Over the past several years, there have been significant changes to the immunization schedules for children, adolescents, and adults. The approval of multiple new vaccines has increased the opportunities for preventive care for both children and adults. Yet coverage with some vaccines remains far below national goals, and outbreaks of vaccine-preventable diseases continue to occur. The introduction of new vaccines and additional changes to the immunization schedules make it increasingly more difficult for physicians to ensure that patients receive the recommended preventive care. This course will focus on the immunization schedules for children, adolescents, and adults, with an emphasis on vaccinations that are routine for most healthy persons. It will address the current recommendations, the rationale for the addition of new vaccines and for several potential new changes, contraindications, and precautions as identified by the Centers for Disease Control and Prevention (CDC) and the Advisory Committee on Immunization Practices (ACIP), and methods to increase vaccination coverage in outpatient practice.
This course is designed for healthcare professionals working in all practice settings who may encourage patients to receive appropriate vaccinations and improve the overall vaccination rates.
There have been significant changes to the immunization schedules for children, adolescents, and adults, and the approval of multiple new vaccines has increased the opportunities for preventive care for both children and adults. However, coverage with some vaccines remains far below national goals, and outbreaks of vaccine-preventable diseases continue to occur. The purpose of this course is to provide healthcare professionals with the information necessary to identify patients who should be vaccinated and methods to increase vaccination coverage in outpatient practice.
Upon completion of this course, you should be able to:
- Outline the current child, adolescent, and adult immunization schedules.
- Discuss possible adverse events and barriers to vaccination, including methods for maximizing vaccination coverage.
John J. Whyte, MD, MPH
John M. Leonard, MD
Margo A. Halm, RN, PhD, NEA-BC, FAAN
Randall L. Allen, PharmD
The division planners have disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Sarah Campbell
The Director of Development and Academic Affairs has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
The purpose of NetCE is to provide challenging curricula to assist healthcare professionals to raise their levels of expertise while fulfilling their continuing education requirements, thereby improving the quality of healthcare.
Our contributing faculty members have taken care to ensure that the information and recommendations are accurate and compatible with the standards generally accepted at the time of publication. The publisher disclaims any liability, loss or damage incurred as a consequence, directly or indirectly, of the use and application of any of the contents. Participants are cautioned about the potential risk of using limited knowledge when integrating new techniques into practice.
It is the policy of NetCE not to accept commercial support. Furthermore, commercial interests are prohibited from distributing or providing access to this activity to learners.
Supported browsers for Windows include Microsoft Internet Explorer 9.0 and up, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Supported browsers for Macintosh include Safari, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Other operating systems and browsers that include complete implementations of ECMAScript edition 3 and CSS 2.0 may work, but are not supported. Supported browsers must utilize the TLS encryption protocol v1.1 or v1.2 in order to connect to pages that require a secured HTTPS connection. TLS v1.0 is not supported.
The role of implicit biases on healthcare outcomes has become a concern, as there is some evidence that implicit biases contribute to health disparities, professionals' attitudes toward and interactions with patients, quality of care, diagnoses, and treatment decisions. This may produce differences in help-seeking, diagnoses, and ultimately treatments and interventions. Implicit biases may also unwittingly produce professional behaviors, attitudes, and interactions that reduce patients' trust and comfort with their provider, leading to earlier termination of visits and/or reduced adherence and follow-up. Disadvantaged groups are marginalized in the healthcare system and vulnerable on multiple levels; health professionals' implicit biases can further exacerbate these existing disadvantages.
Interventions or strategies designed to reduce implicit bias may be categorized as change-based or control-based. Change-based interventions focus on reducing or changing cognitive associations underlying implicit biases. These interventions might include challenging stereotypes. Conversely, control-based interventions involve reducing the effects of the implicit bias on the individual's behaviors. These strategies include increasing awareness of biased thoughts and responses. The two types of interventions are not mutually exclusive and may be used synergistically.
#91744: Child, Adolescent, and Adult Immunization Schedules
Since the mid-1990s, a childhood vaccination schedule approved by the Advisory Committee on Immunization Practices (ACIP), the American Academy of Pediatrics, and the American Academy of Family Physicians has been published annually by the Centers for Disease Control and Prevention (CDC). A standardized adult immunization schedule has been published each year since 2002.
Over the years, there have been significant changes to the immunization schedules for children, adolescents, and adults. The approval of multiple new vaccines has increased the opportunities for preventive care for both children and adults. Yet, coverage with some vaccines remains far below national goals, and outbreaks of vaccine-preventable diseases continue to occur. The introduction of new vaccines, plus additional changes to the immunization schedules, makes it increasingly difficult for healthcare professionals to ensure that patients receive the recommended preventive care.
Changes in disease incidence illustrate the successes of widespread vaccination. Between 2000 and 2015, the incidence of acute hepatitis B declined in all age groups. Between 2015 and 2022, the rate remained low and steady in most age groups. In 2022, the rate was highest (though still decreased from 2015) among persons 40 to 49 years of age and lowest among adolescents and children 19 years of age and younger[1]. Five years after the introduction of the heptavalent pneumococcal conjugate vaccine (PCV), the incidence of invasive pneumococcal disease (IPD) decreased by 82% among children 1 year of age and by 77% in children younger than 5 years of age [2]. The introduction of this vaccine in children appears to have reduced the incidence of IPD caused by covered strains in older adults as well [3].
However, for vaccines against communicable diseases to have the greatest impact, large proportions of the population must be covered. On a national scale, more than 90% of children have received age-appropriate doses of inactivated polio vaccine (IPV), measles/mumps/rubella (MMR), Haemophilus influenzae type b (Hib), hepatitis B (HepB), and varicella (VAR) vaccines by 24 months of age [4]. The Healthy People 2030 goal is to maintain a high level of coverage for these vaccines [114].
