Prior to April 2022, human mpox had been a rare zoonotic viral exanthem occasionally encountered in persons living in West or Central Africa, or in persons having had contact with imported animals or recent travel to countries where the disease occurs. Within the span of six weeks, single case and clusters of confirmed human mpox were reported in 28 countries on multiple continents, including the United States. Given the historical rarity of mpox encountered in the United States, clinical care providers may be unaware of the clinical features, modes of transmission, risk factor assessment, and approaches to disease management and prevention. The goal of this course is to address these knowledge gaps, enabling timely diagnosis and management of mpox, thereby promoting public health strategies to improve clinical and public health outcomes.
This course is designed for physicians, physician assistants, nurses, pharmacy professionals, and other healthcare professionals who may identify and care for patients with suspected or confirmed human monkeypox infection.
The purpose of this course is to provide health professionals with a review of the global outbreak of mpox (formerly known as monkeypox), emphasizing the epidemiology, clinical features, treatment, and public health strategies for preventing infection and limiting spread of the outbreak.
Upon completion of this course, you should be able to:
- Outline the epidemiology and background of mpox globally and in the United States.
- Describe the transmission, clinical presentation and course of mpox disease.
- Recognize, assess and manage a patient with suspected mpox.
- Apply appropriate patient isolation precautions and promote public health measures designed to prevent the spread of mpox.
John M. Leonard, MD, Professor of Medicine Emeritus, Vanderbilt University School of Medicine, completed his post-graduate clinical training at the Yale and Vanderbilt University Medical Centers before joining the Vanderbilt faculty in 1974. He is a clinician-educator and for many years served as director of residency training and student educational programs for the Vanderbilt University Department of Medicine. Over a career span of 40 years, Dr. Leonard conducted an active practice of general internal medicine and an inpatient consulting practice of infectious diseases.
Contributing faculty, John M. Leonard, MD, has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
John V. Jurica, MD, MPH
Mary Franks, MSN, APRN, FNP-C
Randall L. Allen, PharmD
The division planners have disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Sarah Campbell
The Director of Development and Academic Affairs has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
The purpose of NetCE is to provide challenging curricula to assist healthcare professionals to raise their levels of expertise while fulfilling their continuing education requirements, thereby improving the quality of healthcare.
Our contributing faculty members have taken care to ensure that the information and recommendations are accurate and compatible with the standards generally accepted at the time of publication. The publisher disclaims any liability, loss or damage incurred as a consequence, directly or indirectly, of the use and application of any of the contents. Participants are cautioned about the potential risk of using limited knowledge when integrating new techniques into practice.
It is the policy of NetCE not to accept commercial support. Furthermore, commercial interests are prohibited from distributing or providing access to this activity to learners.
Supported browsers for Windows include Microsoft Internet Explorer 9.0 and up, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Supported browsers for Macintosh include Safari, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Other operating systems and browsers that include complete implementations of ECMAScript edition 3 and CSS 2.0 may work, but are not supported. Supported browsers must utilize the TLS encryption protocol v1.1 or v1.2 in order to connect to pages that require a secured HTTPS connection. TLS v1.0 is not supported.
The role of implicit biases on healthcare outcomes has become a concern, as there is some evidence that implicit biases contribute to health disparities, professionals' attitudes toward and interactions with patients, quality of care, diagnoses, and treatment decisions. This may produce differences in help-seeking, diagnoses, and ultimately treatments and interventions. Implicit biases may also unwittingly produce professional behaviors, attitudes, and interactions that reduce patients' trust and comfort with their provider, leading to earlier termination of visits and/or reduced adherence and follow-up. Disadvantaged groups are marginalized in the healthcare system and vulnerable on multiple levels; health professionals' implicit biases can further exacerbate these existing disadvantages.
Interventions or strategies designed to reduce implicit bias may be categorized as change-based or control-based. Change-based interventions focus on reducing or changing cognitive associations underlying implicit biases. These interventions might include challenging stereotypes. Conversely, control-based interventions involve reducing the effects of the implicit bias on the individual's behaviors. These strategies include increasing awareness of biased thoughts and responses. The two types of interventions are not mutually exclusive and may be used synergistically.
#94041: Mpox: The Ongoing Global Outbreak
Mpox (formerly known as monkeypox) is an acute infectious disease caused by an orthopoxvirus of the same genus and family as Variola virus, the cause of smallpox. Illness is characterized by acute onset of a painful skin eruption (rash), enlarged lymph nodes and fever; illness is variable but rarely fatal though healing skin lesions may produce scars. Infection with mpox virus (MPXV) is acquired through handling an infected animal or from close personal contact with a human case having active mpox lesions. Before April 2022, mpox was considered a rare viral zoonotic disease endemic to West and Central Africa [1,2].
The viral agent causing mpox was first isolated in 1958, following a pox-like disease outbreak among captive monkeys housed within the State Serum Institute in Copenhagen, Denmark [2]. The first recognized human case of mpox was reported in 1970; however, sporadic cases likely occurred well before then but were mistaken for mild cases of smallpox. It soon became apparent that smallpox vaccination protects against mpox, as most reported cases involved unvaccinated children younger than 10 years of age. Secondary spread to close contacts was distinctly uncommon (5%), unlike that encountered during outbreaks of smallpox (25% to 40%) [3]. Cases of mpox remained sporadic, and reports of secondary infection among susceptible contacts were limited, indicating insufficient transmissibility for sustaining propagation of MPXV infection within population groups. Thus, mpox disease was not considered a serious threat to public health [3].
