [182,183]. Vectra DA has been independently verified and found to correlate well to disease activity measured with rheumatoid arthritis assessment tools (e.g., Disease Activity Score in 28 joints using the CRP level). The test is validated for use in adults already diagnosed with rheumatoid arthritis but is not intended to diagnose rheumatoid arthritis [184].
There are several other laboratory tests used in the differential diagnosis of rheumatoid arthritis. Complete blood count may reveal mild normochromic and either normocytic or microcytic anemia (hemoglobin 10 g/dL); white blood cell count and differential may reveal thrombocytosis [24,29]. Although baseline evaluation of renal and hepatic function is not sensitive or specific for rheumatoid arthritis, it is recommended because the findings will guide medication choices.
Popular imaging tests for rheumatoid arthritis include joint ultrasound, MRI, and joint x-rays. Imaging studies may show normal findings or osteopenia and erosions near joint spaces in early disease; wrist and ankle films are useful as baselines for comparison with future studies [24,185]. Implementing the modern treatment strategy in rheumatoid arthritis (i.e., early initiation and optimal adjustments of aggressive therapies) requires methods for early diagnosis and sensitive monitoring of the disease process.
A number of different medical conditions may be considered in the differential diagnosis of rheumatoid arthritis [181,186,187,188]. These include:
Connective tissue diseases (e.g., lupus, scleroderma, polymyositis)
Fibromyalgia
Hemochromatosis
Infectious endocarditis
Lyme arthritis
Osteoarthritis
Polyarticular sepsis
Sarcoidosis
Thyroid disease
Viral arthritis
Therapeutic Measures
Rheumatoid arthritis has no known prevention or cure. Lifelong treatment is usually required, including medication, physical therapy, exercise, and possibly surgery. In order to provide the best outcomes, patients should be educated regarding the most appropriate treatment regimens for their disease manifestations, as earlier rheumatoid arthritis diagnosis can assist in aggressive early treatment for rheumatoid arthritis (when indicated), thereby delaying joint destruction. The 2010 ACR/EULAR Classification Criteria for Rheumatoid Arthritis is now a well-established diagnostic and prognostic tool; as such, guidelines (e.g., the 2016 update of the EULAR Recommendations for the Management of Rheumatoid Arthritis with Synthetic and Biological Disease-Modifying Antirheumatic Drugs) recommend that patients start treatment with a disease-modifying antirheumatic drug (DMARD) immediately following a rheumatoid arthritis diagnosis [189]. Therapeutic goals include preservation of function and quality of life, minimization of pain and inflammation, joint protection, and control of systemic complications, with the ultimate aim being low disease activity or remission [23,24,27,189,190].
Today, the recommended standard of treatment is a tightly controlled, aggressive strategy tailored to each patient, with modifications to the individual medication regimen to achieve a particular target (remission, or alternatively, low disease activity) in a specific period of time (usually six months) [189,191]. The "treat-to-target" approach for a patient with early high disease activity and poor prognostic features typically involves initiation of methotrexate and/or another DMARD(s) immediately upon diagnosis [189,190,191]. Initial combination therapies with DMARDs, particularly those including a biologic anti-TNF agent, appear to provide earlier clinical improvement and less joint damage progression in patients with early moderate or highly active disease; they can be withdrawn successfully, and fewer treatment adjustments are needed than with initial monotherapies [189,191,192,193,194]. Patients with active disease are monitored closely (every one to three months), and it is recommended that treatment adjustments be made if there is no improvement at three months (or if the six-month target has not been reached) [189,191]. Patients with low-to-moderate disease activity or high disease activity without poor prognostic features are typically started on DMARD monotherapy. NSAIDs, glucocorticoids, or COX-2 inhibitors are often used concurrently to treat rheumatoid arthritis-associated joint pain and inflammation. However, they do not alter the disease course and should not be used as single therapy.
Occasionally, surgery is needed to correct severely affected joints. Surgeries serve to relieve joint pain, correct deformities, and modestly improve joint function [23,24,27]. The most successful locations of surgery are those performed on the knees and hips [23,24,27]. The first surgical treatment performed is a synovectomy, which removes part or all of the joint lining (synovium). This procedure may only provide temporary relief, but it can be effective for patients for whom pharmacologic treatment has not resulted in improvements. Surgeries performed in later-onset disease include total joint replacement with a joint prosthesis. In extreme cases, total knee or hip replacement can have enhanced importance, making the difference between a dependent or independent lifestyle for a patient.
Range-of-motion exercises and individualized exercise programs prescribed by a physical therapist can also delay the loss of joint function. Joint protection techniques, heat and cold treatments, and splints or orthotic devices to support and align joints may be of assistance [23,24,27]. Some therapists will use specialized devices to apply deep heat or electrical stimulation to reduce pain and improve joint mobility [23,24,27]. Occupational therapists can construct splints for the hand and wrist and teach patients with rheumatoid arthritis how to protect and use their joints most effectively. In addition to physiotherapy, occupational therapists can also show patients with rheumatoid arthritis how to better cope with limitations that can affect their daily tasks at work and at home. For example, many clinicians have recommended frequent rest periods between activities and proper sleeping habits (e.g., 8 to 10 hours of sleep per night) [195].
In addition to the medical management of rheumatoid arthritis, several lifestyle changes may improve symptom severity and decrease the number of flare-ups. The National Institute of Arthritis and Musculoskeletal and Skin Disorders recommends advising patients regarding rest and exercise, use of orthotic devices, stress reduction, and healthful diet [23].
Infectious arthritis (also known as septic arthritis) is the inflammation of a joint resulting from an invading organism that attacks the synovium and synovial fluid. Viral, bacterial, and fungal infections all predispose susceptible people to arthritis involvement. Pathogens present in the host circulate freely in the bloodstream and become trapped in the richly perfused synovial membrane, leading to inflammation and subsequent degenerative changes. Infectious arthritis is an opportunistic disease that primarily occurs in patients with immunocompromise or who already have joint destruction from another disorder (e.g., rheumatoid arthritis). Early diagnosis and treatment can prevent serious degenerative changes [76,196].
Patients with infectious arthritis undergo repeated arthrocentesis, which can be stressful. Additional treatment will depend on the underlying pathogen, with antibiotics, antiviral, or antifungals prescribed as appropriate [76,196].
The nurse should be available to both the patient and family for psychological support, physical care, health education, and monitoring the patient's response to therapy. The control of pain and protection of the involved joint or joints are priorities of nursing management [76,196].
Therapeutic and Nursing Measures
The patient history is key to diagnosis, and nurses should be careful to obtain a complete and accurate history. This should include any recent viral (e.g., parvovirus, alphavirus, hepatitis, Epstein-Barr virus) and bacterial (e.g., Streptococcus pneumoniae) infection. In young, sexually active patients, the most common causative pathogen is Neisseria gonorrhea. For patients who develop infectious arthritis following trauma, puncture wounds, or injection drug use, Pseudomonas aeruginosa is the most likely cause [18,197].
It is important that the pathogen responsible for the infectious arthritis be identified and treatment begun as quickly as possible to prevent joint destruction. Isolating the organism will guide in the selection of intravenous antimicrobial therapy and the level of aggressiveness needed to control the infection. Pathogens are identified through the aspiration of synovial fluid, synovial fluid cultures, and synovial biopsy. Empiric therapy is started after joint aspiration is complete and cultures are obtained [76]. Other therapeutic measures for infectious arthritis include surgical excision of the affected synovium in instances where destruction of the joint cartilage, tendons, or both appears imminent [76].
To protect the intra- and extra-articular structure from future damage and reduce the patient's discomfort, the involved joint should be immobilized during the acute stage. However, after two to three days, aggressive physical therapy is recommended to prevent long-term damage and disability [18].
The involved joint should be assessed frequently for drainage and any change in condition. Sterile technique should be maintained with any dressing changes [18]. For patients receiving parenteral fluids, intake and output should be measured and documented accurately. Laboratory test results will be monitored daily, especially the results of culture and sensitivity tests [18].
