Pressure injuries are among the most common conditions encountered in patients who suffer prolonged hospitalization or require long-term institutional care. The prevalence varies widely by clinical setting, age, and geographical region. Although generally preventable, not all pressure injuries can be classified as preventable or potentially curable, due to impaired blood circulation, sensory loss, and immobility, causing some patients to become more vulnerable to them. Several dynamics give rise to the occurrence of pressure injuries, but compression leading to ischemia is the ultimate communal culprit. Furthermore, cognitive impairment of some patients has made some preventive measures extremely challenging to employ. This course will outline the etiology, pathogenesis, identification, prevention, and treatment of pressure injuries in any practice setting.
- INTRODUCTION
- EPIDEMIOLOGY
- PATHOGENESIS
- RISK FACTORS
- STAGING OF PRESSURE INJURIES
- PHASES OF WOUND HEALING: AN OVERVIEW
- DIAGNOSIS
- PREVENTION AND TREATMENT
- WOUND MONITORING
- COMPLICATIONS
- TREATMENT OF COMORBIDITIES
- ADVANCES IN PRESSURE INJURY CARE
- MEDICO-LEGAL ASPECTS OF INJURY CARE
- EDUCATING PATIENTS
- CONCLUSION
- Works Cited
- Evidence-Based Practice Recommendations Citations
This course is designed for physicians, primary care providers, and physician assistants involved in the care of patients at risk for pressure injury development.
The purpose of this course is to provide physicians, physician assistants, and nurse practitioners a current review of the pathogenesis, diagnosis, and treatment of pressure injuries, with an emphasis on clinical recognition and staging, risk factor assessment and prevention, and management strategies for collaborative care to improve patient outcomes.
Upon completion of this course, you should be able to:
- Discuss the epidemiology, etiology, and pathogenesis of pressure injuries.
- Identify patients at risk based on extrinsic and intrinsic factors important to pathogenesis.
- Recognize and define the severity and progression of pressure injuries by stage.
- Analyze techniques available for the diagnosis of pressure injuries.
- Develop an effective strategy for skin care and prevention of pressure injuries.
- Choose appropriate options for wound cleansing, debridement, and dressing based on wound types.
- Manage other aspects of the care of patients with pressure injuries, including pain management and infectious complications.
- Create individual treatment plans based on patient characteristics and pressure injury stage.
- Identify the qualities of a pressure injury that should be monitored.
- Outline possible complications and comorbidities of pressure injuries and their treatment.
- Describe the medico-legal aspects of pressure injuries and the significance of correct documentation and patient education.
John M. Leonard, MD, Professor of Medicine Emeritus, Vanderbilt University School of Medicine, completed his post-graduate clinical training at the Yale and Vanderbilt University Medical Centers before joining the Vanderbilt faculty in 1974. He is a clinician-educator and for many years served as director of residency training and student educational programs for the Vanderbilt University Department of Medicine. Over a career span of 40 years, Dr. Leonard conducted an active practice of general internal medicine and an inpatient consulting practice of infectious diseases.
Contributing faculty, John M. Leonard, MD, has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
John V. Jurica, MD, MPH
Ronald Runciman, MD
The division planners have disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Sarah Campbell
The Director of Development and Academic Affairs has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
The purpose of NetCE is to provide challenging curricula to assist healthcare professionals to raise their levels of expertise while fulfilling their continuing education requirements, thereby improving the quality of healthcare.
Our contributing faculty members have taken care to ensure that the information and recommendations are accurate and compatible with the standards generally accepted at the time of publication. The publisher disclaims any liability, loss or damage incurred as a consequence, directly or indirectly, of the use and application of any of the contents. Participants are cautioned about the potential risk of using limited knowledge when integrating new techniques into practice.
It is the policy of NetCE not to accept commercial support. Furthermore, commercial interests are prohibited from distributing or providing access to this activity to learners.
Supported browsers for Windows include Microsoft Internet Explorer 9.0 and up, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Supported browsers for Macintosh include Safari, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Other operating systems and browsers that include complete implementations of ECMAScript edition 3 and CSS 2.0 may work, but are not supported. Supported browsers must utilize the TLS encryption protocol v1.1 or v1.2 in order to connect to pages that require a secured HTTPS connection. TLS v1.0 is not supported.
The role of implicit biases on healthcare outcomes has become a concern, as there is some evidence that implicit biases contribute to health disparities, professionals' attitudes toward and interactions with patients, quality of care, diagnoses, and treatment decisions. This may produce differences in help-seeking, diagnoses, and ultimately treatments and interventions. Implicit biases may also unwittingly produce professional behaviors, attitudes, and interactions that reduce patients' trust and comfort with their provider, leading to earlier termination of visits and/or reduced adherence and follow-up. Disadvantaged groups are marginalized in the healthcare system and vulnerable on multiple levels; health professionals' implicit biases can further exacerbate these existing disadvantages.
Interventions or strategies designed to reduce implicit bias may be categorized as change-based or control-based. Change-based interventions focus on reducing or changing cognitive associations underlying implicit biases. These interventions might include challenging stereotypes. Conversely, control-based interventions involve reducing the effects of the implicit bias on the individual's behaviors. These strategies include increasing awareness of biased thoughts and responses. The two types of interventions are not mutually exclusive and may be used synergistically.
#48853: Pressure Injuries: Prevention and Management
A pressure injury is a localized skin erosion and subcutaneous crater, usually over a bony prominence, caused by the mechanical effect of unrelenting pressure [1,2]. The skin and soft tissues become vulnerable when extrinsic factors, such as prolonged pressure, shearing forces, friction, and moisture, coincide with intrinsic (host) factors, such as immobility, poor nutritional state, and incontinence. The most common areas where pressure injuries occur include the sacrum, coccyx or tailbone, hips, heels, and elbows. These injuries can range from superficial to deep and may even penetrate through deep muscle layers to bone. Without proper attention to risk factors, preventive measures, and early vigorous care and treatment, superficial areas of pressure injury to the skin can evolve into deep soft tissue necrosis and ulceration, from which life-threatening complications may ensue.
In 400 B.C.E., Hippocrates was the first physician to document a pressure injury, but it is assumed they have existed for all of human history. The problem is commonly associated with the frail elderly, those with neurologic injury or disease, and with prolonged hospitalization and nursing home care. In fact, anyone with limited mobility, confined to bed or chair, and unable to shift position with relative frequency is at risk.
Pressure injuries are among the most common conditions encountered in patients who suffer prolonged hospitalization or require long-term institutional care. The prevalence varies widely by clinical setting, age, and geographical region. Between the years 2000 to 2010, the reported prevalence of pressure injuries in the United States ranged from 0.4% to 38% in acute care settings, 2.2% to 23.9% in long-term care facilities, and 0.1% to 17% in the course of home care [3,4,5]. In general, the rate of newly acquired pressure injury in patients hospitalized for acute illness is estimated to be 3% to 11%. Approximately 8% to 40% of intensive care unit (ICU) patients develop pressure injuries [2]. The annual rate of pressure injuries in persons with neurologic impairment, such as spinal cord injury, stroke, or dementia, is estimated to be 5% to 8%, and the lifetime risk is 25% to 85%. Pressure injuries are listed as a primary cause of death in 7% to 8% of patients with spinal cord injury [6]. Unfortunately, in spite of modern advances in clinical care, the recurrence rate in patients with healed pressure injuries remains around 90% [6].
A large-scale nation-wide epidemiologic study of the incidence and clinical outcomes of hospital-acquired pressure injuries was conducted utilizing the Medicare Patient Safety Monitoring System (MPSMS) database [7]. The MPSMS is designed to monitor adverse events within the hospitalized Medicare population. The MPSMS pressure injury study analyzed 51,842 inpatient discharges across 50 states between 2006 and 2007. The prevalence rate of pressure injury on admission was 5.8%, and the incidence rate for hospital-acquired pressure injury was determined to be 4.5%. Of 2,999 patients who entered the hospital with a pressure injury, 16.7% developed at least one new injury at a different location during the inpatient stay. The majority of hospital-acquired pressure injuries were located on the coccyx or sacrum (41%), the hip and buttock region (23%), and the heels (23%). Underlying chronic disease, corticosteroid use, and obesity were identified as significant risk factors. In contrast to patients without pressure injuries, the development of hospital-acquired pressure injuries was significantly associated with longer length of stay (11.6 days vs. 4.9 days), higher in-hospital mortality (11.2% vs. 3.3%), and higher mortality within 30 days of discharge (15.3% vs. 4.4%) [7].
The incidence of pressure injuries acquired in the outpatient setting is derived primarily from clinical studies and surveys conducted by home health agencies. In one cohort study of 1,711 non-hospitalized patients older than 60 years of age who did not have a pressure injury at initiation of home care, 108 (6.3%) subsequently developed a stage 1 to 4 pressure injury during the period of active home care [8].
According to data compiled by the Agency for Healthcare Research and Quality, an estimated 2.5 million patients develop pressure injuries each year, and approximately 60,000 die as a direct result of this condition. The individual cost of care ranges from $20,000 to $150,000 per case; the total aggregate annual health care cost is estimated to be between $9.1 billion and $11.5 billion [9].
In susceptible individuals, the combination of immobility and extended periods of pressure or friction over bony prominences leads to reduction in capillary blood flow, tissue hypoxia, and ischemic tissue injury. This in turn evokes an inflammatory response that further impairs perfusion and augments soft tissue and skin injury. Current understanding favors a "bottom-up" model of tissue damage beginning deep in the muscle layer [10]. Muscle is more sensitive to pressure injury than skin because it is the more metabolically active layer and thus more susceptible to ischemic injury.
The age and health of overlying skin determine the ease with which ulceration of the superficial layers occurs. In the elderly, skin and subcutaneous tissue gradually lose regenerative, protective, and sensory functions. Chronic conditions or intercurrent illness such as diabetes, arthritis, incontinence, neurologic impairment, cigarette use, and hypotension are all associated with increased susceptibility and prevalence of pressure injuries [6]. As noted, pressure injuries develop most commonly over the sacrum and coccyx, hips and buttocks, and heels [7].
The development of pressure-induced skin injury and subsequent ulceration usually arises in the setting of failing health and loss of independent mobility. In a community-based study of 12,650 patients older than 60 years of age enrolled in a primary care panel, 366 (2.9%) subsequently developed a pressure injury during the 40-month surveillance period [11]. The two most prominent risk factors were prior history of pressure injury and placement in a long-term care facility, followed by comorbid conditions such as diabetes, falls, cataracts, renal insufficiency, and cardiovascular disease. In a national study of 1,524 adults residing in 95 long-term care facilities, 443 (29%) developed a new pressure injury during the 12-week observational period; factors associated with increased risk included higher initial severity of illness, history of previous pressure injury, weight loss, feeding difficulty, use of catheters, and use of positioning devices [12].
For a given patient, immobility that leads to unrelieved pressure to the skin over a boney prominence is the most important factor in the development of pressure injuries [5,10]. Individual risk factors for pressure injuries may be categorized as extrinsic or intrinsic. Extrinsic factors are external conditions in the immediate environment that place a vulnerable individual at risk (e.g., moisture, compression from an applied device). Intrinsic factors are conditions and comorbidities peculiar to the individual that confer risk (e.g., advanced age, poor nutrition, smoking history).
Pressure that results in injury and subsequent skin breakdown is defined as compression of soft tissues between two rigid surfaces. For example, skin, muscle, subcutaneous fat, and vasculature may be compressed between an underlying boney prominence and a rigid external surface, such as a bed or chair. All the tissues between the two points of pressure are affected, but the tissue closest to the bony prominence suffers the greatest damage. It is important to note that low-intensity pressure over a long period of time can create tissue damage, just as high-intensity pressure over a short period of time can result in damage [10].
The capillary level is the end point of circulation. From the capillaries, oxygen and nutrients diffuse into the tissues, and carbon dioxide and waste products are removed. A collapsed capillary bed is nonfunctioning and useless to the tissues. The minimal amount of pressure required to collapse a capillary is referred to as the capillary closing pressure [13]. Studies have shown that an average of 32 mm Hg will collapse the arterial side of the capillary circulation, and 18 mm Hg of pressure will collapse the venous end. However, these values cannot be accepted as universal; capillary pressures vary among persons, sites, and times [10]. Furthermore, the studies that elicited these values were done on healthy adult males, not debilitated or elderly patients. Other studies have shown that the functional capillary pressure in the peripheral tissues is around 17 mm Hg [14]. Extended pressure resulting in capillary collapse will cause tissue damage.
Shear is the result of gravity pushing down on the body and resistance (friction) between the patient and a surface, such as the bed or the chair, holding the skin in place [10]. For example, when the head of the bed is raised (e.g., high Fowler's position), gravity facilitates forward slide, pulling the body down toward the foot of the bed. The skin on the patient's lower back and gluteal area resists the motion and is held in place by the bed's surface while the bones and tissues beneath the area begin to slide. This causes puckering of the skin, stretching and angulation of small vessels, impedance of blood flow, and traction on subcutaneous tissue and muscle. Left unchecked, the net effect may result in ischemic injury to tissues at the fascia layer. When the head of the bed is elevated more than 30 degrees, shear force occurs over the sacrum and coccyx. Shear injury is not usually visible at the skin level, but shear is responsible for much of the damage associated with initiation of pressure injuries [15]. The areas of the body most vulnerable to shearing forces are shoulder blades, elbows, sacrum, ischial tuberosities, and heels. Signs of shear injury include irregular deep lesions, undermining, and tunneling.
As noted, friction occurs when one surface moves across another surface, such as when a patient's skin slides across a bed sheet. This can result in the "sanding away" of the epidermal layer and upper part of the dermis, causing abrasions [10]. Friction injuries often present as erythema and tenderness followed by skin loss and usually appear under restraints, braces, and on the elbows, or with repetitive rubbing or repetitive cleansing. Patients with uncontrollable movements or spasticity are also at high risk for friction injury, often referred to as "sheet burn." Friction injury occurs more frequently when the skin is fragile or macerated, and tissues subjected to friction are more susceptible to pressure injury damage [15].
Moisture weakens the resilience of the epidermis to external forces. Maceration causes softening of the connective tissue, and a macerated epidermis erodes more easily, as overhydrated skin has decreased tensile strength. Skin can appear "water-logged," with areas of denuded skin and fissure formation. Shear and friction damage is increased when there is a moderate amount of moisture present, but it has been reported that shear and friction decrease in the presence of high levels of moisture. The role moisture plays in pressure injury development is an area of ongoing research [16,17,18].
Major sources of moisture are incontinence, wound drainage, tube leakage, and sweating. Urinary and fecal incontinence expose the skin to excessive amounts of moisture and chemical irritation. There is a higher risk for skin breakdown and infection with fecal incontinence than urinary incontinence because of the pathogens in stool.
Patients older than 65 years of age experience pressure injuries most frequently [13]. With aging, the skin becomes more fragile; the skin layers adhere less securely to each other and can appear paper thin and almost transparent. There is also evidence of increased dryness, decreased vascularization, and increased vascular fragility.
In elderly individuals, there is a decrease in surface barrier function. The ability of the soft tissue to evenly distribute the mechanical load without compromising blood flow is impaired. There is less subcutaneous tissue to cushion boney prominences. This, in addition to decreased sensory perception, makes elderly skin more vulnerable to pressure, shear, and friction [10]. Research has shown that, in the geriatric population, blood flow in the area of the ischial tuberosity while sitting on an unpadded surface is lower than in younger adults [15].
Although much less common, children can also develop pressure injuries. These injuries usually develop in the occipital region in infants and toddlers and on the sacrum in young children [13].
Immobile individuals carry the greatest risk of developing pressure injuries. While sleeping, a healthy individual changes body position approximately once every 10 to 12 minutes. This constant change of position maintains healthy blood circulation, stimulates body organs, and ensures movement of body fluids. However, when an individual is immobile, decreased vascularization and prolonged pressure pair with possible loss of sensation to make the patient extremely vulnerable to pressure injuries. Immobility may be the result of multiple traumas or injuries, spinal cord injuries, stroke, prolonged hospitalization, coma, recovery after surgery, or cognitive deficits [15].
Patients with spinal cord injury, neurologic disease, or even advanced diabetes carry an increased risk of developing pressure injuries as a result of loss of protective sensation. Patients with sensory loss may not feel discomfort or the need to be repositioned.
Poor nutrition, intravascular volume depletion, and peripheral vascular disease can each lead to unhealthy skin and impaired wound healing, which in turn increases the risk of developing pressure injuries. Low body weight is also a concern. Weight less than 119 pounds or a body mass index (BMI) less than 20 indicates increased risk for pressure injury development [19].
Recent weight loss, decreased nutritional intake, inadequate dietary protein, and impaired ability to feed oneself have been identified as risk factors for pressure injury development. An estimated 50% of elderly patients admitted to hospitals have suboptimal protein nutrition [19]. When there is a sustained deficit of protein as an energy source, skin and soft tissues become more vulnerable to injury. In managing patients with pressure injury, or those at risk, the amount of protein in the diet appears to influence prognosis for recovery and prevention. In one study, patients who received a 24% increase in protein intake had significant improvements in pressure injury healing and prevention of new skin injury compared to those who received a 14% increase [20]. The potential role of nutritional supplementation on pressure injury management and prevention is an area of ongoing research [21,22].
Vitamin A, C, and E deficiencies have been associated with pressure injury formation. Vitamin A works in the body to maintain epithelial integrity and is involved in collagen synthesis. It also plays a role in protection against infection. A deficiency of vitamin A can inhibit collagen synthesis, delay re-epithelialization, and decrease cellular cohesion. Vitamin C is also involved in collagen synthesis, immune function, and wound repair. A deficiency of vitamin C can result in capillary fragility. Vitamin E deficiency often decreases the immune function of the skin.
Both urinary incontinence and bowel incontinence can result in excessive moisture on the skin, which decreases the tensile strength and increases skin breakdown. In addition, infection may occur more frequently.
Maintenance of tissue health requires an adequate perfusion pressure in the systemic circulation for delivery of oxygen and nutrients to the cells and removal of waste products. Healthy tissue (in persons with normal sensation and movement) is able to tolerate brief, intermittent periods of inadequate perfusion; however, sustained ischemia leads to tissue damage and, with regard to skin, potentiates the development of pressure injuries.
Low arterial blood pressure (hypotension), defined as systolic blood pressure less than 100 mm Hg and diastolic pressure less than 60 mm Hg, has been linked to increased risk for pressure injury development. In response to hypotension, the body redirects blood flow to the vital internal organs at the expense of the peripheral vascular system, which serves the skin. As the perfusion level drops, so does the skin's ability to tolerate external pressure. Capillaries subsequently close at lower levels of interface pressure, and there is an increased risk of damage due to ischemia [13].