Certain vaccines remain significantly underutilized. For children birth to 24 months of age, completion of four doses of the heptavalent PCV (added to the immunization schedule in 2001) has been increasing but had reached only 83.5% in 2019, with no significant improvements since 2010 [4]. In 2019, full coverage with the hepatitis A vaccine (HepA) for all young children (by 35 months of age) was approximately 79.6%. Coverage with vaccines against rotavirus (by 8 months of age) was approximately 77.1% [4]. The influenza vaccination rate among children younger than 24 years of age remains low (63.9%), although this is higher than the overall rate for the U.S. population (48.1%). In 2018, 48% of adolescents were up to date on the human papillomavirus (HPV) vaccine [114]. This vaccine was considered too new to expect coverage to have met the national goal of 90% by 2020, particularly for teenage boys, for whom the recommendation was added in 2012. The Healthy People 2030 goal is for at least 80% coverage among all adolescents. As of 2022, an estimated 58.6% of adolescents are vaccinated [14].
Undervaccination remains a concern among children even when national data show broad coverage. Coverage varies geographically and among different socioeconomic groups. Not all children receive their vaccinations on time, leaving them unnecessarily vulnerable [5]. Some parents opt out of vaccination entirely because of concerns about adverse effects or because they assume that the vaccine-preventable diseases are no longer a threat. There is also considerable misinformation about vaccine safety. However, recent measles outbreaks confirm that vaccination is still an important public health measure [4,6,124].
In the adult population, vaccines are significantly underutilized (Table 1). For many years, the 23-valent pneumococcal polysaccharide vaccine (PPSV) has been recommended as a routine vaccination for adults 65 years of age and older, and multiple studies confirm that it can reduce the risk of IPD in this population. Yet according to estimates from the 2018 National Health Interview Survey, only 69.0% of adults in this age group have been vaccinated with PPSV [7]. Similarly, only about 47% of adults 50 to 64 years of age and about 69% of adults 65 years of age and older recalled receiving an influenza vaccination within the previous 12 months [7]. Even more than in the pediatric population, special effort may be needed to ensure that adults are aware of and have access to newer vaccines. In the first year after the herpes zoster vaccine was approved, only 2% of adults 60 years of age and older were vaccinated [8]. Attention to disparities is also needed. For example, Hispanics and non-Hispanic Black individuals are substantially less likely than White individuals to receive the influenza vaccine.
U.S. ADULT IMMUNIZATION RATES, 2018
| Vaccine | Subgroup | Percent Vaccinated |
|---|---|---|
| Influenza | 50 to 64 years of age | 46.9% |
| 65 years of age and older | 68.8% | |
| Pneumococcal disease | 19 to 64 years of age, high risk | 23.3% |
| 65 years of age and older | 69.0% | |
| Human papillomavirus | Women 19 to 26 years of age | 52.8% |
| Men 19 to 26 years of age | 26.3% | |
| Herpes zoster (shingles) | 50–64 years of age | 11.5% |
| 65 years of age and older | 39.5% | |
| Td or Tdap | 19 years of age and older | 62.9% |
| Hepatitis A | 19 years of age and older | 11.9% |
| Hepatitis B | 19 years of age and older | 30.0% |
| Td = diphtheria and tetanus toxoids, Tdap = diphtheria and tetanus toxoids and pertussis. | ||
The following course will focus on the immunization schedules for children, adolescents, and adults, with an emphasis on vaccinations that are routine for most healthy persons. It will address the recommendations as of 2025, the rationale for the addition of new vaccines and for several potential new changes, contraindications and precautions as identified by the CDC and the ACIP, and methods to increase vaccination coverage in outpatient practice. The full schedules, including recommendations for patients with specific risk factors and catch-up schedules for patients who have missed doses, are available from the CDC.
Of note, the decision to vaccinate any individual patient should be based on a careful review of the patient's history and of current recommendations regarding each specific vaccine. The recommendation to vaccinate "all" children or adults with a given vaccine should not be interpreted to include those with contraindications or those for whom risks would outweigh benefits.
It is helpful to understand how vaccines are approved and then recommended as part of a schedule. The U.S. Food and Drug Administration's (FDA) Center for Biologics Evaluation and Research (CBER) is responsible for regulating vaccines in the United States. Vaccine clinical development follows the same general pathway as drugs and other biologics. A sponsor who wishes to begin clinical trials with a vaccine must submit an investigational new drug application (IND) to the FDA. The IND describes the vaccine, its method of manufacture, and the types of quality control testing done prior to administering the vaccine to humans. Also included is information about the vaccine's safety and ability to elicit an immune response in animal testing. In addition, the IND contains the proposed clinical protocol.
If the clinical trials are considered successful, a manufacturer will then submit a biologics license application. To be considered, the license application must provide the multidisciplinary FDA reviewer team with the efficacy and safety information necessary to make a risk/benefit assessment and to recommend or oppose the approval of a vaccine. In some cases, the FDA may present their findings to the Vaccines and Related Biological Products Advisory Committee. This non-FDA expert committee (consisting of scientists, physicians, biostatisticians, and a consumer representative) provides advice to the FDA regarding the safety and efficacy of the vaccine for the proposed indication. The FDA makes the final decision for/against approval but relies heavily upon the recommendation of its advisory committees.
It is also important to note that vaccine approval requires the provision of adequate product labeling to allow healthcare providers to understand the vaccine's proper use, including its potential benefits and risks. This information allows healthcare providers to communicate with patients and parents and to safely deliver the vaccine to the public.
FDA approval, however, does not guarantee that a vaccine will be considered routine. Rather, the CDC plays a critical role in determining the schedule. The ACIP consists of 15 experts in fields associated with immunization who have been selected by the Secretary of the U.S. Department of Health and Human Services to provide advice and guidance on the control of vaccine-preventable diseases. The Committee develops written recommendations for the routine administration of vaccines to children and adults in the civilian population; recommendations include age for vaccine administration, number of doses and dosing interval, and precautions and contraindications. The ACIP is the only entity in the federal government that makes such recommendations. These recommendations create the immunization schedules.
