The life-threatening ramifications of human immunodeficiency virus (HIV) infection have overshadowed other STIs and the implications for oral and systemic involvement. Diseases such as gonorrhea, syphilis, human papillomavirus, infectious mononucleosis, genital herpes, chlamydia, and trichomoniasis have a high incidence among the general population. Delayed treatment of these diseases can lead to sterility, pelvic inflammatory disease, and an increased risk of HIV infection if ulcerative lesions are present. Untreated syphilis can lead to complications of the nervous and cardiovascular systems, which can cause serious health complications and even death. Many of these infections manifest in the oral cavity, and dental professionals can intervene to assist with early diagnosis and effective treatment.
This course is designed for all dental professionals.
The purpose of this course is to introduce dental professionals to the pathophysiology of STIs, their oral manifestations, systemic complications, available treatment options, and any modifications required for dental treatment.
Upon completion of this course, you should be able to:
- Outline the spectrum of diseases that are transmitted sexually and their impact on public health.
- Describe the underlying pathogenesis and treatment of gonorrhea and syphilis.
- Identify oral lesions that are associated with genital herpes, human papillomavirus, and Epstein-Barr virus.
- Analyze the systemic implications of chlamydia.
- Review dental treatment modifications that are required for patients with HIV/AIDS.
Mark J. Szarejko, DDS, FAGD, received his dental degree from the State University of New York at Buffalo in 1985. He received fellowship from the Academy of General Dentistry in 1994.
Contributing faculty, Mark J. Szarejko, DDS, FAGD, has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Sarah Campbell
The Director of Development and Academic Affairs has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
The purpose of NetCE is to provide challenging curricula to assist healthcare professionals to raise their levels of expertise while fulfilling their continuing education requirements, thereby improving the quality of healthcare.
Our contributing faculty members have taken care to ensure that the information and recommendations are accurate and compatible with the standards generally accepted at the time of publication. The publisher disclaims any liability, loss or damage incurred as a consequence, directly or indirectly, of the use and application of any of the contents. Participants are cautioned about the potential risk of using limited knowledge when integrating new techniques into practice.
It is the policy of NetCE not to accept commercial support. Furthermore, commercial interests are prohibited from distributing or providing access to this activity to learners.
Supported browsers for Windows include Microsoft Internet Explorer 9.0 and up, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Supported browsers for Macintosh include Safari, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Other operating systems and browsers that include complete implementations of ECMAScript edition 3 and CSS 2.0 may work, but are not supported. Supported browsers must utilize the TLS encryption protocol v1.1 or v1.2 in order to connect to pages that require a secured HTTPS connection. TLS v1.0 is not supported.
The role of implicit biases on healthcare outcomes has become a concern, as there is some evidence that implicit biases contribute to health disparities, professionals' attitudes toward and interactions with patients, quality of care, diagnoses, and treatment decisions. This may produce differences in help-seeking, diagnoses, and ultimately treatments and interventions. Implicit biases may also unwittingly produce professional behaviors, attitudes, and interactions that reduce patients' trust and comfort with their provider, leading to earlier termination of visits and/or reduced adherence and follow-up. Disadvantaged groups are marginalized in the healthcare system and vulnerable on multiple levels; health professionals' implicit biases can further exacerbate these existing disadvantages.
Interventions or strategies designed to reduce implicit bias may be categorized as change-based or control-based. Change-based interventions focus on reducing or changing cognitive associations underlying implicit biases. These interventions might include challenging stereotypes. Conversely, control-based interventions involve reducing the effects of the implicit bias on the individual's behaviors. These strategies include increasing awareness of biased thoughts and responses. The two types of interventions are not mutually exclusive and may be used synergistically.
#54073: Oral Manifestations of Sexually Transmitted Infections
Sexually transmitted infections (STIs) are a group of diverse diseases that are a major public health threat in the United States and throughout the world. The systemic complications that accompany STIs can range from those with minimal symptoms and consequences to those with systemic involvement leading to serious morbidity and even death. Bacterial and viral pathogens are the primary etiologic agents of STIs, but parasitic infestations, such as trichomoniasis, can also occur. Oral manifestations are a common feature of STIs, so it is important that members of the dental profession are able to recognize the oral lesions that may develop secondary to these diseases and provide appropriate referral for medical treatment. In addition, many STIs are transmitted via saliva, blood, and/or direct contact with oral lesions, so compliance with Standard Precautions is essential to minimize the transference of the pathogenic micro-organisms between an infected patient and members of the dental care team.
Treatment of STIs has become more complex due to an increasing number of bacterial strains becoming resistant to empiric antibiotic therapy. As such, education of the public regarding the prevention of STIs is of utmost importance, as all of these diseases can be prevented, but not all can be cured. Further, even if an STI is cured, the cumulative systemic damage that occurs during its progression may be irreversible.
This course will highlight the pathogenesis, oral manifestations, systemic complications, treatment options, and impact upon oral health and dental treatment associated with the most common STIs. Participants should develop an appreciation for the prevalence of STIs and the manner by which they compromise oral and systemic health.
The Centers for Disease Control and Prevention (CDC) estimates that approximately 2.5 million new cases of syphilis, chlamydia, and gonorrhea were reported in the United States in 2022[1]. Young adults 15 to 24 years of age account for nearly one-half of the reported cases [1]. The exact number of cases is difficult to ascertain, as these diseases do not always result in the affected patient seeking medical care, especially if symptoms are mild or absent. Some STIs, such as human papillomavirus (HPV), herpes simplex virus, and Trichomonas vaginalis, are not routinely reportable to the CDC [2]. Lack of access to medical care and the stigma associated with STIs are also obstacles that may prevent patients from seeking medical treatment, and disruptions in the availability of care and screening services during the COVID-19 pandemic have likely caused an increase in undiagnosed infections. Patients who are untreated remain infectious and can transmit the STI to others [1].
Although these factors contribute to the under-reporting of STIs to the CDC, the cases that are reported reflect an ongoing public health problem. The CDC has maintained surveillance for the number of reported cases of syphilis and gonorrhea since 1941 and for reported cases of chlamydia since 1984 (Table 1). The incidence of chlamydia infection has increased significantly through the years, with 7,594 cases (6.5 per 100,000 U.S. population) reported in 1984, compared with 1,649,716 cases (495 per 100,000 population) in 2022 [3].
CASES OF SEXUALLY TRANSMITTED INFECTIONS REPORTED BY STATE HEALTH DEPARTMENTS, UNITED STATES
| Infection | 1984 | 1992 | 2015 | 2022 | ||||
|---|---|---|---|---|---|---|---|---|
| Cases | Ratea | Cases | Ratea | Cases | Ratea | Cases | Ratea | |
| Syphilis (all stages) | 69,872 | 29.6 | 114,730 | 44.7 | 74,709 | 23.2 | 207,255 | 62.2 |
| Chlamydia | 7,594 | 6.5 | 409,697 | 182.3 | 1,533,577 | 471.6 | 1,649,716 | 495 |
| Gonorrhea | 878,556 | 372.5 | 502,858 | 196.0 | 395,216 | 123.0 | 648,056 | 194.4 |
| aRate per 100,000 population. | ||||||||
In 2000 and 2001, there were 2.1 reported cases of syphilis per 100,000 population, the lowest since 1941. However, the rate has increased almost every year since 2001. In 2022, 207,255 primary and secondary syphilis cases (62.2 per 100,000 population) were reported, which was a 17.3% increase from 2021 [3]. While men who have sex with men (MSM) still comprise a plurality of cases at 47.7%, the number of cases among women has increased by 876.8% between 2013 and 2022. There has also been an increase of 146.7% in cases among men who have sex with women only (MSW) during the same time period, indicating that the syphilis epidemic in heterosexual couples continues to surge. National rates of congenital syphilis have also increased, with 102.5 cases per 100,000 live births in 2022, the most since 1991, and a reported 282 congenital-related syphilis stillbirths and deaths [3].
In 2009, the rate of reported gonorrhea cases (301,174) was historically low at 98.1 per 100,000 population. The rate increased slightly each year from 2009 to 2012, when it reached 106.7 cases per 100,000 population and has continued to increase each year until 2021, which showed a 9.2% decrease (from 214 to 194.4 per 100,000 population). In 2022, gonorrhea was still the second most common nationally notifiable STI in the United States [3].
The rate of reported chlamydia remained relatively stable from 2021 to 2022, at around 495 per 100,000 population. Is it currently the most common STI in the United States, with total of 1,649,716 reported cases, and the CDC estimates that these numbers may actually be much higher given the disruptions in STI-related care and screenings during the COVID-19 pandemic [3].