Nicotine impedes blood flow to the tissues in two ways: it is a potent vasoconstrictor, and it increases the adhesiveness of platelets, resulting in clot formation. Carbon monoxide contained in cigarette smoke prevents oxygen from attaching to the hemoglobin molecule. This significantly reduces the amount of oxygen circulating in the blood stream. The same reaction occurs to some extent in people exposed to secondhand smoke. Studies have shown that cigarette smoking is associated with a higher incidence of pressure injury development, and patients who smoke also have a higher rate of recurrence of pressure injuries [15,23].
Stress is a primitive response to injury or anticipated injury. Research has shown that during periods of stress, blood vessels in the peripheral tissues constrict. In a study designed to mimic the body's response to stress, healthy subjects were given an infusion of exogenous epinephrine [13]. The increased levels of epinephrine decreased the levels of subcutaneous tissue oxygen by 45%. Other studies have shown that psychologic stress has a negative impact on healing [13].
In the United States in 2017–2018, the age-adjusted prevalence of obesity among adults was 42.4% [24]. Obesity is defined as a BMI of 30 or greater; morbid ("severe") obesity is defined as a BMI greater than 40 [25]. Factors that contribute to pressure injury development in obese individuals include decreased blood supply in adipose tissue, difficulty in turning and repositioning, moisture within skin folds, incontinence, skin-to-skin friction, immobility, and poor nutrition. Obese patients are particularly at risk for "unusual" pressure injuries resulting from pressure within skin folds. Obese patients may have large panniculi ("aprons") weighing up to 50 pounds, and the abdominal panniculus must be regularly repositioned in order to prevent pressure injury. This can be accomplished by placing the patient on his or her side and lifting the panniculus away from the underlying skin surface in order to simultaneously relieve pressure and allow air to reach the area.
People with severe mental health conditions, such as uncontrolled schizophrenia or severe depression, have an increased risk of pressure injuries. This is thought to be related to these patients having little interest in self-care and nutrition. In addition, patients may have comorbid health conditions, such as diabetes or incontinence, that compound the risk of pressure injury development.
Chronic disease often confers multiple risk factors for developing a pressure injury and for prolonging injury healing as well. Conditions that lead to low tissue perfusion, reduced sensation, poor cognition, and altered posture predispose a patient to the development and/or recurrence of injuries. Common medical conditions associated with an increased risk for pressure injury include:
Diabetes
End-stage renal disease (ESRD)
Thyroid disease
Congestive heart failure
Peripheral vascular disease
Collagen vascular disorders and vasculitis
Immune deficiency states
Malignancies
Chronic obstructive pulmonary disease
Depression and psychosis
Drugs that delay healing
Joint contractures
Tubes or catheters can also cause pressure by burrowing into skin folds. Poorly fitting bed, chairs, or wheelchairs may also be a source of friction, shear, and sustained pressure [26].
No step is more important in preventing pressure injuries than understanding a patient's risk. Risk assessment is used to identify:
Populations at risk
Level of risk
Type of risk
An informal risk assessment cannot take the place of a formal risk assessment, such as the one conducted using the Braden Scale. Research shows that without formal risk assessment, clinicians tend to intervene consistently only at the highest levels of risk [27]. In some studies, repositioning or turning, an important part of pressure injury prevention, was prescribed for fewer than 50% of the patients at mild-to-moderate risk for pressure injury development [15]. Although several scales/tools have been developed to assess pressure injury risk, the Braden Scale is probably the most widely used.
The Braden Scale was developed in 1987 by Barbara Braden and Nancy Bergstrom [27]. Since then, it has undergone testing in several clinical settings, and its validity has been established by expert opinion. It is considered one of the most reliable tools for identifying patients at risk for pressure injury development. The Braden Scale scores factors that contribute to prolonged pressure and factors that result in diminished tissue tolerance for pressure (Table 1) [27]. There are six items scored in the assessment [27]:
Sensory perception
Moisture
Activity
Mobility
Nutrition
Friction and shear
THE BRADEN SCALE FOR PREDICTING PRESSURE ULCER RISK
| Domain | Scorea | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Sensory perception: The ability to respond meaningfully to pressure-related discomfort | Completely limited: Unresponsive (does not moan, flinch, or grasp) to painful stimuli due to diminished level of consciousness or sedation OR limited ability to feel pain over most of body surface. | Very limited: Responds only to painful stimuli and cannot communicate discomfort except by moaning or restlessness OR has a sensory impairment that limits the ability to feel pain or discomfort over half of body. | Slightly limited: Responds to verbal commands, but cannot always communicate discomfort or need to be turned OR has some sensory impairment that limits ability to feel pain or discomfort in one or two extremities. | No impairment: Responds to verbal commands and has no sensory deficit that would limit ability to feel or voice pain or discomfort. |
| Moisture: Degree to which skin is exposed to moisture | Constantly moist: Skin is kept moist almost constantly by perspiration, urine, etc. Dampness is detected every time patient is moved or turned. | Very moist: Skin is often, but not always, moist. Linen must be changed at least once a shift. | Occasionally moist: Skin is occasionally moist, requiring an extra linen change approximately once a day. | Rarely moist: Skin is usually dry. Linen only requires changing at routine intervals. |
| Activity: Degree of physical activity | Bedfast: Confined to bed | Chairfast: Ability to walk severely limited or non-existent. Cannot bear own weight and/or must be assisted into chair or wheelchair. | Walks occasionally: Walks occasionally during day, but for very short distances, with or without assistance. Spends majority of each shift in bed or chair. | Walks frequently: Walks outside the room at least twice a day and inside the room every two hours during waking hours. |
| Mobility: Ability to change and control body position | Completely immobile: Does not make even slight changes in body or extremity position without assistance. | Very limited: Makes occasional slight changes in body or extremity position but unable to make frequent or significant changes independently. | Slightly limited: Makes frequent though slight changes in body or extremity position independently. | No limitations: Makes major and frequent changes in position without assistance. |
| Nutrition: Usual food intake pattern | Very poor: Never eats a complete meal. Rarely eats more than one-third of any food offered. Eats two servings or less of protein per day. Takes fluids poorly. Does not take a liquid dietary supplement. OR is nothing by mouth and/or maintained on clear liquids or intravenous for more than five days. | Probably inadequate: Rarely eats a complete meal and generally eats only about half of any food offered. Protein intake includes only three servings of meat or dairy products per day. Occasionally will take a dietary supplement. OR receives less than optimum amount of liquid diet or tube feeding. | Adequate: Eats more than half of most meals. Eats a total of four servings of protein each day. Occasionally will refuse a meal but will usually take a supplement if offered. OR is on a tube feeding or total parental nutrition regimen that probably meets most of nutritional needs. | Excellent: Eats most of every meal. Never refuses a meal. Usually eats a total of four or more servings of protein. Occasionally eats between meals. Does not require supplementation. |
| Friction and shear | Problem: Requires moderate-to-maximum assistance in moving. | Potential problem: Moves feebly or requires minimum assistance. During a move, skin probably slides to some extent against sheets, chair restraints, or other devices. Maintains relatively good position in chair or bed most of the time, but occasionally slides down. | No apparent problem: Moves in bed and in chair independently and has sufficient muscle strength to lift up completely during move. Maintains good position in bed or chair at all times. | — |
| aA lower Braden Scale Score indicates a lower level of functioning and, therefore, a higher level of risk for pressure ulcer development. Risk levels assigned to each score range: ≤9 is very high risk, 10–12 is high risk, 13–14 is moderate risk, and 15–18 is mild risk. Scores of 19 or greater are considered very low or no risk. | ||||
Each item is scored on a scale between 1 and 4, with the exception of friction and shear, which is scored between 1 and 3. The lower the score, the more severe the impairment or problem in that area. Therefore, the lower the overall score, the higher the patient's risk for pressure injury development. Various studies have shown cut-off scores from 15 to 18 as being at risk [10]. Although cut-off scores vary, usually a score of 13–14 is considered moderate risk, 10–12 indicates high risk, and 9 or less is very high risk.
The Braden Scale should be used for assessment on admission to a care facility or after return from a hospital. Research shows that a repeat assessment done 48 hours to 72 hours after admission further defines pressure injury risk. In nursing home populations, the majority of pressure injuries develop during the first two weeks following admission [13]. Most facilities set their own policies regarding reassessment frequency (e.g., quarterly). However, it is important to note that any change in a patient's condition warrants reassessment.
Braden Scale assessment is completed by licensed personnel familiar with the patient and is shared with all staff caring for the patient; good communication is essential to ensure a meaningful assessment [15]. Licensed and unlicensed staff must have a basic knowledge of Braden scores and how it directs patient care. Accuracy of scoring is very important to determining the appropriate intervention.
The Norton Scale was developed in the 1960s and also is used to assess the risk for pressure injury in adults (Table 2). The five items in the assessment are scored from 1 to 4, with 1 indicating a low level of functioning and 4 indicating the highest level of functioning. A score of 14 or less generally indicates the patient is at risk [28]. The Norton Scale should be used in conjunction with the clinical assessment [28].
The National Pressure Ulcer Advisory Panel (NPUAP), in conjunction with a consensus conference format involving 400 health professionals, redefined the definition of pressure injuries in 2016 and provided an illustrated staging scheme that classifies pressure injuries by the depth and extent of tissue injury into six stages [29,30]. The NPUAP announced that it was changing its preferred terminology from pressure ulcer to pressure injury on the grounds that the latter term better described this injury process in both intact and ulcerated skin [31]. The term "pressure injury" will be used throughout this course as appropriate.
In November 2019, the NPUAP changed its name to the National Pressure Injury Advisory Panel (NPIAP) [31]. The NPIAP defines pressure injury as [30]:
... localized damage to the skin and underlying soft tissue usually over a bony prominence or related to a medical or other device. The injury can present as intact skin or an open ulcer and may be painful. The injury occurs as a result of intense and/or prolonged pressure or pressure in combination with shear. The tolerance of soft tissue for pressure and shear may also be affected by microclimate, nutrition, perfusion, comorbidities, and condition of the soft tissue.
In addition to redefining the definition of pressure injuries, the NPUAP added two additional pressure injury types [30]:
Medical device-related pressure injury
Mucosal membrane pressure injury
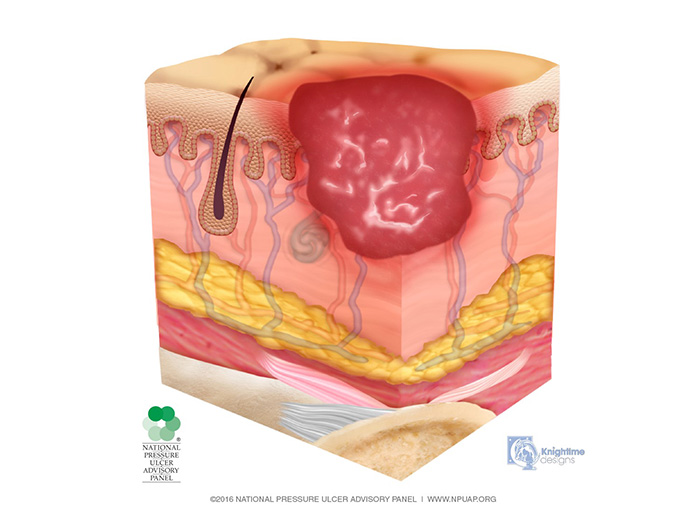
Deep tissue injury is described as a purple or maroon localized area of discolored, intact or non-intact skin or a blood-filled blister caused by damage of underlying soft tissue from pressure and/or shear (Image 1). The area may be preceded by tissue that is painful, firm, mushy, boggy, warmer, or cooler as compared to adjacent tissue.
Deep tissue injury may be difficult to detect in individuals with dark skin tones. The injury may also present as a thin blister over a dark wound bed. The wound may further evolve and become covered by thin eschar. Evolution may be rapid, exposing additional layers of tissue even with optimal treatment. If necrotic tissue, subcutaneous tissue, granulation tissue, fascia, muscle, or other underlying structures are visible, this indicates a full thickness pressure injury.
Stage 1 is characterized by intact skin with nonblanchable redness of a localized area, usually over a bony prominence (Image 2). Darkly pigmented skin may not have visible blanching, making detection difficult, but its color may differ from the surrounding area. The area may be painful, firm, soft, warmer, or cooler as compared to adjacent tissue.
Stage 1 lesions may indicate "at risk" persons (a heralding sign of risk). No tissue destruction occurs, and it is a reversible condition.
Stage 2 injuries present with partial-thickness skin loss into the dermis, presenting as a shallow, open injury with a red-pink wound bed, without slough (Image 3). This stage of injury may also present as an intact or open/ruptured, serum-filled or serosanguineous-filled blister.
Stage 2 injuries present as shiny or dry, shallow injuries without slough or bruising; bruising indicates suspected deep tissue injury. This stage should not be used to describe skin tears, tape burns, perineal dermatitis, maceration, or excoriation. Patients with stage 2 injuries now have an entry point for pathogens; therefore, cleaning the wound and providing some type of dressing is of utmost importance.
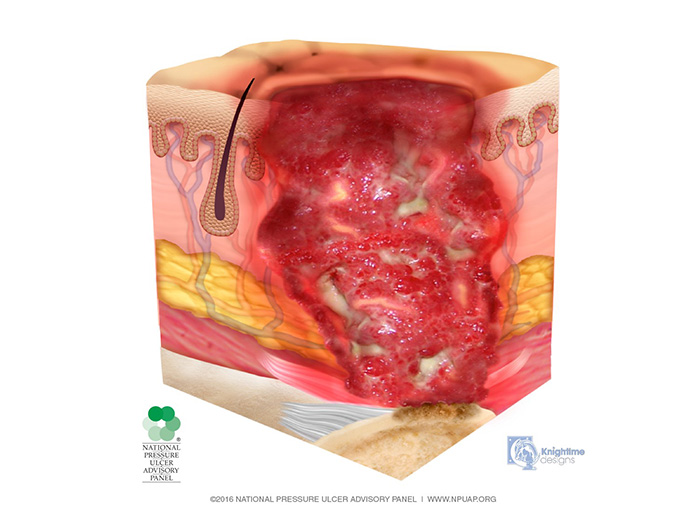
In the stage 3 injury, there is penetration into subcutaneous tissue but not through fascia; fat tissue may be visible, but bone, tendon, and muscle are neither exposed nor directly palpable (Image 4). Slough and/or eschar may be present but does not obscure the depth of tissue loss. These wounds may include undermining and tunneling. The depth of a stage 3 injury varies by anatomical location. The bridge of the nose, ear, occiput, and malleolus do not have subcutaneous tissue, and stage 3 injuries in these areas can be shallow. In contrast, areas of significant adiposity can develop extremely deep stage 3 injuries. Because infection is a very strong risk at this stage, irrigation of the wound is done each time the dressing is changed. Wound debridement may be necessary for healing.
In stage 4 pressure injuries, slough or eschar may be present on some parts of the wound bed (Image 5). Undermining and tunneling are often present. The depth of a stage 4 injury varies by anatomical location. As with stage 3 injuries, wounds on the bridge of the nose, ear, occiput, and malleolus may be shallow. Stage 4 injuries can extend into muscle and/or supporting structures (e.g., fascia, tendon, or joint capsule), making osteomyelitis possible. Exposed bone/tendon is visible or directly palpable. At this stage, the pressure injury is often infected, with deep ulceration and tissue loss; therefore, the patient may need repair with myocutaneous flaps to close the defect. If slough or eschar obscures the extent of tissue loss, this is an unstageable pressure injury.
Unstageable pressure injuries are defined as full-thickness skin and tissue loss in which the base of the injury is covered by slough (yellow, tan, gray, green, or brown) and/or eschar (tan, brown, or black), obscuring the wound bed (Image 6). Until enough slough and/or eschar is removed to expose the base of the wound, the true depth, and therefore stage, cannot be determined.
Medical device-related pressure injuries describe injuries or ulcers that result from the use of devices designed and applied for diagnostic or therapeutic purposes. The injury is generally in the shape or pattern of the device and should be staged using the staging system.
There are three phases of wound healing: inflammation, proliferation, and maturation. Wounds heal by two possible mechanisms: regeneration or scar tissue formation. The depth of the wound (i.e., the number of tissue layers involved) will determine the mechanism by which the wound will heal. Partial-thickness wounds and stage 1 and 2 injuries usually heal by tissue regeneration. Stage 3 and 4 injuries and full-thickness wounds heal by scar formation and contraction. In one study, 20% shrinkage in wound size in a period of two weeks was a reliable predictive indicator of healing [32].
Pressure injuries are notoriously difficult to heal. Only 75% of stage 2 injuries and 17% of stage 3 or 4 injuries heal in eight weeks [33]. Up to 23% of stage 2 pressure injuries and 48% of stage 4 injuries remain unhealed after one year [34].
The standard signs and symptoms of inflammation are erythema, swelling, increased temperature, and pain. In normal healing, these signs are only minimally noticeable, and during the inflammatory phase of wound healing, they are considered a normal response [35]. In general, this phase occurs in the first 0 to 3 days after injury development but may last longer if healing is impaired.
The first part of inflammation is hemostasis, which begins soon after the wound develops. During the inflammatory phase, vasoconstriction results in platelets adhering to damaged endothelium, causing clumping of the thrombocytes and stopping bleeding. Polymorphonuclear leukocytes engorge the wound and clear the debris. Macrophages continue the cleansing process and stimulate growth factors, including cytokines, interleukin-1 (IL-1), tumor growth factor, tumor necrosis factor, and platelet-derived growth factor.
The proliferation phase lasts approximately 3 to 12 days. During this phase, angiogenesis results in a new network of blood vessels in the wound. Production of epithelial cells starts. Collagen synthesis and improved vascularity ensure healthy granulation tissue. Wounds in the proliferation phase are usually pink in color and do not bleed easily.
In full-thickness wounds, the process of re-epithelialization occurs only from the wound edges [10]. Margin basal cells attached to the dermis eventually loosen and start migrating across the wound. The horizontal movement comes to a halt when the cells meet, which is referred to as contact inhibition. Wound contraction is the final part of the proliferative phase. Fibers in the wound contract to bring the wound edges closer together.
Maturation and remodeling of the wound involves rearranging collagen fibers from type III to type I, and increasing the tensile strength of scar tissue. The number of blood vessels in the wound regresses and cellular activity is reduced. Scar tissue regains about 80% of normal tissue strength within three months, but it never achieves the full strength of the original tissue [10]. Therefore, the healed site of an old wound is vulnerable to further breakdown.
Diagnosis of pressure injuries involves careful assessment of symptoms, medical history, physical examination, and certain medical tests. Usually, individuals will have a history of decreased mobility. Patients may complain about the appearance of a wound that may or may not be painful. In cases of infected wounds, fever may be present.