In 1995, the first year that a harmonized childhood immunization schedule was published, there were only five items on the childhood immunization schedule, incorporating protection against nine diseases. Even then, a comment in the journal Pediatrics noted that the schedule's complexity could be confusing for both physician and patient [10]. The recommended shots were [11]:
HepB
Diphtheria and tetanus toxoids and whole-cell pertussis vaccine (DTP), diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP), or tetanus and diphtheria toxoids vaccine (Td), depending on age
Hib
Oral polio vaccine (OPV)
MMR
To achieve full coverage, children required a total of 15 shots and four oral doses spread out over at least six visits. DTaP has since replaced DTP and IPV replaced OPV without any changes in the necessary visits.
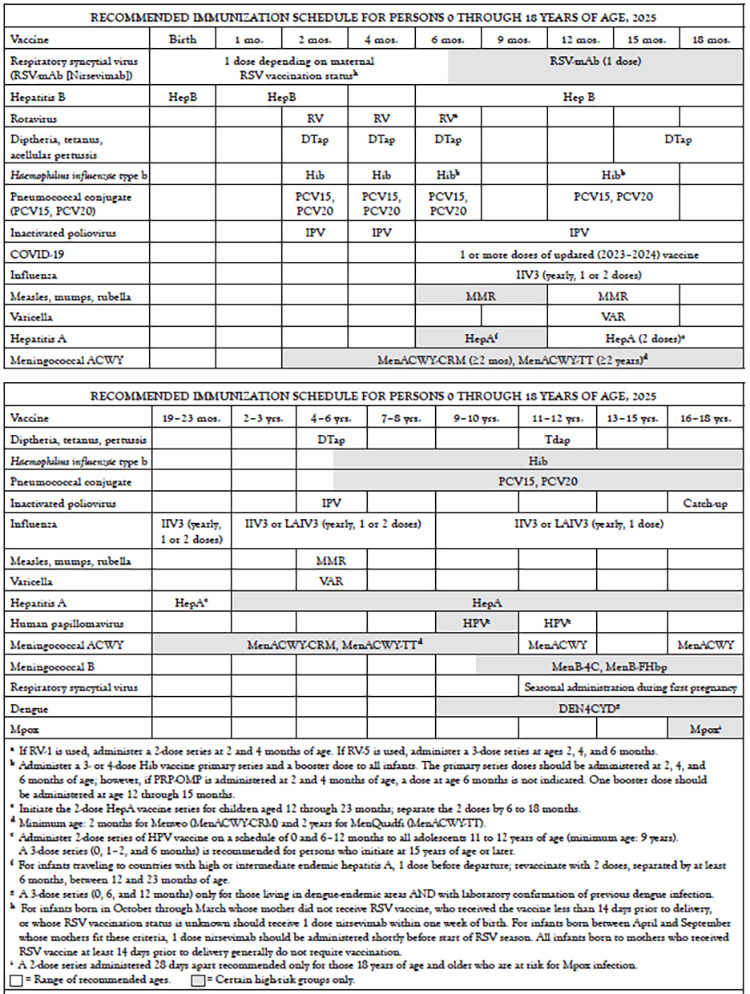
However, with the many new changes that have occurred, parents may be taken by surprise by the number of doses and visits their youngest children need. In 2013, the child and adolescent schedules were combined for the first time, resulting in one schedule for persons 0 to 18 years of age, a format that continues today (Table 2). This combined schedule contains vaccines against up to 16 infectious agents. Expansion of flu vaccine recommendations means annual visits. Other vaccines require multiple visits in the first year of life and at 11 or 12 years of age. Depending on the specific options used, full coverage can involve more than three dozen shots. A "catch-up" schedule for children and adolescents who fall behind on immunizations has also been established (Table 3).
CATCH-UP IMMUNIZATION SCHEDULE FOR PERSONS AGED 4 MONTHS THROUGH 18 YEARS WHO START LATE OR WHO ARE MORE THAN 1 MONTH BEHIND, 2025(Continued)
| Vaccine | Minimum Age for Dose 1 | Minimum Interval Between Doses | |||
|---|---|---|---|---|---|
| Dose 1 to 2 | Dose 2 to 3 | Dose 3 to 4 | Dose 4 to 5 | ||
| Inactivated poliovirus | 6 weeks | 4 weeks | 4 weeks if current age <4 yrs. 6 mos. (as final dose) if current age>4 yrs. | 6 mos. (Minimum age for final dose: 4 years) | –– |
| Meningococcal ACWY | 2 months for MenACWY-CRM, 2 years for MenACWY-TT | 8 weeksa | a | a | –– |
| Measles, mumps, rubella | 12 mos. | 4 weeks | –– | –– | –– |
| Varicella | 12 mos. | 3 mos. | –– | –– | –– |
| Hepatitis A | 12 mos. | 6 mos. | –– | –– | –– |
| Persons 7 through 18 years of age | |||||
| Tetanus, diphtheria; tetanus, diphtheria, acellular pertussis | 7 years | 4 weeks | 4 weeks if first dose DTaP/DT before 1st birthday. 6 mos. (as final dose) if first dose of DTaP/DT or Tdap/Td at ≥12 mos. | 6 mos. if first dose DTaP/DT before 1st birthday | –– |
| Human papillomavirus | 9 years | Routine dosing intervals are recommended. | |||
| Hepatitis A | –– | 6 mos. | –– | –– | –– |
| Hepatitis B | –– | 4 weeks | 8 weeks and at least 16 weeks after first dose | ||
| Inactivated poliovirus | –– | 4 weeks | 6 mos. A fourth dose is not necessary if the third dose was administered at ≥4 years and at least 6 months after the previous dose. | A fourth dose indicated only if all previous doses administered <4 yrs. OR if third dose administered <6 mos. after second dose | –– |
| Meningococcal ACWY | –– | 8 weeks | –– | –– | –– |
| Measles, mumps, rubella | –– | 4 weeks | –– | –– | –– |
| Varicella | –– | 3 mos. if age <13 years OR 4 weeks if age ≥13 years | –– | –– | –– |
| Dengue | 9 years | 6 mos. | 6 mos. | ||
| aAdminister MenACWY vaccine at age 13 through 18 years if not previously vaccinated. If the first dose is administered at age 13 through 15 years, a booster dose should be administered at age 16 through 18 years with a minimum interval of at least 8 weeks between doses. If the first dose is administered at age 16 years or older, a booster dose is not needed. | |||||
Major changes to the annually published childhood schedule in the last decade have included [9,11]:
2003: Influenza vaccination was to be "encouraged" for all children 6 to 23 months of age.