When cases of human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS), HPV, herpes simplex virus, and trichomoniasis are added to the cases of syphilis, gonorrhea, and chlamydia, the public health burden of STIs is readily apparent. In total, it is estimated that 68 million men and women in the United States have an STI on any given day [5]. The annual direct medical costs for the treatment of STIs in the United States are estimated to be $16 billion. This does not take into account the costs associated with lost productivity [5].
Transmission of STIs can occur during unprotected sexual contact or during the exchange of bodily fluids during any type of activity. Intact mucous membranes prevent infection with pathogenic micro-organisms such as those that cause STIs. But when the mucous membranes of the mouth, vagina, or perianal tissues are torn or ulcerated, transmission of STIs is facilitated. Depending on the patient population, other risk factors for STIs include sex in conjunction with illicit drug use, multiple partners, a new sexual partner, and the prevalence of the infection in the community [6].
There have been many public health campaigns that have highlighted the risk factors for STIs, transmission methods, and how transmission of these diseases can be prevented. Despite this, many people continue to engage in high-risk sexual activities, and the rates of infection remain elevated. As such, it is likely that STIs will remain a public health problem and that most dental practitioners will treat patients that have one or more of these diseases. Clinicians should be knowledgeable about the nature of these diseases, their course and the consequences of their clinical progression, and the direct and indirect impact upon oral and systemic health.
Gonorrhea (the result of infection with Neisseria gonorrhoeae) is the second most common reportable STI in the United States [3]. In 2022, 648,056 cases of gonorrhea were reported to the CDC, with more than half occurring in people 15 to 24 years of age [3,7]. N. gonorrhoeae is a gram-negative aerobic bacterium that thrives in a warm, moist environment. The humidity, temperature range, and pH level of the mucous membranes of the oral cavity, genitals, and perirectum make these areas ideal environments for the replication of N. gonorrhoeae and a reservoir for its transmission.
N. gonorrhoeae is a fragile bacterium that cannot tolerate a dry environment, so it is rarely transmitted by contact with contaminated fomites (on inanimate objects). The transmission of gonorrhea occurs almost exclusively by sexual activity that involves genital-to-genital, oral-to-genital, or rectal-to-genital contact. Uncommonly, an infected mother may transmit the disease to her child during childbirth [8].
The histology of certain tissues makes them more susceptible to infection with N. gonorrhoeae. For example, the columnar epithelium that makes up the mucosal lining of the cervix and urethra and the transitional epithelium of the mucosal lining of the oropharynx and rectum are common entry points for gonococcal infection. In contrast, the stratified squamous epithelium that composes the skin and the mucosal lining of the oral cavity proper appears more resistant to N. gonorrhoeae [9].
Gonorrhea may be asymptomatic in both men and women, which increases the risk of the transmission to uninfected partners. The pathogenesis of gonorrheal infections is ultimately the result of the host response to this pathogen. The cuboidal and columnar epithelia are the primary cells most susceptible to N. gonorrhoeae infection [9]. These bacteria penetrate these cells 24 to 48 hours after exposure and infiltrate the submucosal layer. The systemic immune response is characterized by marked neutrophilic infiltration, causing epithelial sloughing, the formation of microabscesses, and the development of suppuration [10]. In men, this immune response can lead to dysuria (painful urination) and a white, yellow, or green urethral discharge 1 to 14 days after the initial infection. Women who are symptomatic may exhibit a urethral or vaginal discharge and dysuria [7,11].
While symptoms may seem benign and self-limiting, untreated gonorrhea infections can lead to serious health problems for both men and women. Untreated gonorrhea in men can extend from the urethra into the bladder, epididymis, prostate gland, or the seminal vesicles. Inflammation of the epididymis (the coiled tube in which sperm mature), also referred to as epididymitis, may be caused by untreated gonococcal infections and can ultimately lead to infertility. In women, N. gonorrhoeae can ascend into the endometrium, fallopian tubes, ovaries, and/or pelvic peritoneum. This can result in pelvic inflammatory disease, the consequences of which include scarring of the fallopian tubes, ectopic pregnancy, and infertility [7].
Oral lesions are not a uniquely identifying feature of gonorrhea, but gonococcal infections of the pharynx may occur following oral-genital contact with an infected person. If present, patients may complain of a sore throat, and redness or white spotting (exudates) may be apparent; however, up to 90% of cases are asymptomatic [14]. A pharyngeal nucleic acid amplification test (NAAT) is preferred in suspected cases of pharyngeal infection with N. gonorrhoeae [15].
In 0.5% to 3% of cases, gonorrhea infection may become disseminated throughout the body [16]. The major signs of disseminated infection are skin lesions and joint pain (e.g., arthralgia, tenosynovitis, septic arthritis).
Treatment of uncomplicated gonorrhea usually consists of a single-dose antibiotic regimen that is compatible with the patient's medical history. The CDC recommends an intramuscular (IM) dose of 500 mg ceftriaxone. Other regimens of cephalosporin (e.g., ceftizoxime, cefotaxime), while potentially safe and effective against uncomplicated urogenital and anorectal infections, have not been shown to have any advantage over ceftriaxone, and there is evidence that their effectiveness may be waning [11]. Oral azithromycin is no longer recommended due to a widespread increase in macrolide resistance [17]. The CDC currently recommends the use of ceftriaxone 500 mg IM in a single dose for persons weighing <150 kg (1g IM in a single dose for persons weighing >150 kg) for the treatment of uncomplicated gonorrhea of the pharynx [11]. Alternative treatments for pharyngeal gonorrhea are unreliable.
Another essential component in the treatment of gonorrhea or any STI is the notification of potentially exposed partners. These contacts should be screened, and if gonorrhea is diagnosed, treatment should be initiated to prevent subsequent spread of the disease. There are no contraindications for dental treatment for patients that have been successfully treated for gonorrhea. As noted, the non-specific diffuse inflammation that accompanies oropharyngeal gonorrhea is not by itself an identifying symptom of this disease. However, it is prudent for dental professionals to defer treatment and to refer any patient with a diffuse inflammatory response in the oropharyngeal area to a physician for further evaluation and treatment.
Syphilis is an ancient disease prevalent since the Middle Ages, when it was known as "pox" or "lues." The spirochete bacterium Treponema pallidum is the etiologic agent of this disease. T. pallidum can gain access to the body through intact mucosal tissues and through minute abrasions in the skin. Syphilis is typically transmitted between people by direct contact of syphilis lesions (chancres) during vaginal, oral, or anal sex. T. pallidum lacks the metabolic capability of synthesizing its required nutrients and therefore cannot survive outside the body.
In 2022, there were 203,500 new cases of syphilis in the United States and an estimated 8 million cases globally. While rates remain lower than gonorrhea and chlamydia, syphilis reporting to the CDC has increased 78.9% since 2018 [3,18]. It is essential that dental clinicians are familiar with the pathogenesis of this disease and the oral manifestations associated with its different phases.
Syphilis has been called the "Great Imitator," as its symptoms may mimic many other diseases and occur in many body systems. The course of syphilis occurs in three stages if left untreated: the primary stage, the secondary stage, and the late (tertiary) stage. Compared to the nonspecific inflammatory response of oropharyngeal gonorrhea, syphilis has pronounced oral lesions that should be included in the differential diagnosis of the disease. The oral lesions that form during the various stages of syphilis are infectious to others. However, patients with any oral lesion of unknown origin should be referred for evaluation and diagnosis [22].
As noted, syphilis occurs in distinct stages characterized by unique systemic symptoms and oral lesions. These stages are also associated with specific treatment needs and differing prognosis.
The primary stage of syphilis infection occurs when T. pallidum infiltrates the mucosa or abraded skin. The incubation period between the initial infiltration and the emergence of symptoms can range from 3 to 90 days and depends upon the amount of T. pallidum inoculated into the tissues. Dissemination to distant sites, including of the central nervous system, occurs by hematogenous and lymphatic routes [23].
The classic lesion associated with the primary stage of syphilis is a chancre located at the site of inoculation. These painless lesions are firm and round and can occur on the external genitals, vagina, anus, rectum, lips, tongue, or oral mucosa. These infectious lesions may be solitary or multiple and can exhibit variance in their size. An estimated 40% to 75% of extragenital chancres occur in the mouth [24]. The chancre can appear two to three weeks after the initial exposure and may be associated with a lymphadenopathy. The surface of the chancre commonly ulcerates and forms a hemorrhagic covering that harbors a large reservoir of T. pallidum.
These lesions usually resolve without scarring. Patients may be unaware of the origin and nature of this lesion and equate its disappearance with a permanent resolution of a benign process. While the chancre will resolve, lack of appropriate medical treatment at this time allows progression to secondary syphilis [21].