In 2022, the epidemiology of mpox changed. In the short period from mid-April to June 2022, cases and case clusters of confirmed mpox were reported to the World Health Organization (WHO) from 28 countries across multiple regions of the globe, including the United States. Demographic data showed significant deviation from the usual epidemiologic characteristics of endemic mpox; more than 90% of reported cases were men, and intimate sexual contact appeared to be the predominant mode of transmission. In July 2022, responding to an alarming increase in the numbers and affected regions, the WHO declared that the emerging global mpox outbreak constituted a Public Health Emergency of International Concern (PHEIC) [1].
In May 2022, the Centers for Disease Control and Prevention (CDC) began tracking cases of mpox in the United States. During the first seven months of 2022, 21,985 confirmed mpox/orthopoxvirus cases were reported to the CDC. The cumulative number of cases reported by state varied directly with the population density, with more than 1,000 cases per state were reported from New York, Illinois, Florida, Georgia, Texas, and California. Although the outbreak diminished following introduction of preventive measures, transmission is ongoing, and new cases of mpox are reported daily. In the first half of 2025, mpox reporting to CDC has numbered 100 to 150 cases per month [10].
Given the historical rarity of mpox encountered in the United States, clinical care providers may be unaware of the clinical features, modes of transmission, risk factor assessment, and approaches to disease management and prevention. The goal of this course is to address these knowledge gaps, enabling timely diagnosis and management of mpox, thereby promoting public health strategies to improve clinical and public health outcomes.
MPXV is a large, double-stranded DNA viral agent within the genus Orthopoxvirus, family Poxviridae. Orthopoxviruses include Variola virus, Vaccinia virus, Monkeypox virus, and Cowpox virus. Infection with one orthopoxvirus or immunization with an orthopoxvirus vaccine induces immunologic cross-protection against other species of virus in the genus [6]. The source of MPXV in nature is unknown, though African rodents, including squirrels, are considered likely reservoirs. A variety of animals have been identified as susceptible to MPXV infection, including squirrels, Gambian pouched rats, dormice, non-human primates, and other species [1]. The pattern of MPVX circulation in nature and human behaviors that facilitate zoonotic transmission are not entirely clear.
Genomic sequencing has identified the existence of two geographically distinct clades of MPXV: the central African (Congo Basin) clade, which is more severe and has a case fatality rate near 10%; the West African clade, which is less severe and has a mortality of <1% to 3.6% [1]. Both MPXV clades spread the same way and can be prevented using the same methods. In November 2022, the WHO updated citations used in reference to MPXV clades; the Congo Basin clade is now referred to as clade I and West African derivative clade II, with subclades IIa and IIb [1]. All human mpox acquired outside Africa, including the current global outbreak, has been caused by MPXV clade IIb.
In the 10-year period from 1970–1979, 47 cases of a smallpox-like disease were detected in smallpox-free villages near tropical rain forests of West and Central Africa [3]. The clinical features began with malaise, fever, and lymphadenopathy followed by a papulovesicular rash. Absent complications (e.g., pneumonitis, encephalitis, secondary bacterial skin and soft tissue infection), the illness tended to resolve in two to four weeks. Most cases resembled a mild case of smallpox and case fatalities were rare. Virologic studies revealed the source of infection as MPXV, known to circulate among small animals and non-human primates. In subsequent years, sporadic reports of mpox were attributed to zoonotic (animal-to-human) transmission; rare cases reported in persons living outside Africa were linked to contact with imported animals or recent travel to countries where the disease occurs [1]. From 2018 to 2022, cases of mpox were observed among travelers from Nigeria to Israel, the United Kingdom, Singapore, and the United States [1].
During this period of prevailing, widespread attention to smallpox, clinical recognition of other pox-like infections was obscured, and the occasional intercurrent case of mpox was often attributed to mild smallpox [1].
While human-to-human transmission of MPXV was known to occur among household contacts and prison populations, chains of transmission were short and unsustainable [2,3]. An analysis of data on mpox case reports in the Democratic Republic of Congo (DRC, formerly Zaire) for the period 1981– 1986 found that among 2,278 persons who had close contact with 245 patients infected from an animal source, 93 subsequently developed mpox and were presumed to have acquired their infection from a human source [4]. Of the 93 secondary infections, 69 were spread in the first generation, 19 in the second generation, and the remaining 5 cases in the third and fourth generations. The overall probability of acquiring mpox from exposure to a known human source was 3%. Household spread was the predominant locus of secondary transmission, and the highest attack rate (11.7%) occurred in children younger than 5 years of age. These findings indicated that human spread of MPXV was limited and appeared to require prolonged or repeated exposure to open skin lesions or virus-laden debris in the environment.
In 2003, the first recognized mpox outbreak outside Africa was reported from the United States. An investigation identified 47 confirmed cases, including many children, linked to contact with MPXV-infected pet prairie dogs recently housed with small animals imported from Ghana [1]. This outbreak demonstrated that MPXV circulating in intermediate hosts could be transferred from one animal to another and subsequently to humans. The case series included two cases of suspected human spread, though the possibility of transmission from an infected pet could not be excluded. In 2018, a case of secondary infection was reported in the United Kingdom, involving a healthcare worker who had been exposed to a traveler whose infection was acquired before entering the country. Transmission was attributed to physical contact with contaminated bedding, and infection control and contact precautions were implemented. An investigation of 134 potential contacts identified 4 additional secondary cases of mpox, all of whom survived [5].
Prior to eradication of smallpox and cessation of routine smallpox vaccination, the prevailing natural and vaccine-induced immunity to Variola virus provided immune cross-protection against related orthopoxvirus infections, including MPXV [2,3]. Following eradication of smallpox and termination of routine vaccination in 1980, cross-protection against orthopoxviruses gradually diminished, and mpox emerged as a public health issue of broader concern [6]. Population groups in countries endemic for MPXV became more susceptible to MPXV infection, evidenced by a progressive increase in reported cases and localized mpox outbreaks. After the initial reports of human mpox in 1970, 48 confirmed cases in six African countries were reported over the next decade (1970–1979); this increased to 343 cases in the 1980s and 520 cases in the 1990s [7].