Patient education should include instruction about range-of-motion exercises to maintain joint mobility; dressing change techniques and wound care, if appropriate; and adherence to prescribed medications. The patient should be advised of symptoms and signs of repeated infection (e.g., increased pain, fever, swelling, redness, drainage) and to avoid any trauma to the joint [18].
In this section, the discussion of masses and tumors of the joints and surrounding structures will be limited to the benign and malignant lesions generally included in a differential diagnosis for arthritis.
Patients with a benign lesion of the joint may experience years of intermittent minor problems with the involved joint, with a history of discomfort and joint instability. Because the symptoms of a benign tumor can remain innocuous for long periods, joint damage may result prior to diagnosis. No matter the extent of damage, joint surgery is required to resolve the issue and prevent further deterioration [37].
Lipoma
A lipoma of a joint is a lobulated fatty mass. Lipomas develop frequently in the elbow or knee joint of patients with osteoarthritis [37]. In many cases, patients seek medical attention when the involved joint begins locking or when pain, decreased motion, or an effusion occurs. Effusion aspirate is clear, and x-rays are non-diagnostic [37].
Hemangioma
Hemangiomas are rare vascular tumors often associated with arteriovenous malformations of skin vascular disease. They tend to affect younger individuals, often teenage girls who have been symptomatic since childhood. The knee is the most commonly involved joint [37].
In patients with joint hemangioma, there is a history of episodic, unilateral "doughy" joint swelling; pain; limitation of motion; and locking, buckling, or both. Aspiration of the joint repeatedly produces serosanguineous fluid in the absence of trauma. X-rays early in the course of the hemangioma may appear normal; ultrasound is often more helpful. With more advanced disease, enlarged epiphyses, joint narrowing, and enlargement of the intercondylar notch layer may be visualized [37].
Surgical removal yields good results in the treatment of a localized hemangioma. If accessible, therapeutic embolization of a major feeder vessel may be effective as well. Because of the vascular nature of this tumor, a diffuse form may involve the entire joint capsule and make resection impossible. For these patients, radiation therapy is the usual treatment [37].
Synovial Chondromatosis
Synovial chondromatosis is a condition of unknown etiology in which numerous cartilaginous nodules form. These nodules involve the joint, bursae, and in some cases the tendon sheaths of a knee, hip, elbow, shoulder, or ankle. It is a self-limiting disease that most frequently affects young and middle-aged men [37].
Synovial chondromatosis has an insidious onset, and many years often pass before the patient seeks evaluation for the problem. Presenting symptoms usually consist of swelling, pain, stiffness, limitation of motion, and joint locking. Particularly with synovial chondromatosis, the patient experiences joint crepitation or a grating sensation from the multiple intrasynovial nodules. X-ray findings may demonstrate calcified, free-floating bodies in the synovium [37].
Excision of the involved synovium and removal of all loose bodies is the treatment of choice. Surgical therapy has a good prognosis, although the condition may recur if removal is incomplete [37].
Pigmented Villonodular Synovitis
Pigmented villonodular synovitis is a condition in which the synovial lining cells have a marked proliferation that results in the appearance of numerous villi and folds. The formation of the finger-like projections can affect not only the synovial lining but also the tendon sheath, bursa, and bone. Unilateral involvement of the knee, hip, ankle, or elbow joint of young adults is most common [37].
There are two forms of pigmented villonodular synovitis: localized and diffuse. The diffuse type causes pain and mild, episodic joint swelling over a period of months to years. Occasionally, the patient may note an acutely painful, warm, swollen joint with limited motion. Repeated aspirations of joint fluid yield dark serosanguineous fluid in the absence of trauma [37].
The localized type of pigmented villonodular synovitis occurs in either the medial or lateral knee compartment as a solitary nodule. It, too, may begin with episodic pain and mild swelling and can be misdiagnosed as torn meniscus. Serosanguineous fluid is rarely aspirated with this type of lesion [37]. Imaging studies may show soft tissue density, but angiographies are more useful because the vascularity of these enable the condition to be diagnosed [37].
Surgical resection is the treatment for both types of pigmented villonodular synovitis. The localized form is usually cured with simple excision. With the diffuse type, lesions may recur if synovectomy is incomplete [37].
Specific Nursing Measures
During the diagnostic phase, the health history is important. The nurse should concentrate on any significant joint trauma and inquire regarding any past history of arthritis. The patient should be asked for a detailed description of swelling, pain, limitation of motion, and/or joint instability [7].
In cases of significant joint instability, a cane or crutches may be necessary. Patients should also receive education on appropriate pain management measures, including thermotherapy, over-the-counter medications, and biofeedback. In most cases, the patient will also require education to prepare for surgery and postoperative recovery [7].
Postoperative care includes monitoring for wound drainage or other signs of infection, dressing changes (as necessary), and splinting. Discharge planning includes instruction in the care of the surgical site, activity restrictions, and follow-up appointments [7].
Malignant tumors of the joint are rare. However, if a patient has a slow-growing monoarticular mass, malignancy should be suspected [198].
Synovial Sarcoma
Though it is the most common primary tumor of the joint, synovial sarcoma is rare. This malignancy can appear at any age, although it seems to predominate in young adults. The growth generally appears on a lower extremity, but synovial sarcomas can also develop in an upper extremity, the neck, or the chest [198].
Patients with synovial sarcoma often present with a slow-growing mass that may have been present for months to years, depending on how deeply seated it is in tissue. Pain may be present, or the patient may have a vague sensation of discomfort over the involved area. There may also be localized swelling. In cases involving the neck, tumor invasion may produce hoarseness, dysphagia, or dyspnea [198].
As with most cancer, survival time is dependent on the size of the tumor, site in the body, and age at diagnosis. The five-year survival rate is approximately 60%; this increases to 75% in patients 30 years of age or younger. If the tumor is in an extremity, five-year survival is about 65%; if the tumor is present in the trunk, the rate decreases to 40% [199].
The goals of treatment for synovial sarcoma are to eliminate the tumor, preserve a functional limb, and minimize mortality and morbidity. Preoperative chemotherapy and radiation therapy may be undertaken to decrease the size of the tumor [198]. Wide surgical resection is typically undertaken, followed by continued radiation therapy.
Clear Cell Sarcoma
Clear cell sarcoma is a rare tumor that involves tendons rather than joint spaces. It can occur in any age group and usually is found in an extremity, particularly the foot. However, it can develop in the trunk, head, genitals, stomach, and intestines. Because of this lesion's location and its predisposition to metastasis, it can be difficult to remove entirely, making treatment complicated and prognosis poor [198]. The average age at diagnosis is 25 years [200].
The primary treatment of clear cell sarcoma is radical resection of the tumor. In some cases, an extremity may be amputated. Preoperative and postoperative radiation therapy are employed. In some cases, chemotherapy may be used, but it is not particularly effective [200].
Permanent structural changes may occur in a joint as a result of cartilage and capsular tears, detachment of menisci, hemorrhagic effusions, articular fractures, or repetitive trauma. Because of the realignment of involved bone, bursa, and tendons, a mechanical deterioration of articular cartilage results in osteoarthritis [201,202].
Traumatic arthritis may result from unexpected force (e.g., sports, motor vehicle accidents) or from repetitive trauma—a chronic injury resulting from repeated smaller stresses to a joint through vibrations, blows, abnormal strain, or position. Injury resulting from repetitive stress is often related to occupation and lifestyle, for example, the stress placed on the metatarsophalangeal joints of a ballet dancer or the knees of a jogger. Over time, repetitive trauma may realign the joint and lead to the same result as an acute injury [201,202].
The patient with traumatic arthritis must make lifestyle changes. In order to continue participating in chosen sports or occupations, patients may require braces, splints, or special equipment; in some cases, they may need to halt participation or seek accommodations in their workplace. Although these options are effective in halting the progression of traumatic arthritis, they are often undesired or impossible for patients. The responsibility of the nurse is to provide accurate information about the alternatives available [201,202].