Careful examination in patients with pressure injuries reveals skin ulceration surrounded by erythema. The size and depth of the injury should be determined and documented at this point. Bleeding, malodor, and fluids or debris in the wound indicate severe infection.
Blood tests may be ordered to assess nutritional status and overall health status. No laboratory study of nutritional status can absolutely predict pressure injuries; however, monitoring a patient's protein status is of value. There are many serologic markers used to assess a patient's nutritional status; prealbumin level is one of the most sensitive. Prealbumin is a protein with a much shorter half-life than the other serologic markers; therefore, its level gives a more accurate picture of current conditions.
If infection is suspected, culture of the pressure injury is important to determine the pathogen. In some cases, a wound biopsy is performed to rule out vasculitis and skin cancers. An x-ray is done if bone infection is suspected and to rule out osteomyelitis. A bone scan is carried out when x-ray findings are equivocal.
The principles and management strategies discussed in this section are applicable to prevention in high-risk patients, control of early stage injury, and treatment of established pressure injuries. The NPIAP, in conjunction with two international agencies, published evidence-based guidelines for risk assessment, prevention, and management of pressure injuries in 2014 and released an update to this guideline in 2019 [29,36]. This guideline is available online at https://npiap.com/page/2019Guideline.
The primary objectives for prevention and arrest of progression are:
Preventive skin care
Pressure reduction, minimizing or eliminating friction and shear forces
Adequate nutrition
Exudate management
Prevention of wound infection
Managing moist wound environments
Decreasing the frequency of dressing changes
The general treatment of established injuries involves:
Pressure-relieving strategies
Optimal nutritional support, including protein and micronutrient supplementation
Intense injury care
Pain management
Prevention/eradication of infection
Adjunctive treatment or surgery
The actual treatment plan is individualized based on the injury stage, patient health, and short- and long-term goals.
The principles of preventive skin care include [29,36]:
Avoid positioning the patient over an area of erythema.
Keep the skin clean and dry.
Do not massage or vigorously rub skin that is at risk for pressure injury.
Develop an individualized continence plan.
Protect the skin from exposure to excessive moisture and friction.
For the at-risk patient, a strategy to avoid or relieve pressure is essential for the prevention and treatment of pressure injuries [37]. This is best accomplished by a three-pronged approach of:
Careful patient positioning
Use of protective devices
Judicious use of support surfaces
Bedbound Patients
Bedbound patients should be properly positioned and frequently repositioned, at least every two hours. When in lateral decubitus position, patients' heads should be maintained at an angle of 30 degrees in order to mitigate pressure in the trochanteric region. Pillows or foam wedges should be placed between the legs, at the knees and ankles, to prevent pressure at these sites when patients have little or no ability to move legs and feet.
To the extent the patient is able, regular physical activity should be encouraged and assisted. Even a few steps done frequently will help maintain current activity level, mobility, and range of motion. Lifting devices, such as an overhead trapeze or bed linen, are helpful when moving patients. It is important to avoid or minimize dragging the patient during transfers and position changes. Patients require protection from environmental factors leading to skin drying, such as low humidity (less than 40%) and exposure to cold [15]. Posting an individualized turning schedule in patient rooms can be helpful to healthcare professionals and families.
The heels are especially vulnerable, and pressure injuries at this location are very painful, difficult to heal, and prone to infection with easy access to adjacent bone. Heel pressure injuries can develop infection, and advanced cases may lead to amputation of the foot. To protect the heels, place a pillow under the calf to float the heels off the bed. There are also devices available that eliminate pressure on heels and prevent foot drop (e.g., suspension boots). Current guidelines state that heels are to be kept off the bed [10,36].
Chairbound Patients
Chairbound patients require special attention to positioning as well. The risk of pressure injuries from prolonged sitting is greater than that from reclining in bed, as sitting puts the patient's weight on the relatively small surface areas of the buttocks, thighs, and soles of the feet. Much of this weight is centered over the small area of tissue covering the ischial tuberosities. It is important for patients who sit in a chair to regularly change position. A dependent patient must have his/her position changed in a chair at least every hour. Patients who are able to move themselves should shift their weight (even slightly) every 15 minutes.
A patient should be properly positioned in a chair for postural alignment, distribution of weight, balance, and stability. Patients should sit with their back erect and against the back of the chair, thighs parallel to the floor, knees comfortably parted, and arms horizontal and supported by the arms of the chair. This position distributes weight evenly over the available body surface area. Slouching can cause shearing and friction and places undue pressure on the sacrum and coccyx. Feet should be kept flat on the floor to protect the heels from pressure and distribute the weight of the legs over the largest available surface area. The thighs and arms should remain parallel to ensure that weight is evenly distributed instead of being focused on the ischial tuberosities and elbows. Parting the knees will prevent the knees and ankles from rubbing together. If a patient uses a footstool, it is vital that his or her knees are not above hip level, because this shifts the weight from the back of the thighs to the ischial tuberosities. This same problem can occur if the chair is too short for the patient.
Protective padding and pillows should be utilized for pressure reduction whenever possible. Heel protectors, foam, and pillows can be helpful for patients in supine positions. Cutting a window through the cast can greatly reduce pressure at certain sites in patients immobilized by fractures. Patients should be provided soft seat cushions when sitting in a chair. Sheepskin and donut-shaped devices should not be used for treating pressure injuries; ring cushions can reduce blood flow to an even wider area of tissue.
Support surfaces are indicated when patients are not able to reposition themselves or when periodic repositioning care is not available. In a comprehensive literature review researchers founds good evidence that specially designed support surfaces effectively prevent pressure injuries [38].
An ideal support surface will manage microclimate, tissue loads, and other curative functions. Seat cushions, overlays, mattresses, and integrated bed systems are commonly used to prevent pressure injuries. The type of device or surface selected is based on level of risk as well as degree of assistance necessary for repositioning or mobility (Table 3).
THERAPEUTIC SUPPORT SURFACE SELECTION TOOL
| Validated Risk Assessment Category or Pressure Ulcer Description | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
At risk OR Redness present that fades quickly when pressure is removed |
Moderate risk OR One pressure ulcer (excluding the heels) where the patient can be positioned off the ulcer |
High risk OR One pressure ulcer (excluding the heels) and redness over another area |
Very high risk OR Multiple pressure ulcers (excluding the heels) or the patient cannot be positioned off of an ulcerated area | ||||||
| Ability to change position in bed (e.g., bed mobility) | Total assist to change position in bed. | Reactive support surface (non-powered) (e.g., air/gel/foam overlay) | Reactive support surface (non-powered) (e.g., air/gel/foam overlay) |
|
| ||||
| Moderate assistance with bed mobility required. | Reactive support surface (non-powered) (e.g., air/gel/foam overlay or high-density foam mattress) | Reactive support surface (non-powered) (e.g., foam overlay with air section inset in the area of the wound) | Reactive support surface (non-powered) (e.g., foam overlay with air section inset in the area of the wound) |
| |||||
| Patient independent with or without a device with bed positioning. (Light assist may be required.) | Reactive support surface (non-powered) (e.g., high-density foam mattress) | Reactive support surface (non-powered) (e.g., foam overlay with air section insert) | Reactive support surface (non-powered) (e.g., air/gel/foam overlay) | Reactive support surface (powered if the control is within the patient's reach) | |||||
| Instructions for use of this clinical tool: Determine the patient's level of risk and level of mobility in bed and follow the column-and-row intersection to determine the appropriate reactive or active support system. | |||||||||
Types of Support Surfaces
Overlays are filled with water, air, gel, or foam (or a combination of these products) and are applied to the top of a mattress. Foam overlays should be a minimum of 3 inches thick. If a patient's weight completely compresses an overlay, it is not effective.
Pressure relief mattresses are made of a combination of foam, water, or gel or layers of varying foam densities. They are usually indicated in place of standard mattresses used in hospitals and at home. Studies have shown that people at high risk of developing pressure injuries should use higher-specification foam mattresses instead of standard hospital foam mattresses [39].
Air-fluidized beds are embedded with tiny, silicone-coated beads suspended by pressurized, temperature-controlled air. They are recommended for immobile patients who carry higher risk for posterior pressure injuries. These beds reduce pressure against the patient's skin surface, resulting in increased capillary blood flow to the skin. This greatly increases granulation and healing of injuries. These beds are beneficial in patients with multiple large injuries; however, they are unsuitable for patients who are ambulatory, have pulmonary disease, or have spinal instability. They are usually used as an adjunct to comprehensive care.
Low-air-loss beds are made of numerous interconnected air-filled pillows designed to lose air through the cushions at a controlled rate. These beds distribute the patient's weight evenly, which in turn reduces friction, provides pressure relief, and increases capillary blood flow. They are indicated in patients with stage 3 or 4 injuries or stage 1 injuries with hyperemia.
Combination air-fluidized/low-air-loss beds combine the benefits of both types of beds. The low-air-loss component is placed on the upper half of the bed, while the air-fluidized component is placed on the lower half of the bed.
The most important consideration when choosing a support surface is ease of use. Patient goals and overall care plan are also considerations when selecting a support surface. Pressure injury risk, bed mobility, transfer, posture and positioning, financial resources, and advantages and disadvantages of the support surfaces should all be taken into account prior to coming to a decision.
The role of nutrition, including evidence-based strategies for management, has recently been reviewed in connection with the NPIAP 2019 practice guideline for prevention and treatment of pressure injury [36]. Consultation with a nutritionist should be considered for every patient with, or at high risk for, a pressure injury.
Malnutrition has been shown to increase the risk of developing a pressure injury and to delay wound healing [40,41]. For this reason, nutritional assessment, including food intake and recent weight loss, is an essential component of effective prevention and care. The goal of the assessment is to identify the patient's current nutritional status and any changes that have occurred in recent months or weeks, including the patient's overall level of functioning. The patient's state of hydration should also be assessed, as blood volume depletion impairs circulation and the delivery of oxygen and nutrients to healing wounds [35,36].
A number of clinical and laboratory parameters have been used to screen for poor nutritional status and increased risk of pressure injuries, particularly in elderly patients admitted to a hospital and nursing home. These include serum albumin, prealbumin, and weight less than 80% of ideal. Other signs of malnutrition thought to be useful are:
Loss of subcutaneous tissue
Muscle wasting
Generalized edema
Dry, pluckable hair
Dry, flaky, itchy skin
Cracks in the mucous membranes
Delayed wound healing/failure to granulate
The provision of an optimal diet (e.g., 30–35 kcalories/kg body weight for adults who are at risk for malnutrition), including the addition of supplemental protein, amino acids, zinc, and vitamins, has been shown to reduce risk of pressure-induced skin injury and to speed wound healing. The recommended daily protein intake for healthy adults (0.8 g/kg of body weight) may not be adequate in the frail elderly or under conditions of chronic inflammation and loss of lean body mass. For dietetic management of adults at high risk of pressure injury or delayed wound healing, the recommended intake is 1.25–1.5 g protein/kg body weight daily [29,42].
Protein is synthesized from amino acids, and specific amino acids (e.g., arginine and glutamine) become essential during periods of protracted metabolic stress (e.g., recovery from trauma, sepsis, pressure injuries). A deficiency in micronutrients, specifically vitamin A, vitamin C, zinc, and copper, is thought to impede injury healing. Zinc is important for the synthesis of protein and nucleic acid, for epithelial cell proliferation, and, along with copper, is essential for healthy collagen formation [42].
The benefit of a high-protein, arginine- and micronutrient-rich diet was evaluated in a prospective study among institutionalized, older adults with pressure injuries. Patients were randomized to receive either a standard diet with 16% energy from protein (control group) or a standard diet plus a 500-calorie supplement with 34 g protein, 6 g arginine, 500 mg vitamin C, and 18 mg zinc (treatment group). A standard protocol to measure rate of wound healing and reduction in injury size was used to monitor the progress of patients in each group. A significant difference in injury area (favoring the treatment group) was evident at week 8; an improved rate of healing, as evidenced by a difference in wound healing score (favoring treatment), reached significance at week 12 [43].
For patients with inadequate nutrition, strategies should be employed to increase oral intake. The preferred route of nutritional support is oral; whenever possible, the gastrointestinal tract should be used for feeding. It is the easiest and most comfortable way to provide supplementation, and it is also the least expensive and most convenient way. Patients should have diets prescribed with protein and caloric content sufficient to meet metabolic needs, with consideration of the patient's preferences and special needs (e.g., mechanical soft diets) [15]. Daily multivitamin supplementation may be implemented. Mouth care should be performed prior to eating. Additionally, toileting and hand washing should be offered prior to meals.
It is important to provide an environment conducive to eating. Position the patient properly; an upright position is preferred. Make sure the food is at the right temperature for the patient. Do not rush eating, particularly if the patient is elderly and requires more time to be oriented. Many patients benefit from the inclusion of snacks high in calories and protein in the diet (e.g., a peanut butter sandwich with milk). Consider adding powdered milk to yogurt and puddings to maximize caloric intake and protein levels. Commercial nutritional supplements, such as breakfast shakes, are also a common adjunct.
It is vital to maintain patient control as far as medically feasible. Some patients may not like ice in their water; others may prefer soup lukewarm. Patient preferences should be accommodated as much as possible.
Remind the patient to chew food thoroughly. If necessary, liquids may be offered between bites; some patients require this to help swallow their food.
Assuring adequate fluids and the maintenance of intravascular volume homeostasis are equally important. Vigilance is required to detect early signs of volume depletion or dehydration (e.g., change in weight, loss of skin turgor, falling urine output, hypernatremia); patients identified to be at risk should be listed on assignment/report sheets as a reminder to monitor these patients closely. Fluids should be scheduled between meals at least three times a day. Patient preferences for fluids (e.g., straws, temperature, ice, etc.) should be observed and noted. Refill water pitchers frequently and keep them within reach of patients, especially those with restricted mobility. Patients should be offered something to drink at every interaction. Ambulatory patients should be provided with a water bottle. As with nutrition and positioning, it is necessary to educate patients/families about the importance of regular fluid intake. When, despite these measures, patients are unable to consume adequate levels of water or nutrients, tube feeding or parenteral feeding should be considered. Patient and family preferences and the overall goals of treatment guide these decisions [19].
Careful, regular wound cleansing and meticulous skin care is essential. Cleaning and gentle debridement are necessary to remove necrotic debris, contaminants, bacteria, and remnants of previous dressings from the wound surface and adjacent area, usually with the help of fluids (irrigation). This process helps accelerate the healing process and decreases the likelihood of infection [31,44]. One must remember that cleansing is "clearing" a wound, not sterilizing it. Minimal mechanical force is used while cleansing the wound in order to minimize trauma to the wound bed and surrounding healthy tissue. Irrigation at a pressure in the 4 to 5 pounds per square inch range should be used.
The injury and surrounding skin should be cleansed at least daily. If the dressing is being changed more than once daily, wound cleansing should be done during each dressing change.
Generally, normal saline is used for cleansing pressure injuries. In injuries with necrotic tissue, debris, or confirmed or suspected infection, antimicrobials or surfactants should be considered. For infected wounds, diluted povidone-iodine may be used as the irrigation fluid. However, it should not be used during the granulation phase of healing. Acetic acid (0.5%) is highly effective in fungating lesions, especially against Pseudomonas aeruginosa. There are various cleansing agents available in the market, but normal saline is usually the best option [31,45]. Normal saline also should be used as a rinse after other solutions are used to irrigate the wound and minimize fluid shifts within newly forming tissue. Normal saline solution can reduce the drying effects that some irrigants may have on tissue [31].
Debridement has been shown to accelerate the healing process in some patients with advanced injuries. In addition to helping move the wound through the stages of healing, debridement is often necessary to visualize the wound bed and to stage the wound; a wound covered with necrotic tissue cannot be staged [15,31]. An exception is eschar on the heels, which acts as a natural biologic cover and should not be removed unless infection is present.
The method of debridement used depends on the amount of necrotic tissue present, the location of the wound, and the patient's overall condition [35]. Patients with stage 3 or 4 pressure injuries who have undermining and/or tunneling or extensive necrotic tissue should have a surgical evaluation for possible surgical debridement of the wound, if this is consistent with their condition and goals of care [29]. Infected wounds may require systemic antibiotic treatment and immediate surgical debridement [15]. Maintenance debridement should be continued until there is a covering of granulation tissue in the wound bed and the wound is free of necrotic tissue [29]. Debridement is contraindicated if there is inadequate blood supply to support wound healing.
Autolytic debridement uses the body's own enzymes and moisture to heal the injury. To be successful, there must be sufficient white blood cells available to the wound and a moist environment [13]. A layer of wound exudate should be kept in contact with the surface of the wound, usually using a moisture-retaining dressing [10,15,35]. This allows fluid to accumulate in the wound, rehydrating necrotic tissue and making it possible for enzymes in the wound to digest the dead tissue [35]. For a wound covered with dry eschar, it is appropriate to crosshatch the eschar, as this allows a faster build-up of moisture in the wound [35]. In their clinical practice guidelines for pressure injury treatment, the Agency for Healthcare Research and Quality recommends autolytic and enzymatic debridement as the preferred approach for patients in long-term care or home care and for patients who cannot tolerate other methods of debridement [35,46]. In general, this type of debridement is ideal for patients with stage 3 or 4 injuries with light-to-moderate exudates.
Autolytic debridement is highly selective; healthy tissue is spared and only necrotic tissue is liquefied. It is considered very safe, as it uses the patient's own immune system to promote healing and clean the wound of necrotic tissue. Autolytic debridement is easy to perform, very effective, and can be combined with other approaches. It is almost painless for the patient, making it a very attractive option.
However, there are disadvantages as well. It is comparatively slow in efficacy compared to surgical debridement, with progress usually seen in about one week [35]. Close monitoring of the injury is necessary to detect signs of infection. There is a risk of anaerobic growth when an occlusive hydrocolloid dressing is used, and this approach should not be used in infected injuries.
Mechanical debridement is a nonselective type of debridement that loosens and removes devitalized tissue and debris as well as viable tissue. This method is usually accomplished by means of mechanical force, such as wet-to-dry dressings, pulsatile lavage, or wound irrigation [31]. Pulse lavage may require special knowledge or training on equipment. Although it is a low-cost procedure, mechanical debridement is time consuming and can be painful.
Mechanical debridement is indicated for both acute and chronic wounds with moderate-to-large amounts of necrotic tissue, regardless of the presence of an active infection [47]. Depending on the modality used, the contraindications to mechanical debridement include the presence of granulation tissue in a higher amount than the devitalized tissue, inability to control pain, patients with poor perfusion, and an intact eschar with no gross clinical evidence of an underlying infection [47]. Mechanical debridement also is not indicated for clean wounds.