Multiple factors are thought to account for the increasing incidence of reported MPXV infection, including rain forest exploitation, climate change, armed conflicts in disease areas, and waning herd immunity. [6]. Using ecological niche modeling techniques in conjunction with climate predictions and remote sensing variables, investigators were able to forecast animal reservoir distribution and the spread of MPXV in Africa's Congo Basin. Results show that forest clearing, and climate change are major factors driving the transmission of MPXV from wildlife to humans under current climate conditions [8]. As deforestation and urbanization has gradually enhanced incidental human contact with infected rodents, populations have also become more susceptible to MPXV because of waning herd immunity following cessation of smallpox vaccination in 1980. Previously, vaccination against smallpox with first-generation vaccinia-based vaccine was shown to be 85% effective in preventing mpox [1]. An analysis of data collected on 91 human mpox cases during the 1980s found that the secondary attack rate among contacts having no vaccination scar (4.3%) differed significantly from those who had been vaccinated in the past (0.7%) [3].
During the first eight months of 2022, 54,707 confirmed cases of mpox, including 18 deaths were reported to WHO from 102 Member States across all WHO regions [9]. This was the first time that mpox cases and sustained chains of MPXV transmission had been reported in countries without direct epidemiologic links to areas of West or Central Africa. The most affected countries globally were the United States (n=19,833), Spain (n=6,749), Brazil (n=5,523), France (n=3,646), Germany (n=3,505), and the United Kingdom (n=3,484). From January 2022 to January 2025, the ongoing global mpox outbreak totaled more than 100,000 reported cases and more than 220 reported deaths (case fatality rate: 0.2%). Although the number of mpox cases worldwide has declined steadily since the peak of the outbreak in 2023, ongoing human transmission has sustained the outbreak; in 2024, the number totaled 9,000. The outbreak has impacted 122 countries, including 115 countries where no case of mpox had been previously reported.
Data reported to the WHO revealed several key epidemiological findings [9]. The outbreak affected young people of male gender; of those cases with available data on gender, 98% were males, with a median age of 36 years. Among cases with sexual orientation reported, 96% identified as men who have sex with men. Of all reported modes of transmission, a sexual encounter was the most (85%) often suspected transmission event. Those infected were likely exposed in a party setting involving sexual contacts, which accounted for 61% of all likely exposure categories reported. Among cases with known human immunodeficiency virus (HIV) status, 45% are HIV positive. Of all reported mpox cases having available data, 1.9% were female; the exposure setting cited most often was the household (32%) and most common form of transmission was via sexual encounters [9].
Multiple factors of public health importance account for the global mpox outbreak: mutations in the viral genome that alter transmissibility; waning herd immunity to smallpox; changes in human behavior, such as relaxation of COVID-19 preventive measures and resumption of international travel; and sexual interactions occurring in the setting of large gatherings, suggesting an amplification of transmission through sexual networks [11]. Women and children account for a small (2%) subset of cases, indicating the potential for wider spread and the propensity for anyone to become infected who has been in close contact with someone who has mpox.
At the onset of reported mpox cases in the United States, the CDC in concert with local and state health agencies adapted surveillance systems, diagnostic testing, vaccines and therapeutics originally developed for smallpox preparedness to meet the unique needs of the outbreak. From May 2022 to May 2023, approximately 140,000 diagnostic specimens were tested, 30,000 cases of mpox reported, >1.2 million vaccine doses administered, and 6,900 patients received treatment with an antiviral agent active against orthopoxviruses such as Variola virus (smallpox) and MPXV [26]. Although the size and pace of the outbreak has rapidly diminished, largely in response to vaccination of high-risk adults, ongoing MPXV activity and reported new cases of mpox persist in this country, particularly in large urban areas.
As of 2025, differing epidemiologic patterns have emerged in reference to mpox disease activity. The ongoing, low-level global outbreak of clade II mpox is spread predominantly by sexual transmission among men having sex with men; this accounts for almost all newly diagnosed cases acquired in the United States and other countries outside Africa. Since the 1980s, reports of endemic mpox within regions of Central Africa have steadily increased. MPXV clade Ia, which accounts for many sporadic mpox cases, spreads by contact with infected dead or live wild animals, household transmission, or care-giver exposure to infected patients. A high proportion of cases are in children younger than 15 years of age.
In 2024, the WHO reported an alarming upsurge of mpox cases in Africa, averaging 500 cases per week, with the highest case burden in the DRC. From January 2022 to August 2024, 45,652 mpox cases were clinically diagnosed and laboratory confirmed from 12 African countries, resulting in 1492 mpox deaths (case fatality rate 3.3%) [17]. The case fatality rate among children younger than 1 year of age was 8.6%, compared with 2.4% among persons older than 15 years of age. Mpox in persons living with HIV and low CD4 counts may incur a mortality risk near 10%. Cases reported from DRC accounted for 88% of the total African mpox burden in 2024. Genomic testing showed that the new upsurge of mpox in DRC and nearby countries is caused by a new clade variant (Ib), which was likely acquired from an animal reservoir but now is spreading through sustained human transmission, including heterosexual transmission involving sex trade workers. An analysis of data from 108 confirmed mpox cases reported in a mining community with a highly mobile population of migrant workers, showed the median age was 22 years, 51.9% of patients were female, and 29% were sex workers [18].