Nurses also play an important role in the therapeutic and preventive care of traumatic disorders. If they are the first person on the scene of an accident, nurses may be able to prevent any residual damage by splinting, elevating the area, and not allowing weight on the area to protect the joint. It may be possible to identify tasks that expose employees to repetitive trauma; employees then can rotate through the jobs rather than be assigned permanently to potentially harmful tasks [201,202].
Joint effusions can occur as a result of simple trauma or secondary to fractures, internal derangements, or severe sprains. Within 24 hours after a blow to the joint, synovial fluid accumulates. If blood vessels in the synovium are broken, hemarthrosis also occurs. The knee is most commonly affected by this injury, although it can occur in other joints as well [75].
Clinical Manifestations
In simple cases of traumatic synovitis, joint swelling with mild pain occurs. Aspiration of the joint produces clear fluid with elevated protein content and decreased viscosity. Hemarthrosis, which usually develops within 15 minutes to 2 hours after the trauma, is usually more painful than clear effusion and is accompanied by low-grade fever. Diagnosis of traumatic synovitis is primarily by physical examination, but x-ray examination is done to rule out fracture [75].
Therapeutic Measures
Immediately following injury, patients should be advised to apply cryotherapy (e.g., ice) for 30 minutes; this can be repeated up to four times per day to reduce swelling and relieve pain. After the first 24 hours, patients should switch to moist heat to relax surrounding muscles and reduce pain. If fluid accumulates in the joint, repeated joint aspirations may be necessary. Compression dressings applied to the joint, along with limited weight bearing, may be used, depending on the severity of the injury [75].
Dislocation is the complete displacement of a joint's articulating surfaces following trauma. Partial displacement of the articulating surfaces results in subluxation. Both subluxations and dislocations can damage soft tissues, nerves, or blood vessels if not attended to promptly. The joints most often affected are shoulders, wrists, elbows, fingers, hips, knees, and toes [75].
Clinical Manifestations
After injury, the joint appears deformed; it is tender, and motion is limited. The involved extremity may be visibly shortened. Joint pain may be intense, especially if articular surface fractures are present. With immediate treatment, there is a good prognosis. However, bone necrosis can result if reduction of the subluxation or dislocation is delayed [75]. Diagnosis is made through physical examination and patient history, with x-rays taken to evaluate joint displacement and to determine whether fractures are present [75].
Therapeutic Measures
The longer the delay in correcting a joint displacement, the more difficult the procedure becomes because of edema and muscle spasms. Two types of procedures can correct this injury. Closed reduction is manual traction done under local or general anesthesia. The pain associated with this procedure can be intense, and pain management techniques (including strong analgesics) are necessary. If muscle spasms are an issue, tranquilizers and/or muscle relaxants may be administered. Open reduction is done when wire fixations of the joint or repair of torn ligaments is also necessary [75].
If present at the site of injury, the joint should be splinted—even if crooked—to prevent further damage. Cold compresses can be applied to decrease pain and swelling [35]. The area distal to the injury should be observed for evidence of vascular damage (e.g., pallor, absent pulse, abnormal coolness) and nerve damage (e.g., paresthesia, paralysis) [35].
If analgesics, muscle relaxants, or tranquilizers are administered, it is important to monitor the patient's respiratory status. Any dressings or casts should be checked for pressure that may impair blood flow. Patients should receive education on gradual mobilization and return to activities [7].
Patient A is a woman, 29 years of age, with two small children. She presents to her primary care provider with complaints of rashes developing on her arms and legs whenever she spends time in the sun. She also reports several small patches of hair loss on her head that she attributes to the stress of new motherhood and to a recent trip and her fear of flying. She reports a lack of energy, being easily fatigued, and always needing to nap during the day. Patient A also reports mild pain in her fingers and elbows but attributes the joint discomfort to caring for the children. She states that these problems have been ongoing for approximately four months.
Patient A has no known allergies and takes no prescription or over-the-counter medication aside from occasional naproxen for joint pain and antacid for heartburn. She neither smokes nor drinks alcohol. Her youngest child is 2 years of age, and she reports unremarkable childbirths and postpartum periods. Aside from the current complaints, the patient's medical history is unremarkable.
She has four brothers and three sisters. The family history indicates an older sister with rheumatoid arthritis, an aunt with pernicious anemia, and mother with hyperthyroidism.
The primary care provider conducts a full physical assessment (Table 2). Several laboratory tests are ordered, with the following results:
Hematocrit (HCT): 23%
Red blood cell (RBC) count: 3.5 million cells/mcL
White blood cell (WBC) count: 5,500 cells/mcL
Platelets: 350,000 cells/mcL
ESR: 25 mm/hour
Urinalysis: Normal
ANA: 1:640
Anti-DNA antibody test: Elevated
Complement assay: Decreased C3 level at 43 mg/dL and decreased C4 level at 14 mg/dL
PATIENT A'S FIRST PHYSICAL EXAM RESULTS
| Parameter | Findings | ||||
|---|---|---|---|---|---|
| General appearance |
| ||||
| Skin and nails |
| ||||
| Head and nose |
| ||||
| Eyes |
| ||||
| Ears | Tympanic membranes intact | ||||
| Neck |
| ||||
| Chest |
| ||||
| Abdomen |
| ||||
| Extremities |
| ||||
| Genitourinary system | Normal female | ||||
| Neurologic status |
| ||||
| Cardiovascular system |
| ||||
| Vital Signs | |||||
| Blood pressure | 110/70 mm Hg | ||||
| Temperature | 99.8° F | ||||
| Heart rate | 70 beats per minute with regular rhythm | ||||
| Respiratory rate | 15 breaths per minute | ||||
Further, a tissue biopsy of one of the lesions is taken and reveals vasculitis (i.e., white blood cells within the walls of blood vessels).
Based on the results of the assessment and laboratory studies, Patient A is diagnosed with SLE.
A one-month course of prednisone with tapered doses is prescribed. Nabumetone, an anti-inflammatory, is added to the regimen prior to the prednisone being weaned off. After one month of treatment, all signs and symptoms of lupus have resolved.
However, 13 years later, Patient A again presents to her primary care provider, this time with complaints of a productive cough and transient stiffness and pain in her hands and feet (migratory polyarthritis). She is afraid that she is developing rheumatoid arthritis like her sister. The provider conducts a physical examination (Table 3) and is concerned that the patient may be showing signs of pneumonia. A chest x-ray revealed mild pulmonary edema but no white blood cell infiltrates in the terminal airways. Laboratory tests reveal:
HCT: 43%
Platelet: 330,000 cells/mcL
WBC count: 1,200 cells/mcL
Urinalysis: Within normal limits
PATIENT A'S SECOND PHYSICAL EXAM RESULTS
| Parameter | Findings | |||
|---|---|---|---|---|
| General appearance |
| |||
| Skin and nails | No lesions or abnormalities noted | |||
| Head and nose |
| |||
| Eyes |
| |||
| Ears | Tympanic membranes intact | |||
| Neck |
| |||
| Chest |
| |||
| Abdomen |
| |||
| Extremities |
| |||
| Genitourinary system |
| |||
| Neurologic status |
| |||
| Cardiovascular system |
| |||
| Vital Signs | ||||
| Blood pressure | 140/90 mm Hg | |||
| Temperature | 100.0° F | |||
| Heart rate | 105 beats per minute with regular rhythm | |||
| Respiratory rate | 15 breaths per minute | |||
Patient A is diagnosed as experiencing a lupus flare and is prescribed a one-month course of prednisone along with a 10-day course of antibiotics to prevent pneumonia. Within three months, all signs and symptoms have resolved.
Five years later, Patient A returns to her primary care provider complaining of fatigue, anorexia, weight loss (25 pounds in the last four months), and significant swelling in her abdomen, face, and ankles. The nurse practitioner notes a "butterfly-shaped" rash present across the bridge of the patient's nose and cheeks. Blood tests reveal an HCT of 24% and a WBC count of 2,400 cells/mcL. A dipstick examination of the urine reveals an abnormal protein concentration, and microscopy indicates the presence of significant numbers of red and white blood cells. A 24-hour urine protein collection reveals excretion of 2.5 g protein in 24 hours.