2004: Influenza vaccination was recommended for all children 6 to 23 months of age and close contacts of children 0 to 23 months of age.
2006: Tetanus and diphtheria toxoids and acellular pertussis (Tdap) vaccine replaced Td for adolescents, meningococcal conjugate vaccine (MCV) was recommended for certain age groups, and HepA was expanded to include all children, not just those in selected areas.
2007: Rotavirus and HPV vaccines were added. Influenza vaccination was expanded to all children 6 to 59 months of age. A second VAR dose was recommended for all children.
2008: The recommendation for MCV was expanded to include immunization of all children 11 years of age and older at the earliest opportunity.
2009: The recommendation for influenza vaccination was expanded to include children 6 months to 18 years of age (beginning with the 2008–2009 season).
2012: HPV vaccination recommendation extended to include boys 11 or 12 years of age.
2016: Meningococcal B vaccine added for high-risk children and adolescents 10 years of age and older.
2022: Dengue vaccine added for children and adolescents 9 to 16 years of age living in endemic areas who have had a laboratory-confirmed dengue infection.
2024: RSV vaccine added for infants, shortly after birth, if the mother was not vaccinated when the infant was in the womb.
Other changes to the childhood schedule have added to the potential for confusion. For example, there are two different rotavirus vaccines, with different numbers of doses. Understanding the differences is essential to these vaccines' safe and effective use.
As noted, the adult immunization schedule was created in 2002 to bring together the recommendations for routine vaccination of adults and to help healthcare professionals recall the specific needs of patients in certain chronic disease groups. The intention was to provide an up-to-date tool for providers to use in assessing patients' vaccination needs, creating standing orders and reminder systems, and otherwise reducing missed opportunities for vaccination [15].
The original adult schedule had a relatively short list of routine vaccinations for healthy persons, including [11]:
Td every 10 years
Annual influenza vaccine for adults 50 years of age and older
PPSV for adults 65 years of age and older, with 1 booster for certain patients
MMR (up to age 49 years) and varicella for those who are susceptible
Since that time, several changes have been made (Table 4 and Table 5). The recommendation for routine vaccination against influenza was temporarily changed to age 65 years and older due to a vaccine shortage, but it has now returned to include all patients 6 months of age and older. Tdap is now recommended in lieu of one Td dose for adults up to 64 years of age. HPV vaccine is recommended for women and men up to 26 years of age, and the herpes zoster vaccine is routine for adults 60 years of age and older.
RECOMMENDED ADULT IMMUNIZATION SCHEDULE BY VACCINE AND AGE GROUP, 2025
| Vaccine | 19–23 years | 24–26 years | 27–49 years | 50–64 years | 65 years and older | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| COVID-19 | 1 or more doses of updated vaccine | ||||||||||
| Influenza (IIV3, RIV3, LAIV3) | 1 dose (IIV3, RIV3, or LAIV3) annuallya | 1 dose (IIV3 or RIV3) annuallya | 2 or more doses of updated vaccine | ||||||||
| Respiratory syncytial virus | Seasonal RSV during first pregnancy only | — | RSVc | RSVa | |||||||
| Tetanus, diphtheria, pertussis (Td or Tdap) | One dose of Tdap, then boost with Tdap or Td every 10 years | ||||||||||
| One dose Tdap during each pregnancy; one dose Td for wound prophylaxisa | |||||||||||
| Varicella | 2 doses (if born in 1980 or later)a | 2 dosesb | |||||||||
| Human papillomavirus | 2 or 3 dosesa | 2 or 3 dosesc | — | — | |||||||
| Zoster (RZV) | 2 doses (if immunocompromised)b | 2 doses | |||||||||
| Measles, mumps, rubella | 1 or 2 doses (if born 1957 or later)a | — | |||||||||
| Pneumococcal 13-valent conjugate (PCV15, PCV20,PCV21) | 1 dose PCV20 or PCV21 OR 1 dose PCV15 followed by PPSV23b | 1 dose PCV20 or PCV21 OR 1 dose PCV15 followed by PPSV23a | |||||||||
| Hepatitis A | 2,3, or 4 dosesb | ||||||||||
| Hepatitis B | 2, 3, or 4 dosesa | 2, 3 or 4 dosesb | |||||||||
| Meningococcal ACWY | 1 or 2 doses, then boosterb every 5 years | ||||||||||
| Meningococcal B (MenB) | 2 or 3 dosesc | 2 or 3 dosesb | |||||||||
| Haemophilus influenzae type b (Hib) | 1 or 3 dosesb | ||||||||||
| Mpox | 2 dosesb | ||||||||||
| Inactivated polio virus (IPV) | Complete 3-dose series if incompletely vaccinateda | ||||||||||
| |||||||||||
VACCINES THAT MIGHT BE INDICATED FOR ADULTS BASED ON MEDICAL AND OTHER INDICATIONS, UNITED STATES, 2025
| Vaccine | Pregnancy | Immunocompromised (excluding HIV) | HIV infection | Men who have sex with men (MSM) | |
|---|---|---|---|---|---|
| CD4+ <200 cells/mcL | CD4+ ≥200 cells/mcL | ||||
| COVID-19 | 1 or more doses of updated vaccinea | ||||
| Tetanus, diphtheria, pertussis (Td or Tdap) | 1 dose Tdap each pregnancy | 1 dose Tdap, then boost with Td every 10 yearsa | |||
| Human papillomavirus (HPV) | Delay | 2 or 3 doses through 26 years of agea | |||
| Varicella | Contraindicated | 2 dosesa | |||