The WHO publishes a monthly report updating the global mpox epidemiologic situation, based on information provided the mpox global surveillance system. From January 1, 2022, through April 30, 2025, a total of 142,151 confirmed cases of mpox, including 328 deaths, were reported to WHO from 133 countries in all six WHO regions (19). Of 3,915 new cases reported in April 2025 (12.5% decline from the previous month), most were reported from the African Region (88.75), followed by the European Region (7.5%) and the Region of the Americas (2.3%). Most regions outside Africa continue to report circulation of MPXV clade IIb, and the most affected population continues to be men who have sex with men, primarily exposed through sexual contact. In instances where women and children have been affected, it has not led to sustained transmission of MPXV.
Animal-to-human (zoonotic) MPXV transmission results from direct contact with the blood, bodily fluids, or cutaneous or mucosal lesions of infected animals [1]. Eating inadequately cooked meat and other animal products of infected animals is a possible risk factor [1].
Unlike sporadic mpox cases previously reported in central Africa, human-to-human transmission is the predominant mode of transmission observed in the current global outbreak and accounts for the scope and rapidity of spread. Human-to-human transmission of MPXV requires close, sustained physical contact that results in direct exposure to body fluids or material emanating from open skin and mucosal lesions. Examples of intermediate- and high-risk exposures associated with the 2022 mpox outbreak include [10]:
Direct contact with infectious skin lesions or scabs (including during sexual contact, kissing, cuddling, or holding hands)
Exposure to large respiratory droplets during prolonged face-to-face or mouth-to-mouth contact, or by proximity to coughing or sneezing of an individual with active infection
Contact with contaminated clothing, bed linens, or towels used by an infected person (fomite transmission)
If contaminated clothing or linens are shaken, infectious particles may be dispersed into the air and inhaled or land on mucosal membranes. The precaution of wearing an N95 mask or equivalent respirator is recommended during any procedure that may create aerosols from oral secretions, skin lesions, or suspensions of dried exudates [10]. Patients with mpox are considered infectious from the time of symptom onset until the skin lesions have crusted.
Published clinical case studies have further characterized the role of intimate physical contact via sexual activity in the spread of mpox associated with the 2022 global outbreak. In a clinical case series of mpox involving 528 patients at 43 sites across 16 countries, diagnosed over a two-month period in the spring of 2022, 98% of the patients were gay or bisexual men [11]. The suspected mode of MPXV transmission reported by the examining clinician was sexual close contact in 95% of cases. The likelihood of sexual transmission was supported by the presence of primary genital, anal, and oral mucosal lesions, considered to represent the inoculation site. Although MPXV DNA was detectable by polymerase chain reaction (PCR) in seminal fluid from 29 of 32 cases in which samples were submitted, it is not yet known whether the presence of viral DNA in semen reflects replication-competent virus capable of transmitting the infection. Among 406 patients with a recorded sexual history, 147 (28%) reported they had travelled abroad in the month before diagnosis, and 103 (20%) had attended large gatherings (more than 30 persons), such as Pride events [11].
Mpox during pregnancy, like smallpox, is associated with severe disease, miscarriage, and stillbirth. A published report citing two cases of miscarriage and one of fatal congenital mpox disease following maternal infection during pregnancy indicates the possibility of vertical (transplacental) transmission of MPXV from mother to the unborn fetus [12]. A 2024 systematic review of seven studies identified 32 pregnant women with clade IIb MPVX infection between 6 and 31 weeks of gestation; of 12 cases with reported gestational outcomes, half resulted in fetal demise [18].
The incubation period of mpox is 5 to 21 days [1]. Illness usually begins with a prodromal phase, including fever, malaise, headache, myalgias, and lymphadenopathy, followed in one to five days by onset of a maculopapular skin eruption and/or mucosal lesions, most pronounced on the face and extremities. According to WHO 2022 outbreak data, the most common sites for skin and mucosal lesions are face (95%), palms and soles (75%), oral mucus membranes (70%), genitalia (30%), and conjunctivae (20%) [1]. Skin lesions progress in stages from maculopapular to papulovesicular to umbilicated pustules before crusting over and desquamating within a period of two to four weeks. Several unusual features of the mpox rash have been observed in patients from the current outbreak: mucosal lesions more numerous than previously described; lesions confined to atypical locations, such as the genital or perineal/perianal area, as well as the mouth and eyes; and development of rash or mucosal lesions prior to onset of constitutional symptoms [1].
In the 2022 clinical review of 528 mpox cases across multiple countries, common systemic symptoms preceding onset of rash included fever (62%), lethargy (41%), myalgia (31%), and headache (27%); lymphadenopathy was reported in 56% [11]. Seventy (13%) patients were admitted to a hospital for infection control purposes or management of complications. The most common medical reasons for admission were management of pain (21 persons, mostly for severe anorectal pain) and treatment of soft-tissue superinfection [18]. Other reasons included severe pharyngitis limiting oral intake (5 persons), eye lesions (2 persons), acute kidney injury (2 persons), and myocarditis (2 persons).
The possibility of mpox should be considered in any patient with compatible clinical syndrome (e.g., fever, rash, lymphadenopathy) combined with epidemiologic risk factors for MPXV exposure such as travel or animal exposure connected to endemic areas of virus circulation or recent history of sexual activity involving persons known or suspected of mpox disease. The differential diagnosis includes other infections that present with generalized or focal skin lesions (e.g., herpes, varicella zoster, secondary syphilis, acute streptococcal or meningococcal infection). The presence of lymphadenopathy helps distinguish mpox from other, similar viral exanthems (e.g., varicella zoster, chickenpox). The laboratory diagnosis of MPXV infection relies on DNA PCR testing of specimens (scrapings) obtained from skin or mucosal lesions. PCR testing of blood samples are usually inconclusive because of the short duration of viremia relative to the timing of specimen collection [1]. Serologic testing is unreliable for mpox-specific confirmation because of common cross-reactivity with other orthopoxviruses and vaccination.