What is the significance of the patient's family history?
Is this patient underweight, normal weight, overweight, or obese?
What underlying pathologic process is responsible for Patient A's hair loss? What is the relevance of the abnormal ESR?
Vasculitis in lupus results from the trapping of antigen antibody complexes in blood vessel walls followed by an intense inflammatory response to the immune complexes. Why is prednisone effective in relieving vasculitis?
What is the most likely cause of jaundice in this patent?
What pathophysiology underlies lymph node enlargement in this patient?
The patient's WBC differential was: 75% neutrophils, 15% lymphocytes, 5% monocytes/macrophages, 4% eosinophils, and 1% basophils. Which one of these white blood cell types has been specifically targeted by the patient's immune system?
Why was Patient A experiencing swelling throughout her body?
Patient B is a woman, 35 years of age, who has worked as a housekeeper for the past 10 years. She is 5 foot 3 inches in height with a weight of 178 pounds. She presents to her primary care provider with complaints of low back pain. She reports having had this pain intermittently for several years; however, for the past two days, it has been worse than ever. The recent exacerbation started after vacuuming a rug (i.e., pulling and twisting at the waist). Patient B reports that the pain is primarily on the right lower side and radiates down her posterior right thigh to her knee; it is not associated with any numbness or tingling. The pain can be relieved by lying flat on her back with her legs slightly elevated and is lessened somewhat when she takes ibuprofen 400 mg. Except for moderate obesity and difficulty maneuvering onto the examination table because of pain, the patient's examination is fairly normal. The only abnormalities noted are a positive straight leg raise test, with raising the right leg eliciting more pain than the left. Her strength, sensation, and deep tendon reflexes in all extremities are normal.
Patient C is a woman, 50 years of age, who presents to her primary care provider for her annual exam. She reports having been very tired for the past month and also experiencing stiffness, pain, and swelling in multiple joints. She states, "I ache all over, and I have pain in different places all the time. One day it is in my right shoulder, the next day in my right wrist, and the following day my left wrist. I'm stiff everywhere when I get up in the morning or if I sit for any length of time. And I feel so tired, like I have a case of the flu that won't go away."
The patient has been diagnosed with hypothyroidism in the past, for which she is taking levothyroxine. She is also prescribed venlafaxine to treat major depressive disorder, and she indicates that her mood has been good, despite the fatigue. She is also taking an over-the-counter multivitamin and calcium supplement. Patient C reports rarely using alcohol and never smoking. There is no family history of autoimmune disorders.
The primary care provider does a complete physical exam (Table 4) and orders laboratory tests. The laboratory blood test results are:
Sodium: 140 meq/L
ANA: Negative
HCT: 43%
Uric acid: 2.9 mg/dL
Potassium: 3.7 meq/L
ESR: 38 mm/hour
WBC count: 15,100 cells/mcL
Cholesterol: 189 mg/dL
Chloride: 104 meq/L
Creatinine: 1.0 mg/dL
Platelets: 270,000 cells/mcL
Albumin: 4.0 g/dL
Bicarbonate: 23 meq/L
Blood glucose: 94 mg/dL
RBC count: 4.7 million cells/mcL
Thyroid stimulating hormone (TSH): 1.7 mcU/mL
Blood urea nitrogen: 18 meq/L
Hemoglobin: 14.9 g/dL
Calcium: 8.8 mg/dL
Rheumatoid factor: Positive
PATIENT C'S PHYSICAL EXAM RESULTS
| Parameter | Findings | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| General appearance |
| ||||||||
| Skin and nails |
| ||||||||
| Head and nose | Head atraumatic | ||||||||
| Eyes |
| ||||||||
| Ears | Tympanic membranes intact | ||||||||
| Neck |
| ||||||||
| Chest |
| ||||||||
| Abdomen |
| ||||||||
| Extremities |
| ||||||||
| Genitourinary system |
| ||||||||
| Neurologic status |
| ||||||||
| Cardiovascular system |
| ||||||||
| Vital Signs | |||||||||
| Blood pressure | 125/80 mm Hg | ||||||||
| Temperature | 100.0° F | ||||||||
| Heart rate | 80 beats per minute with regular rhythm | ||||||||
| Respiratory rate | 15 breaths per minute | ||||||||
A urinalysis is performed and is normal, with no RBCs, WBCs, or protein. A chest x-ray finds no fluid, masses, infection, or cardiomegaly. An x-ray of the hand shows soft tissue swelling and bone demineralization but no erosions. Synovial fluid removed from the left knee (7.4 mL) is cloudy and pale yellow in appearance; analysis indicates 14,000 white blood cells/mcL (primarily neutrophils) and a glucose level of 60 mg/dL.
Based on these findings, Patient C is diagnosed with rheumatoid arthritis and referred to a rheumatologist for follow-up.
Which of Patient C's vital signs is consistent with a diagnosis of rheumatoid arthritis and why?
Are there any other abnormal findings from the patient's physical exam that are consistent with a diagnosis of rheumatoid arthritis?
What is the association between the fixed nodules at pressure points on the left wrist/right elbow and a diagnosis of rheumatoid arthritis?
Why is it reasonable that this patient has no stiffness, pain, or swelling in the DIP joints of the fingers?
Which of these patient's laboratory test results are consistent with a diagnosis of rheumatoid arthritis?
In terms of the progression of the disease, what do the results of the hand x-ray suggest?
Which findings in the examination of the synovial fluid are consistent with a diagnosis of rheumatoid arthritis?
What causes limitation of joint motion that occurs early in the clinical course of rheumatoid arthritis? What causes limitation of joint motion that occurs late in the clinical course of rheumatoid arthritis?
With knowledge of the structures and function of the muscles, joints, and connective tissue and the dynamic pathology that intrudes and impedes normal function, nurses can readily provide quality and often life-saving actions. An awareness of why symptoms appear leads to quicker reporting to physicians of changes in the patient's condition. Nurses can also perform immediate interventions based on standing orders and recognition of what needs to be done in order to provide safe quality care. This knowledge changes what could be only technical care to professional care through the use of decision-making skills built upon the knowledge of pathophysiology.
1. National Institutes of Health. Complete Medical Guide for Disease Volume V: Ankylosing Spondylitis. Online: MedHealth; 2012.
2. Grossman SC, Porths CM. Porth's Pathophysiology: Concepts of Altered Health States. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013.
3. Bruyere HJ Jr. 100 Case Studies in Pathophysiology. Philadelphia, PA: Lippincott Williams & Wilkins; 2010.
4. Brashers VL. Clinical Applications of Pathophysiology: Assessment, Diagnostic Reasoning, and Management. 2nd ed. St. Louis, MO: Mosby; 2002.
5. Huether SE, McCance KL, Brashers VL. Understanding Pathophysiology. 7th ed. St. Louis, MO: Mosby; 2019.
6. LeMone P, Burke KM. Medical-Surgical Nursing: Critical Thinking in Client Care. 4th ed. Upper Saddle River, NJ: Pearson Prentice Hall; 2007.
7. Bullock BL, Henze RL. Focus on Pathophysiology. Philadelphia, PA: Lippincott Williams & Wilkins; 1999.
8. Lazenby RB. Handbook of Pathophysiology. 4th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2010.
9. Price SA, Wilson LM. Pathophysiology: Clinical Concepts of Disease Processes. 6th ed. Mosby, 2002.
10. de Boer P, Morgan SJ, van der Werken C. AO Handbook: Orthopedic Trauma Care. New York, NY: Thieme; 2011.
11. Thompson SR, Zlotolow DA. Handbook of Splinting and Casting: Mobile Medicine Series. 1st ed. Philadelphia, PA: Mosby; 2011.
12. El Miedany Y. Musculoskeletal Ultrasonography in Rheumatic Diseases. Cham, Switzerland: Springer International; 2015.