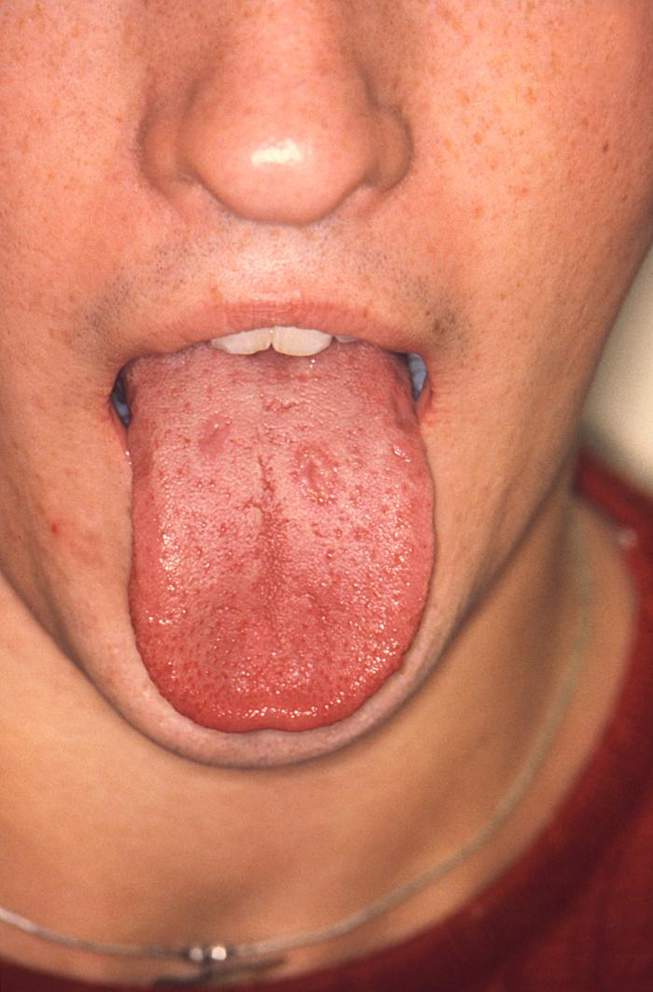
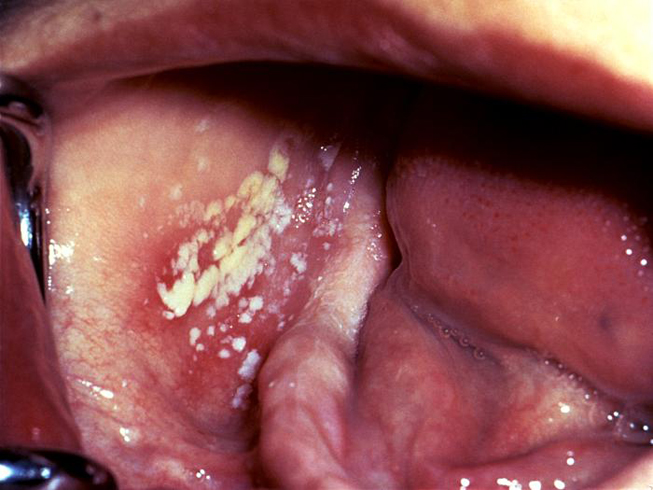
The secondary stage of syphilis usually develops 2 to 12 weeks after initial exposure [24]. The systemic manifestations of secondary syphilis are the result of the hematogenous dissemination of T. pallidum. Patients may experience fever, painful joints, malaise, and generalized lymphadenopathy. Mucocutaneous involvement is also common and features the development of a non-pruritic rash. The rash often develops on the palms of the hands and soles of the feet, but the symmetrical, red-brown lesions can occur anywhere in the body [24]. Oral lesions occur in approximately 30% of patients with secondary syphilis. The classic oral lesion at this stage is a grayish-white erosion with an erythematous base known as a mucous patch (Image 1). These shallow ulcerations can extend to 1 cm in diameter and usually occur bilaterally, most often involving the mobile surfaces of the oral cavity (e.g., the tongue) [25]. Extension of these lesions onto the gingiva and hard palate is rare. However, the hard palate may develop macular lesions that appear as firm, flat or minimally elevated erythematous lesions. Although the oral and cutaneous lesions that occur during secondary syphilis are more pronounced and more widespread than those of primary syphilis, they will resolve without medical intervention, again leading many patients to believe that the disease is "cured." However, syphilis will continue to progress in the absence of medical treatment.
If medical treatment has not been completed, syphilis will enter a latent stage. As with any stage of syphilis, the time of the onset and the duration of the latent stage are variable among patients. This stage can be further divided into early and late latent phases. The first 12 months of an extended period of latency are considered the early latent stage. During this period, mucocutaneous relapses may occur and patients are considered infectious. After 12 months, patients enter the late latent stage, during which time mucocutaneous relapses do not occur and patients are considered noninfectious except via blood transfusions and vertical transmission during pregnancies [8,21]. Because the mucocutaneous rash can recur several years after the initial inoculation with the T. pallidum bacteria, patients may not equate this symptom with syphilis or an STI. While some patients will remain in the latent stage, others will progress to the most serious stage of this disease, tertiary syphilis.
Approximately one-third of patients who have not undergone medical treatment will advance to the tertiary stage of syphilis [25]. This stage can occur decades after the initial infection. Patients with late-stage syphilis are noninfectious, but the disease that results can be widespread, serious, and potentially fatal [21]. The characteristic lesion of tertiary syphilis is a gumma, which may appear on the skin, mucous membranes, neural tissue, bone, and/or any visceral organ. The gumma is a long-standing granulomatous lesion with a necrotic central core. Oral gummata are not common, but when they do occur, they usually involve the tongue and the palate. The necrotic core of palatal gummata can be expansive and perforate the nasal cavity or the maxillary sinus. These lesions can range in size from a few millimeters to large masses more than 1 cm in diameter. As with the oral lesions of the primary and secondary stages of syphilis, a gumma cannot be identified by visual means only. Referral for a medical evaluation and a biopsy is necessary to distinguish it from other lesions, such as squamous cell carcinoma. Dental treatment should be deferred until the identification of the lesion is ascertained. Syphilitic involvement of the tongue can manifest as an interstitial glossitis. The tongue may appear erythematous, with a loss of surface papillae and the development of a fissured and lobulated appearance.
Patients who use maxillary dentures will have difficulty if a gumma appears on the hard palate, alters the tissue topography, and/or interferes with the proper tissue adaptation. The denture base may also irritate the contiguous inflamed tissue of a gumma and preclude the ability of the prosthesis to function properly. Scar formation that accompanies the healing of a gumma may also change the palatal tissue surface enough that a reline or remake of the maxillary denture is required in order to re-establish the proper adaptation of the denture to the tissue surface.
The systemic manifestations of tertiary syphilis present the greatest threat to the general health of the patient. Neurosyphilis is a serious complication of tertiary syphilis involving the brain and the spinal cord. Symptoms include muscle spasms, blindness, dementia, and paralysis [26]. Cardiovascular syphilis is rare but can cause aneurysm of the ascending aorta and potentially death. Ocular syphilis can occur at any stage of infection and involve any eye structure, most commonly the posterior uveitis and panuveitis. Symptoms include vision changes, decreased visual acuity, and in some cases, permanent blindness [21].
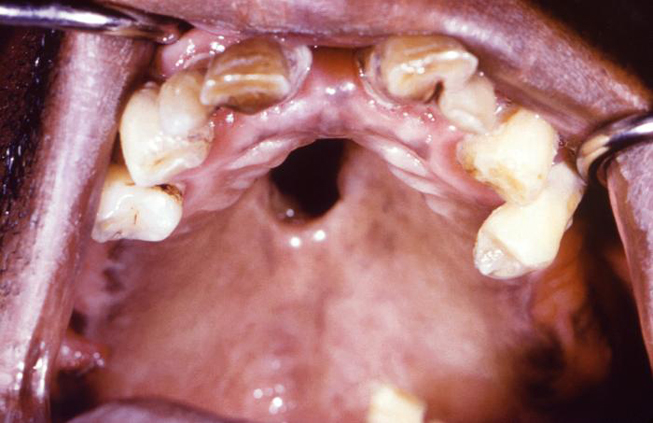
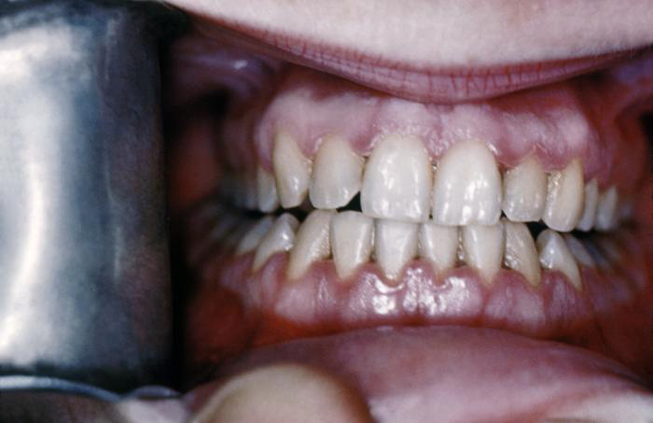
Syphilis can be transferred from an infected mother directly to the developing fetus in utero via the placenta or via direct contact with a genital lesion during childbirth. The highest incidence of mother-to-child transmission occurs during the primary stage, with risk progressively decreasing during the advanced stages of the disease. Syphilitic involvement of the developing fetus can result in spontaneous abortion, stillbirth, and neonatal disease. Untreated syphilis in pregnant women results in infant death in up to 40% of cases [4]. Congenital syphilis can also lead to specific oral and facial manifestations in affected children, including perforations in the hard palate (Image 2) and Hutchinson triad. The triad consists of interstitial keratitis, eighth nerve deafness, and Hutchinson teeth. The dental malformation known as Hutchinson teeth is caused by an inhibition of the proper developmental function of the ameloblasts (enamel-forming cells) via an inflammatory process mediated by T. pallidum [27]. Disruption in the formation of the crowns of the teeth occurs during later development of the teeth, which spares the deciduous teeth from this anomaly [28]. This condition affects the permanent incisors, resulting in characteristic semilunar notches on their incisal edges.