The CDC recommends careful attention to the following epidemiologic criteria, within 21 days of illness onset, when assessing a patient with suspected mpox [13]:
Reports having contact with a person or people with a similar appearing rash or who received a diagnosis of confirmed or probable mpox
Had close or in-person contact with individuals in a social network experiencing mpox activity; this includes men who have sex with men and meet partners through an online website, digital application ("app"), or social event/gathering
Traveled outside the United States to a region with known confirmed cases or country where mpox is endemic
Had contact with a dead or wild animal or exotic pet known to be an African endemic species or used a product derived from such animals (e.g., game meat, creams, lotions, powders)
For purposes of reporting and public health response to the 2022 outbreak, the CDC defines mpox cases as suspect, probable, or confirmed [13]. A suspect case is one with new-onset characteristic rash or meets one of the epidemiologic criteria and has high clinical suspicion for mpox. A probable case is defined as having no suspicion of other recent orthopoxvirus exposure in which there is laboratory evidence of active orthopoxvirus infection demonstrated by PCR, immunochemical, or electron microscopy, or the presence of detectable anti-orthopoxvirus immunoglobulin M (IgM) antibody within 4 to 54 days after onset of rash. A confirmed case of mpox requires demonstration of the presence of MPXV DNA by PCR testing or next-generation sequencing of a clinical specimen or isolation of the virus in culture from a clinical specimen [13].
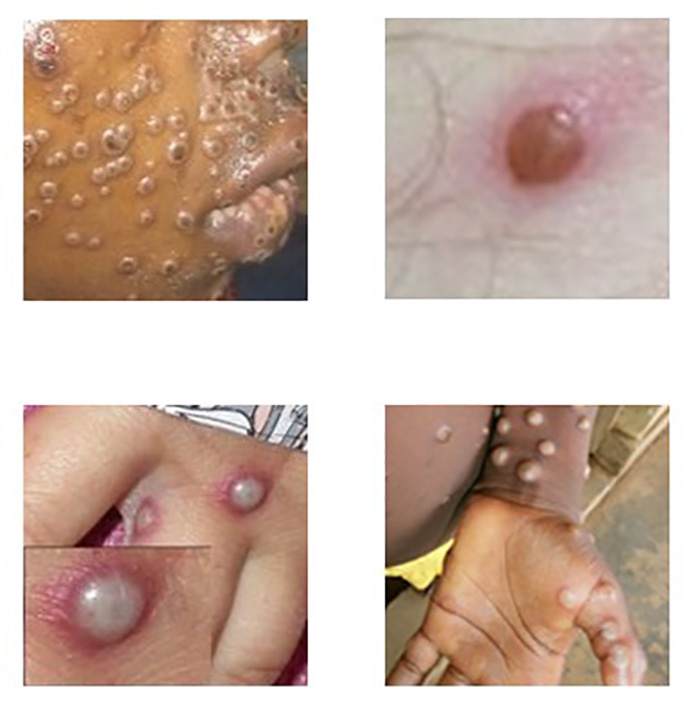
The characteristic skin eruption of mpox disease exhibits the following features: deep-seated, firm or rubbery, well-circumscribed lesions, that often develop central umbilication (Image 1) [14]. The lesions progress through sequential stages—macules, papules, vesicles, pustules, and scabs. The skin lesions are sometimes confused with those seen in other infections more commonly encountered in clinical practice (e.g., secondary syphilis, herpes, varicella zoster). Historically, sporadic accounts of patients co-infected with MPXV and other infectious agents (e.g., varicella zoster, syphilis) have been reported, so patients with skin lesions typical for mpox should be considered for testing, even if diagnostic testing for other conditions has returned positive [13]. Key characteristics for identifying mpox rash, including instructive photographic images of skin lesions in sequential stages, are provided at the CDC website, accessible at https://www.cdc.gov/mpox/hcp/clinical-signs [14].
Procedures for safe collection and transport of specimens, including specimen collection printouts for testing patients suspected of mpox, are available through the CDC [15]. Effective communication and precautionary measures between collection teams and laboratory staff are emphasized. When collecting specimens, personnel should wear personal protective equipment (PPE) in accordance with recommendations for healthcare settings.
For diagnostic purposes, clinical specimens should be submitted to Laboratory Response Network (LRN) laboratories or authorized commercial laboratories; the recommended specimen type is lesion material (i.e., swab of lesion surface or crust from healing lesion). Specific guidelines on acceptable specimen types within laboratory facilities may vary. Providers should contact the appropriate public health department or commercial laboratory to determine acceptable specimens. The CDC has prepared two 1-page illustrated printouts (in PDF form for posting in office or clinic) with instructions for mpox diagnostic testing and techniques for specimen collection. These printouts are accessible at https://www.cdc.gov/mpox/hcp/diagnosis-testing/collecting-specimens.html.
In general, specimens should be collected in the following manner, using a plastic, sterile, leak-proof container for specimen collection [15]. Select two or three lesions from different locations on the body or from lesions that differ in appearance. Two swabs from each lesion should be collected for testing. Only sterile synthetic swabs with plastic, wood, or thin aluminum shaft should be used; swab the lesion vigorously at least two to three times on each side of the swab to collect adequate material for DNA testing. Do not use cotton swabs. The CDC suggests it is not necessary to de-roof the lesion before swabbing, but if the lesion ruptures while swabbing, ensure that swab collects lesion fluid. Break off the end of each swab's applicator into a 1.5- or 2-mL screw-capped tube with O-ring or place the entire swab into a sterile container that has a gasket seal in order to be shipped under required conditions [15].
Specimen collection, storage, and shipping of human specimens is subject to certain restrictions and requires a designated request form with each specimen. Clinician providers should consult with appropriate laboratory personnel to assure proper procedure [15].