Surgical debridement is indicated for wounds with thick exudate and necrotic tissue. Approaches include hydrotherapy, laser, maggot debridement therapy (MDT), ultrasound, whirlpool, or excision (with a scalpel or scissors). Extensive or deep injuries should be debrided in operating rooms, although a moderate amount of necrotic tissue can be debrided at the bedside. Newer approaches, such as laser debridement, limit collateral damage, but these approaches can be prohibitively expensive [48]. Hydrotherapy can result in tissue maceration, and there is a risk of infection. Therefore, it is considered a less attractive option. The use of ultrasound waves to debride wounds also has been explored. With this technique, low-frequency ultrasound creates small bubbles in the wound that implode, causing the necrotic tissue to liquefy [49]. This method is generally less painful and less traumatic than traditional methods, with faster healing rates compared with other types of mechanical debridement [50]. However, further comparative evidence is necessary before it can be recommended as a replacement for established treatment modalities. Sharp surgical debridement is contraindicated in patients with intact eschar and no clinical evidence of an underlying infection [47]. The decision to attempt inpatient surgical debridement should take into account the patient's surgical risk stratification [47].
Debridement with maggots, an approach popular at the early 20th century, is finding a new place in wound care debridement [15]. MDT is an efficient, simple, cost-effective and reliable biosurgery method that uses mostly larvae of the Lucilia sericata species of the green bottle fly [51]. The larvae stimulate wound healing by activating molecular processes in the wound area through proteolytic enzymes, including collagenase, in their excretions/secretions that break down the necrotic tissue, ingest and digest micro-organisms, and inhibit bacterial growth by releasing ammonia into the would bed, which increases the wound pH [13,47]. Studies have shown that the excretions/secretions have antibacterial, antifungal, anti-inflammatory, and tissue-regenerating effects; the enzymes are still mostly unidentified [51]. Maggots have the ability to access moist tissue throughout the wound bed and clean small areas without harming healthy tissue [52]. MDT involves the application of sterilized larvae to the wound bed every two to three days [15]. The maggots can be applied to the wound directly or in a containment pouch [15]. The most appropriate dressing for wounds treated with maggots is one that keeps the larvae in place, allows for a flow of oxygen, and is suitable for the characteristics of the wound [35].
MDT is indicated for pressure injuries with necrotic tissue with or without infection [52]. Maggots work well in wounds in which moisture and oxygen are readily available and the pH is fairly stable [35]. The therapy is considered mainly for the treatment of wounds for which other forms of treatment are either not appropriate or not successful, and it is contraindicated in patients who have bleeding abnormalities or deep tunneled wounds [10,15,47].
Precautions should be taken to prevent the larvae from coming in contact with healthy skin, as there is a possibility of enzymatic damage [13]. Otherwise, there are no reported side effects from MDT [13]. However, some patients complain of a crawling or tingling feeling [13]. MDT also may cause psychologic distress for many patients, and its use should be discussed thoroughly with the patient and/or family prior to commencing therapy [15]. This therapy should only be used with appropriately informed consent.
Enzymatic debridement is a selective method of debridement in which concentrated enzymes (e.g., collagenase, papain, becaplermin, trypsin) attack collagen and liquefy necrotic wound debris without damaging viable tissue. Enzymatic debridement is used either alone or in combination with other techniques to remove necrotic tissue and promote wound healing [53,54]. For instance, collagenase and moisture retentive dressings can work in synergy, thereby enhancing debridement [47].
Usually, stage 3 or 4 injuries are considered candidates for enzymatic debridement. Application of the enzyme should be discontinued when the wound is free of necrotic tissue. If eschar is present, it should be crosshatched prior to introduction of the enzyme to improve efficacy, as enzymes are not active on a dry surface.
Enzymatic debridement is relatively fast acting, with progress evident in 48 to 72 hours. However, complete debridement may be a long process, so other methods (e.g., surgical removal of loosely adherent necrotic tissue) are often used in conjunction.
Enzymatic debridement should not be used for heavily infected wounds or in conjunction with silver-based products or Dakin solution [47].
Wound dressings are a cardinal component in the treatment of pressure injuries. There is a variety of available dressings, each with its own benefits and drawbacks (Table 4) [55]. The selection of dressing for an injury is determined by several parameters. These include:
Condition of the injury and wound bed
Size and depth
Necrosis/slough
Dry/exudating
Epithelializing
Presence of tunneling
Infection
Over-granulating
Malodorous
Malignant
Pain
Aim of treatment
Facilitate healing
Promote debridement
Combat infection
Relieve pain
Absorb exudates or add moisture
Prevent or treat scarring
Combat odor
Cosmetic/provide concealment
Condition of surrounding skin
Fragile
Macerated
Anatomical location
Difficult to dress
Dressing affects use of normal clothing or shoes
Etiology
External compression
Prospect of healing
Palliation
Indication of specific topical therapies (e.g., enzymatic debridement, antibiotics)
Economics/cost
Availability (reimbursement issues)
OVERVIEW OF DIFFERENT DRESSINGS FOR PRESSURE ULCERS
| Dressing Type | Description | Indication | Advantages | Disadvantages | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transparent film | Adhesive, semipermeable, polyurethane membrane that allows water to vaporize and cross the barrier | Management of stage 1 and 2 pressure ulcers with light or no exudates; may be used with hydrogel or hydrocolloid dressings for full-thickness wounds |
|
| ||||||||||
| Hydrogel | Water- or glycerin-based amorphous gels, impregnated gauze, or sheet dressings; amorphous and impregnated gauze fill the dead tissue space and can be used for deep wounds | Management of stage 2, 3, and 4 ulcers; deep wounds; and wounds with necrosis or slough |
|
| ||||||||||
| Alginate | Derived from brown seaweed; composed of soft, nonwoven fibers shaped into ropes or pads | May be used as primary dressing for stages 3 and 4 ulcers, wounds with moderate-to-heavy exudate or tunneling, and infected or noninfected wounds |
|
| ||||||||||
| Foam | Provides a moist environment and thermal insulation; available as pads, sheets, and pillow dressings | May be used as primary dressing (to provide absorption and insulation) or as secondary dressing (for wounds with packing) for stage 2 to 4 ulcers with variable drainage |
|
| ||||||||||
| Hydrocolloid | Occlusive or semiocclusive dressings composed of materials such as gelatin and pectin; available in various forms (e.g., wafers, pastes, powders) | May be used as primary or secondary dressing for stage 2 to 4 ulcers, wounds with slough and necrosis, or wounds with light to moderate exudates; some may be used for stage 1 ulcers |
|
| ||||||||||
| Moistened gauze | 2×2- or 4×4-inch square of gauze soaked in saline for packing | May be used for stage 3 and 4 ulcers and for deep wounds, especially those with tunneling or undermining | Accessible |
|
One of the most important factors in the selection of a dressing is moisture maintenance in and around the injury. A dressing that absorbs but does not dry out the wound is needed for injuries with excessive exudates. For injuries with minimal drainage, a dressing that restores moisture and prevents drying is needed. Frequency of dressing change also depends on the quantity of drainage.
For injuries with limited exudates, transparent films should be used; however, their use over cavities is contraindicated. These dressings should be changed every three to seven days, and application of triple antibiotic ointment is also recommended by some experts [55].
Hydrogel dressings are useful for patients with very shallow, dry, or minimally exuding injuries and for painful pressure injuries [29]. These gels, sheets, sprays, or ribbons of cross-linked polymers require a secondary dressing to affix them to the patient. Hydrogels not only rehydrate the wound bed but also aid autolytic debridement and reduce pain. These dressings can be used in infected injuries and are easily applied and removed from the wound. However, they are not recommended for pressure injuries with heavy exudation. They also dehydrate easily and can cause maceration.
Hydrocolloid dressings contain a gel that promotes the growth of new skin and are indicated for clean, uninfected stage 2 and shallow stage 3 injuries in body areas where they will not roll or melt [29]. They are available as pastes, powders, or wafers composed of gelatin, pectin, and carboxymethylcellulose. These dressings promote angiogenesis, autolysis, and granulation. Some hydrocolloids are self-adhering, but secondary dressings are required when powders and pastes are used. These dressings are especially beneficial on heels and sacral injuries that require contouring. They are recommended for partial and full-thickness injuries with or without necrotic tissue.
Hydrocolloid dressings can produce an odor upon removal [15]. In the absence of other signs of clinical infection, this is not an abnormal finding and should be explained to concerned patients and staff [15]. Hydrocolloids may also leave a residue in the wound bed, which should be gently irrigated out at dressing change [13]. These dressings lose their effectiveness if they are changed too frequently and should not be used for wounds that must be monitored daily [13].
Calcium alginate dressings, or alginates, are polysaccharide seaweed derivatives containing alginic acid. Alginate dressings either form a moist gel when they come in contact with exudates or retain their original shape while absorbing exudates. They are hemostatic, protect surrounding skin from maceration, promote healing, and may reduce risk of infection. These dressings are available in the form of ribbons, pads, and ropes.
Alginate dressings should be considered for the treatment of moderately and heavily exuding pressure injuries and for clinically infected injuries when there is concurrent antimicrobial therapy [29]. They may also be used to control bleeding after surgical debridement. A secondary dressing is necessary to secure these dressings, and they should be changed every 12 hours to four days. Wounds treated with alginate dressings may smell fishy or like "low tide" [15].
Foam dressings are protective and absorbent and potentiate wound healing by providing a moist environment. They are also permeable to gas and provide good insulation. These dressings are a good choice for stage 2 and shallow stage 3 injuries with drainage and for painful injuries.
In patients with incontinence, waterproof versions of foam dressings are prescribed in order to protect the skin. They can also be used under compression to prevent damage from shear. However, foam dressings are not effective in removing necrotic tissue, and after the dressing becomes saturated, maceration of skin around the wound can develop quickly. Therefore, these dressings should be paired with alginates for wounds with a high level of exudate.
Silver-impregnated dressings are a treatment option for infected or heavily colonized wounds or wounds that are at increased risk for infection [29]. Silver has an antimicrobial effect on a broad spectrum of organisms and has been shown to reduce the bacterial count in wounds [13]. Sustained-release sliver dressings are toxic to bacteria and fungi but do not adversely affect healthy wound tissue [10]. However, silver-resistant organisms do exist, and the judicious use of silver is advised, similar to the approach adopted with antibiotics [13]. It is recommended that the use of silver dressings be limited to a two- to four-week period [13]. As stated, silver-based products should not be used for enzymatic debridement [47].
Cadexomer iodine dressings are used for wounds with moderate-to-high amounts of drainage. They should not be used for patients who are sensitive to iodine or individuals with thyroid disease [29]. Cadexomer iodine dressings are antimicrobial and maintain a moist environment for wound healing [15]. Although these dressings are capable of reducing bacteria counts in wounds, they do not replace the need for systemic antibiotic therapy and are regarded as an adjunct in the treatment of wound infections [15].
Gauze packing was used frequently in the past, but it is now regarded as less effective in coping with wound drainage than calcium alginate or hydrocolloid fiber dressings [10]. Gauze dressings do not provide a barrier against bacteria, lower wound temperature, and can pull healthy granulating tissue out of the wound on removal [10]. When other forms of moisture-retentive dressings are not available, continually moist gauze is preferable to dry gauze, and one should avoid the use of wet-to-dry gauze dressings [29]. Gauze dressings are also labor intensive, requiring several dressing changes daily, which adds to the cost of the overall care [15].
Composite dressings are a combination of more than one material used to fulfill several important functions in the wound [15]. They provide an effective barrier to bacterial contamination of the wound and include an absorptive layer and either foam, hydrocolloid, or hydrogel [15]. Composite dressings can have either a semi-adherent or non-adherent surface placed in contact with the wound bed [15]. Composite dressings are comfortable and are available in various shapes and sizes [13]. These dressings should not be cut, as this compromises the structure of the dressing [15].
Some dressings incorporate collagen, which is an important protein involved in wound healing and repair [13]. The dressings can be 100% collagen or combined with other products, such as alginates [15]. They provide a high level of absorption while keeping the wound bed moist and are easily removed [15]. For wounds with very little drainage, collagen gel can be applied in a layer one-quarter inch thick [13]. Collagen used in most wound dressings is derived from cowhide [15]. Therefore, it should not be used in patients who are allergic to bovine products [15].
A research study concluded that severity of pain was correlated to pressure injury stage, and patients with later-staged pressure injuries may experience excruciating pain [56]. The goal of pain management in the pressure injury patient is to eliminate the cause of pain and to provide analgesia. There are several interventions and practice modifications that can prevent or manage wound-associated pain.
Skin care and assessments should be performed at a time of day when the patient is less fatigued [2]. All procedures should be thoroughly explained before they are performed. If a patient has questions, this should be addressed, and healthcare professionals should be encouraging and provide positive reinforcement. It is important to avoid trauma (shearing and tear injuries) to fragile skin during transferring, positioning, or holding a patient. If necessary, adjunctive medications may be administered to improve sleep and reduce anxiety, which can contribute to experiences of pain.
Dressing changes are often very painful. An analgesic may be administered 30 minutes before dressing changes, and if possible, the number of daily changes should be kept to a minimum. Tape should always be avoided on fragile skin. If patients are able, they should be allowed to remove their own dressings or set the pace of dressing changes. All patients should be assessed for pain before, during, and after dressing changes, and these findings must be documented [2].
Physical therapy and occupational therapy may be helpful to decrease contractures and muscle spasm. Of course, ensuring proper seating and positioning can improve pain scores and decrease the risk for further pressure injuries.
For mild-to-moderate pain, nonsteroidal anti-inflammatory drugs (NSAIDs) or acetaminophen may be used. Opioids should be avoided as much as possible, as the sedative effects boost immobility; however, they may be necessary during dressing changes and/or debridement.
Surgical closure is required for large defects, particularly when musculoskeletal structures are exposed. In case of large but shallow defects, skin grafts may be beneficial. The most widely used reconstructions performed for pressure injuries are local flaps. In this reconstruction, well-vascularized skin is transferred with underlying structures (such as subcutaneous tissue, fascia, and muscle) to the wound area. These procedures are classified as [57]:
Simple cutaneous: Includes skin and subcutaneous tissue only
Fasciocutaneous: Includes skin, subcutaneous tissue, and fascia
Myocutaneous: Includes all soft tissue layers from skin to muscle
There are benefits of surgical closure of pressure injuries, including more rapid healing of the wound and short-term resolution of complications [58]. Flaps containing muscle provide a physiologic barrier to infection, eliminate dead space in the wound, and improve vascularity [31]. Surgery may be difficult for some patients, however, particularly elderly and frail persons. The patient should be medically stable, of good nutritional status, and able to benefit from the procedure [31]. Complications as a result of surgical closure are considerable and include hematoma, seroma, wound dehiscence, and wound infection [31]. In addition, injuries frequently recur, even in younger patients.
Continuous assessment of pressure injuries for infection is vital. Signs of infection include erythema, fever, increased drainage, and increased leukocyte count, and these parameters should be monitored periodically. Topical treatment with silver sulfadiazine, triple antibiotic, or metronidazole is recommended for patients with infected injuries or who are at risk for infection. In general, noninfected wounds produce little or no odor.
Systemic antibiotic therapy is indicated when there are signs of cellulitis, purulent wound drainage, fever, or osteomyelitis. The choice of systemic agent should be supported by clinical assessment, imaging studies, and deep tissue culture.
Prophylaxis of infection is also important, as infected wounds are associated with pain, longer healing times, and greater impairments in patient functioning. Topical application of silver sulfadiazine and oral antibiotics are effective in infection prevention in pressure injuries [59,60].
There are many adjunctive treatments used in wound management. They include electrical stimulation, growth factors, hyperbaric oxygen, normothermic infrared and temperature therapy, and negative pressure wound therapy.
Electrical stimulation is effective in increasing the healing rate of chronic pressure injuries [61]. It is generally indicated for persons with spinal cord instability and difficult-to-heal injuries. In one study of individuals with spinal cord injury and pressure injuries, low-frequency pulsed current (i.e., alternating current) was compared to direct current and control groups [62,63]. Researchers found that injuries treated with electrical stimulation healed faster compared to controls and to standard treatment. Electrical stimulation is recommended by the ACP as adjunctive therapy in patients with pressure injuries to accelerate wound healing [64,65].
This therapy consists of the placement of a high-voltage, pulsed electrical current onto the wound bed (direct) or near the wound (induced), usually once daily for several weeks. The electrical settings (e.g., the polarity, amplitude and voltage, amperage) are established according to wound and patient characteristics. The Institute for Clinical Systems Improvement 2012 guideline recommends considering the use of direct contact electrical stimulation in the management of recalcitrant stage 2 as well as stage 3 and 4 pressure injuries to facilitate wound healing [61]. Although electrical stimulation is used for the treatment of pressure ulcers, a 2020 Cochrane review found that available evidence is insufficient to support its widespread use apart from its use in research [66].
Fluids taken from chronic pressure injuries have a significant degradation of growth factor activity compared to acute wounds [67,68,69]. The application of topical gels containing platelet-derived growth factor can increase fibroblast activity and accelerate healing for chronic wounds [68,70]. The application of 100 mcg/g becaplermin gel once daily has been found to increase the incidence of complete healing compared to placebo [71].
The use of hyperbaric oxygen in the treatment of pressure injuries is controversial. Hyperbaric oxygen therapy consists of 100% oxygen administered at a pressure of 2 to 3 atmosphere for a duration of one to two hours daily. This results in hemoglobin becoming saturated and oxygen spilling into the blood plasma. The vasoconstrictive effects result in reduction of edema in compromised flaps, pressure injuries, burns, and crush injuries. Improved oxygenation causes increased fibroblast activity and antimicrobial activity, with the stimulation of the phagocytic activity of the white blood cells [72]. It has been hypothesized that hyperbaric oxygen therapy could promote healing of pressure injuries; however, more research is necessary to evaluate the risks, benefits, and associated costs [73].
Studies have shown that pressure injuries treated with radiant heat heal faster and result in shrinking of the injury [74]. Because this requires special equipment and trained clinicians, however, it is not yet widely used.
Theoretically, therapeutic ultrasound can be beneficial in healing pressure injuries based on both thermal and non-thermal effects (Table 5). The NPIAP recommends that high-frequency ultrasound be considered as an adjunct for the treatment of infected pressure injuries. However, both a meta-analysis and a comprehensive review of available data on the efficacy of ultrasound in the treatment of pressure injuries were inconclusive [75,76].
THERMAL AND NON-THERMAL EFFECTS OF THERAPEUTIC ULTRASOUND
| Thermal | Non-Thermal | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
Pulsed electromagnetic field therapy can be an alternative treatment when pressure injuries are resistant to conventional therapies. This technique has been found to increase synthesis of DNA, improve revascularization, reduce bacterial growth, and increase neurotransmitter receptor and hormone receptor activity [77,78,79]. Researchers have demonstrated complete healing within a few weeks of initiating this treatment in patients with stage 3 and 4 injuries [80,81].