13. Mitchell RN, Kumar V, Fausto N, Abbas AK, Aster JC. Pocket Companion to Robbins and Cotran Pathologic Basis of Disease. 8th ed. Philadelphia, PA: Saunders; 2011.
15. Ehrman JK, Gordon PM, Visich PS, Keteyian SJ. Clinical Exercise Physiology. 5th ed. Champaign, IL: Human Kinetics, Inc; 2022.
16. Barkauskas V, Baumann LC, Darling-Fisher C. Health and Physical Assessment. 3rd ed. Mosby; 2002.
17. Flynn Makic MB, Martinez-Kratz M (eds). Ackley and Ladwig's Nursing Diagnosis Handbook: An Evidence-Based Guide to Planning Care. 13th ed. Maryland Heights, MO: Mosby; 2022.
18. Ladwig GB, Ackley BJ, Flynn Makic MB, Martinez-Kratz M, Zanotti M. Mosby's Guide to Nursing Diagnosis. 6th ed. Maryland Heights, MO: Mosby; 2021.
19. Ferrell BR, Coyle N. Oxford Textbook of Palliative Nursing. 5th ed. New York, NY: Oxford University Press; 2019.
20. Berg S, Bittner EA. The Massachusetts General Hospital Review of Critical Care Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2013.
21. Lupus Foundation of America. Lab Tests for Lupus. Available at https://www.lupus.org/resources/lab-tests-for-lupus. Last accessed September 28, 2022.
22. Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569-2581.
23. National Institute of Arthritis and Musculoskeletal and Skin Diseases. Rheumatoid Arthritis. Washington, DC: National Institutes of Health, U.S. Department of Health and Human Services; 2009.
24. Rindflesich JA, Muller D. Diagnosis and management of rheumatoid arthritis. Am Fam Physician. 2005;72(6):1037-1047.
25. Firestein GS. Etiology and pathogenesis of rheumatoid arthritis. In: Firestein GS, Budd RC, Gabriel SE, McInnes IB, O'Dell JR (eds). Kelley's Textbook of Rheumatology. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2013: 1059-1069.
26. Smith HR, Brown A. Rheumatoid Arthritis. Available at https://emedicine.medscape.com/article/331715-overview. Last accessed September 28, 2022.
27. O'Dell JR. Rheumatoid arthritis. In: Goldman L, Schafer AI (eds). Goldman-Cecil Medicine. 25th ed. Philadelphia, PA: Saunders Elsevier; 2015.
28. Andersson ML, Svensson B, Petersson IF, et al. Early increase in serum-COMP is associated with joint damage progression over the first five years in patients with rheumatoid arthritis. BMC Musculoskelet Disord. 2013;14:229.
29. Pincus T, Sokka T. Laboratory tests to assess patients with rheumatoid arthritis: advantages and limitations. Rheum Dis Clin N Am. 2009;35(4):731-734.
30. Lozada CJ, Culpepper SS. Osteoarthritis Workup. Available at https://emedicine.medscape.com/article/330487-workup#c11. Last accessed September 28, 2022.
31. Radiological Society of America, American College of Rdaiology. Musculoskeletal MRI. Available at https://www.radiologyinfo.org/en/info/muscmr. Last accessed August 25, 2022.
32. American Academy of Physical Medicine and Rehabilitation. Ultrasound Imaging of Musculoskeletal Disorders. Available at https://now.aapmr.org/ultrasound-imaging-of-musculoskeletal-disorders. Last accessed August 25, 2022.
33. Ono K, Dvořác J, Dunn E. Cervical Spondylosis and Similar Disorders. Hackensack, NJ: World Scientific; 1998.
34. National Human Genome Research Institute. About Marfan Syndrome. Available at https://www.genome.gov/Genetic-Disorders/Marfan-Syndrome. Last accessed August 26, 2022.
36. The Ehlers-Danlos Society. 2017 EDS International Classification. Available at https://www.ehlers-danlos.com/2017-eds-international-classification. Last accessed August 26, 2022.
37. McMahon P, Skinner HB. Current Diagnosis and Treatment in Orthopedics. 6th ed. New York, NY: McGraw-Hill Medical; 2021.
38. Dangers JE. Carpal Tunnel Syndrome in Emergency Medicine. Available at https://emedicine.medscape.com/article/822792-overview. Last accessed September 28, 2022.
39. American Society for Surgery of the Hand. de Quervain's Tendonitis. Available at https://www.assh.org/handcare/condition/dequervains-tenosynovitis. Last accessed September 28, 2022.
40. American Society for Surgery of the Hand. Trigger Finger. Available at https://www.assh.org/handcare/condition/trigger-finger. Last accessed September 28, 2022.
41. Dylan Morrissey. Guidelines and pathways for clinical practice in tendinopathy: their role and development. J Orthop Sports Phys Ther. 2015;45(11):819-822.
42. Ohio State University. Tendinopathy Clinical Practice Guideline. Available at https://wexnermedical.osu.edu/-/media/files/wexnermedical/patient-care/healthcare-services/sports-medicine/education/medical-professionals/other/tendinopathy.pdf. Last accessed August 26, 2022.
44. National Institute of Arthritis and Musculoskeletal and Skin Diseases. Polymyalgia Rheumatica and Giant Cell Arteritis. Available at https://www.niams.nih.gov/health-topics/polymyalgia-rheumatica-giant-cell-arteritis. Last accessed August 26, 2022.
45. Dejaco C, Singh YP, Perel P, et al. 2015 recommendations for the management of polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Arthritis Rheumatol. 2015;67(10):2569-2580.
46. Jones L. Scleroderma: Your Guide to Scleroderma Symptoms and Treatments. Learning Life eBooks; 2011.
47. National Institute of Allergy and Infectious Diseases. Lupus: A Patient Care Guide for Nurses and Other Health Professionals. 3rd ed. NIH Publication No. 06-4262. Bethesda, MD: U.S. Department of Health and Human Services; 2006.
48. Falvo DR. Medical and Psychosocial Aspects of Chronic Illness and Disability. 6th ed. Sudbury, MA: Jones and Bartlett Publishers; 2017.
49. Wallace DJ. The Lupus Book: A Guide for Patients and Their Families. 5th ed. New York, NY: Oxford University Press; 2012.
50. Phillips RH. Coping with Lupus: A Practical Guide to Alleviating the Challenges of Systemic Lupus Erythematosus. 3rd ed. New York, NY: Penguin Putnam; 2001.
51. National Institute of Arthritis and Musculoskeletal and Skin Diseases. Handout on Health: Systemic Lupus Erythematosus. Available at https://www.niams.nih.gov/health-topics/lupus. Last accessed September 28, 2022.
52. Centers for Disease Control and Prevention. Systemic Lupus Erythematosus. Available at https://www.cdc.gov/lupus/facts/detailed.html. Last accessed September 28, 2022.
53. Lupus Foundation of America. What is Lupus? Available at https://www.lupus.org/resources/what-is-lupus. Last accessed September 28, 2022.
54. U.S. Department of Health and Human Services, Office on Women's Health. Lupus. Available at https://www.womenshealth.gov/lupus?from=AtoZ. Last accessed September 28, 2022.
55. Lupus Research Alliance. About Lupus. Available at https://www.lupusresearch.org/understanding-lupus/what-is-lupus/about-lupus. Last accessed September 28, 2022.
56. Mayo Clinic. Lupus. Available at https://www.mayoclinic.org/diseases-conditions/lupus/symptoms-causes/syc-20365789. Last accessed September 28, 2022.
57. Lupus Foundation of America. Risk Factors for Developing Lupus. Available at https://www.lupus.org/resources/risk-factors-for-developing-lupus. Last accessed September 28, 2022.
58. Alexander LL, LaRosa JH, Bader H, Garfield S. New Dimensions in Women's Health. 8th ed. Sudbury, MA: Jones and Bartlett Publishers; 2020.
59. Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71(9):1400-1412.