Many patients with mpox follow a mild, self-limited course, requiring only symptomatic treatment combined with measures aimed at limiting transmission of infection. The prognosis for mpox depends on multiple factors, such as previous vaccination status, general health status, presence of comorbidities and concurrent medications. As of June 2025, there is no antiviral therapeutic agent approved specifically for treatment of MPXV infection. However, antiviral drugs developed for use in patients with smallpox may be beneficial for mpox as well. CDC's interim clinical guidance may assist clinicians in managing patients who have or are at risk for severe, protracted, or life-threatening manifestations of mpox [16].
Patients considered for treatment following consultation with the CDC include: those with severe disease and/or complications requiring hospitalization; persons at high risk of severe disease because of immunocompromise (e.g., HIV, hematologic malignancy, immunosuppressive therapies); patients with aberrant infections involving anatomic areas that pose a specific hazard, such as the eyes (e.g., keratoconjunctivitis), genitals, and anus [16]. Other population groups possibly at risk for severe disease include pediatric patients, especially those younger than 8 years of age, pregnant or breastfeeding women, and persons with the history or presence of serious skin disorders (e.g., atopic dermatitis, eczema, herpes zoster). Healthcare providers should consult CDC Interim Clinical Guidance for the Treatment of Mpox, which includes the use of medical countermeasures available through the Strategic National Stockpile (SNS) [16]. Treatment options available through the SNS include two antiviral agents (tecovirimat and cidofovir) and vaccinia immune globulin intravenous (VIGIV).
Tecovirimat (TPOXX) is an antiviral medication approved by the U.S. Food and Drug Administration (FDA) for the treatment of smallpox in adults and children. Data on tecovirimat effectiveness for treating mpox are limited, but animal studies have shown it to be effective against infections caused by orthopoxviruses. Human clinical trials have found the drug to be safe with only minor side effects. Small case studies of individuals infected with MPXV suggest that tecovirimat may shorten the duration of illness and viral shedding. However, initial data from a clinical trial in the DRC indicated that tecovirimat treatment of children and adults for clade I mpox did not reduce the duration of skin manifestations. The CDC holds an expanded access protocol (sometimes called compassionate use) that allows for dispensing stockpiled tecovirimat to treat patients with severe illness or complications, and those considered at risk for same such as patients with HIV and other immunodeficiency states [16]. Tecovirimat is formulated as an oral capsule (200 mg) and as a solution for intravenous administration.
The CDC, in partnership with the FDA, has streamlined the process of obtaining tecovirimat, allowing healthcare providers to start treatment before paperwork is completed. Multiple state health and territorial departments are pre-positioning supplies of tecovirimat within their jurisdictions. Clinicians and care facility pharmacists requesting tecovirimat should contact their state/territorial health department. For questions regarding urgent situations after hours and on weekends, providers may contact the CDC through the CDC Emergency Operations Center (770-488-7100) to talk with a clinician. Additional information on the process for requesting tecovirimat and the CDC guidance regarding patient selection, efficacy, formulations, adverse reactions and drug-drug interactions may be accessed at: https://www.cdc.gov/mpox/hcp/clinical-care/index.html [16].
VIGIV is licensed by the FDA for the treatment of complications due to vaccinia vaccination including eczema vaccinatum and generalized vaccinia, among other complications. The CDC holds an expanded access protocol that allows the use of VIGIV for the treatment of orthopoxviruses (including mpox) in an outbreak. Data are not available on the effectiveness of VIG in the treatment of monkeypox, and it is unknown whether a patient with severe disease will benefit from treatment [16]. However, healthcare providers may consider its use in severe cases.
VIG can be considered for prophylaxis against monkeypox in an exposed person with severe immunodeficiency in T-cell function for which smallpox vaccination following exposure to mpox virus is contraindicated [16].
When properly administered before an exposure, vaccines previously developed and stockpiled for prevention of smallpox are effective at protecting people against MPXV infection. Vaccines may also be effective for post-exposure prophylaxis if given within four days from the date of exposure [1,20]. When administered between 4 to 14 days after exposure, vaccination may reduce symptoms and severity of mpox disease. ACAM2000 and JYNNEOS (also known as Imvamune or Imvanex) are the two licensed smallpox vaccines in the United States. The CDC has prepared vaccine guidance for healthcare professionals on prevention of mpox, including interim clinical considerations for using JNNEOS and ACAM2000 vaccines during the mpox outbreak [20,21].
ACAM2000 is an attenuated, self-replicating live vaccine. It carries some risk of dissemination and is not safe for use in persons with immunodeficiency syndromes. JYNNEOS is a third-generation vaccine based on an attenuated, non-replicating live orthopoxvirus, Modified Vaccinia Ankara (MVA) [20]. MVA does not replicate efficiently in humans and has a better safety profile. The JYNNEOS vaccine is the primary vaccine being used during the 2022 mpox outbreak in the United States. Although JYNNEOS is routinely administered subcutaneously, a smaller dose administered by intradermal injection is considered equally effective and conserves the limited available supplies of this vaccine. No clinical trial data are available on the effectiveness of either vaccine in the current global outbreak; the effectiveness of JYNNEOS in preventing or ameliorating mpox disease has been concluded from a clinical study on the immunogenicity of JYNNEOS and efficacy data from animal studies [20]. People who are vaccinated should continue to take steps to protect themselves from infection by avoiding close, skin-to-skin contact, including intimate contact, with someone who has mpox.