Negative pressure wound therapy is useful for advanced (stage 3 or 4) pressure injuries and poorly healing wounds with copious drainage. In general, it consists of a foam sponge dressing placed into the injury and connected by a drainage tube to a pump that exerts intermittent or continuous negative pressure on the wound bed. The negative pressure reduces edema and facilitates removal of excessive fluid and infectious debris from the bed of the injury. The effect is to increase local blood flow, promote granulation tissue, and speed wound healing
Negative pressure wound therapy has cost-saving advantages. It reduces the frequency of dressing change to three or less times a week, decreases nursing time and cost, and often permits earlier discharge and follow-up care in the ambulatory setting. Patients experience less of the pain associated with manipulation of the wound.
When considering negative pressure therapy for wound management, patient selection and plan of care should be made with caution and always in consultation with wound care specialists and/or experienced surgeons. Adverse events, such as serious bleeding and unrecognized deep infection, have been reported [82]. Moreover, this modality is not effective for injuries and wounds with necrotic tissue or malignancy in the margins.
An individualized treatment plan for the patient with a pressure injury is based on the stage of the injury, the patient's physical and psychosocial status, and whether infection is present (Figure 1) [83]. The following sections outline the general goals of treatment based on the stage of the wound, but it is important to individualize treatment as well.
At this stage, injuries often heal with proper care and treatment, but some will deteriorate further. The aim is to quantify the extent of the wound, increase blood circulation, and prevent further breakdown of skin. Treatment should focus on:
Pressure redistribution
Prevention of shear and friction
Moisture maintenance
Physical therapy and nutritional support
Periodic assessment of the patient
Pain management
Prevention and treatment of infection
The aim for patients with stage 1 pressure injuries is to prevent further deterioration of the injury and improve circulation. The same treatment plan implemented for suspected deep tissue injury should be the starting point. Skin should be cleansed and lightly moisturized. Massage on the affected area is contraindicated as it can lead to further tissue injury. If indicated, hydrocolloid dressings and transparent film dressings should be used. Surgical treatment and debridement are not indicated.
Promoting healing is the main goal for stage 2 injuries, with a focus on prevention of progression to full-thickness injury. Beginning with the same treatment plan implemented for previous stages, steps should be added to protect against infection. An appropriate dressing should be applied to promote healing and keep the wound bed clean. The skin is fragile at this stage, so adhesives should be avoided. Nutritional status should be re-evaluated, and supplements added, if necessary. Surgical treatment is not indicated, and debridement is rarely necessary.
Maintenance of a clean, moist wound bed to prevent infection and promote healthy granulation tissue is the prime goal in the treatment of stage 3 injuries. Infection impedes healing and may lead to complications such as sepsis and osteomyelitis. Careful assessment and follow-up are important at this stage; at the first clinical sign of infection, deep wound cultures should be obtained and appropriate antimicrobial therapy initiated promptly.
Along with the measures described for the previous stages, debridement should be carried out if indicated. Autolytic or enzymatic debridement is recommended for stage 3 injuries with light-to-moderate exudate; surgical debridement is necessary if there is necrotic tissue and infection [83].
Wound gels may be used if the injury is partially or completely covered with necrotic tissue. Foam dressings and cavity fillers are indicated if the injury bed is free of necrotic tissue and debris. Alginate dressings can also be applied if the wound has excessive exudates.
Stage 3 injuries usually heal spontaneously with appropriate cleaning and dressing. However, when treated conservatively, they have a recurrence rate of between 32% and 77% [84]. Surgical management can reduce the rate of recurrence in some patients [84]. However, according to a Cochrane review, randomized evidence neither supports nor refutes the role of surgery in the management of stage 2 or above pressure injuries [85].
For patients with stage 4 injuries, the focus of treatment is to provide an environment for new tissue growth. This can also involve removal of necrotic tissue and drainage reduction.
First, the injury should be assessed for bone involvement and signs of infection. If indicated, antibiotics should be started. Surgical management is often indicated in stage 4 injuries to address or prevent complications due to the large size of the wound. Debridement is often necessary to remove necrotic tissue.
When treating patients with unstageable pressure injury, practitioners should focus on providing a moist environment and preventing further deterioration. Injury stage should be reassessed when the base is visible and the treatment plan adjusted accordingly. The wound should be assessed for signs of infection, and antibiotics may be indicated. As with all stages, pain management and patient comfort should be ensured.
The wound should be debrided of nonviable tissue in the wound bed. One exception is the eschar on the heels, which should never be debrided unless infected.
During the treatment of pressure injuries, routine evaluation of improvement or wound progression is essential and wounds should be assessed at each dressing change. In addition, a holistic patient assessment is indicated, which encompasses systemic factors, psychosocial factors, and local factors. Systemic factors include assessment of etiology, duration, and blood flow to the injury; infection; medications; and comorbidities. Determination of the patient's knowledge, beliefs, social support, and financial health (psychosocial factors) is also important. Finally, factors specific to the wound itself, such as amount of exudate, recurrent injury, and tissue necrosis, must be evaluated and documented.
When evaluating the wound, the most important factor to consider is whether the wound is progressing toward the goals established at the onset of treatment [13]. Clinical signs of improvement are expected to appear within two to four weeks [10]. If the wound is not progressing, further assessment and adjustment of the treatment approach are warranted [13].
For nonhealing wounds, the first factor to evaluate is the quality of wound care. This includes determining if dressing changes are being carried out at the recommended intervals, if the dressings are applied appropriately, and if the manufacturer's instructions for product use are being followed [13]. Factors affecting the patient's condition should be taken into consideration and addressed appropriately [10]. Failure of a wound to improve is often due to systemic factors, such as ischemia, infection, or malnutrition, or continuation of the causative factors [10]. These issues must be addressed first to achieve optimum wound healing. A change in the dressing treatment is indicated if any of the following problems occur [10]:
Maceration of the surrounding skin
Inadequate control of wound drainage
A change in the amount of drainage or the depth of the wound
Reverse staging of pressure injuries is not an acceptable approach to gauging the level of wound healing. Healed pressure injuries do not replace lost muscle, subcutaneous fat, or dermis [29]. Tools that appropriately measure degrees of healing include the Bates-Jensen Wound Assessment Tool and the Pressure Ulcer Scale for Healing (PUSH) tool [15,86]. The Bates-Jensen Wound Assessment Tool has thirteen variables that provide a composite picture of the status of the wound [15]. The PUSH tool uses scores in three domains (i.e., size, exudate amount, and tissue type) to indicate improvement or deterioration of the injury (Table 6) [87]. When using this tool, surface area is calculated by multiplying the greatest length (head to toe) by the greatest width (side to side) in centimeters. After removal of the dressing and before applying any topical agent to the injury, the amount of exudate is estimated as none, light, moderate, or heavy. Finally, the type(s) of tissue present in the wound bed is evaluated (i.e., necrotic, slough, granulation, epithelial, or closed). A score of 0 on the PUSH tool indicates the wound has healed, whereas the highest score of 17 indicates wound degeneration [15]. Results of the assessment should be recorded; a decrease in score over time indicates improvement.
THE PRESSURE ULCER SCALE FOR HEALING (PUSH) TOOL
| Factor | Points |
|---|---|
| Size (surface area) | |
| 0 cm2 | 0 |
| <0.3 cm2 (but more than 0 cm2) | 1 |
| 0.3–0.6 cm2 | 2 |
| 0.7–1.0 cm2 | 3 |
| 1.1–2.0 cm2 | 4 |
| 2.1–3.0 cm2 | 5 |
| 3.1–4.0 cm2 | 6 |
| 4.1–8.0 cm2 | 7 |
| 8.1–12.0 cm2 | 8 |
| 12.1–24.0 cm2 | 9 |
| >24.0 cm2 | 10 |
| Exudate amount | |
| None | 0 |
| Light | 1 |
| Moderate | 2 |
| Heavy | 3 |
| Tissue in wound bed | |
| Closed wound | 0 |
| Epithelial tissue | 1 |
| Granulation tissue | 2 |
| Slough | 3 |
| Necrotic tissue | 4 |
Wound assessment also includes wound location, size and depth, signs of infection, and exudates. The wound is examined for presence of tissue debris, base tissues, quality and amount of exudate, odor, and pain.
Documentation of location is paramount for proper monitoring of pressure injuries. Placement can affect healing and treatment decisions. Wounds that may be exposed to urine or feces should be provided special attention and care, and peripheral wounds may require more time to resolve.
The location of the wound should be stated in a manner that is clearly understood, such as the sacrum or right or left ischium [49]. Anatomical markings should be used when possible.
Accurate measurement of the wound is probably the most important feature of wound assessment [35]. It provides information on the initial size and progression or non-progression of healing, allowing for valuable feedback on the effectiveness of clinical interventions [15]. Decreasing wound size is generally regarded as a sign of wound healing, but an increase in wound area is not necessarily indicative of deterioration [15].
Wounds should always be measured in centimeters, using a plastic or paper ruler. Wound length is measured from head to toe; width is measured from hip to hip [13]. The depth of the wound can be obtained by gently inserting a sterile cotton-tipped applicator into the wound bed and marking it at skin level. The applicator is then measured using a metric ruler [13].
Sinus tracts and undermining impair healing, and it is important to immediately identify their presence. A sinus tract is a tunnel that extends from any part of the wound and can bore through subcutaneous tissue and muscle. This tunnel creates dead space, which can result in abscess formation and further impede the healing process. A sinus tract can be measured using a sterile cotton swab.
Undermining is defined as destruction of the tissue under the skin around the edges of the wound. This frequently occurs in pressure injuries that have been subjected to shear force as well as pressure. It is important to document the location and extent of undermining.
The easiest way to measure and describe undermining is by using the face of the clock [15]. With the patient's head representing 12 o'clock, sweep the area of undermining or probe the tunneling to ascertain the depth. For example, undermining along the right border would be recorded as extending from 1 o'clock to 5 o'clock with a depth of 4 cm. It is important to check around the entire perimeter of the wound, as undermining can occur in more than one location.
It is also vital to assess and document the appearance of the wound bed (Table 7). If the wound bed has a mixture of tissue in it, this should be documented by an approximate percentage (e.g., 75% granulation tissue and 25% slough). Granulation results in "beefy" red tissue consisting of new capillaries, fibroblasts, and collagen fibers with a shiny, moist granular appearance. Grey or purple granulation is a sign of poor vascularization. Granulation present in the wound denotes healing.
WOUND BASE COLOR DESCRIPTIONS
| Color | Description and Clinical Implications | ||||
|---|---|---|---|---|---|
| Red |
| ||||
| Yellow |
| ||||
| Black or brown |
|
Necrosis is gray, brown, or black unviable tissue that usually must be removed in order for healing to take place. Eschars are typically gray to black and dry or leathery in appearance. Slough tissue is yellow/white to gray in color. It may be stringy or thick and appear as a layer over the wound bed. Epithelial tissue will often begin to grow in from the edges over the wound surface. This tissue is generally pink and shiny. As a quick reference color guide, red is associated with normal healing, yellow indicates slough or dead tissue, and black is necrosis.
Exudates are an indication of inflammation in the wound. The presence of large amount of exudate can delay healing and increase the risk of infection. As such, this factor has a significant impact on treatment decisions, particularly dressing type. The amount and quality of any exudates should be noted. Amount may be denoted as:
Large/copious: Extends beyond dressing
Moderate: Contained within the dressing
Small/slight: Small amount of exudate in the center of dressing
None: Absence of exudate
The quality of the exudates may be described as [88]:
Purulent: Thick exudate that may be malodorous and tan, yellow, green, or opaque in appearance and denotes presence of infection
Serosanguineous: Thin and pink or light red in color
Serous: Thin/watery and clear or straw colored, with no blood or debris
Hemorrhagic: Red and thick, consisting mainly of blood
Fibrinous: Cloudy and thin with strands of fibrin
Malodor with purulent exudates suggests mixed infection often combined with ischemic necrosis. However, the majority of wounds (even those free of infection) do have some odor. As previously discussed, gram-negative and gram-positive infections have distinct odors. If present, an injury's odor (quality and strength) should be documented.
Wound edges are open and closed. Healthy wound edges are open and allow cell migration. Closed wound edges prevent cell migration and may delay healing. These edges may be described as calloused, approximated, or rolled. The presence or absence of erosion, papules, excoriation, denudement, pustules, or other lesions should be noted.
The condition of the surrounding skin surface up to 4 cm from the edge of the wound circumferentially must also be assessed and documented. Its characteristics should be noted, particularly color and integrity [10]. Maceration from excessive drainage may indicate that the dressing used is not appropriate and a different product is needed. Circumferential erythema and/or induration up to 2 cm from the wound are indicative of cellulitis.
Pressure injuries cause considerable pain and suffering, ranging from sore to excruciating [30]. In one study, 75% of patients rated their pain as mild, discomforting, or distressing; 18% rated their pain as horrible or excruciating [89]. Pain and odor control are a major concern for patients, and studies have shown that patients rank pain control as more important than healing [15]. The level of pressure injury pain depends both on the stage of the injury and on manipulation of the area (e.g., if a dressing change is done at the time of assessment), although the majority of patients report pressure injury pain at rest as well as with dressing changes. Pressure injury pain may be due to tissue trauma, inflammation, damaged nerve endings, infection, and procedures such as debridement and dressing changes [90].
The gold standard for assessing pain intensity is self-reporting using standard pain intensity instruments. Two of the most widely used pain assessment scales are the numeric pain intensity scale and the Wong-Baker Faces Pain Rating Scale [91]. The numeric pain intensity scale consists of ratings from 0 (no pain) to 10 (worst possible pain). This scale can be used for pain assessment with adults and children older than 7 years of age [92]. Visual presentation of the numeric pain intensity scale is helpful with hearing impaired patients, and the scale has been translated into many languages.
The Wong-Baker Faces Pain Rating Scale consists of six faces ranging from a happy smiling face (no pain), to a crying, frowning face (worst pain). The patient is asked to choose the face that best reflects his or her pain. The Faces Pain Rating Scale is the preferred scale for use with children and may also be used with the geriatric population, cognitively impaired patients, and those for whom English is a second language.
After the initial pain assessment has been completed, reassessment should be done at regular intervals. As noted, pain intensity should be rated by the patient, not a healthcare professional. The following questions may be used to help determine patients' pain levels:
What kind of pain are you experiencing?
What word(s) would you use to best describe it (e.g., burning, aching, shooting)?
What makes the pain better?
What makes it worse?
Where is the pain located?
Does the pain radiate?
Would you describe your pain as none, mild, moderate, severe, or excruciating?
How would you rate your pain on a scale of 0 to 10, with 0 representing no pain and 10 being the worst imaginable pain?
What is the pain intensity at its worst, best, and now?
Is the pain better or worse at any particular time of the day or night?
When does it start and when does it stop?
Infections remain a significant cause of both pain and poor outcomes in patients with pressure injuries. At each wound assessment, the patient should be evaluated for signs of local or systemic infection. Common signs and symptoms include:
Systemic signs (e.g., fever, chills, sweats)
Local signs (e.g., rubor, calor, color of adjacent tissues)
Unusual odor
Friable or dysmorphic granulation
Purulent exudates
If any of these are present, infection should be suspected and treated. Culturing the wound may also be helpful, particularly for patients with refractory or recurring wound infections. Obtaining a good culture requires that a semi-quantitative swab collection also be taken. The gold standard is quantitative biopsy, but this procedure is invasive and expensive.
The prevention of complications in pressure injuries begins with injury recognition. As soon as an injury is diagnosed, pressure relief and prevention and control of infection begin. Without proper care and treatment, a superficial stage 1 or 2 injury can evolve to a more serious and severe stage 3 or 4 injury. The near-term complications of pressure injuries include cellulitis, abscess, sepsis, and osteomyelitis. Indolent, non-healing deep injuries may eventually lead to malignancy.
Cellulitis can occur when infection spreads from the site of the injury to a deeper layer of skin, causing acute infection of connective tissue and possibly leading to sepsis. Staphylococci and streptococci are the most common causative agents of cellulitis.
Physical exam may reveal signs of infection, including erythema, edema, warmth, and possibly increased drainage. Lymphadenopathy may be present near the area of cellulitis. Diagnosis may be confirmed by blood culture, complete blood count (CBC), or fluid or exudate culture from the affected area.
Treatment usually involves a course of antibiotics. Analgesics may be necessary if the area is painful.
Sepsis is one of the most serious complications of a pressure injury that has become secondarily infected. Sepsis occurs when bacteria or the products of bacterial infection in the wound enter the bloodstream, producing a systemic inflammatory response syndrome (SIRS) marked by fever, hemodynamic changes, and organ dysfunction. The SIRS designation is not specific to infection and may be used in reference to any serious, ongoing inflammatory process resulting in end-organ damage and multisystem failure. While sepsis is a common and important cause, SIRS may be encountered in association with noninfectious insults such as trauma, burns, pancreatitis, anaphylaxis, adrenal insufficiency, and pulmonary embolism.
In 2014, a task force of the European and American societies of critical care medicine was convened to reexamine current concepts and definitions of sepsis and septic shock in light of improved understanding of the pathophysiology and management of sepsis. The task force concluded that previous definitions are limited by excessive focus on inflammation, and that the SIRS criteria have inadequate specificity and sensitivity for defining sepsis. The task force report and new, consensus definitions for sepsis and septic shock were published in 2016 [93].
Sepsis is now defined as life-threatening organ dysfunction caused by a dysregulated host response to infection [93]. This definition emphasizes the loss of adaptive homeostasis in response to infection and the potential lethality of infection when any degree of organ dysfunction is present. Even modest organ dysfunction has been found to confer a mortality risk in excess of 10%, hence the importance of urgent assessment and prompt treatment [7]. The presence and extent of organ dysfunction can be assessed with various scoring systems that rely on clinical and laboratory parameters, such as the following [7,94,95]:
Acute lung injury: a ratio of arterial oxygen tension to fraction of inspired oxygen of 280 or less
The presence of a metabolic acidosis, e.g. lactate >2 mmol/L
Oliguria (urinary output of less than 0.5 mL/kg body weight/hour for at least two hours in a patient with a urinary catheter in place)
Coagulation abnormalities (international normalized ratio [INR] >1.5)
Thrombocytopenia (platelet count <100,000/mcL)
Bilirubin >2 mg/dL
An acute alteration in mental status
The scoring system currently utilized in most critical care units is the Sequential Organ Failure Assessment (SOFA) score, which grades abnormality by organ system and accounts for clinical interventions [95]. A higher SOFA score is associated with an increased probability of mortality. Organ dysfunction can be identified by an acute change in SOFA score ≥2 points consequent to the infection. This score reflects an overall mortality risk of approximately 10% in a general hospital population with suspected infection [7].