| Zoster (RZV) | — | 2 doses at 19 years of age | — | 2 dosesa | |
| Measles, mumps, rubella | Contraindicated | 1 or 2 dosesa | |||
| Influenza | 1 dose annuallya(LAIV contraindicated) | 1 dose annuallya | |||
| Respiratory syncytial virus (RSV) | Seasonal administrationa | Seasonal administrationc | |||
| Pneumococcal (PCV15, PCV20,PCV21) | — | 1 dose PCV15 followed by PPSV23 or 1 dose PCV20 or PCV21a | Vaccinate if other risk factorsb | ||
| Hepatitis A | 2 or 3 dosesb | — | 2 or 3 dosesa | ||
| Hepatitis B | 3 dosesb | 3 dosesa | |||
| Meningococcal ACWY | — | 1 or 2 doses, then booster every 5 yearsa | — | ||
| Meningococcal B (MenB) | Exercise precaution | — | |||
| Haemophilus influenzae type b (Hib) | — | 3 doses post-stem cell transplant recipients onlya | — | ||
| Mpox | 2 dosesb | ||||
| Inactivated polio virus (IPV) | Complete 3-dose series if incompletely vaccinated | ||||
VACCINES THAT MIGHT BE INDICATED FOR ADULTS BASED ON MEDICAL AND OTHER INDICATIONS, UNITED STATES, 2025
| Vaccine | Heart disease, lung disease, chronic alcoholism | Asplenia, complement deficiencies | Chronic liver disease | Diabetes, end-stage renal disease, hemodialysis | Healthcare personnel | |||
|---|---|---|---|---|---|---|---|---|
| COVID-19 | 1 or more doses of updated vaccinea | |||||||
| Tetanus, diphtheria, pertussis (Td/Tdap) | 1 dose Tdap, then boost with Td or Tdap every 10 yearsa | |||||||
| Human papillomavirus (HPV) | 2 or 3 doses through 26 years of agea | |||||||
| Varicella | 2 dosesa | |||||||
| Zoster (RZV) | 2 doses ≥50 yearsa | |||||||
| Measles, mumps, rubella | 1 or 2 dosesa | |||||||
| Influenza | 1 dose annually (exercise precaution with LAIV)a | 1 dose annually (LAIV contraindicated)a | 1 dose annually (exercise precaution with LAIV)a | 1 dose annuallya | ||||
| Respiratory syncytial virus (RSV) | Seasonal administrationc | |||||||
| Pneumococcal polysaccharide (PCV15, PCV20,PCV21) | 1 dose PCV15 followed by PPSV23 or 1 dose PCV20 or PCV21a | 1 dose PCV15 followed by PPSV23 or 1 dose PCV20 or PCV21b | ||||||
| Hepatitis A | 2 or 3 dosesb | 2 or 3 dosesa | 2 or 3 dosesb | |||||
| Hepatitis B | 2, 3, or 4 doses depending on vaccine or conditiona | |||||||
| Meningococcal ACWY | — | 1 or 2 doses, then booster every 5 yearsa | — | |||||
| Meningococcal B (MenB) | — | 2 or 3 dosesa | — | |||||
| Haemophilus influenzae type b (Hib) | — | 1 dose for asplenia only | — | |||||
| Mpox | 2 dosesb | |||||||
| Inactivated polio virus (IPV) | Complete 3-dose series if incompletely vaccinated | |||||||
| ||||||||
Since 2009, annual influenza vaccination has been recommended for all persons 6 months of age and older. The 2024–2025 trivalent influenza vaccine contained an H1N1-like antigen as well as H3N2 and one B antigen [20]. For many years the vaccine has been quadrivalent and included an antigen of the B/Yamagata lineage; however, viruses from this lineage have been undetected since 2020.
Given the large number of vaccines now recommended, both parents and adult patients often have concerns about whether all the doses are needed. The following review of the rationale behind the changes to the child, adolescent, and adult immunization schedules is intended to help clinicians improve their own understanding and explain the rationale to patients.
Recommendation for Children: Influenza vaccine is recommended annually for children 6 months through 18 years of age. Two doses, separated by at least four weeks, should be given to children if they are receiving influenza vaccine for the first time. Also give two doses if the child was vaccinated for the first time the prior season but received only one dose. For the 2024–2025 season, use of live attenuated influenza vaccine (LAIV) may be considered for children 2 years of age and older.
Recommendation for Adults: Vaccination is recommended annually for all adults without a contraindication with inactivated influenza vaccine (IIV), recombinant influenza vaccine (RIV), or live attenuated influenza vaccine (LAIV). Other options include high-dose or adjuvanted IIV for adults 65 years of age or older. Women who are or may become pregnant should not receive LAIV.
The expansion of the recommended ages for the vaccination of children and adults against influenza is one of the most significant changes to the schedule in recent years. It requires an annual visit to a healthcare provider, including among older children and young adults who typically have low rates of physician visits.
The ACIP considered multiple factors in making this recommendation. First, according to accumulated evidence, the influenza vaccine appears to be both safe and effective, with the benefits of vaccination outweighing the small risk of adverse effects [21]. Widespread vaccination is also intended to lower the social and economic impact of influenza, including hundreds of thousands of hospitalizations and tens of thousands of premature deaths each year. The number of missed days of school for children and missed days of work for parents is substantial during flu season. Physician visits for the flu may lead to a prescription for antibiotics—treatment that is unnecessary and potentially dangerous.