Congenital syphilis may also cause malformation of the enamel of the permanent molars, resulting in a condition known as mulberry molars. Affected molars have an occlusal surface with non-anatomical depressions that alternate with rounded nodules of enamel that are actual rudimentary cusps. Congenital syphilis may also cause doming of the first permanent molars, also referred to as Moon's molars.
Congenital syphilis can also lead to the premature loss of deciduous teeth, with subsequent delays in the development of speech patterns, difficulties in eating and swallowing, and problems with the emergence of a healthy self-image. Restorative and orthodontic treatment to correct these developmental problems is possible but can present a financial challenge.
The antibiotic of choice to treat all stages of syphilis is penicillin G administered parenterally [20]. The dosage, duration of treatment, and preparation (benzathine, aqueous crystalline, or aqueous procaine) are selected based on the stage and manifestations of the disease [20]. Extended treatment is necessary for patients in latent and tertiary stages. Pregnant persons and patients with a penicillin allergy whose compliance with therapy or follow-up cannot be ensured should be desensitized and treated with benzathine penicillin G. Where available, skin testing for penicillin allergy should be used to differentiate those with a true allergy to penicillin [20]. Azithromycin should never be used to treat syphilis, as macrolide resistance and treatment failure are well documented.
Patients should be advised that these treatment regimens do not confer immunity against future syphilitic infections and any systemic damage cannot be reversed. Cardiovascular syphilis may damage the valves of the heart, requiring surgical repair or replacement. Consultation with the patient's physician is necessary to determine if premedication with antibiotics is required prior to initiation of dental procedures. Aside from this, modification of dental treatment is usually not required for patients with syphilis unless there is significant systemic involvement that poses a medical concern.
HPV is a family of more than 200 genotypes, of which more than 40 types infect the mucosal epithelium [29]. Unlike gonorrhea and syphilis, HPV infection is not reportable to the CDC, and most cases are asymptomatic and subclinical. The CDC estimates that 13 million people acquire HPV infections each year in the United States and that 42 million Americans are currently infected, making it the most common STI in the country [30]. Orogenital contact is the primary means by which the HPV is transferred to the structures in and around the oral and maxillofacial complex. Approximately 90% of this viral pathogen is cleared by the immune system within two years, but the remainder can cause oral and systemic problems with a high degree of morbidity, including squamous papilloma, verruca vulgaris, condyloma acuminatum, and certain cancers [29].
Squamous papilloma is the most common benign neoplasm of oral epithelial origin. It is usually associated with HPV types 6 and 11, although HPV type 16 has occasionally been isolated from these lesions [29,30]. The lesions can occur anywhere within the oral cavity but have a predilection for the soft palate, the uvula, and the tongue. The size of squamous papilloma lesions can vary but are usually less than 1 cm in diameter. They may be attached to the underlying tissue by a movable, stalk-like base (pedunculated) or a fixed, broad base (sessile). The lesions are generally asymptomatic and usually have the same color as the contiguous oral mucosa; however, they may appear white due to keratinization of the superficial layer. The lesions cannot be diagnosed by visual means alone, so histopathologic examination is essential to rule out a malignant neoplasm. Surgical excision is the treatment of choice, and recurrence of these lesions is uncommon.
HPV types 2, 4, 6, and 40 are the primary etiologic agents for the development of verruca vulgaris, also known as the common wart [32]. Verruca vulgaris is a relatively common cutaneous lesion, but oral lesions can also occur. When these lesions develop intra-orally, they are most likely to affect keratinized tissues such as the lip, the hard palate, and the gingiva. Verruca vulgaris lesions are contagious, and their origin in and around the mouth is usually via autoinoculation (e.g., when a cutaneous wart on a finger is brought into contact with the oral or peri-oral structures). Transmission of an existing oral lesion to the genitals is infrequent but can occur through oral-genital contact.
The lesions are typically solitary, with cauliflower-like appearance that may appear similar to squamous papillomas. Their color is usually similar to the adjacent oral mucosa, but keratinization of the superficial layer can give a white appearance. Histopathologic examination is required for a definitive diagnosis. Occasionally, these lesions resolve spontaneously. If not, surgical excision may be necessary.
Condyloma acuminatum, also referred to as anogenital wart, accounts for 20% to 30% of all STIs in the United States [33]. It is usually associated with HPV types 6 and 11, although HPV types 16 and 18 have also been isolated from the lesions [29,33]. The lesions typically affect the anogenital epithelium and are transmitted to the oral cavity by oral-genital contact. Condyloma acuminatum lesions are larger than squamous papillomas and are often located on the labial mucosa, the soft palate, the lingual frenum, and the tongue [34]. It is a benign lesion that is firmly attached to the underlying mucosa [33].
Individual lesions coalesce to form a larger lesion whose surface layer can have a cauliflower-like appearance and finger-like projections. They are asymptomatic, although larger lesions on the labial mucosa or the tongue may become ulcerated via occlusal trauma. Nearly all infections are the result of sexual contact. When a definitive histologic diagnosis of a condyloma acuminatum lesion is made in a child, there should be a strong suspicion of sexual abuse and the appropriate authorities should be notified [35].
Treatment involves the eradication of all individual oral lesions to prevent autoinoculation of the adjacent tissues. Patients with oral involvement should also receive treatment for genital lesions, if present. Notification, evaluation, and treatment of all sexual partners is necessary to prevent the further propagation of this disease in the community. The lesions of condyloma acuminatum may be removed by surgical excision, chemical cauterization, or cryoablation. Because the virus is present in a latent state in the basal cell layer of the epidermis, the lesion often recurs, requiring retreatment [36].
As with other oral lesions, identification of condyloma acuminatum cannot be accomplished by visual means and is dependent on histologic examination. Dental treatment should be deferred until the identification of the lesion is established. The area of the lesion should be recorded carefully and examined for recurrence at each dental appointment. After the area of the excised lesion has healed, dental treatment may commence.
So far, the HPV-associated lesions discussed have been benign. The role of this viral pathogen in the development of malignancies is of even greater concern. HPV types 16 and 18 are associated with approximately 70% of cervical cancers throughout the world [37]. In addition, the oncogenic forms of HPV types 16 and 18 have a role in the development of some malignancies of the head, neck, oropharynx, and oral cavity [38]. Research indicates that approximately 10% of men and 3.6% of women in the United States are infected with oral HPV [39]. In total, HPV DNA is present in more than 50% of oral carcinomas [40]. It is not clear if long-term infection with HPV alone is the cause of oropharyngeal malignancy or if it has an oncogenic synergistic effect in the presence of other factors, such as cigarette smoking, smokeless tobacco, or long-term alcohol abuse. HPV genes can induce the development of proteins that attach to and inactivate compounds from tumor-suppressor genes key in the development of a malignancy [41].
Because HPV is not curable once contracted, the best approach is the prevention of infection through safe sex practices and vaccination. A vaccine is available to prevent or decrease the potential of acquiring HPV. It is important to note that the vaccine will not reverse the effects of an HPV-related disease or stop the progression of HPV. In 2014, a nine-valent HPV recombinant vaccine (Gardasil 9) was approved that adds protection to HPV types 31, 33, 45, 52, and 58 in addition to those types covered by the original Gardasil [42,43,44]. As of 2016, Gardasil 9 is the only available HPV vaccine in the United States and is indicated in individuals 9 to 45 years of age. With the increased coverage, the nine-valent vaccine has the potential to prevent up to 90% of cervical, vulvar, vaginal, and anal cancers [44]. Initial studies indicate that vaccination is also effective in preventing oral vaccine-type HPV infections [31,45].