ACAM2000 is licensed as a single dose. It is administered as a live vaccinia virus preparation that is inoculated into the skin by pricking the skin surface [20]. Following a successful inoculation, a lesion will develop at the site of the vaccination (i.e., a "take"). Replicating virus from the inoculation site may spread to other parts of the body or even to other people; therefore, individuals who receive vaccination with ACAM2000 must take precautions to prevent spread [20]. Adverse events following ACAM2000, including myopericarditis or vaccinia virus transmission to household contacts, can be serious [21]. ACAM2000 will be made available for individuals who decide in consultation with their healthcare provider that the potential benefits of vaccination outweigh potential risks from adverse events [21].
The JYNNEOS vaccine is licensed as a series of two doses administered 28 days (4 weeks) apart for use in adults 18 years of age and older. There is no visible "take" and no risk of spread to other parts of the body or other people [20]. The standard regimen involves a subcutaneous injection volume of 0.5 mL. People who receive JYNNEOS are not considered vaccinated until two weeks after they receive the second dose of the vaccine.
In the context of the current national Public Health Emergency (PHE), an alternative regimen to subcutaneous JYNNEOS vaccine administration may be used. The authorized alternative regimen involves an intradermal route of administration with an injection volume of 0.1 mL. This approach could increase the number of available JYNNEOS vaccine doses by up to five-fold. Results from a clinical study showed that the lower intradermal dose was immunologically non-inferior to the standard subcutaneous dose [21].
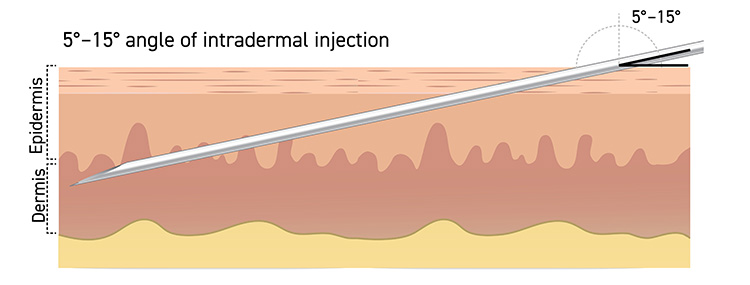
Intradermal administration involves injecting the vaccine superficially between the epidermis and the hypodermis layers of the skin, typically of the volar aspect (inner side) of the forearm. After an appropriate location is located, it should be cleaned. The skin is pulled taut, and the needle is positioned with the bevel up. The needle should be inserted at a 5- to 15-degree angle into the dermis (Figure 1). The solution should then be slowly injected into the dermis. This should produce a noticeable pale elevation of the skin (wheal). All patients should be observed for at least 15 minutes following vaccination; patients with a history of anaphylaxis to chicken, egg protein, gentamicin, or ciprofloxacin should be observed for 30 minutes. The technique requires prior experience or training in order to ensure it is done properly; otherwise, such a small volume of vaccine delivered into the subcutaneous layer may not elicit an optimal immune response.
Recipients should be informed of the risks and benefits of JYNNEOS prior to vaccination. Healthcare providers should review CDC guidance on pre- and post-vaccination counseling, including side effects and safety considerations [21]. Local side effects (e.g., pain, erythema, edema) are more severe with intradermal than subcutaneous administration. It is important to determine the medical and allergy history of recipients to appropriately decide whether to administer the vaccine subcutaneously or intradermally. JYNNEOS vaccine contains small amounts of gentamicin and ciprofloxacin and is produced using chicken embryo fibroblast cells [20]. Recipients should be counseled about possible side effects from vaccination and be provided with a JYNNEOS vaccine information statement or FDA JYNNEOS EUA Fact Sheet, as applicable.
During a one-year period (May 2022—May 2023), 1.2 million doses of JYNNEOS were administered in United States public health jurisdictions. An analysis of vaccine effectiveness demonstrated that the subsequent incidence of mpox among unvaccinated persons was 9.6 times higher than that among persons who received two doses of vaccine [26]. A matched case control study found an adjusted VE of 85.9% for two doses, compared to 75.2% for one dose, across all routes of administration; no new or unexpected safety concerns were identified. In 2023, the Advisory Committee on Immunization Practices voted in favor of the use of JYNNEOS for adults who at risk for acquiring mpox during an outbreak [26].
Control the current global mpox outbreak requires a concerted effort to interrupt human-to-human transmission. The WHO has issued interim guidance for disease surveillance, case investigation, and contact tracing for mpox [22]. The objectives are rapid identification of active cases; provision of optimal patient care and adherence to isolation precautions; identification and follow-up of contacts to assure rapid identification of secondary cases; protection of frontline health workers; and tailoring of effective control and prevention measures [22].
Clinicians should promptly report suspected, probable, and confirmed mpox cases to local or state public health authorities. A minimum dataset of epidemiologically relevant information should be included that enables contact tracing to be initiated as quickly as possible. Contacts of cases should be monitored (or self-monitor) daily for signs or symptoms of mpox for a period of 21 days [22].
The CDC recommends that patients with mpox remain isolated at home or at another location for the duration of illness, but this may not be possible in all situations [23]. Current data suggest that risk of transmitting MPXV begins with onset of symptoms and continues until all symptoms have resolved, including full healing of the rash with formation of a fresh layer of skin. Ideally, people with mpox should remain in isolation for the duration of illness, typically for two to four weeks. If unable to remain fully isolated throughout the period of illness, a patient with mpox should be encouraged to adhere to the following precautions [23]:
Remain isolated at home while symptomatic.
Avoid close or physical contact with others, including sexual and/or close intimate contact.
Cover lesions.
Wear a well-fitting face mask when interacting with others.
Avoid public transportation when leaving home.
While rash persists, cover all parts with clothing, gloves, or bandages.
Until all signs and symptoms have resolved, do not share items that have been worn or handled with other people or animals and avoid sharing utensils, cups, and other personal use items.