The recommendation is also intended to simplify the decision to advise vaccination for children [21]. In previous years, vaccination was recommended for a number of groups with specific risk factors. These included older children with certain medical conditions and children who were close contacts of people who should be immunized. Making vaccination routine for all children is expected to lead to a 50% increase in coverage for those children who have a specific risk-based or contact-based indication.
Another change, for both children and adults, was the development of LAIV, a nasal-spray vaccine that can be easier for some patients to accept than an injection [22]. Data from the 2015–2016 flu season found an only 3% efficacy rate with LAIV (compared with 63% with IIV), and LAIV was not recommended between the 2015 and 2018 seasons [19,20]. However, the 2018–2019 influenza guideline reintroduced LAIV as an option for persons 2 to 49 years of age for whom it is appropriate, and it remains an option in the 2024–2025 guideline [20]. This excludes women who are pregnant and those with HIV, immunocompromise, asplenia, and/or complement deficiencies.
In the past, egg allergy (beyond urticaria) was considered contraindication or cause for additional safety measures when influenza vaccination was considered. However, it is now recommended that all persons 6 months of age or older with egg allergy should receive any influenza vaccine (egg-based or non-egg-based) that is otherwise appropriate for the recipient's age and health status [20]. It is no longer recommended that persons who have had an allergic reaction to egg involving symptoms other than urticaria should be vaccinated in an inpatient or outpatient medical setting supervised by a healthcare provider who is able to recognize and manage severe allergic reactions if an egg-based vaccine is used.
Recommendation for Children: DTaP is recommended at 2, 4, 6, and 15 to 18 months of age (or as early as 12 months, if 6 months have passed since the last dose) and at 4 to 6 years of age. Tdap is recommended at 11 to 12 years for children who have completed the recommended childhood DTP/DTaP vaccination series and have not received a Td booster dose and for older children who have not received a dose. If a child has already received Td, a five-year interval before Tdap is encouraged unless pertussis protection is specifically needed.
Recommendation for Adults: Td or Tdap booster every 10 years. Tdap replaces one Td dose for adults who have not already received Tdap. (See immunization schedule for special situations, including adults who have not received primary childhood vaccination and pregnant women.)
The inclusion of Tdap on the adult immunization schedules may create confusion because it replaces a dose of Td that was previously routine and patients may be uncertain about which vaccine they received. However, Tdap also has the potential to make an important impact on the public's health [23]. In the past, vaccination against pertussis was given only during young childhood. However, immunity against pertussis declines within about 5 to 10 years [23,116]. Reported cases of pertussis increased steadily from the 1980s to a peak in 2014. In 2022, 3,044 cases were reported—an increase of 44% compared with 2021—but many more go undiagnosed and unreported [24]. Infants younger than 1 year of age are at highest risk and continue to have the highest reported rate of pertussis; nearly half require hospitalization. Adolescents 11 to 19 years of age and adults 20 years of age and older accounted for approximately 54.8% of reported cases in 2022; cases among children 7 to 10 years of age accounted for approximately 6.9% of reported cases [24]. Adults may also have complications including pneumonia, rib fracture, and loss of consciousness ("cough syncope") [25]. The true risks are somewhat unclear, however, because cases without a classic presentation are less likely to be diagnosed and reported.
The primary objective of the ACIP in recommending Tdap for adolescents is to protect individual adolescents against pertussis while continuing the standard protection against tetanus and diphtheria [23]. An important secondary goal is to reduce the reservoir of pertussis within the population as a whole. This may be particularly important for infants. The recommendation for adults was put in place primarily to protect individual adults against pertussis and also to reduce the reservoir of pertussis [25]. Widespread immunization of adults may also reduce the impact of pertussis on healthcare facilities and other institutional settings.
The recommended timing of Tdap vaccination takes into account recommendations for the administration of other tetanus and/or diphtheria toxoid-containing vaccines, including MenACWY, because of an association between frequent doses of such vaccines and a risk of increased local and systemic reactogenicity [23].
Recommendation for Adolescents: HPV vaccine is recommended for girls and boys 11 to 12 years of age and for older adolescents who have not yet been vaccinated. Children 9 to 10 years of age may also be vaccinated.
Recommendation for Adults: HPV vaccine is recommended for adults up to 26 years of age who have not completed the vaccine series. HPV vaccine is also recommended for those 27 to 45 years of age if desired or if a risk factor is present.
When it was first added, there was significant public controversy over the inclusion of the HPV vaccine on the adolescent immunization schedule. Some parents remain concerned about the vaccine's safety or about the possibility of promoting sexual activity among young teens. Meanwhile, in some places this vaccine is now required for school attendance, although exemptions are generally allowed [27].
Statistics regarding HPV infection and cancer illustrate the rationale behind the vaccine itself. About 13,360 cases of cervical cancer will be diagnosed in the United States in 2025, and more than 4,320 will die from the disease [28]. The CDC estimates that more than 37,800 cancers attributable to HPV occur each year, including (in order of frequency) cancer of the oropharynx, cervix, anus, vulva, penis, and vagina [16].
There is one HPV vaccine available in the United States: Gardasil 9, which is approved for use in individuals 9 to 45 years of age [12,19,113]. Cervarix was a bivalent vaccine covering HPV types 16 and 18; however, this vaccine is no longer available in the United States [12,30]. Quadrivalent Gardasil (no longer available in the United States) was a quadrivalent vaccine covering types 6, 11, 16, and 18 [31]. In 2014, a 9-valent HPV recombinant vaccine (Gardasil 9) was approved for use in individuals 9 to 26 years of age and added protection to HPV types 31, 33, 45, 52, and 58 in addition to those types covered by the original Gardasil [108,113]. In 2018, the FDA approved expanded use of Gardasil 9 to include women and men up to 45 years of age [113]. Three-fourths of cervical cancers are squamous cell tumors, and HPV 16 and 18 account for about 68% of these [32]. The rest are adenocarcinomas, and HPV types 16 and 18 account for about 83% of these tumors [32]. The increased coverage of the 9-valent vaccine has the potential to prevent up to 90% of oropharyngeal, cervical, anal, vulvar, penile, and vaginal cancers [108].