Lesions of the oral mucosa caused by infection with HPV subtype 13 or 32 is known as focal epithelial hyperplasia or Heck disease. The lesions appear as small, single or multiple papules. They may appear anywhere in the oral cavity, especially on the labial and buccal mucosa, lower lip, and tongue, and less often on the upper lip, gingiva, and palate [46]. Removal is usually not necessary, and the lesions often resolve on their own. However, if the papules are impairing the patient's ability to eat or maintain good oral hygiene or if they are causing aesthetic concern, they may be removed via excisional biopsy or cryosurgery [46].
It is essential that all patients who have had oral lesions caused by any HPV type understand the increased risk for oral malignancy. Patient education should stress the importance of regular oropharyngeal cancer screening by a dentist as a critical component in the maintenance of oral and systemic health.
Epstein-Barr virus (EBV) is a member of the herpes virus family, specifically human herpesvirus-4 (HHV-4). This virus has a global distribution, and approximately 90% of adults have antibody titers demonstrating current or past infection with EBV [47]. Most initial infections are asymptomatic or may present with mild symptoms that resemble those of the common cold. EBV is frequently transmitted via bodily fluids, most notably saliva. This viral pathogen can also be transmitted by blood and semen during sexual relations, blood transfusions, and organ transplantations.
EBV shares the characteristic of latency with other members of the herpes virus family. After the acute infection resolves, EBV remains latent in B lymphocytes for the balance of the patient's life, with reactivation and the emergence of symptoms an unpredictable event.
During adolescence, infection with the EBV is the primary cause of infectious mononucleosis, also known as "mono" or the "kissing disease." This is not a classic STI, but it is included in this section because it is transmitted through intimate interpersonal contact.
Patients with infectious mononucleosis develop a classic triad of symptoms: fever, pharyngitis, and lymphadenopathy. Possible oral manifestations of infectious mononucleosis include palatal petechiae, tonsillar enlargement, and pharyngitis, although these physical findings alone are not diagnostic. While other viral pathogens, such as cytomegalovirus, can cause similar symptoms, EBV has been implicated as the etiologic factor in 90% of the cases of infectious mononucleosis [48]. The diagnostic criteria for infectious mononucleosis include laboratory testing of a blood sample. In a normal blood smear, approximately 1% to 2% of the cells evaluated are large reactive lymphocytes, while these cells comprise approximately 10% to 40% of blood smears of patients with infectious mononucleosis [49]. Antibody testing may be useful in conjunction with the assessment of somatic and oral manifestations to confirm the diagnosis of infectious mononucleosis; however, the CDC recommends that the Monospot rapid latex agglutination test should no longer be used due to high numbers of false-positive and false-negative results [47,50].
This disease has an incubation period of one to two months and a typical duration of one to two weeks. Some patients have a prodromal period characterized by malaise, fatigue, arthralgia, and myalgia. Complications of infectious mononucleosis are rare but can include neurologic conditions (e.g., meningitis), enlarged or ruptured spleen, cranial nerve palsies, upper airway obstruction, and opportunistic bacterial infections that can lead to sepsis and death [50].
Treatment of infectious mononucleosis involves palliative relief of the symptoms with analgesics and antipyretic medications, bed rest, and adequate fluid and nutritional intake. Elective dental treatment is contraindicated during the active course of the disease, and emergency dental treatment should be limited to the relief of pain and the control of acute odontogenic infections; definitive dental treatment should be deferred until the active disease has resolved. The use of amoxicillin or ampicillin is contraindicated in patients with acute infectious mononucleosis as there is an increased risk of an antibiotic-induced rash (maculopapular exanthems), which could be mistaken for an allergic reaction [51]. If an antibiotic is required to treat an odontogenic infection while a patient is still experiencing symptoms of infectious mononucleosis, an alternate antibiotic compatible with the patient's medical history should be used.
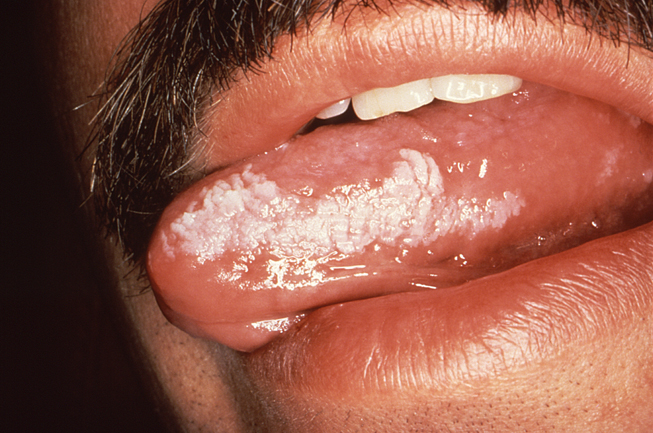
Oral hairy leukoplakia (OHL) is an opportunistic infection caused by a reactivation of the EBV among immunosuppressed patients. This condition occurs most frequently among patients with HIV/AIDS, but it can also occur among organ or bone marrow transplant recipients who require lifelong immunosuppression. The classic presentation of OHL features corrugated white lesions on the lateral surfaces of the tongue (Image 3). Occasional involvement of the buccal mucosa, gingiva, and dorsum of the tongue is also possible. The lesions are firmly adherent to the underlying tissue and lack erythema or edema of the contiguous tissue. The hair-like projections that extend from the surface of the lesions are hyperkeratotic extensions. The lesions of OHL are asymptomatic and are usually discovered during a routine examination. Chronic hyperplastic candidiasis can have a similar appearance, so biopsy may be required to provide an accurate differential diagnosis.
Among patients with HIV, the appearance of OHL often reflects a progressive deterioration of the immune system and advancement toward AIDS. The use of highly active antiretroviral therapy (HAART) has deferred the onset of opportunistic infections such as OHL among many patients with HIV. Spontaneous resolution of OHL is possible, but recurrence is an ongoing threat. In addition, the use of antiviral medications such as acyclovir, valacyclovir, and famciclovir will cause OHL lesions to resolve temporarily, but they will return after the medication is stopped [52].
The presence of OHL alone is not an absolute contraindication for dental treatment. Their discovery during a comprehensive oral examination and a thorough review of the patient's medical history should prompt diagnosis by a dentist and/or referral to a dental specialist or physician for further evaluation and diagnosis.
Herpes simplex viruses and the infections they cause are among the most common in the world [53]. Herpes simplex virus-1 (HSV-1) causes recurrent herpes labialis, also known as "cold sores" or "fever blisters." The typical clinical presentation involves individual vesicles that coalesce to form larger vesicles, usually at the mucocutaneous junction of the skin and the lips. These vesicles rupture to form shallow ulcers surrounded by an erythematous border. These initial lesions contain a large amount of live viral particles that can be transferred to another person through direct contact or indirect exposure (e.g., sharing utensils or towels). HSV-1 may be transmitted to the genitals during oral-genital sexual contact, and slightly less than 50% of genital herpetic infections are caused by HSV-1 [54]. These lesions usually heal without scarring within two weeks, but patients with immunosuppression may experience expansive involvement and a protracted healing time.
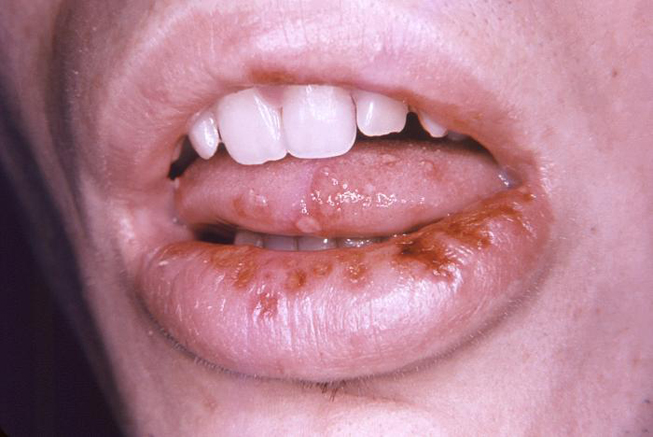
An initial infection with HSV-1 (primary herpetic gingivostomatitis) often occurs in children, and intraoral manifestations are most prominent at this stage. Lesions may occur on the hard and soft palate, gingival tissues, tongue, or lips and surrounding skin (Image 4). Oral ulcerations usually have an erythematous border while the gingival tissues appear edematous with a bright red margin. Oral involvement may complicate the ability to eat, swallow, and maintain oral hygiene.