Working from a model derived from a large inpatient data base, the sepsis task force was able to identify, and validate, a simple "bedside" clinical measure that can be used to identify which patients with suspected infection are at risk for developing sepsis. Designated the qSOFA (for quick SOFA), this measure consists of three elements [93,96]:
Respiratory rate ≥22/min
Altered mentation (Glasgow Coma Scale <15)
Systolic blood pressure ≤100 mm Hg
The data analysis demonstrated that patients with infection who are positive for two or more of these elements are likely to have a prolonged ICU stay (i.e., three or more days) or die in the hospital.
Physicians and nurses can employ the qSOFA in the office, emergency department, or inpatient facility to quickly identify which patients with an infection (e.g., pressure injury) are on the clinical threshold of sepsis and thus at risk of further clinical deterioration. The task force suggests that positive qSOFA criteria be used to prompt clinicians to further investigate for organ dysfunction, to initiate or escalate therapy as appropriate, and to consider referral to critical care [7].
Septic shock is a subset of sepsis in which underlying circulatory and cellular/metabolic abnormalities are profound enough to substantially increase mortality. Within the clinical construct of sepsis, the patient with septic shock can be identified by the presence of the following two criteria [93]:
Persisting hypotension requiring vasopressors to maintain mean arterial blood pressure (MAP) ≥65 mmHg
Blood lactate >2 mmol/L despite adequate volume resuscitation
The hospital mortality rate for patients meeting these criteria is in excess of 40%, or four times greater than for patients with sepsis [7].
For patients with an infected pressure injury, the confirmation and etiology of sepsis is determined by blood cultures, wound culture and Gram staining of exudate and/or tissue obtained from the base of the injury, and/or by selective application of the polymerase chain reaction.
In addition to fluid and metabolic resuscitation, treatment of sepsis requires parenteral antibiotics and, when necessary, surgical debridement and/or drainage of deep tissue suppurative fluid collections. Vasopressors and inotropic therapy may be necessary to restore adequate blood pressure and perfusion. The initial choice of antibiotics will depend on the most likely pathogens associated with the source of infection as well as the prevalent micro-organisms in the local community and health facility. The anticipated susceptibility profile of prevalent local pathogens and the ability of the antibiotic to penetrate to the source of the infection should also be considered. A combination of drugs with activity against all likely pathogens should be administered initially, but the regimen should be reassessed in light of culture results, the goal being to identify a single, narrow-spectrum antibiotic that will best control the infection [97]. A common approach is to initiate empiric therapy with a carbapenem or extended-spectrum penicillin/beta-lactamase inhibitor (e.g., ticarcillin/tazobactam) to cover gram-negative enteric bacilli, anaerobes, and Pseudomonas, often in combination with vancomycin to cover Staphylococcus aureus pending culture results.
Proper support for organ failure is indicated for patients with severe sepsis, and sedation, mechanical ventilation, and analgesia are often required in late-stage disease. Adequate nutrition (usually administered parenterally) and glucose control are necessary. The patient should be stabilized prior to restarting injury treatments.
When the infection from a full-thickness tissue injury, generally stage IV, spreads to bone and joints, periosteitis, septic or infectious arthritis, or osteomyelitis can result. If the injury crater can be probed to bone (i.e., one can see or palpate bone at the base of the crater), then the likelihood of bone infection approaches 80%. Confirmation of the diagnosis relies on imaging studies and needle aspiration or bone biopsy for histopathology and culture.
Plain bone radiographs are useful for diagnosing chronic osteomyelitis when infection has been present for many weeks; however, false-positive or false-negative results are common. Computed tomography scans are helpful for demonstrating fluid collections, bony erosion, and joint involvement. Radionuclide bone scanning (with technetium methylene-99m diphosphate) can be diagnostic, but it only has a moderate degree of sensitivity and specificity. Magnetic resonance imaging is the most sensitive and specific imaging modality for identifying the presence of osteomyelitis.
Bone biopsy is the definitive diagnostic test. Bone biopsy for histologic examination and culture not only verifies bone involvement but also helps to establish the bacterial etiology and antibiotic sensitivities upon which optimal treatment depends.
In the presence of underlying osteomyelitis, satisfactory healing of the pressure injury is usually not possible unless the bone infection is eradicated. This requires parenteral followed by oral antimicrobial therapy for a minimum of six to eight weeks, combined, if necessary, with surgical debridement of necrotic bone. Any surgical approach to (presumed) infected bone, whether needle aspiration, biopsy, or debridement, should include submission of bone specimens for culture.
In the surgical management of stage 4 injury cases with little or no overt clinical or radiographic sign of bone infection, submission of bone specimens for culture is useful for identifying early, limited osteomyelitis and thus the need for post-operative antimicrobial treatment. Examples are cases requiring partial ostectomy or ablative surgery of stage 4 injuries with resection of bone from the base of the injury crater.
Long-standing pressure injuries can develop into malignant tumors called Marjolin ulcers. These tumors were named for surgeon Jean-Nicolas Marjolin, who first described the condition in 1820 [98,99]. They are very aggressive ulcerating squamous cell carcinoma found in the area of injuries and other long-standing indolent wounds. It can develop many years after the initial trauma.
The first sign of Marjolin ulcer is a change in the character of the wound. Drainage increases, and the odor of the drainage becomes putrid. In some cases, there is frank bleeding. Diagnosis is made after histologic examination of a specimen removed from the injury, usually at the time of a flap closure. Confirmation of the diagnosis requires a preoperative tissue biopsy; wedge biopsy is the method of choice.
Excision of the lesion with 1-cm margin is required. Oncologic assistance is also necessary in the management of this condition, and extensive treatment is often necessary.
Gas gangrene is a rare but serious form of Clostridium perfringens infection. These obligate anaerobes release gas and harmful toxins that can result in gas gangrene, sepsis, and septic shock. The most common symptoms are severe pain and rapid swelling of the skin around or near the injury.
Surgical debridement is usually necessary [100]. In very serious cases, excision with amputation is required to prevent spread of the infection [101]. Penicillin is administered as adjuvant therapy. Hyperbaric oxygen therapy is effective in growth inhibition and killing C. perfringens [102,103]. However, results of a Cochrane review could not determine the efficacy and safety of hyperbaric oxygen therapy for the treatment of gas gangrene [101].
People with stage 3 and 4 injuries are at a risk for contracting the rapidly progressive infection necrotizing fasciitis. Necrotizing fasciitis is defined as a group A streptococcal infection of the fascia with accompanying necrosis of the subcutaneous tissues. It is an uncommon consequence of pressure ulceration [104,105].
Initial signs of this condition are fever, pain, and massive swelling. Visual and microscopic evaluation of the tissues confirms the diagnosis. Emergency treatment is required, and aggressive surgical debridement is vital to prevent spreading [106]. In some cases, early amputation of affected tissues and maximum intensive care treatment (e.g., in case of sepsis) are required [106].
As soon as necrotizing fasciitis is suspected, antibiotics should be started. A combination of intravenous antibiotics, usually clindamycin, vancomycin, and penicillin, is administered. One review recommended aminopenicillin plus sulbactam in combination with clindamycin and/or metronidazole as initial treatment [106]. Hyperbaric oxygen therapy can also be effective.
Recurrence of a pressure injury is a common complication of treatment. As noted, as many as 90% of patients with a healed wound will experience a recurrence [6,9]. Compared to normal tissue, scar tissue has lower tensile strength, poor blood supply, and poor ability to withstand trauma, making it vulnerable to recurrent episodes. If an injury recurs at the same site within four months of the initial injury, it is likely due to incomplete healing rather than a true recurrence [107]. Risk factors for recurrence include male sex, younger age, African/black race, lower socioeconomic status, nursing home residence, and previous pressure injury surgery [107].
Comorbid conditions both increase susceptibility to pressure injuries and impair the healing process. Therefore, comorbid conditions should be addressed as part of the overall treatment plan. Common comorbid conditions in patients with pressure injuries include:
Diabetes
Incontinence
Peripheral vascular disease (PVD)
Hypotension and hypoxia
Dehydration, poor nutritional status, or malnutrition
Cancers
ESRD
Depression and cognitive impairment
Even with the best medical care and preventive measures, pressure injuries can occur [108]. It is unclear which combination of comorbid conditions can lead to injury formation, but changes in skin with comorbidities are believed to result in injuries. Recent acute illnesses or acute exacerbation of comorbid conditions may cause lethargy and reduced mobility, increasing the risk of pressure injuries. In patients with comorbid conditions, special attention to the drugs being used is necessary. Chemotherapeutic agents, systemic corticosteroids, and NSAIDs can impair healing.
In patients with long-standing diabetes, both macro- and microvasculature can be severely damaged, particularly if the disease is poorly controlled. Diabetes also causes neuropathy, and the resultant loss of sensation and protective reflexes is a risk factor for pressure injury progression. Patients with diabetes have elevated glucose levels that result in rigid blood cell walls and decreased blood flow through microvasculature. The disease also impairs oxygen release by the hemoglobin, resulting in hypoxia. Leukocytes have less effective chemotaxis and phagocytosis, which makes the patient susceptible to infection. Diabetics are prone to fungal rashes that can result in perineal irritation and skin breakdown. Impaired granulocyte function inhibits wound healing. All of these factors contribute to the increased risk of pressure injury development in a patient with diabetes.
The aim of diabetes management is to keep blood glucose levels close to normal (i.e., glycosylated hemoglobin less than 7%). Usually, pharmacotherapy and/or insulin therapy is necessary for optimum disease control. In addition, eating habits should be modified and physical activity should be increased.
Approximately 10 to 13 million Americans have transient or chronic urinary incontinence [109]. It is also estimated that 50% to 70% of patients with urinary incontinence fail to seek medical intervention or treatment [109]. The high prevalence of incontinence among certain populations (e.g., nursing home residents) makes this an important factor in pressure injury development and healing.
Extended exposure of skin to urine and feces can result in breakdown, making the patient more susceptible to injuries [110]. Urinary incontinence results in maceration, which causes increased skin irritation and fragility. Coliform bacteria and C. difficile contamination of existing wounds can lead to severe infections.
The treatment of these patients is dependent on determining the incontinence etiology. General approaches include regular assessment of skin; hydration and infection should not be ignored. Patients should be checked for incontinence every two hours. For patients who are cooperative and aware of bladder filling, a toilet program should be instituted, including planned voiding every two hours. For patients who are uncooperative or unaware of bladder filling, consider the use of absorptive products or condom catheters for men. It is important to use diapers and underpads that wick moisture away from patients' skin. Incontinent patients should be cleaned as soon as possible after soiling using specialized incontinence skin cleansers or soaps.
For patients with severe diarrhea, all potential causative factors should be explored and addressed. A rectal pouch may be useful for these patients. In cases of chronic incontinence, an every other day suppository or enema may be considered. In addition, barrier ointments help protect the skin from incontinent episodes. If used, apply a thick coat of ointment, wipe off the soiled top layer, and apply another layer. Do not clean off the paste to skin level when bathing or cleaning.
PVD is an important contributory factor in the development of vascular injuries, particularly diabetic foot ulcerations (as the cause of 10% to 30% of all cases) [111]. The wounds of patients with severe PVD heal poorly as a result of inadequate blood supply, and minor trauma or pressure often leads to ulceration.
When conducting a thorough physical examination, signs and symptoms consistent with PVD should be documented and ankle-brachial index or pulse volume recordings should be recorded. The ankle-brachial index is not specific in patients with advanced calcific atherosclerotic disease, and for patients with this condition, photoplethysmography or magnetic resonance angiogram is more accurate [112,113].
Treatment of PVD first relies on assessment and management of modifiable risk factors (e.g., smoking, physical activity). Pharmacologic management with antiplatelets is the gold standard for patients whose disease is not well controlled with lifestyle changes [114]. In addition, comorbidities should be treated aggressively.
Prolonged hypotension and hypoxia can cause tissue hypoperfusion, especially in anatomical locations already at risk for developing injuries. Patients with septic shock have microvascular endothelial dysfunction, which results in tissue hypoxia despite adequate oxygenation [115,116]. Tissue hypoxia and hypoperfusion may also be caused by hemorrhagic shock. Anemia and low cardiac output limit the blood's oxygen-carrying capacity and perpetuate tissue hypoperfusion. It is necessary to check vital signs, CBC, blood gas, and echocardiogram and manage accordingly.
As discussed, optimal nutrition is important for the prevention and treatment of pressure injuries. Therefore, intestinal diseases and acquired disorders that adversely affect nutrient absorption should be identified and treated as part of a management plan. Conditions like celiac disease, Crohn disease, and gastrointestinal malignancy often lead to malabsorption and micronutrient deficiencies. C. difficile colitis can cause severe diarrhea and hypoalbuminemia, making patients nutritionally and hemodynamically compromised. Patients who have undergone surgical bowel resections and fecal diversion are also at risk for malabsorption, fluid depletion, and electrolyte deficiency.
In addition to correction of nutritional deficiencies, the primary cause of malabsorption should be identified and treated. The plan of care will include interventions to increase nutritional intake, keeping in mind that the patient's and family's preferences play an important role in establishing a diet plan.
Malignancy often leads to cachexia, a syndrome characterized by weight loss, malnutrition, weakness, and anemia. However, there is limited research exploring the link between pressure injuries and cancer [117]. Radiation therapy can cause dermatitis and desquamation and increase the risk for skin breakdown. Both the malignancy and associated treatments may cause immunosuppression and increase the likelihood and severity of infections.
Patients with cancer who are also experiencing skin breakdown should be assessed for nutritional status, and medications may be prescribed to combat the anorexia associated with chemotherapy. Optimum skin care, particularly in areas affected by radiation therapy, is a necessity.
Severe uremia associated with ESRD necessitates the use of dialysis to remove uremic toxins and prevent or control complications, and pruritus and xerosis are common in these patients. Malnutrition and dehydration are also risks. Dietary management of patients with ESRD should be aimed at control of electrolytes (including calcium, phosphorus, and potassium), prevention of malnutrition, and maintenance of acceptable fluid volume status.
The National Institute of Mental Health estimates that 8.4% of adults in the United States are suffering from depression, and major depression is the leading cause of disability worldwide [118]. Depressed patients often have little interest in self-care and nutrition, both of which may predispose an individual to pressure injuries [15]. Pressure injuries have also been found to contribute to depressive symptoms [119]. Patients should be regularly assessed for depression or other psychologic illnesses and referred to mental health care.
Loss of cognition is associated with increased risk for pressure injuries, and impaired mental status leads to a lack of awareness of discomfort or pressure and may be associated with incontinence. The ability to respond appropriately or to inform others of the need for assistance may be lost completely. As such, these patients often require intensive care.
Research and technical innovations are producing sophisticated technology and analytic devices to diagnose, prevent, and treat pressure injuries. These new innovations are changing the way injuries are cared for and prevented in a variety of settings.
Studies have shown that patients with less nocturnal periodic movements are at risk for pressure injuries, but sleeping patients may be unaware of their lack of movement [120]. Alert systems have been implemented to remind staff to reposition the patient every two hours. Because repositioning is one of the basic hallmarks of pressure injury prevention, alerts may help address this risk factor better.
Early detection is of utmost importance in pressure injury management. Advanced technology and devices can be used to achieve better sensitivity and specificity in the diagnosis of injuries. These techniques include laser Doppler, visible and near-infrared spectroscopy, ultrasound, and pulse oximetry [121]. Each of these approaches ascertains clinical parameters associated with blood flow and hemoglobin concentration, and combining such measures with clinical information allows for a high degree of sensitivity and specificity in identifying impending chronic wounds in patients with any skin color [121].
New methodologies for documenting and measuring wounds can help both with monitoring treatment and preventing legal complications. Highly advanced computerized documentation is being used by many organizations. Techniques are now available to thoroughly assess the geometric, physiologic (i.e., pressure and degree of wound perfusion), and biochemical (i.e., concentrations of various enzymes involved in tissue degradation and healing) aspects of the wound.
Electronic records and images are preferred by many institutions. The role of wound photography is growing and is a vital aspect of monitoring response to treatment and in defending against possible future litigation. Approximately 75% of home health agencies in the United States now include wound photography as part of the patient's medical record [35].
Ultrasound has been explored as a diagnostic and monitoring tool. High-frequency ultrasound provides greater resolution than other ultrasound techniques, but the depth of penetration of the sound waves is less. Therefore, it is ideal for imaging near-surface pathology. High-resolution ultrasound can easily detect the fluid content of tissue and measure skin thickness. More research is necessary to determine the efficacy and cost effectiveness of ultrasound for the routine care of pressure injuries.
Although the available skin substitutes are not perfect, they do offer benefits when treating pressure injuries, particularly those resistant to healing. Research in the field of skin tissue engineering focuses on improving the skin barrier properties and the structural interaction of the epidermal and dermal equivalent of composite grafts. There are two types of bioengineered skin replacements available: biosynthetic skin substitutes and skin grafts.
Biosynthetic skin substitutes are developed without allogenic cells and consist of a mesh coated with a dermal analogue (bilayer) or an acellular dressing (monolayer). They act as starting points to promote ingrowth of tissue in the wound. The bilayer options also include a removable silicone layer that functions as a protective dressing.
Skin grafts may be categorized as xenografts, autografts, or allografts. A xenograft is derived from a non-human source, usually porcine skin. An autograft is a graft taken from the patient, while an allograft is transplanted from a genetically non-identical individual, often from a cadaver donor. Grafts may be further identified as dermal or epithelial/epidermal (or both) and as cultured or processed. Within these categories, there are several specific options, each with their own benefits and drawbacks [122]. It has been suggested that the properties inherent to skin grafts, such as the presence of hair follicles, may make them a good option to accelerate wound healing [123]. Selection of the best option is dependent on the features of the wound, the goals of treatment, and patient preferences.
Gene therapy has a tremendous potential, but use of the technology in the treatment of pressure injuries is still in its infancy. Gene therapy has been proposed to accelerate wound healing and also to reduce healing complications such as keloid formation or chronic ulceration.
Genetically modified skin grafts have a potential use for treatment of large wounds. Smaller wounds may be amenable to in vivo delivery of genetic material using a variety of approaches including gene injection, gene gun, microseeding, and liposomal gene delivery. The preclinical studies of genetically modified skin grafts are promising, but more tests are necessary to determine the effectiveness of this treatment option [124,125].
Negative pressure wound devices provide an environment that resolves edema and hematoma and results in increased local perfusion. Newer negative pressure wound devices are smaller and more portable than earlier devices, and some deliver continuous subatmospheric pressure to the wound bed to promote healing. It should be noted that these devices have been associated with extensive bleeding, particularly in patients with blood vessel grafts in the leg, with breastbone or groin wounds, or who are receiving anticoagulant therapy [126]. Hemorrhage may also occur when dressings attached to the tissues are removed.