Epidemiologic data on HPV incidence and age of sexual debut suggest that the pre-teen years are an appropriate time to begin HPV protection [32]. Genital HPV is the most common sexually transmitted infection in the United States, with 13 million new infections among people ≥15 years of age each year [95]. Teens and young adults are particularly at risk; about half of those infections occur in individuals 15 to 24 years of age [32]. One multisite, clinic-based study of sexually active females found the highest prevalence of HPV in girls 14 to 19 years of age. In another study, using a representative, population-based sample, HPV prevalence was 26.9% among sexually active women 18 to 25 years of age [33]. The prevalence of types 16 or 18 was 7.8%. Another study, also intended to be representative of the general population, found that the prevalence of HPV was 26.8% for women 14 to 59 years of age and nearly 45% among women 20 to 24 years of age [34]. In the overall study population, the prevalence of type 16 was 1.5%, and type 18 was 0.8%.
An important consideration in protecting adolescents who are not yet sexually active is that HPV infection is common within the first few years after sexual debut [32]. In addition, studies have shown high antibody titers with vaccination at age 11 to 12 years. The projected impact of vaccinating girls at 12 years of age is a 20% to 66% reduction in lifetime cervical cancer risk, depending on the effectiveness of the vaccine and the duration of protection. Vaccination could also lead to a 21% reduction in low-grade abnormalities on Pap tests over the life of a cohort of vaccinated females. A comparison of HPV prevalence data from the vaccine era (2009–2012) and the prevaccine era (2003–2006) found that the prevalence of the HPV types included in the quadrivalent vaccine decreased by 64% (from 11.5% to 4.3%) among girls 14 to 19 years of age [17]. Considering the modest uptake of this vaccine, the potential impact is significant.
The recommendation to vaccinate young adults takes into account the fact that many will already be sexually active and may have been exposed to one or more types of HPV. Young adults who are not yet sexually active can receive the full benefit of vaccination. In addition, it is likely that many individuals who are infected have not yet encountered each of the vaccine-covered types, so they can receive at least partial benefit [35,36]. The recommendation to vaccinate adults to the age of 26 years reflects the safety and efficacy testing on which the initial vaccines' approvals were based [30,31,37]. Use in older individuals is also effective, and many patients will benefit from vaccination at 27 to 45 years of age. Medical professionals can inform patients of the option to receive the vaccine series or to complete the series, help assess the benefits and individual risk factors, and facilitate decision-making. As noted, the HPV vaccine remains significantly underutilized as of 2024.
Recommendation for Children: Rotavirus vaccine is recommended for infants 6 weeks to 14 weeks of age (maximum age for first dose: 14 weeks, 6 days). The last dose should be given by age 8 months, 0 days.
A rotavirus vaccine was first added to the immunization schedule in 1999 but was quickly taken off the market due to concerns about intussusception. The two available vaccines have each been tested in hundreds of thousands of infants [38,39]. A large-scale study completed in 2014 found a slight increase in risk with RV5 (1.5 excess cases of intussusception per 100,000 recipients of the first dose) and some evidence of an elevated risk with RV1 [38]. However, these data should be considered in light of the benefits of vaccination. In an effort to maximize safety, these vaccines have a narrow age range for administration, reflecting the ages of the children in the large safety studies.
In adding rotavirus vaccination to the routine immunization schedule, the ACIP observed that rates of illness are similar in industrialized and less developed countries, suggesting that public health measures such as clean water supplies and good hygiene are not enough to control rotavirus disease [40]. Further, there is a high level of morbidity due to rotavirus in the United States in spite of available medical care. In the years before vaccination was available, rotavirus was responsible for approximately 20 to 60 deaths each year, 55,000 to 70,000 hospitalizations, more than 200,000 emergency department visits, 400,000 physician visits, and direct and indirect costs of approximately $1 billion [40,41].
The vaccines are designed to mimic the effect of a first bout of rotavirus, which is usually the most serious [40]. Subsequent bouts of symptomatic infection can occur after a first natural infection, but they tend to be milder. As such, vaccination is not expected to prevent disease entirely but to reduce the severity of symptoms, the need for medical care, and the risk of serious sequelae, including hospitalization and death.
In 2009, the age parameters for vaccine administration were adjusted to harmonize the schedules of the two approved rotavirus vaccines [40]. One is a pentavalent reassortant vaccine based on a bovine rotavirus, often abbreviated as RV5. The other is a live, attenuated human rotavirus vaccine, often abbreviated as RV1. RV5 has a three-dose schedule, while RV1 requires two doses [41]. The maximum ages for these vaccines are somewhat different, according to their prescribing information, but an ACIP workgroup has concluded that safety and efficacy are unlikely to be affected if the same age limits are used for both [40].
Recommendation for Children: MenACWY is recommended routinely for children 11 to 18 years of age, for older children who have not yet been vaccinated, and for children 6 weeks to 10 years of age in certain risk groups. MenB vaccination is recommended for children 10 to 18 years of age in certain risk groups. In addition, young adults 16 to 23 years of age (preferred age range: 16 to 18 years) may be vaccinated to provide short-term protection against most strains of serogroup B meningococcal disease.
Recommendation for Adults: MenACWY vaccine is recommended for adults 19 years of age and older with increased risk for meningococcal disease, including military recruits, freshmen college students living in dormitories, persons without a spleen or with a damaged spleen, those with terminal complement deficiency, and persons traveling to or residing in countries in which the disease is common. Revaccination with MenACWY every five years is recommended for adults previously vaccinated who remain at increased risk of infection. MenB vaccine is recommended for adults with certain risk factors, including all adults with anatomical or functional asplenia or persistent complement component deficiencies.