60. Kauffman CL. Drug-Induced Lupus Erythematosus. Available at http://emedicine.medscape.com/article/1065086-overview#a0156. Last accessed September 28, 2022.
61. Lupus Foundation of America. How Lupus Affects the Bones. Available at https://resources.lupus.org/entry/how-lupus-affects-the-bones. Last accessed September 28, 2022.
62. Lupus Foundation of America. What You Need to Know About Anemia. Available at https://www.lupus.org/resources/what-you-need-to-know-about-anemia. Last accessed September 28, 2022.
63. Lupus Foundation of America. Cognitive Dysfunction in Lupus is Prevalent and Not Associated with Disease Activity. Available at https://www.lupus.org/news/cognitive-dysfunction-in-lupus-is-prevalent-and-not-associated-with-disease-activity. Last accessed September 28, 2022.
64. Lyons RF, Duck S, Langille L, Sullivan MJL. Mobilizing support in chronic illness: a relationship perspective. In: Stewart MJ (ed). Chronic Conditions and Caregiving in Canada. Toronto: University of Toronto Press; 2000: 223-246.
65. Keller SM. Social Support and Psychological Distress in Women with Systemic Lupus Erythematosus [dissertation]. Cleveland, OH: Case Western Reserve University; 1999.
67. Darner GF. Self-Concept and Psychosocial Adjustment in Women with Systemic Lupus Erythematosus [dissertation]. Long Island, NY: Adelphi University; 1991.
69. Kowal-Bielecka O, Fransen J, Avouac J, et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis. 2017;76(8):1327-1339.
70. Anderson BC. Office Orthopedics for Primary Care: Treatment. 3rd ed. Philadelphia, PA: Saunders; 2005.
71. Mueller-Wohlfahrt H-W, Haensel L, Mithoefer K, et al. Terminology and classification of muscle injuries in sport: the Munich consensus statement. Br J Sports Med. 2013;47(6):342-350.
72. Torres PA, Helmstetter JA, Kaye AM, Kaye AD. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15(1): 58-69.
73. Malkina A. Rhabdomyolysis. Available at https://www.merckmanuals.com/professional/genitourinary-disorders/acute-kidney-injury/rhabdomyolysis. Last accessed August 26, 2022.
75. Eiff MP, Hatch R. Fracture Management for Primary Care. 3rd ed. Philadelphia, PA: Elsevier; 2017.
76. Brown TE, Cui Q, Mihalko WM, Saleh K. Arthritis and Arthroplasty: The Hip. 1st ed. Philadelphia, PA: Saunders; 2009.
77. Singh JA, Guyatt G, Ogdie A, et al. Special article: 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol. 2019;71(1):5-32.
78. Rothschild BM. What is the Prevalence of Gout in the US? Available at https://www.medscape.com/answers/329958-10236/what-is-the-prevalence-of-gout-in-the-us. Last accessed July 28, 2022.
80. National Institute of Arthritis and Musculoskeletal and Skin Diseases. Gout. Available at https://www.niams.nih.gov/health-topics/gout. Last accessed July 28, 2022.
81. Centers for Disease Control and Prevention. Arthritis: Gout. Available at https://www.cdc.gov/arthritis/basics/gout.html. Last accessed July 28, 2022.
83. Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64(10):1431-1446.
84. Martinon F, Glimcher LH. Gout: New insights into an old disease. J Clin Invest. 2006;116(8):2073-2075.
85. Al-Ashkar F. Gout and Calcium Pyrophosphate Deposition Disease. Available at http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/rheumatology/gout-and-pseudogout. Last accessed July 28, 2022.
86. Chohan S, Becker MA, MacDonald PA, Chefo S, Jackson RL. Women with gout: efficacy and safety of urate-lowering with febuxostat and allopurinol. Arthritis Care Res (Hoboken). 2012;64(2):256-261.
87. Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and anti-inflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res. 2012;64(10):1447-1461.
88. FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res. 2020;72(6):744-760.
89. LexiComp Online. Available at https://online.lexi.com. Last accessed July 28, 2022.
90. Nuki G. Colchicine: its mechanism of action and efficacy in crystal-induced inflammation. Curr Rheumatol Rep. 2008;10(3):218-227.
92. National Institute of Neurological Disorders and Stroke. Low Back Pain Fact Sheet. Available at https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Low-Back-Pain-Fact-Sheet. Last accessed September 28, 2022.
93. American Medical Association. Module 8 Pain Management: Assessing and Treating Persistent Nonmalignant Pain: Common Persistent Pain Conditions. Available at https://cme.ama-assn.org/Activity/1729078/Detail.aspx. Last accessed September 28, 2022.
94. Stevans JM, Saper RB. Chronic low back pain. In: Rakel D (ed). Integrative Medicine. 3rd ed. Philadelphia, PA: Elsevier Inc.; 2012.
95. Corwell BN. The emergency department evaluation, management, and treatment of back pain. Emerg Med Clin North Am. 2010;28(4):811-839.
96. Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147(7):478-491.
97. Zhou Y. Principles of pain management. In: Daroff RB (ed). Bradley's Neurology in Clinical Practice. 6th ed. Philadelphia, PA: Elsevier Inc.; 2012.
98. Mayo Clinic. Myofascial Pain Syndrome. Available at https://www.mayoclinic.org/diseases-conditions/myofascial-pain-syndrome/symptoms-causes/syc-20375444. Last accessed September 28, 2022.
99. Cedars-Sinai. Facet Joint Syndrome. Available at https://www.cedars-sinai.edu/Patients/Health-Conditions/Facet-Joint-Syndrome.aspx. Last accessed September 28, 2022.
100. National Institute of Neurological Disorders and Stroke. Low Back Pain Fact Sheet. Available at https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Low-Back-Pain-Fact-Sheet#top. Last accessed September 28, 2022.
101. Balagué F, Mannion AF, Pellisé F, Cedraschi C. Non-specific low back pain. Lancet. 2012;379(9814):482-491.
102. American College of Occupational and Environmental Medicine. Cervical and thoracic spine disorders. In: Hegmann KT (ed). Occupational Medicine Practice Guidelines: Evaluation and Management of Common Health Problems and Functional Recovery in Workers. Elk Grove Village, IL: American College of Occupational and Environmental Medicine; 2011: 1-332.
103. Manchikanti L, Singh V, Datta S, et al. Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Physician. 2009;12(4):E35-E70.
104. American Society of Anesthesiologists. Practice guidelines for chronic pain management. Anesthesiology. 2010;112(4):810-833.
105. Institute for Clinical Systems Improvement. Guidelines: Low Back Pain, Adult Acute and Subacute. Available at https://www.icsi.org/guideline/low-back-pain. Last accessed September 28, 2022.
106. Chou R, Loeser JD, Owens DK, et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine. 2009;34(10):1066-1077.
107. National Institute for Health and Clinical Excellence. Low Back Pain in Adults: Early Management. Available at https://www.nice.org.uk/guidance/cg88. Last accessed September 28, 2022.
108. Venable S. Springhouse Nurse's Drug Guide 2008. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007.
109. U.S. Preventive Services Task Force. Adolescent Idiopathic Scoliosis: Screening. Available at https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/adolescent-idiopathic-scoliosis-screening. Last accessed August 26, 2022.
111. Ratzlaff CR, Gillies JH, Koehoorn MW. Work-related repetitive strain injury and leisure-time physical activity. Arthritis Rheum. 2007;57(3):495-500.
112. American College of Rheumatology. Carpal Tunnel Syndrome. Available at https://www.rheumatology.org/I-Am-A/Patient-Caregiver/Diseases-Conditions/Carpal-Tunnel-Syndrome. Last accessed September 28, 2022.
113. American Academy of Family Physicians. Carpal Tunnel Syndrome: What is Carpal Tunnel Syndrome? Available at https://familydoctor.org/condition/carpal-tunnel-syndrome/. Last accessed September 28, 2022.
114. Mayo Clinic. Carpal Tunnel Syndrome. Available at https://www.mayoclinic.org/diseases-conditions/carpal-tunnel-syndrome/symptoms-causes/syc-20355603. Last accessed September 28, 2022.