Biologic wound products accelerate healing by augmentation or modulation of inflammatory mediators. Growth factors that hold great promise in wound healing include eicosanoids, prostaglandin E1, cytokines, and interleukin-1.
Lasers may also accelerate the process of tissue repairing [127]. The lasers appear to simulate fibroblastic activity, although the mechanism is not yet fully understood.
Nanoparticles containing chitosan have been shown to have effective antimicrobial activity against Staphylococcus saprophyticus and Escherichia coli [127]. These materials could be used to prevent infection and facilitate wound healing.
Researchers have shown that a novel peptide (UN3) created by the combination of two naturally occurring peptides found in platelet-rich plasma stimulates wound vascularization and promotes epithelial proliferation. This could lead to new treatments for pressure injuries [128]. The use of novel peptides derived from platelet-derived growth factor (PDGF-BB) also shows promise for wound healing and treatment [129]. PDGF stimulates bone cell replication and DNA synthesis.
Electrical stimulation may also accelerate healing in pressure injuries, but its effect on time to complete healing is uncertain compared with no electrical stimulation [130,131]. Free radical scavengers and special drug delivery systems can also be effective in prevention of injuries [132,133,134].
Pressure injuries are the second most common medicolegal claim after wrongful death, resulting in more than 17,000 lawsuits annually in the United States [135]. Results of a retrospective study published in 2019 examined 141 cases related to pressure injuries over a 32-year period from 1987 to 2019 [136]. Of the 141 cases, 75.9% were for negligence and 22.7% were for malpractice. Hospitals were listed as the defendant 61.7% of the time, nursing homes 31.2% of the time, and individual providers 7.1% of the time. Hospitals lost 63% of the cases brought against them, nursing homes lost 75%, and individual providers lost 20%. Hospitals paid out approximately $1.6 million to plaintiffs, nursing homes $4 million, and individual providers an average of $400,000 [136].
As litigation is becoming more common, adherence to established standards of care is very important and has legal implications [137]. Some experts have suggested that if institutional neglect is responsible for development of pressure injuries, caregivers could be liable to criminal prosecution.
Federal law establishes standards for long-term care facilities [138]. Based on the comprehensive assessment of a resident, the facility must ensure that 1) a resident who enters the facility without pressure sores does not develop pressure sores unless the individual's clinical condition demonstrates that they were unavoidable; and 2) a resident having pressure sores receives necessary treatment and services to promote healing, prevent infection, and prevent new sores from developing.
As noted, despite best patient care and treatment, not all pressure injuries are avoidable [139]. In long-term care, the NPIAP defines an unavoidable injury as one that occurs even though "the facility had evaluated the individual's clinical condition and pressure injury risk factors; defined and implemented interventions that are consistent with individual needs, goals, and recognized standards of practice; monitored and evaluated the impact of the interventions; and revised the approaches as appropriate" [140]. In addition to establishing the definition for long-term care facilities, in 2014 the NPIAP sought to establish consensus (80% agreement among conference delegates) on whether pressure injury development may be unavoidable in some individuals and whether there is a difference between pressure injuries and end-of-life skin changes. Unanimous consensus was reached for the following statements [141]:
Most (but not all) pressure injuries are avoidable.
Comorbid conditions can contribute to unavoidable pressure injuries.
Some situations render pressure injury development unavoidable, including:
Hemodynamic instability worsened by physical movement
Poor nutrition/hydration status and/or advanced directive that prohibits artificial nutrition/hydration
Pressure redistribution surfaces cannot replace turning/repositioning
Skin cannot always survive even when pressure from external body skin is alleviated
In light of the significant changes to pressure injury prevention in acute care hospitals brought about by the COVID-19 pandemic, the NPIAP additionally published position statements on pressure injuries that develop during COVID-19 crisis situations in these healthcare settings [141].
Facilities should institute adequate measures to prevent wounds, and in the case an injury develops, staff should respond appropriately to prevent worsening of the wound. Wounds detected in the early stages have a greater chance of cure than later stage wounds that are infected or necrotic. Therefore, regular assessment and early intervention are required for all injuries, avoidable or not. The presence of risk factors, including comorbidities, inability to maintain adequate nutrition and hydration status (e.g., if an advance directive prohibits artificial feeding), certain drugs, and immobility, is an indicator of avoidability. After assessment of risk factors, the next step is to ascertain if the response was timely according to documentation of all interventions. If any skin ulceration develops in spite of timely, appropriate interventions, the injury may be considered unavoidable.
Suspicion that improper medical care resulting in the development and/or worsening of a pressure injury requires a full, document-intensive investigation. Because many factors can be indicative of medical neglect, each domain of care should be assessed (Table 8). Furthermore, the best defense against a claim of neglect is complete documentation of appropriate care.
New or worsening pressure injuries are generally due to poor administrative organization, understaffing, and poor training. The medico-legal implications of proper care cannot be overlooked, as those injuries are responsible for a significant proportion of healthcare litigation.
The medical chart should be examined to determine the level of care planning, assessment, and interventions. Wound neglect is usually associated with improper treatment, inadequate nutrition, and/or poor survey results.
Family diaries, stage surveys, in-service records, complaint files, minutes of quality assurance meetings, and photographs can also be helpful for identification of deviations from standard injury care.
Documentation of pressure injury care must be timely and detailed. Regular and consistent descriptions of the injury should be documented, as should responses to treatment. Improper, irregular, or "late entries" are absolutely impermissible and unacceptable.
In patients with multiple injuries, progression of each wound should be documented and tracked. With the growing awareness of the possibility of litigation in wound care, many facilities have instigated a policy of photo documentation. Wounds present on admission are photographed, and some facilities require serial photographs to track wound progress and status at the time of discharge. Signed consent is required before wound photographs can be taken, and in most instances, the photographs become a permanent part of the patient's medical record.
It has been found that 30% to 85% of nursing home residents are malnourished and 30% to 50% are considered underweight [112]. Pressure injury treatment requires proper nutritional assessment and intervention during every stage. Weight loss, lab results, protein consumption, and vitamin levels should be documented, as should attempts to address abnormalities. If tube feeding is indicated, it should be discussed with the family prior to initiation.
Treatment administration records should be examined to ensure that treatments were carried out per orders. Documentation should also include staffing records and time-sheets. Dates and times should always be included.
Minimum data sets are required for all residents of skilled nursing facilities. Improper and inaccurate entries in the minimum data sets indicate a facility with under-staffing and disorganized structure providing poor treatment to the resident. Scrutiny of this document can lead to litigation.
A vital component of any pressure injury program is patient/family education, with an overall goal of decreasing the incidence of injury development or recurrence. If possible, pressure injury prevention should not be a passive process for the patient and his/her family members. Rather, it should be a dialogue in which the patient and family feel comfortable asking questions and discussing problems. Patients should have as much control as possible in the plan of care. Empowerment is very important in maintaining the patient's physical and emotional well-being, and the plan of care should be explained thoroughly to cognitively aware patients and/or their family. It is important for everyone involved to appreciate that the prevention of pressure injury formation is a lifelong process [142].
At the same time, it is necessary to evaluate the patient's/family's existing knowledge regarding pressure and pressure injuries. Healthcare professionals should show patients what they can do to facilitate pressure relief (e.g., how to make small position changes while in the chair). If possible, teach patients how to do simple range-of-motion exercises. Take time to train the patient as often as is appropriate; not everyone will absorb the information the first time they hear it [4]. It is important not to let noncompliance or a bad attitude from the patient or family discourage the teaching process. The subject should be approached as often as is reasonable. Include the family members and caregivers in the instructions; as well as assisting with care, they can encourage compliance. All efforts at patient and family/caregiver education should be documented, along with the patient's response (both verbal and behavioral).
Different methods of teaching, such as photographs, videos, charts, diagrams, and written materials in the patient's native language, should be used. Education should be reinforced regularly and consistently [143]. The information provided to patients and/or their families should be specific to the individual treatment plan and goals.
Patient education programs should include all of the following areas:
Etiology of pressure injuries
Reduction of risk
Reduction of friction and shear
Skin protection and inspection
Importance of nutrition
Proper and safe cleansing procedures and agents
Procedure for recurrence(s)
Management approaches
Proper dressing change procedure
Patients should be provided information about factors involved in pressure injury development. The role of pressure, friction, shear, and moisture in the development of injuries should be explained. Other conditions that can impact pressure injury development should be outlined, including:
Immobility (partial or total)
Excessive moisture and/or incontinence
Poor local or systemic circulation
Uncontrolled diabetes
Severe trauma
Dehydration
Hypoxia
Malnutrition
Sepsis
Previous history of pressure injury
Pressure reduction techniques should be thoroughly explained. Proper use of mattresses, cushions, and overlays and the placement of pillows, heel protectors, and wedges are essential. The role of repositioning and turning of the patient should be stressed. If the patient will be sitting in a chair for a long period of time, pressure release tactics should be included. Repositioning should be done every one to two hours, depending on the patient's condition [49].
Patients and/or caregivers should have a clear understanding of measures to decrease friction and shear. Education should include the proper body alignments that should be maintained when sitting in a chair or lying on a bed. The importance of using lifting devices for repositioning or transferring should be stressed.
Careful skin inspection is paramount in the care of pressure injuries. The patient or caregiver should carefully inspect the skin for new openings or breaks in the skin at every repositioning. Any discoloration or redness of the skin that does not resolve within 30 minutes after changing position should be reported.
Maintaining skin cleanliness and moisturizing frequently can protect skin integrity. The skin should be cleaned with water and a gentle soap, preferably a pH-balanced cleanser. Alkaline products remove skin lipids, which increases water loss and weakens the barrier function of the skin [2]. Avoid hot water for bathing and scrubbing or using harsh cleaning agents. A soft cloth should be used to pat rather than rub the skin dry. Thromboembolic deterrent hose should be removed when bathing, and the nurse or physician should be notified of any redness, discoloration, or skin breakdown.
It is important to individualize the frequency of skin cleansing based on the patient's age, skin texture, and dryness or excessive oiliness of the skin. A daily bath may not be needed for all patients.
The epidermis is about 30% water, but through a process called trans-epidermal water loss, skin can lose its natural moisture. Without sufficient moisture, skin can become dry, brittle, and vulnerable to breakdown [10]. Therefore, products should be used to keep the skin supple. Emollients, such as mineral oil, petrolatum, and lanolin, penetrate into the stratum corneum to increase the lipid component and add softness to the skin. The oil film on the skin surface also prevents water loss and helps to rehydrate the stratum corneum [10]. Moisture barriers such as dimethicone can prevent water loss and help to retain lipids and water within the skin cells [10].
Humectants, such as glycerin, urea, and lactic acid, increase the water content of the stratum corneum by pulling water from the environment. All moisturizers should be applied to clean, slightly moist skin. Special attention should be paid to bony prominences, heels, ears, and the back of the head.
Adequate nutrition is an essential area of patient education, as malnutrition makes individuals susceptible to the development of pressure injuries and at increased risk for infections, including sepsis, necrotizing fasciitis, and gangrene. Patients must also understand that adequate hydration is essential to improve tissue perfusion and excreting waste products. Patients/caregivers should make sure they comply with dietitian recommendations for diet, adequate fluid intake, and nutritional supplements.
Patients should be advised to take the following actions should a pressure injury develop:
Increase the frequency of repositioning and turning.
Note the size, location, odor, color, and drainage of the wound and adjacent tissue.
Notify the physician immediately about this new development.
If the patient is receiving home care services, the home health nurse should be notified immediately.
After a treatment plan is set, all aspects should be explained to the patient or caregiver again. Key education points include:
Products being used for wound prevention or healing
Where to procure dressings and pressure redistribution surfaces
Signs and symptoms of pressure injury deterioration
The importance of compliance to the established plan
Patients should be advised to look for new necrotic tissue in the wound bed, wound drainage with odor, and erythema and induration around the injury. If a clean wound enlarges or becomes deeper, the patient should be instructed to contact his or her physician immediately.
Proper dressing change technique should be demonstrated. The patient/caregiver should be able to change the dressing without any assistance from the supporting staff, and clean technique should be used. Hand washing, use of gloves, and infection prevention should all be a part of basic patient education.
Pressure injuries are a common but preventable condition frequently seen in elderly individuals and those with comorbid conditions. In the United States, the incidence of pressure injuries ranges from 0% to 40%, depending on the site of care [2].
Pressure injuries are a major psychologic, physical, and social burden to patients and often result in significantly decreased quality of life. In addition, they are a major source of healthcare expenditures. The healthcare costs related to the care of patients with pressure injuries exceeds $10 billion annually in the United States [9].
Evolution of pressure injuries is multifactorial, and individuals with specific risk factors are more susceptible to injury development. Without proper care and treatment, superficial injuries progress to more serious deep tissue wounds, often with life-threatening complications. Satisfactory resolution of a well-established pressure injury is difficult, time-consuming, and costly; it is far better to prevent the injury in the first place. An interdisciplinary approach of creating a care plan that includes steps for the prevention of injuries is the best practice.
1. Thomas DR. The new F-tag 314: prevention and management of pressure ulcers. J Am Med Dir Assoc. 2006;7(8):523-531.
2. The National Pressure Ulcer Advisory Panel. Pressure ulcers prevalence, cost and risk assessment: consensus development conference statement. Decubitus. 1989;2(2):24-28.
3. The National Pressure Ulcer Advisory Panel. Pressure ulcers in America: prevalence, incidence, and implications for the future. An executive summary of the National Pressure Ulcer Advisory Panel monograph. Adv Skin Wound Care. 2001;14(4):208-215.
4. Reddy M, Gill SS, Rochon PA. Preventing pressure ulcers: a systematic review. JAMA. 2006;296(8):974-984.
6. Tauqir SF, Mirza S, Gul S, Ghaffar H, Zafar A. Complications in patients with spinal cord injuries sustained in an earthquake in northern Pakistan. J Spinal Cord Med. 2007;30(4):373-377.
7. Lyder CH, Wang Y, Metersky M, et al. Hospital-acquired pressure ulcers: results from the national Medicare Patient Safety Monitoring System study. J Am Geriatr Soc. 2012;60:1603-1608.
8. Bergquest S, Frantz R. Braden Scale validity in community-based older adults receiving home health care. Appl Nurs Res. 2001;14(1):36-43.
9. Agency for Healthcare Research and Quality. Preventing Pressure Ulcers in Hospitals: A Toolkit for Improving Quality of Care. Available at https://www.ahrq.gov/topics/pressure-ulcers.html. Last accessed April 20, 2022.
10. Emory University School of Medicine Wound, Ostomy and Continence Nursing Education Center. Skin and Wound Module. 6th ed. Atlanta, GA: Emory University WOCNEC; 2006.
11. Takahashi PY, Chandra A, Cha SS. Risk factors for pressure ulceration in an older community-dwelling population. Adv Skin Wound Care. 2011;24(2):72.
12. Horn SD, Bender SA, Ferguson ML, et al. The National Pressure Ulcer Long-Term Care Study: pressure ulcer development in long-term care patients. J Am Geriatr Soc. 2004;52(3):369.
13. Bryant RA, Nix DP. Acute and Chronic Wounds: Current Management Concepts. 5th ed. St. Louis, MO: Elsevier; 2015.
14. Hall JE (ed). Guyton and Hall Textbook of Medical Physiology. 13th ed. Philadelphia, PA: Saunders Elsevier; 2015.
15. Baranoski S, Ayello EA. Wound Care Essentials: Practice Principles. 4th ed. Ambler, PA: Lippincott Williams & Wilkins; 2015.
16. Dolynchuk K, Keast D, Campbell K, et al. Best practices for the prevention and treatment of pressure ulcers. Ostomy Wound Manage. 2000;46(11):38-52.
17. Shaked E, Gefen A. Modeling the effects of moisture-related skin-support friction on the risk for superficial pressure ulcers during patient repositioning in bed. Front Bioeng Biotechnol. 2013;1:9.
18. Schwartz D, Magen YK, Levy A, Gefen A. Effects of humidity on skin friction against medical textiles as related to prevention of pressure injuries. Int Wound J. 2018;15(6):866-874.
19. Stasik L. Hospital-Acquired Pressure Ulcers: Making the Nutrition Connection. Columbus, OH: Abbott Nutrition; 2008.
20. Breslow RA, Hallfrisch J, Guy DG, Crawley B, Goldberg AP. The importance of dietary protein in healing pressure ulcers. J Am Geriatr Soc. 1993;41(4):357-362.
21. Langer G, Fink A, Nutritional interventions for preventing and treating pressure ulcers. Cochrane Database Syst Rev. 2014;(6):CD003216.
22. Yap JW, Holloway S. Evidence-based review of the effects of nutritional supplementation for pressure ulcer prevention. Int Wound J. 2021;18(6):805-821.
23. Smith BM, Guihan M, LaVela SL, Garber SL. Factors predicting pressure ulcers in veterans with spinal cord injuries. Am J Phys Med Rehabil. 2008;87(9):750-757.
24. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS Data Brief, No 360. Hyattsville, MD: National Center for Health Statistics; 2020.
25. Centers for Disease Control and Prevention. Overweight and Obesity: Defining Adult Overweight and Obesity. Available at https://www.cdc.gov/obesity/adult/defining.html. Last accessed April 20, 2022.
26. Gallagher S. The Challenges of Caring for the Obese Patient. Edgemont, PA: Matrix Medical Communications; 2005.
27. Bergstrom N, Braden BJ, Laguzza A, Holman V. The Braden Scale for Predicting Pressure Sore Risk. Nurs Res. 1987;36:205-210.
28. Agency for Healthcare Research and Quality. Preventing Pressure Ulcers in Hospitals: Section 7: Tools and Resources. Available at https://www.ahrq.gov/patient-safety/settings/hospital/resource/pressureulcer/tool/pu7b.html. Last accessed April 20, 2022.
29. Haesler E (ed). Prevention and Treatment of Pressure Ulcers: Quick Reference Guide. Osborne Park: Cambridge Media; 2014.
30. National Pressure Injury Advisory Panel. Pressure Injury Stages: Pressure Injury Illustrations. Available at https://npiap.com/page/PressureInjuryStages. Last accessed April 20, 2022.
31. Kirman CN. Pressure Injuries (Pressure Ulcers) and Wound Care Treatment and Management. Available at https://emedicine.medscape.com/article/190115-treatment. Last accessed April 20, 2022.
32. Flanagan M. Improving accuracy of wound measurement in clinical practice. Ostomy Wound Manage. 2003;49(10):28-40.
33. Ferrell BA, Osterweil D, Christenson P. A randomized trial of low-air-loss beds for treatment of pressure ulcers. JAMA. 1993;269(4):494-497.
34. Brandeis GH, Morris JN, Nash DJ, Lipsitz LA. The epidemiology and natural history of pressure ulcers in elderly nursing home residents. JAMA. 1990;264(22):2905-2909.