Aside from the oral lesions, primary HSV-1 infection may present with cervical lymphadenopathy, malaise, fever, and irritability. While the initial infection is self-limited in immunocompetent patients and resolves within two weeks, the virus is not eliminated from the body. Instead, the virus migrates to the trigeminal ganglion, where it remains in a state of latency. The reactivation of HSV-1 (recurrent herpes labialis) occurs secondary to psychologic or emotional stress, overexposure to sunlight, illness, injury, or immunosuppression. Prior to an outbreak, most patients experience a prodromal sensation of itching, burning, or tightness of the skin when viral replication causes the death of the target host cell followed by ballooning degeneration of the affected host cells. HSV-1 infections are not strictly considered an STI, though the virus can be transmitted through close contact with saliva (e.g., kissing) and sexual activity (e.g., oral sex) during prodrome and outbreaks.
There is no vaccine or cure for HSV-1. Treatment with topical or systemic antiviral medications such as acyclovir may decrease the duration of an outbreak [20].
Dental treatment should be deferred during periods of active outbreaks, as retracting and stretching the tissue of involved areas may cause rupture of the vesicles and additional inoculation of the virus into the adjacent skin. Aerosols or droplets created with the use of high-speed hand pieces during restorative procedures or air polishing systems during prophylaxis may contain viral particles that can infect the dental staff and/or other areas on the patient (e.g., the conjunctiva of the eye). Live viral particles may also be transmitted through porosities or small tears in gloves to infect the finger(s) of the clinician, causing a condition known as herpetic whitlow (i.e., recurrent herpetic lesions on the affected finger). Clinicians who develop this condition should refrain from clinical practice until the lesions are completely healed.
Patients should be advised that the lesions of recurrent labialis should not be touched or squeezed in order to prevent autoinoculation of their fingers and the adjacent skin. Topical application of an antiviral ointment should be done with a disposable cotton tip applicator to avoid cross contamination and autoinoculation.
Herpes simplex virus type-2 (HSV-2) is the etiologic pathogen associated with most cases of genital herpes. The CDC estimates that 11.9% of persons 14 to 49 years of age in the United States is infected with HSV-2 [20]. Although genital herpes is an STI, its exact incidence is unknown because it is not a reportable disease. The primary mode of transmission of HSV-2 is via genital-to-genital contact. It may also be transmitted by oral-genital contact or self-inoculation. Oral herpetic lesions that are caused by HSV-2 are clinically indistinguishable from those caused by HSV-1. About 1% to 3% of individuals infected with HSV-2 are asymptomatic and can shed the virus at any time, even without the presence of active lesions [55]. The characteristic of latency also applies to genital HSV-2 infection, as the virus migrates to the sacral ganglion at the base of the spine between outbreaks.
As with HSV-1, there is no vaccine or cure for HSV-2. Antiviral medications are used to control the duration, frequency, and severity of outbreaks as much as possible. These medications do not have interactions with the medications frequently used for dentistry. Patients who have more than five outbreaks per year may use these medications on a daily basis for suppressive therapy, which can also reduce asymptomatic viral shedding [48]. Acyclovir, valacyclovir, and famciclovir have been shown to be safe and effective for long-term use [20]. The long-term use of valacyclovir can cause a reduction in platelets (thrombocytopenia) in a small percentage of patients [18]. A complete blood count should be obtained for all patients taking long-term valacyclovir prior to any dental procedure in which the ability to achieve hemostasis is essential. Patients with an active outbreak of HSV-2 may experience regional lymphadenopathy, generalized malaise, fever, myalgia, and arthralgia, which may require deferral of elective dental treatment. Resolution of active HSV-2 infections can take several weeks, with recurrent infections a possibility at any time.
Chlamydia is an STI caused by the bacterium Chlamydia trachomatis. It is the most commonly reported STI in the United States, with more than 1.6 million cases reported to the CDC in 2022 [3]. In addition, many cases of chlamydia go unreported and undetected due to the lack of symptoms. When symptoms are present, they may develop many weeks after the initial infection. Chlamydial infections in women can develop in the cervix and/or the urethra, with associated vaginal discharge or pain during urination. Men may experience a discharge from the urethra, a burning sensation during urination, or a painful swelling in one or both testicles (epididymitis). A urine sample or a swab of fluid from the penis or the vagina is taken to definitively diagnose a chlamydial infection. Without treatment, chlamydial infection can spread to the uterus and the fallopian tubes, leading to pelvic inflammatory disease, endometriosis, and an increased risk of premature births and infertility [56]. Occasionally, untreated chlamydial infections in men can cause the development of reactive arthritis (Reiter syndrome), which features inflammation of the joints, the urethra, and the eyes [57].
Because C. trachomatis infects the genitals, transmission occurs most often during sexual intercourse, although transmission during oral sex can also occur. There are no specific oral manifestations of chlamydial infections. Patients may complain of a sore throat or have a generalized inflammation of the pharynx and/or tonsils. It is not possible to diagnose a chlamydial infection based strictly upon these oropharyngeal manifestations. However, the presence of these symptoms should prompt referral to a physician for further evaluation.
After a chlamydial infection has been diagnosed, it can be treated and cured with conventional antibiotic therapy. Doxycycline is the antibiotic of choice for the treatment of chlamydial infections; penicillin is ineffective [20]. Treatment of chlamydia also involves treating all partners with whom the infected person has had sexual contact. Patients who have completed antibiotic therapy successfully should be advised that this does not confer lifelong immunity against chlamydial infections and that reinfection can occur.
There are no contraindications to dental procedures for patients with chlamydial infections. Patients who are symptomatic may experience enough discomfort that an appointment for an elective dental procedure should be deferred.
AIDS and its etiologic viral pathogen HIV have transformed the medical, political, and social conventions of the United States and the global community. From a historical perspective, HIV/AIDS has been a relatively recent disease. In June 1981, the CDC reported five cases of Pneumocystis jiroveci pneumonia (PJP) in five young homosexual men in Los Angeles. This rare disease, caused by airborne inoculation of the fungal organism P. jiroveci, is easily controlled by a healthy immune system. The local outbreak quickly grew, with health professionals and epidemiologists seeking to identify the etiologic agent impairing patients' immune systems to the point that they developed PJP (and eventually died). By 1983, a retrovirus later named HIV was isolated from a patient with AIDS. As of 2022, the CDC estimates that more than 1.1 million people are currently living with HIV in the United States, with approximately 13% unaware of their infection [58]. In 2022, 37,981 new HIV cases were diagnosed in the United States [58].
HIV is a bloodborne viral pathogen that may be transmitted during unprotected sexual activity, sharing needles during illicit drug use, receiving HIV-infected organ and tissue transplants, or exposure of mucous membranes or non-intact skin with HIV-infected blood. Among healthcare workers from 1985–2013, there were 58 confirmed cases and 150 possible cases of occupational transmission of HIV from the blood or tissue products of HIV-infected patients (only 1 confirmed case occurred from 1999–2013) [59,60]. A healthcare worker who is accidentally exposed to the blood or tissue products of a patient with HIV has a 0.3% chance of becoming infected with HIV [60].
Progression from initial infection with HIV to the development of AIDS can take many years or decades, and the use of HAART may prevent progression indefinitely by suppressing viral loads and by maintaining the levels of the T-helper lymphocytes (CD4+ cells). However, these medications are known to have many adverse effects, making long-term compliance a challenge.
The diagnosis and staging of HIV and AIDS is based on the CDC's case definition for HIV infection [61]. This system uses a combination of laboratory evidence of HIV infection, CD4+ cell count, and the presence of AIDS-defining conditions to assign stages to the infection. Stage 3 HIV infection, designated as AIDS, is defined by laboratory confirmation of HIV infection and a CD4+ T-lymphocyte count less than 200 cells/mcL or less than 14%. At this stage, opportunistic infections are present and can have a high degree of morbidity and even mortality. The most common oral manifestations of AIDS include:
Oral candidiasis
Linear gingival erythema
Oral hairy leukoplakia
Necrotizing ulcerative gingivitis
Kaposi sarcoma
Candida albicans is a fungal organism that naturally occurs in the oral environment. Immunosuppression, such as that seen in patients with AIDS, results in overgrowth of C. albicans and the development of oral candidiasis, the most common oral/maxillofacial manifestation of AIDS. In these patients, oral candidiasis can extend into the esophagus and become disseminated systemically, with the potential for serious morbidity and death. Among those with AIDS, opportunistic oral candidiasis occurs in up to 80% of patients [62].