115. Weber RA, Boyer KM. Consecutive versus simultaneous bilateral carpal tunnel release. Ann Plast Surg. 2005;54(1):15-19.
116. National Institute of Neurological Disorders and Stroke. Carpal Tunnel Syndrome Fact Sheet. Available at https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Carpal-Tunnel-Syndrome-Fact-Sheet. Last accessed September 28, 2022.
117. Mallette P, Zhao M, Zurakowski D, Ring D. Muscle atrophy at diagnosis of carpal and cubital tunnel syndrome. J Hand Surg Am. 2007;32(6):855-858.
118. Cartwright MS, Hobson-Webb LD, Boon AJ, et al. Evidence-based guideline: neuromuscular ultrasound for the diagnosis of carpal tunnel syndrome. Muscle Nerve. 2012;46(2):287-293.
119. Washington State Department of Labor and Industries. Work-Related Carpal Tunnel Syndrome Diagnosis and Treatment Guideline. Olympia, WA: Washington State Department of Labor and Industries; 2014.
120. Blumenthal S, Herskovitz S, Verghese J. Carpal tunnel syndrome in older adults. Muscle Nerve. 2006;34(1):78-83.
121. Baysal O, Altay Z, Ozcan C, Ertem K, Yologlu S, Kayhan A. Comparison of three conservative treatment protocols in carpal tunnel syndrome. Int J Clin Pract. 2006;60(7):820-828.
122. Verdugo RJ, Salinas RA, Castillo JL, Cea JG. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev. 2008;(4):CD001552.
123. Scholten RJPM, Mink van der Molen A, Uitdehaag BMJ, Bouter LM, de Vet HCW. Surgical treatment options for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(4):CD003905.
124. Marshall SC, Tardif G, Ashworth NL. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(2):CD001554.
125. Ettema AM, Amadio PC, Cha SS, Harrington JR, Harris AM, Offord KP. Surgery versus conservative therapy in carpal tunnel syndrome in people aged 70 years and older. Plast Reconstr Surg. 2006;118(4):947-958.
126. American Academy of Orthopaedic Surgeons. Clinical Practice Guideline on the Management of Carpal Tunnel Syndrome. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2016.
127. O'Connor D, Marshall SC, Massy-Westropp N. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst Rev. 2003;(1):CD003219.
128. American Academy of Orthopaedic Surgeons. Carpal Tunnel Syndrome. Available at https://orthoinfo.aaos.org/en/diseases--conditions/carpal-tunnel-syndrome. Last accessed September 28, 2022.
129. Taber's Online Medical Dictionary. Available at https://www.tabers.com/tabersonline. Last accessed September 28, 2022.
130. Herndon CM, Hutchison RW, Berdine HJ, et al. Management of chronic nonmalignant pain with nonsteroidal anti-inflammatory drugs. Pharmacotherapy. 2008;28(6):788-805.
131. Piazzini DB, Aprile I, Ferrara PE, et al. A systematic review of conservative treatment of carpal tunnel syndrome. Clin Rehabil. 2007;21(4):299-314.
132. Hegmann KT. Hand, wrist, and forearm disorders not including carpal tunnel syndrome. In: Hegmann KT (ed). Occupational Medicine Practice Guidelines: Evaluation and Management of Common Health Problems and Functional Recovery in Workers. 3rd ed. Elk Grove Village, IL: American College of Occupational and Environmental Medicine; 2011: 1-188.
133. Work Loss Data Institute. Carpal Tunnel Syndrome (Acute and Chronic). Encinitas, CA: Work Loss Data Institute; 2011.
134. Mount Sinai Medical Center. Carpal Tunnel Syndrome. Available athttps://www.mountsinai.org/health-library/report/carpal-tunnel-syndrome. Last accessed September 28, 2022.
135. Naeser MA, Hahn KA, Lieberman BE, Branco KF. Carpal tunnel syndrome pain treated with low-level laser and microamperes TENS: a controlled study. Arch Phys Med Rehabil. 2002;83(7):978-988.
136. American Society for Surgery of the Hand. Carpal Tunnel Syndrome. Available at https://www.assh.org/handcare/condition/carpal-tunnel-syndrome.. Last accessed September 28, 2022.
137. Centers for Disease Control and Prevention. Osteoarthritis. Available at https://www.cdc.gov/arthritis/basics/osteoarthritis.htm. Last accessed September 28, 2022.
138. Lawrence R, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35.
139. Sayre E, Li LC, Kopec JA, Esdaile JM, Bar S, Cibere J. The effect of disease site (knee, hip, hand, foot, lower back or neck) on employment reduction due to osteoarthritis. PLoS. 2010;5(5):e10470.
140. Kalichman L, Li L, Kim DH, et al. Facet joint osteoarthritis and low back pain in the community-based population. Spine (Phila Pa 1976). 2008;33(23):2560-2565.
141. Lozada CJ. Osteoarthritis. Available at https://emedicine.medscape.com/article/330487-overview. Last accessed September 9, 2019.
142. Millett P, Gobezie R, Boykin RE. Shoulder osteoarthritis: diagnosis and management. Am Fam Physician. 2008;78(5):605-611, 612.
143. Cheung EV, Adams R, Morrey BF. Primary osteoarthritis of the elbow: current treatment options. J Am Acad Orthop Surg. 2008;16(2):77-87.
144. Chou LB, Coughlin MT, Hansen S Jr, et al. Osteoarthritis of the ankle: the role of arthroplasty. J Am Acad Orthop Surg. 2008;16(5):249-259.
145. Brown T, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20(10):739-744.
146. Maffulli N, Longo UG, Gougoulias N, Caine D, Denaro V. Sports injuries: a review of outcomes. Br Med Bull. 2011;97:47-80.
147. National Health Service UK. Osteoarthritis. Available at https://www.nhs.uk/conditions/osteoarthritis. Last accessed September 28, 2022.
148. Altman R, Kuritzky L, Ruoff G. Improving long-term management of osteoarthritis: strategies for primary care physicians. J Fam Pract. 2009;58(2 Suppl):S17-S24.
149. Hinton R, Moody RL, Davis AW, Thomas SF. Osteoarthritis: diagnosis and therapeutic considerations. Am Fam Physician. 2002;65(5):841-848.
150. Cibere J. Do we need radiographs to diagnose osteoarthritis? Best Pract Res Clin. 2006;20:27-38.
152. Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskeletal Disord. 2008;9:116.
153. Kornaat PR, Bloem JL, Ceulemans RYT, et al. Osteoarthritis of the knee: association between clinical features and MR imaging findings. Radiology. 2006;239(3):811-817.
154. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465-474.
155. The New York City Department of Health and Mental Hygiene. Preventing, diagnosing, and managing osteoarthritis of the hip and knee. City Health Information. 2012;31(2):9-16.
156. Altman R, Alarcon G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum. 1990;33:1601-1610.
157. Johns Hopkins Arthritis Center. ACR Diagnostic Guidelines. Available at https://www.hopkinsarthritis.org/physician-corner/education/arthritis-education-diagnostic-guidelines. Last accessed September 28, 2022.
158. Zhang W, Doherty M, Leeb BF, et al. EULAR evidence-based recommendations for the management of hand osteoarthritis: report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT).Ann Rheum Dis. 2007;66(3):377-388.
159. Gramstad G, Galatz LM. Management of elbow osteoarthritis. J Bone Joint Surg Am. 2006;88:421-430.
160. American College of Rheumatology. Position Statement on Interdisciplinary Care for Patients with Rheumatic and Musculoskeletal Diseases, 2015. Available at https://www.rheumatology.org/Practice-Quality/Administrative-Support/Position-Statements. Last accessed September 28, 2022.
161. Langworthy M, Saad A, Langworthy NM. Conservative treatment modalities and outcomes for osteoarthritis: the concomitant pyramid of treatment. Phys Sports Med. 2010;38(2):133-145.