The CDC website provides general guidance for prevention of mpox [24]. The topics addressed include risk assessment and vaccine for prevention, personal protection, safe sex, risks associated with social gatherings and congregate settings, pets and mpox. As of June 2025, data suggest that gay, bisexual, and other men who have sex with men account for most reported cases in the United States and associated with the global mpox outbreak. However, anyone, regardless of sexual orientation or gender, who has been in close personal contact with someone who has mpox is at risk [24].
It is important for healthcare professionals to give patients explicit verbal and written instructions on management of mpox symptoms and prevention of disease transmission. Patients' health literacy is typically low, and patients whose primary language is not English, racial/ethnic minorities, and patients older than 60 years of age have the lowest health literacy. Many patients report that the clinician did not provide information in words they could understand, and some individuals feel shame about their lack of understanding and fail to ask for clarification due to embarrassment. Non-English-proficient patients require a professional translator (i.e., not a family member) to be present at each visit. It is vital that the patient understand the importance of precisely following transmission prevention measures.
| Centers for Disease Control and Prevention |
| https://www.cdc.gov/mpox |
| National Institute of Allergy and Infectious Diseases |
| https://www.niaid.nih.gov/diseases-conditions/monkeypox |
| U.S. Food and Drug Administration |
| https://www.fda.gov/emergency-preparedness-and-response/mcm-issues/fda-monkeypox-response |
| World Health Organization |
| https://www.who.int/emergencies/situations/mpox-outbreak |
1. World Health Organization. Mpox. Available at https://www.who.int/news-room/fact-sheets/detail/monkeypox. Last accessed June 5, 2025.
2. Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4:15-25.
3. Jezek Z, Gromyko AI, Szczeniowski MV. Human monkeypox. J Hyg Epidemiol Microbiol Immunol. 1983;27:13-28.
4. Jezek Z, Grab B, Szczeniowski MV, et al. Human monkeypox: secondary attack rates. Bull World Health Organ. 1988;66:465-470.
5. Vaughan A, Aarons E, Astbury J, et al. Human-to-human transmission of monkeypox virus, United Kingdom, October 2018. Emerg Infect Dis. 2020;26:782-785.
6. Simpson K, Heymann D, Brown CS, et al. Human monkeypox—after 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38:5077-5081.
7. Stacey J. Monkeypox virus outbreak: can evolution guide us to new treatments or vaccines? eBioMedicine 2022;82:104221.
8. Thomassen HA, Fuller T, Asefi-Najafabady S, et al. Pathogen-host associations and predicted range shifts of human monkeypox in response to climate change in central Africa. PLoS One. 2013;8(7):e66071.
9. World Health Organization. 2022 Global Mpox Trends. Available at https://worldhealthorg.shinyapps.io/mpx_global. Last accessed June 5, 2025.
10. Centers for Disease Control and Prevention. Mpox. Available at: https://www.cdc.gov/mpox/index.html. Last accessed June 5, 2025.
11. Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries—April–June 2022. N Engl J Med. 2022;387:679-691.
12. Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis 2017;216:824-828.
13. Centers for Disease Control and Prevention. Mpox Case Definitions. Available at: https://www.cdc.gov/mpox/hcp/case-definitions. Last accessed September 8, 2025.
14. Centers for Disease Control and Prevention. Clinical Features of Mpox. Available at: https://www.cdc.gov/mpox/hcp/clinical-signs. Last accessed June 5, 2025.
15. Centers for Disease Control and Prevention. Guidelines for Collecting and Handling Specimens for Mpox Testing. Available at: https://www.cdc.gov/mpox/hcp/diagnosis-testing/collecting-specimens.html. Last accessed June 5, 2025.
16. Centers for Disease Control and Prevention. Clinical Treatment of Mpox. Available at: https://www.cdc.gov/mpox/hcp/clinical-care. Last accessed June 5, 2025.
17. Ndembi N, Folayan MO, Komakech A, et al. Evolving epidemiology of mpox in Africa in 2024. N Engl J Med. 2025;392:666-676.
18. Nachegaa JB, Mohr EL, Dashraath P, et al. Mpox in pregnancy – risks, vertical transmission, prevention, and treatment. N Engl J Med. 2024;391:1267-1270.
19. World Health Organization. Mpox: Multi-Country External Situation Reports, No. 53 Published 29 May 2025. Available at https://cdn.who.int/media/docs/default-source/documents/emergencies/multi-country-outbreak-of-mpox--external-situation-report--53.pdf. Last reviewed June 5, 2025.
20. Centers for Disease Control and Prevention. Mpox Vaccination. Available at https://www.cdc.gov/mpox/vaccines/index.html. Last accessed June 5, 2025..
21. Centers for Disease Control and Prevention. Vaccine for Mpox Prevention in the United States. Available at: https://www.cdc.gov/mpox/hcp/vaccine-considerations/index.html. Last accessed June 5, 2025.
22. World Health Organization. Surveillance, Case Investigation, and Contact Tracing for Mpox (Monkeypox): Interim Guidance, 20 March 2024. Available at https://www.who.int/publications/i/item/WHO-MPX-Surveillance-2022.3. Last accessed September 14, 2022.
23. Centers for Disease Control and Prevention. Isolation and Prevention Practices for People with Mpox. Available at https://archive.cdc.gov/#/details?url=https://www.cdc.gov/poxvirus/mpox/clinicians/isolation-procedures.html. Last accessed June 5, 2025.
24. Centers for Disease Control and Prevention. Preventing Mpox. Available at https://www.cdc.gov/mpox/prevention. Last accessed June 5, 2025.
25. World Health Organization. WHO Recommends New Name for Monkeypox Disease. Available at https://www.who.int/news/item/28-11-2022-who-recommends-new-name-for-monkeypox-disease. Last accessed December 12, 2022.
Mention of commercial products does not indicate endorsement.