35. Sussman C, Bates-Jensen BM. Wound Care: A Collaborative Practice Manual for Health Professionals. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2012.
36. National Pressure Injury Advisory Panel. 2019 Guideline. Available at https://npiap.com/page/2019Guideline. Last accessed April 20, 2022.
37. Remsburg RE, Bennett RG. Pressure-relieving strategies for preventing and treating pressure sores. Clin Geriatr Med. 1997;13(3):513-541.
38. Agostini JV, Baker DI, Bogardus ST. Prevention of Pressure Ulcers in Older Patients. Available at https://archive.ahrq.gov/clinic/ptsafety/chap27.htm. Last accessed April 20, 2022.
39. McInnes E, Jammali-Blasi A, Bell-Syer SEM, Dumville, JC, Middleton V, Cullum N. Support surfaces for pressure ulcer prevention. Cochrane Database Syst Rev. 2015;(9):CD001735.
40. Thomas DR, Goode PS, Tarquine PH, Allman RM. Hospital-acquired pressure ulcers and risk of death. J Am Geriatr Soc. 1996;44(12):1435-1440.
42. Posthauer ME, Banks M, Dorner B, Schols JM. The role of nutrition for pressure ulcer management: National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel, and Pan Pacific Pressure Injury Alliance white paper. Adv Skin Wound Care. 2015;28:175-188.
43. Cereda E, Gini A, Pedrolli C, Vanotti A. Disease-specific, versus standard, nutritional support for the treatment of pressure ulcers in institutionalized older adults: a randomized controlled trial. J Am Geriatr Soc. 2009;57:1395-1402.
44. Rodeheaver GT, Kurtz L, Kircher BJ, Edlich RF. Pluronic F-68: a promising new skin wound cleanser. Ann Emerg Med. 1980;9(11):572-576.
45. Bergstrom N, Bennett MA, Carlson CE, et al. Treatment of Pressure Ulcers Guideline Panel. Rockville, MD: U.S. Department of Health and Human Services; 1994.
46. AHRQ Panel for the Prediction and Prevention of Pressure Ulcers in Adults. Pressure Ulcers in Adults: Prediction and Prevention. Clinical Practice Guideline Number 3. Rockville, MD: Agency for Healthcare Research and Quality; 1992.
47. Manna B, Nahirniak P, Morrison CA. Wound Debridement. Treasure Island, FL: StatPearls Publishing; 2022.
48. Wynne JJ, Felsenstein JM, Trzcinski R, Zupanski-Nielsen D, Connors DP. Collateral damage-free debridement using 193nm ArF laser. Proc SPIE 7883. Paper presented at: Photonic Therapeutics and Diagnostics VII; February 17, 2011; San Francisco, CA.
50. Butcher G. Low frequency ultrasonic debridement: a new tool in our armoury? J Foot Ankle Res. 2011;4(Suppl 1):P7.
51. Tombulturk FK, Kanigur-Sultuybek G. A molecular approach to maggot debridement therapy with Lucilia sericata and its excretions/secretions in would healing. Wound Repair Regen. 2021;29(6):1051-1061.
52. Pearson C. Something old is new again: debriding and reducing local wound infection with maggots. Wound Care Canada. 2007;5(2):22-26.
53. Kirshen C, Woo K, Ayella EA, Sibbald RG. Debridement: a vital component of wound bed preparation. Adv Skin Wound Care. 2006;19(9):506-517.
54. Ayello EA, Cuddigan JE. Debridement: controlling the necrotic/cellular burden. Adv Skin Wound Care. 2004;17(2):66-75.
55. Bluestein D, Javaheri A. Pressure ulcers: prevention, evaluation, and management. Am Fam Physician. 2008;78(10):1186-1194.
56. Roth RS, Lowery J, Hamill J. Assessing persistent pain and its relation to affective distress, depressive symptoms and pain catastrophizing in patients with chronic wounds: a pilot study. Am J Phys Med Rehabil. 2004;83:827-834.
58. Thomas DR. Prevention and treatment of pressure ulcers: what works? What doesn't? Cleve Clin J Med. 2001;68(8):704-722.
59. Romanelli M, Magliaro A, Mastronicola D, Siani S. Systemic antimicrobial therapies for pressure ulcers. Ostomy Wound Manage. 2003;49(5A Suppl):25-29.
60. Meaume S, Vallet D, Morere MN, Téot L. Evaluation of a silver-releasing hydroalginate dressing in chronic wounds with signs of local infection. J Wound Care. 2005;14:411-419.
61. Institute for Clinical Systems Improvement. Pressure Ulcer Prevention and Treatment Protocol. Bloomington, MN: Institute for Clinical Systems Improvement; 2012.
62. Stefanovska A, Vodovnik L, Benko H, Turk R. Treatment of chronic wounds by means of electric and electromagnetic fields. Part 2: value of FES parameters for pressure sore treatment. Med Biol Eng Comput. 1993;313:213-220.
63. Baker L, Rubayi S, Villar F, Demuth SK. Effect of electrical stimulation waveform on healing of ulcers in human beings with spinal cord injury. Wound Rep Regen. 1996;4(1):21-28.
64. Qaseem A, Mir TP, Starkey M, Denberg TD. Risk assessment and prevention of pressure ulcers: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2015;162(5):359-369.
65. Qaseem A, Humphrey LL, Forciea MA, Starkey M, Denberg TD. Treatment of pressure ulcers: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2015;162(5):370-379.
66. Arora M. Harvey LA, Glinsky JV, et al. Electrical stimulation for treating pressure ulcers. Cochrane Database Syst Rev. 2020;(1):CD012196.
67. Trengove NJ, Stacey MC, MacAuley S, et al. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Rep Regen. 1999;7(6):442-452.
68. Pierce GF, Tarpley JE, Tseng J, et al. Detection of platelet-derived growth factor PDGF-AA in actively healing wounds treated with recombinant PDGF-BB and absence of PDGF in chronic nonhealing wounds. J Clin Invest. 1995;96(3):1336-1350.
69. Jiang L, Dai Y, Cui F, et al. Expression of cytokines, growth factors and apoptosis-related signal molecules in chronic pressure ulcer wounds healing. Spinal Cord. 2014;52(2):145-151.
70. Mast BA, Schultz G. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Rep Regen. 1996;4(4):411-420.
71. Papanas N, Maltezos E. Becaplermin gel in the treatment of diabetic neuropathic foot ulcers. Clin Interv Aging. 2008;3(2):233-240.
73. Kranke P, Bennett MH, Martyn-St. James M, Schnabel A, Debus SE, Weibel S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2015;(6):CD004123.
74. Kloth LC, Berman JE, Dumit-Minkel S, Sutton CH, Papanek PE, Wurzel J. Effects of a normothermic dressing on pressure ulcer healing. Adv Skin Wound Care. 2000;13(2):69-74.
75. Baba-Akbari Sari A, Flemming K, Cullum NA, Wollina U. Therapeutic ultrasound for pressure ulcers. Cochrane Database Syst Rev. 2006;(3):CD001275.
76. Alkahtani SA, Kunwa PS, Jalilifar M, Rashidi S, Yadollahpour A. Ultrasound-based techniques as alternative treatments for chronic wounds: a comprehensive review of clinical applications. Cureus. 2017;9(12):e1952.
77. Pezzetti F, DeMattei M, Caruso A, et al. Effects of pulsed electromagnetic fields on human chondrocytes: an in vitro study. Calcif Tissue Int. 1999;65(5):396-401.
78. Garland DE, Adkins RH, Matsuno NN, Stewart CA. The effect of pulsed electromagnetic fields on osteoporosis at the knee in individuals with spinal cord injury. J Spinal Cord Med. 1999;22(4):239-245.
79. Roland D, Ferder M, Kothuru R, Faierman T, Strauch B. Effects of pulsed magnetic energy on a microsurgically transferred vessel. Plast Reconstr Surg. 2000;105(4):1371-1374.
80. Griffin JW, Tooms RE, Mendius RA, Clifft JK, Vander ZR, el Zeky F. Efficacy of high voltage pulsed current for healing of pressure ulcers in patients with spinal cord injury. Phys Ther. 1991;71(6):433-442.
81. Salzberg CA, Cooper-Vastola SA, Perez F, Viehbeck MG, Byrne DW. The effects of non-thermal pulsed electromagnetic energy on wound healing of pressure ulcers in spinal cord-injured patients: a randomized, double-blind study. Ostomy Wound Manage. 1995;41(3):42-44, 46, 48.
83. Hess CT. Clinical Guide to Skin and Wound Care. 7th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012.
84. Conway H, Griffith B. Plastic surgery for closure of decubitus ulcers in patients with paraplegia, based on experience with 1000 cases. Am J Surg. 1956;91(6):946-975.
85. Wong JKF, Amin K, Dumville JC. Reconstructive surgery for treating pressure ulcers. Cochrane Database Syst Rev. 2016;(12):CD012032.
86. Harris C, Bates-Jensen B, Parslow N, Raizman R, Singh M, Ketchen R. Bates-Jensen wound assessment tool: pictorial guide validation project. J Wound Ostomy Continence Nurs. 2010;37(3):253-259.
87. National Pressure Ulcer Advisory Panel. PUSH Tool 3.0. Available at https://www.sralab.org/sites/default/files/2017-06/push3.pdf. Last accessed April 20, 2022.
89. Szor JK, Bourguignon C. Description of pressure ulcer pain at rest and at dressing change. J WOCN. 1999;26(3):115-120.
91. Wong DL, Hockenberry-Eaton M, Wilson D, Winkelstein ML, Schwartz P. Wong's Essentials of Pediatric Nursing: Wong-Baker Faces Pain Rating Scale. 6th ed. St. Louis, MO: Mosby; 2001.
92. Bergstrom N, Allman R, Alvaraz O, et al. Treatment of Pressure Ulcers: Clinical Guideline Number 15. Publication No. 95-0652. Washington, DC: Agency for Healthcare Policy and Research; 1994.
93. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis 3). JAMA. 2016;315(8):803-810.
94. Society of Critical Care Medicine. Surviving Sepsis Campaign 2021 Adult Guidelines. Available at https://www.sccm.org/SurvivingSepsisCampaign/Guidelines/Adult-Patients. Last accessed April 20, 2022.
95. Vincent JL, de Mendonça A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Crit Care Med. 1998;26(11):1793-1800.
96. MD CALC. qSOFA (Quick SOFA) Score for Sepsis. Available at https://www.mdcalc.com/qsofa-quick-sofa-score-sepsis. Last accessed April 20, 2022.
97. Paul M, Lador A, Grozinsky-Glasberg S, Leibovici L. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev. 2014;(1):CD003344.
100. Leiblein M, Wagner N, Adam EH, Frank J, Marzi I, Nau C. Clostridial gas gangrene – a rare but deadly infection: case series and comparison to other necrotizing soft tissue infections. Orthop Surg. 2020;12(6):1733-1747.
101. Yang Z, Hu J, Qu Y, et al. Interventions for treating gas gangrene. Cochrane Database Syst Rev. 2015;(12):CD010577.
102. Hart GB, Strauss MB. Gas gangrene—clostridial myonecrosis: a review. J Hyperbaric Med. 1990;5(2):125-144.
103. Zamboni WA, Riseman JA, Kucan JO. Management of Fournier's gangrene and the role of hyperbaric oxygen. J Hyperbaric Med. 1990;5(3):177-186.
104. Sugarman B, Hawes S, Musher DM, Klima M, Young EJ, Pircher F. Osteomyelitis beneath pressure sores. Arch Intern Med. 1983;143(4):683-688.
105. Kaplan LJ, Pameijer C, Blank-Reid C, Granick MS. Necrotizing fasciitis: an uncommon consequence of pressure ulceration. Adv Wound Care. 1998;11(4):185-189.
106. Leiblein M, Marzi I, Sander AL, Barker JH, Ebert F, Frank J. Necrotizing fasciitis: treatment concepts and clinical results. Eur J Trauma Emerg Surg. 2018;44(2):279-290.
107. Bates-Jensen BM, Guihan M, Garber SL, Chin AS, Burns SP. Characteristics of recurrent pressure ulcers in veterans with spinal cord injury. J Spinal Cord Med. 2009;32(1):34-42.
108. Lyder CH, Preston J, Grady JN, et al. Quality of care for hospitalized Medicare patients at risk for pressure ulcers. Arch Intern Med. 2001;161(12):1549-1554.
109. Vasavada SP. Urinary Incontinence: Epidemiology. Available at https://emedicine.medscape.com/article/452289-overview#a6. Last accessed April 20, 2022.
110. Levine JM. Historical perspective: the neurotrophic theory of skin ulceration. J Am Geriatr Soc. 1992;40(12):1281-1283.
111. Mills JI. Evaluation and management of peripheral arterial disease. In: Armstrong DG, Lavery LA. Clinical Care of the Diabetic Foot. Alexandria, VA: American Diabetes Association; 2005: 45-51.
112. Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas. 2007;28:R1-R39.
113. Stephens E. Peripheral Vascular Disease Workup. Available at https://emedicine.medscape.com/article/761556-workup#c6. Last accessed April 20, 2022.
114. Sontheimer DL. Peripheral vascular disease: diagnosis and treatment. Am Fam Physician. 2006;73(11):1971-1976.
115. Gutierrez G, Reines HD, Wulf-Gutierrez ME. Clinical review: hemorrhagic shock. Crit Care. 2004;8(5):373-381.
116. Ellis CG, Jagger J, Sharpe M. The microcirculation as a functional system. Crit Care. 2005;9(Suppl 4):S3-S8.
117. Masaki F, Riko K, Seiji H, Shuhei Y, Aya Y. Evaluation of pressure ulcers in 202 patients with cancer. Wounds. 2007;19(1):13-19.
118. National Institute of Mental Health. Statistics. Available at https://www.nimh.nih.gov/health/statistics/index.shtml. Last accessed April 20, 2022.
119. Galhardo VAC, Magalhães MG, Blanes L, Juliano Y, Ferreira LM. Health-related quality of life and depression in older patients with pressure ulcers. Wounds. 2010;22(1):20-26.
120. Barbenel JC, Ferguson-Pell MW, Kennedy R. Mobility of elderly patients in bed: measurement and association with patient condition. J Am Geriatr Soc. 1986;34(9):633-636.
122. Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20(9 Pt 1):493-508.
123. Han I, Shim KJ, Kim JY, et al. Effect of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) nanofiber matrices cocultured with hair follicular epithelial and dermal cells for biological wound dressing. Artif Organs. 2007;31(11):801-808.
124. Jones JE, Nelson EA, Al-Hity A. Skin grafting for venous leg ulcers. Cochrane Database Syst Rev. 2013;1:CD001737.
125. Koyama T, Hackl F, Aflaki P, et al. A new technique of ex vivo gene delivery of VEGF to wounds using genetically modified skin particles promotes wound angiogenesis. J Am Coll Surg. 2011;212(3):340-348.
126. U.S. Food and Drug Administration. Negative Pressure Wound Devices Draw FDA Notice, Advice. Available at https://wayback.archive-it.org/7993/20170112131329/http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm193277.htm. Last accessed April 20, 2022.
127. Leonida MD, Banjade S, Vo T, Anderle G, Haas GJ, Philips N. Nanocomposite materials with antimicrobial activity based on chitosan. Int J Nano Biomater. 2011;3(4):310-334.
128. Demidova-Rice TN, Wolf L, Deckenback J, Hambli MR, Herman IM. Human platelet-rich plasma- and extracellular matrix-derived peptides promote impaired cutaneous wound healing in vivo. PLoS ONE. 2012;7(2):e32146.
129. Deptula M, Karpowicz P, Wardowska, et al. Development of a peptide derived from platelet-derived growth factor (PDGF-BB) into a potential drug candidate for the treatment of wounds. Adv Wound Care (New Rochelle). 2020;9(12):657-675.
130. Levine SP, Kett RL, Gross MD. Blood flow in the gluteus maximus of seated individuals during electrical muscle stimulation.Arch Phys Med Rehabil. 1990;71(9):682-686.
131. Arora M, Harvey LA, Glinsky JV, et al. Electrical stimulation for treating pressure ulcers. Cochrane Database Syst Rev. 2020;(1):CD012196.
132. Salcido R, Carney J, Fisher S, et al. A reliable animal model of pressure sore development: the role of free radicals. J Am Paraplegia Soc. 1993;16:61.
133. Salcido R, Donofrio JC, Fisher SB, et al. Histopathology of pressure ulcers as a result of sequential computer-controlled pressure sessions in a fuzzy rat model. Adv Wound Care. 1994;7(5):23-24, 26, 28.
134. Salcido R, Fisher SB, Donofrio JC, et al. An animal model and computer-controlled surface pressure delivery system for the production of pressure ulcers. J Rehabil Res Dev. 1995;32(2):149-161.
135. Agency for Healthcare Research and Quality. Preventing Pressure Ulcers in Hospitals: Are We Ready For This Change? Available at https://www.ahrq.gov/patient-safety/settings/hospital/resource/pressureulcer/tool/pu1.html. Last accessed April 20, 2022.
136. Jehle CC, Hartnett D, Snapp WK, Schmidt S. Assessment of malpractice claims associated with pressure ulcers. Plast Reconstr Surg Glob Open. 2019;7(8 Suppl):S90.
138. GovInfo. Content Details: 42 CFR 483.25: Quality of Care. Available at https://www.govinfo.gov/app/details/CFR-2011-title42-vol5/CFR-2011-title42-vol5-sec483-25. Last accessed April 11, 2022.
139. Burger SG, Kayser-Jones J, Prince Bell J. Malnutrition and Dehydration in Nursing Home Residents: Key Issues in Prevention and Treatment. Available at https://www.commonwealthfund.org/publications/fund-reports/2000/jul/malnutrition-and-dehydration-nursing-homes-key-issues-prevention. Last accessed April 20, 2022.
140. Black JM, Edsberg LE, Baharestani MM, et al. Pressure ulcers: avoidable or unavoidable? Results of the National Pressure Ulcer Advisory Panel Consensus Conference. Ostomy Wound Manage. 2011;57(2):24-37.
141. NPIAP. Unavoidable Pressure Injury During COVID-19 Pandemic: A Position Paper from the National Pressure Injury Advisory Panel. Available at https://cdn.ymaws.com/npiap.com/resource/resmgr/white_papers/Unavoidable_in_COVID_Pandemi.pdf. Last accessed April 20, 2022.
1. National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel, Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. 3rd ed. Washington, DC: National Pressure Ulcer Advisory Panel; 2019. Available at https://www.internationalguideline.com/static/pdfs/Quick_Reference_Guide-10Mar2019.pdf. Last accessed April 22, 2022.
Mention of commercial products does not indicate endorsement.