162. Jordan K, Arden NK, Doherty M, et al. EULAR recommendations 2003: an evidence-based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2003;62(12):1145-1155.
163. Agency for Healthcare Policy and Research. Managing Osteoarthritis: Helping the Elderly Maintain Function and Mobility. Research in Action, Issue 4. AHRQ Publication No. 02-0023. Rockville, MD: Agency for Health Care Policy and Research; 2002.
164. Stacey D, Hawker G, Dervin G, et al. Improving shared decision making in osteoarthritis. BMJ. 2008;336(7650):954-955.
165. Altman R. Early management of osteoarthritis. Am J Manag Care. 2010;16(Suppl Management):S41-S47.
166. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis. Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137-162.
167. Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635-646.
169. Singh K, Al-Sadawi M, Ortega RR, et al. Interstitial lung disease as the initial manifestation of rheumatoid arthritis: a case report and review of the literature. Am J Med Case Rep. 2019;7(12):342-347.
170. Alunno A, Gerli R, Giacomelli R, Carubbi F. Clinical, epidemiological, and histopathological features of respiratory involvement in rheumatoid arthritis. Biomed Res Int. 2017;7915340.
171. Kremers HM, Crowson CS, Therneau TM, et al. High ten-year risk of cardiovascular disease in newly diagnosed rheumatoid arthritis patients: a population-based cohort study. Arthritis Rheum. 2008;58(8):2268-2274.
172. Balbir-Gurman A, Yigla M, Nahir AM, Braun-Moscovici Y. Rheumatoid pleural effusion. Semin Arthritis Rheum. 2006;35(6):368-378.
173. Helmers R, Galvin J, Hunninghake GW. Pulmonary manifestations associated with rheumatoid arthritis. Chest. 1991;100(1):235-238.
174. Jurik AG, Davidsen D, Graudal H. Prevalence of pulmonary involvement in rheumatoid arthritis and its relationship to some characteristics of the patients: a radiological and clinical study. Scand J Rheumatol. 1982;11(4):217-224.
175. Young S. Ocular involvement in connective tissue disorders. Curr Allergy Asthma Rep. 2005;5(4):323-326.
176. Smith JR, Mackensen F, Rosenbaum JT. Therapy insight: scleritis and its relationship to systemic autoimmune disease. Nat Clin Pract Rheumatol. 2007;3(4):219-226.
177. Michels ML, Cobo LM, Caldwell DS, Rice JR, Haynes BF. Rheumatoid arthritis and sterile corneal ulceration: analysis of tissue immune effector cells and ocular epithelial antigens using monoclonal antibodies. Arthritis Rheum. 1984;27(6):606-614.
178. Schett G, Hayer S, Zwerina J, Redlich K, Smolen JS. Mechanisms of disease: the link between RANKL and arthritic bone disease. Nat Clin Pract Rheumatol. 2005;1(1):47-54.
179. Compston JE, Vedi S, Croucher PI, Garrahan NJ, O'Sullivan MM. Bone turnover in non-steroid treated rheumatoid arthritis.Ann Rheum Dis. 1994;53(3):163-166.
180. Roldan JF, Del Rincon I, Escalante A. Loss of cortical bone from the metacarpal diaphysis in patients with rheumatoid arthritis: independent effects of systemic inflammation and glucocorticoids. J Rheumatol. 2006;33(3):508-516.
181. British Columbia Medical Association. Rheumatoid Arthritis: Diagnosis, Management, and Monitoring. Available at https://www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/bc-guidelines/rheumatoid-arthritis. Last accessed September 28, 2022.
182. Myriad. Vectra. Available at https://myriadrbm.com/products-services/vectra-da/. Last accessed September 28, 2022.
183. Curtis JR, van der Helm-van Mil AH, Knevel R, et al. Validation of a novel multibiomarker test to assess rheumatoid arthritis disease activity. Arthritis Care Res (Hoboken). 2012;64(12):1794-1803.
184. Vectra. Available at https://vectrascore.com. Last accessed September 28, 2022.
185. McGonagle D, Tan AL. What magnetic resonance imaging has told us about the pathogenesis of rheumatoid arthritis: the first 50 years. Arthritis Res Ther. 2008;10(5):222.
186. O'Dell JR. Clinical features of rheumatoid arthritis. In: Firestein GS, Budd RC, Gabriel SE, McInnes IB, O'Dell JR (eds). Kelley's Textbook of Rheumatology. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2013: 1109-1136.
187. Akil M, Amos RS. ABC of rheumatology: rheumatoid arthritis. I: clinical features and diagnosis. BMJ. 1995;310(6979):587-590.
188. Venables PJW, Maini RN. Diagnosis and Differential Diagnosis of Rheumatoid Arthritis. Available at https://www.uptodate.com/contents/diagnosis-and-differential-diagnosis-of-rheumatoid-arthritis. Last accessed September 28, 2022.
189. Smolen JS, Landewé R, Bijilsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960-977.
190. Jurgens MS, Welsing PM, Jacobs JW. Overview and analysis of treat-to-target trials in rheumatoid arthritis reporting on remission. Clin Exp Rheumatol. 2012;30(4 Suppl 73):S56-63.
191. Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2016;68(1):1-26.
192. Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Comparison of treatment strategies in early rheumatoid arthritis: a randomized trial. Ann Intern Med. 2007;146(6):406-415.
193. Pincus T, O'Dell JR, Kremer JM. Combination therapy with multiple disease-modifying antirheumatic drugs in rheumatoid arthritis: a preventive strategy. Ann Intern Med. 1999;131(10):768-774.
195. Wells G, Li T, Maxwell L, MacLean R, Tugwell P. Determining the minimal clinically important differences in activity, fatigue, and sleep quality in patients with rheumatoid arthritis. J Rheumatol. 2007;34(2):280-289.
196. Jaffe HL. Metabolic, Degenerative, and Inflammatory Diseases of Bones and Joints. Philadelphia, PA: Lea & Febiger; 1972.
198. Malawer MM, Sugarbaker PH. Musculoskeletal Cancer Surgery: Treatment of Sarcomas and Allied Diseases. Boston, MA: Kluwer Academic Publishers; 2001.
199. Cancer Research UK. Survival for Soft Tissue Sarcomas. Available at https://www.cancerresearchuk.org/about-cancer/soft-tissue-sarcoma/survival. Last accessed September 1, 2022.
200. National Cancer Institute. Clear Cell Sarcoma. Available at https://www.cancer.gov/pediatric-adult-rare-tumor/rare-tumors/rare-soft-tissue-tumors/clear-cell-sarcoma. Last accessed September 1, 2022.
1. Jacobson JA, Roberts CC, Bencardino JT, et al. ACR Appropriateness Criteria: Chronic Extremity Joint Pain, Suspected Inflammatory Arthritis. Reston, VA: American College of Radiology; 2016. Available at https://acsearch.acr.org/docs/3097211/Narrative. Last accessed September 26, 2022.
2. FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020;72(6)744-760. Available at https://www.rheumatology.org/Portals/0/Files/Gout-Guideline-Final-2020.pdf. Last accessed September 26, 2022.
3. Thorson D, Campbell R, Massey M, et al. Adult Acute and Subacute Low Back Pain. Bloomington, MN: Institute for Clinical Systems Improvement; 2018. Available at https://www.icsi.org/guideline/low-back-pain. Last accessed September 26, 2022.
4. American Academy of Orthopaedic Surgeons. Management of Carpal Tunnel Syndrome: Evidence-Based Clinical Practice Guideline. Available at https://www.aaos.org/globalassets/quality-and-practice-resources/carpal-tunnel/cts_cpg_4-25-19.pdf. Last accessed September 26, 2022.
5. American Academy of Orthopaedic Surgeons. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on Treatment of Osteoarthritis of the Knee. 3rd ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2021. Available at https://www.aaos.org/globalassets/quality-and-practice-resources/osteoarthritis-of-the-knee/oak3cpg.pdf. Last accessed September 26, 2022.
Mention of commercial products does not indicate endorsement.