Oral candidiasis can appear in a variety of forms. Pseudomembranous candidiasis is characterized by white to yellow-white plaques that may be removed to reveal an erythematous or bleeding surface underneath (Image 5). Erythematous candidiasis consists of painful denuded areas of the surface epithelium (red in appearance). Chronic hyperplastic candidiasis presents as white to yellow-white plaques that cannot be removed from the underlying tissue. Fungal infection at the commissures of the lips is referred to as angular cheilitis and features cracking and fissuring lesions at the corners of the mouth. Opening and closing the mouth can stretch these lesions and interfere with their healing. It is possible for a patient with AIDS to have more than one form of oral candidiasis concurrently.
In this patient population, opportunistic fungal infections can be refractory to treatment and require oral or intravenous systemic antifungal medications. Patients who use partial or complete dentures should also disinfect the tissue-bearing surfaces of these prostheses, as the fungal organisms can remain viable in the porosities in the acrylic. The failure to eradicate these organisms will allow for a cycle of re-inoculation of the tissues with C. albicans and a perpetuation of the oral candidiasis. Dental treatment should be deferred until the fungal infection has been eradicated, as systemic dissemination can occur through breaks in the tissue.
Gingivitis and periodontal disease are common oral conditions in the general population, and their progression is slow and innocuous for most patients. The progressive depletion of the immune system during the advancement of HIV and AIDS permits the pathogenic bacteria that cause gingivitis and periodontal disease to progress at an advanced rate, resulting in a more aggressive course that can be refractory to treatment.
The first gingival manifestation unique to HIV-infected patients is linear gingival erythema. This condition features a distinct bright-red demarcation at the free gingival margin in the absence of local irritants (e.g., plaque, calculus) that demonstrates minimal or no resolution with conventional periodontal therapy. Despite the intensity of its color, bleeding and discomfort are not always an accompaniment of this condition. C. albicans may be a contributing factor [48]. It is most frequently seen on anterior teeth, although any tooth can be affected.
Over time, periodontal pathology in patients with HIV/AIDS progresses to necrotizing ulcerative gingivitis. This condition features the rapid destruction of the gingival tissues, with necrosis of the interdental papilla (Image 6). Mild bleeding is present in the involved tissues. The progression from gingivitis to periodontal disease in patients with healthy immune systems is usually a slow process, but in those with AIDS, progression from necrotizing ulcerative gingivitis to necrotizing ulcerative periodontitis is often rapid and aggressive. Necrotizing ulcerative periodontitis is typified by destruction of the supporting alveolar bone, causing moderate-to-severe pain. Occasionally, the bacteria can extend into the alveolar and buccal mucosa and cause a necrotizing stomatitis [12].
The initial treatment of HIV/AIDS-associated periodontal disease includes the use of antibiotics to control the infection and the use of an analgesic to control the pain. Even when the acute symptoms of necrotizing ulcerative periodontitis are controlled, the supporting osseous architecture and the supporting gingival tissues are altered in a fashion that complicates the ability to maintain optimal oral hygiene. The extensive destruction of the supporting alveolar bone leaves many teeth with advanced mobility patterns requiring extraction. It is essential to control necrotizing ulcerative periodontitis, as its extension into the contiguous mucosal tissues can lead to systemic dissemination and bacterial sepsis.
Patients with HIV/AIDS should maintain a dental prophylaxis schedule that reflects the extent of periodontal involvement to decrease the progression of these destructive periodontal patterns. Because the inflammatory lesions of periodontal disease can provide systemic access for multiple pathogenic organisms, it is essential that periodontally involved areas are treated promptly. Teeth with a poor periodontal prognosis should be extracted to eliminate a source of local, regional, and systemic infection.
Kaposi sarcoma is the most common malignant disease in patients with HIV/AIDS [13]. It is caused by infection with human herpesvirus-8, also known as Kaposi sarcoma-associated herpesvirus. Although cutaneous lesions are more common, oral lesions of Kaposi sarcoma occur in approximately 50% of patients. When these lesions appear in the oral cavity, the most common sites are the hard palate, the gingiva, and the tongue. The lesions of Kaposi sarcoma do not blanch upon compression and are generally asymptomatic until they enlarge and interfere with normal function or become traumatized or ulcerated. These singular or nodular lesions can be a combination of blue, red, and purple and are often elevated from the tissue surface. Biopsy is required to confirm diagnosis.
The treatment of Kaposi sarcoma may involve surgical removal or injections with chemotherapeutic agents such as vinblastine sulfate [19]. Unfortunately, appearance of these lesions reflects a progressive deterioration of the immune system and a poor prognosis for the patient. Larger lesions on the hard palate can alter the tissue topography and preclude the ability to wear a partial or complete denture and complicate the patient's ability to eat at a time when adequate nutrition is critical. Given the advanced immunosuppression levels that exist when Kaposi sarcoma lesions are present, consultation with the patient's physician is advisable before any dental treatment is started.
It is beyond the scope of this course to enumerate all of the dental treatment modifications required for patients with HIV/AIDS. Patients with similar viral loads, CD4+ counts, and absolute neutrophil levels can have radically different responses to the same dental procedures, some of which can involve a high degree of morbidity. Laboratory values and any medical information relevant to the patient's HIV/AIDS status should be obtained and their significance discussed with the patient's physician prior to initiating dental treatment. HAART medications used to manage HIV/AIDS can have side effects and adverse interactions with some of the medications used in dental treatment, so this should also be documented. A decrease in the platelet count (thrombocytopenia) can be a complication of HIV/AIDS, which poses obvious risks for coagulation during invasive dental procedures. The psychologic and emotional component of HIV/AIDS should also be considered; depression and anxiety may develop as the patient confronts the realities of living with a life-threatening disease. Opioid analgesics should be avoided or used minimally if a patient is taking antidepressants or anxiolytics, as the synergism between these medications can cause advanced levels of sedation and respiratory depression. Dental clinicians should remain current in the treatment modalities and medication regimens that are used for HIV/AIDS so safe and efficacious dental treatment can be provided.
This course has provided a brief overview of STIs, their oral manifestations, and their potential systemic complications. Diseases such as gonorrhea, syphilis, chlamydia, HPV, EBV, herpes simplex viruses, and HIV can cause problems ranging from a temporary inconvenience to life-threatening illness. Many of the oral manifestations associated with these diseases are not uniquely identifying features, but their presence should prompt referral for further medical evaluation.
1. Centers for Disease Control and Prevention. Sexually Transmitted Infections (STIs). Available at https://www.cdc.gov/std/statistics/2022/default.htm. Last accessed September 11, 2024.
2. Medline Plus. Reportable Diseases. Available at https://medlineplus.gov/ency/article/001929.htm. Last accessed September 10, 2024..
3. Centers for Disease Control and Prevention. Sexually Transmitted Infections Surveillance, 2022. Available at https://www.cdc.gov/std/statistics/2022/overview.htm. Last accessed September 11, 2024.
4. Canto SVE, Araújo MAL, Miranda AE, Cardoso ARP, de Almeida RLF. Fetal and infant mortality of congenital syphilis reported to the Health Information System. PLoS One. 2019;14(1):e0209906.
5. Centers for Disease Control and Prevention. CDC Estimates 1 in 5 People in the U.S. Have a Sexually Transmitted Infection. Available at https://archive.cdc.gov/#/details?url=https://www.cdc.gov/media/releases/2021/p0125-sexualy-transmitted-infection.html. Last accessed September 24, 2024.
6. National Academies of Sciences, Engineering, and Medicine. Sexually Transmitted Infections: Adopting a Sexual Health Paradigm. Washington, DC: The National Academies Press; 2021. Available at https://nap.nationalacademies.org/read/25955/chapter/1. Last accessed September 25, 2024.
7. Centers for Disease Control and Prevention. About Gonorrhea. Available at https://www.cdc.gov/gonorrhea/about. Last accessed September 12, 2024.
8. Frank KM, McAdam AJ. Infectious diseases. In: Kumar V, Abbas AK, Aster JC (eds). Robbins and Cotran Pathologic Basis of Disease. 10th ed. Philadelphia, PA: Elsevier Saunders; 2020.
9. Quillin S, Seifert H. Neisseria gonorrhoeae host adaptation and pathogenesis. Nat Rev Microbiol. 2018;16:226-240.
10. Marrazzo JM, Handsfield HH, Sparling PF. Neisseria gonorrhea. In: Mandel GL, Gordon Douglas R, Bennett JE (eds). Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone; 2010.
11. Centers for Disease Control and Prevention. Gonococcal Infections Among Adolescents and Adults. Available at https://www.cdc.gov/std/treatment-guidelines/gonorrhea-adults.htm. Last accessed September 12, 2024.
12. Tyring SK (ed). Mucosal Immunology and Virology. Singapore: Springer-Verlag London Limited; 2006.
13. Dodd CL, Greenspan D, Greenspan JS. Oral Kaposi's sarcoma in a woman as a first indication of HIV infection. J Am Dent Assoc. 1991;122(4):61-63.
14. Mayor MT, Roett MA, Uduhiri KA. Diagnosis and management of gonococcal infections. Am Fam Physician. 2012;86(10):931-938.
15. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR. 2021;70(RR4):1-187.
16. Miller KE. Diagnosis and treatment of Neisseria gonorrhoeae infections. Am Fam Physician. 2006;73(10):1779-1784.
17. Yonke N, Aragón M, Phillips JK. Chlamydial and gonococcal infections: screening, diagnosis, and treatment. Am Fam Physician. 2022;105(4):388-396.
18. Wynn RL, Meiller TF, Crossley HL. Drug Information Handbook for Dentistry. 24th ed. Hudson, OH: Lexicomp; 2018.
20. Centers for Disease Control and Prevention. About Syphilis. Available at https://www.cdc.gov/syphilis/about. Last accessed September 12, 2024.
21. Singh PV, Ranjit R. Atypical oral manifestations in secondary syphilis. Indian J Dent Res. 2013;24(1):142-144.
22. Sanchez MR. Syphilis. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K (eds). Fitzpatrick's Dermatology in General Medicine. 8th ed. New York, NY: McGraw Hill; 2012.
23. Ficarra G, Carlos R. Syphilis: the renaissance of an old disease with oral implications. Head Neck Pathol. 2009;3(3):195-206.
24. Smith MH, Vargo RJ, Bilodeau EA, et al. Oral manifestations of syphilis: a review of the clinical and histopathologic characteristics of a reemerging entity with report of 19 new cases. Head Neck Pathol. 2021;15(3):787-795.
25. Cohen SE, Klausner JD, Engelman J, Philip S. Syphilis in the modern era: an update for physicians. Infect Dis Clin North Am. 2013;27(4):705-722.
26. Pessoa L, Galvão V. Clinical aspects of congenital syphilis with Hutchinson's triad. BMJ Case Rep. 2011;12:1-3.
27. Hillson S, Grigson C, Bond S. Dental defects of congenital syphilis. Am J Phys Anthopol. 1998;107(1):25-40.
28. Gargano J, Meites E, Watson M, Unger E, Markowitz L, Centers for Disease Control and Prevention. Manual for Surveillance of Vaccine-Preventable Diseases. Chapter 5: Human Papillomavirus (HPV). Available at https://www.cdc.gov/vaccines/pubs/surv-manual/chpt05-hpv.html. Last accessed September 12, 2024.
29. Centers for Disease Control and Prevention. About HPV. Available at https://www.cdc.gov/hpv/about/ Last accessed September 12, 2024.
30. Syrjänen S. Human papillomavirus infections and oral tumors. Med Microbiol Immunol. 2003;192(3):123-128.
31. Schlecht NF, Masika M, Diaz A, et al. Risk of oral human papillomavirus infection among sexually active female adolescents receiving the quadrivalent vaccine. JAMA Netw Open. 2019;2(10):e1914031.
32. Lukes SM, Meneses MB. The dental hygienist's role in HPV recognition. Dimens Dent Hyg. 2010;8(6):72.75-77.
33. University of Washington. Oral Pathology Case of the Month: Condyloma Acuminatum. Available at https://dental.washington.edu/oral-pathology/case-of-the-month-archives/com-jan-2011-diagnosis. Last accessed September 12, 2024.
34. Jackson JL, Muhammad A, Longwe E, Childers Ester LB, Mody V. Condyloma acuminatum: an opportunistic infection found in the oral cavity. New York Medical Journal. 2010;5(2).
35. Weiss A, Dym H. Oral Lesions Caused by the Human Papillomavirus. Available at https://www.clinicaladvisor.com/features/oral-lesions-caused-by-human-papillomavirus. Last accessed September 4, 2024.
36. Fazel N, Wilczynski S, Lowe L, Su LD. Clinical, histopathologic and molecular aspects of cutaneous human papillomavirus infections. Dermatol Clin. 1999;17(3):521-536.
37. Bosch FX, Burchell AN, Schiffman M, et al. Epidemiology and natural history of the human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26(1):K1-K16.
38. Patton L, Glick M. The ADA Practical Guide to Patients with Medical Conditions. 2nd ed. Chichester: Wiley-Blackwell; 2016.
39. Centers for Disease Control and Prevention. HPV and Oropharyngeal Cancer. Available at https://www.cdc.gov/cancer/hpv/oropharyngeal-cancer.html. Last accessed September 12, 2024.
40. National Cancer Institute. Oropharyngeal Cancer Treatment (Adult). Available at https://www.cancer.gov/types/head-and-neck/hp/adult/oropharyngeal-treatment-pdq. Last accessed September 12, 2024.
41. Waldboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12-19.
42. Centers for Disease Control and Prevention. Human Papillomavirus (HPV) Vaccine Safety. Available at https://www.cdc.gov/vaccine-safety/vaccines/hpv.html. Last accessed September 12, 2024.
43. U.S. Food and Drug Administration. Gardasil 9. Available at https://www.fda.gov/vaccines-blood-biologics/vaccines/gardasil-9. Last accessed September 12, 2024.
44. European Society for Medical Oncology. FDA Approves Gardasil 9 for Prevention of Certain Cancers Caused by Five Additional Types of HPV. Available at https://www.esmo.org/oncology-news/archive/fda-approves-gardasil-9-for-prevention-of-certain-cancers-caused-by-five-additional-types-of-hpv. Last accessed September 12, 2024.
45. Herrero R, Quint W, Hildesheim A, et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One. 2013;8(7):e68329.
46. Ozden B, Gunduz K, Gunhan O, Ozden FO. A case report of focal epithelial hyperplasia (Heck's disease) with PCR detection of human papillomavirus. J Maxillofac Oral Surg. 2011;10(4):357-360.
47. Centers for Disease Control and Prevention. About Epstein-Barr Virus (EBV). Available at https://www.cdc.gov/epstein-barr/about/index.html. Last accessed September 12, 2024.
48. Little JW, Falace DA, Miller CS, Rhodus NL. Little and Falace's Dental Management of the Medically Compromised Patient. 8th ed. St. Louis, MO: Elsevier/Mosby; 2013.
50. Shetty K. Epstein-Barr Virus (EBV) Infectious Mononucleosis (Mono) Workup. Available at https://emedicine.medscape.com/article/222040-workup. Last accessed September 12, 2024.
51. Ónodi-Nagy K, Kinyó Á, Meszes A, Garaczi E, Kemény L, Bata-Csörgő Z. Amoxicillin rash in patients with infectious mononucleosis: evidence of true drug sensitization. Allergy Asthma Clin Immunol. 2015;11(1):1.
52. Cade JE. Hairy Leukoplakia. Available at https://emedicine.medscape.com/article/279269-overview. Last accessed September 12, 2024.
53. Centers for Disease Control and Prevention. About Genital Herpes. Available at https://www.cdc.gov/herpes/about. Last accessed September 12, 2024.
54. Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006; 296(8):964-973.
55. Johns Hopkins Medicine. Genital Herpes. Available at https://www.hopkinsmedicine.org/health/conditions-and-diseases/herpes-hsv1-and-hsv2/genital-herpes. Last accessed September 12, 2024.
56. Centers for Disease Control and Prevention. About Chlamydia. Available at https://www.cdc.gov/chlamydia/about/index.html. Last accessed September 12, 2024.
57. Sexually Transmitted Diseases Guide. Chlamydia. Available at https://www.std-gov.org/stds/chlamydia.htm. Last accessed September 12, 2024.
58. Centers for Disease Control and Prevention. HIV in the United States and Dependent Areas. Available at https://www.cdc.gov/hiv-data. Last accessed September 25, 2024.
59. Joyce MP, Kuhar D, Brooks JT. Notes from the field: occupationally acquired HIV infection among health care workers—United States, 1985–2013. MMWR. 2015;63(53):1245-1246.
60. Centers for Disease Control and Prevention. Occupational HIV Transmission and Prevention Among Health Care Workers. Available at https://www.cdc.gov/hiv/workplace/healthcareworkers.html. Last accessed September 16, 2024.
1. World Health Organization. WHO Guidelines for the Treatment of Neisseria gonorrhoeae. Geneva: World Health Organization; 2016. Available at https://www.who.int/publications/i/item/9789241549691. Last accessed September 27, 2024.
2. The U.S. Preventive Services Task Force. Syphilis Infection in Nonpregnant Adults and Adolescents: Screening. Available at https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/syphilis-infection-nonpregnant-adults-adolescents-screening. Last accessed September 27, 2024.
3. Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents With HIV. Washington, DC: Department of Health and Human Services; 2024. Available at https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/adult-adolescent-oi/guidelines-adult-adolescent-oi.pdf. Last accessed September 27, 2024.
Mention of commercial products does not indicate endorsement.