Historically, before widespread vaccination, there were about 1,400 to 2,800 cases of meningococcal disease in the United States each year [42]. Although not a common illness, meningococcal disease has a rapid course and a high degree of mortality, with a case-fatality ratio of about 10% to 14%. Among survivors, 11% to 19% will experience serious sequelae, such as neurologic deficit, deafness, or loss of a limb [43]. The degree of severity means that, in addition to putting the patient's life at risk, each case requires a substantial public health effort to identify additional cases quickly and prevent the disease from spreading [44].
There are two main types of serogroup A, C, W, and Y meningococcal vaccine: MenACWY and MPSV. However, MPSV is no longer available in the United States. The two available vaccines are MenACWY-TT (≥2 years) and MenACWY-CRM (≥2 months) [19,45]. MenACWY vaccines cover serogroups C, Y, A, and W-135 [44]. In the United States, serogroups C, Y, and B have each been responsible for about one-third of cases overall.
Incidence of meningococcal disease also increases during adolescence, and this group is the main focus of the recommendations for vaccination with MenACWY. Among people 11 years of age and older, 75% of cases are caused by group C, Y, or W-135, which are all covered by the vaccine [42]. The original recommendation for the use of MenACWY focused on certain age groups: children 11 to 12 years of age, children entering high school, and college freshmen who would be living in dorms. These specifications were created because of concerns about there being a short supply of vaccine during the first few years of production [47]. Now that supply is expected to be adequate, the recommendation is to vaccinate all children 11 years of age and older who have not previously received vaccination against meningococcus, with a booster at 16 years of age. This broader recommendation is intended to simplify decisions about vaccinating and improve overall coverage. The child and adolescent immunization schedules provide details about revaccinating children who have received MPSV in the past.
Creating a vaccine against serogroup B was particularly challenging because of its immunochemical structure. However, the first vaccine to protect against invasive meningococcal disease caused by Neisseria meningitidis serogroup B was approved by the FDA in 2014 [46]. There are now two MenB vaccines available: MenB-FHbp and MenB-4C [18]. The MenB vaccines are approved for use in persons 10 to 25 years of age; however, because there is no theoretical difference in safety for persons older than 25 years of age compared to those in the approved age-group, MenB vaccine is recommended for use in persons older than 10 years of age who are at increased risk for serogroup B meningococcal disease, including situations and settings in which MCV would be appropriate [19]. MenB vaccine should either be administered as a three-dose series of MenB-FHbp; or a two-dose (or three-dose) series of MenB-4C. If the second dose of MenB-4C is administered earlier than 6 months after the first dose, a third dose should be administered at least 4 months after the second dose; alternately, the vaccine may be administered at 0, 1 to 2, and 6 months [13]. The two vaccines are not interchangeable; the same vaccine product must be used for all doses [18]. MenB vaccine may be administered concomitantly with an MCV vaccine but at a different anatomic site, if feasible [19].
In 2023, a pentavalent vaccine combining MenACWY and MenB coverage (termed MenABCWY) became available [120]. The MenABCWY vaccine consists of substance from MenB-fHbp and MenACWY-TT and is recommended as an option for people 10 years of age or older who are getting MenACWY and MenB vaccines at the same visit [120]. It is administered in two doses at least six months apart. If a patient receives MenABCWY vaccine, MenB-fHbp should be used for additional MenB dose(s) when MenACWY is not indicated; any MenACWY vaccine may be used for booster when given alone. The MenABCWY vaccine can be used only when both MenACWY and MenB vaccines are indicated at the same visit. Otherwise, MenACWY and MenB vaccines should be given separately as appropriate [120].
Recommendation for Children: HepA is recommended for all children 12 to 23 months of age and for unvaccinated children 24 months and older (as catch-up vaccination).
Recommendation for Adults: HepA or combination HepA-HepB is recommended for certain risk groups, for those travelling to countries with endemic hepatitis A, and for those who desire protection (with no risk factor required for vaccination).
Hepatitis A can be a serious disease. According to U.S. surveillance data, an estimated 11% to 22% of people who contract hepatitis A are hospitalized [48]. Adults who are hospitalized lose an estimated 33 days of work, and those who do not require hospitalization lose about 15 days [48]. In the pre-vaccine era, infection was especially common among children. Although young children often had asymptomatic or unrecognized infection, they were an important source of disease transmission.
The ACIP has been pursuing an incremental strategy to increase immunization, with the goal of potentially eliminating indigenous hepatitis A virus transmission entirely [48]. At first, routine vaccination for healthy children was recommended only for areas with high rates of disease. Implementation of vaccination in such regions led to a decline in local disease rates to the lowest levels ever recorded. This left the highest rates in places where routine vaccination was not yet recommended. The next step was the current recommendation to vaccinate all children at 1 year of age [12]. (Some local programs also incorporate vaccination of older children.)
The range to begin routine vaccination, 12 to 23 months of age, was chosen in part because well-child visits are more frequent before 2 years of age. Vaccination is also recommended for older children and adults in certain high-risk groups. Younger children (6 to 12 months of age) may be vaccinated if they will be travelling internationally [12].
Recommendation for Adults: RZV is recommended for individuals 50 years of age and older with no vaccination history and for individuals who previously received the ZVL vaccine. RZV is also recommended for individuals 19 years of age or older who are immunocompromised or who will be immunodeficient/immunosuppressed due to disease or therapy.
There are an estimated 1 million cases of herpes zoster each year, and incidence increases with age [49]. Without vaccination, about one-third of Americans will experience shingles at some point in their lives [49]. In addition to discomfort and inconvenience for the patient, there is also a risk of viral transmission leading to primary varicella in at-risk contacts. Postherpetic neuralgia (PHN) is an unfortunate but fairly common complication. A community-based study in Minnesota looked at the incidence of PHN as defined by various durations of pain [50]. Eighteen percent of patients experienced PHN-type pain for at least 30 days, 13% for at least 60 days, and 10% for at least 90 days [50]. The ACIP added the zoster vaccine to the adult immunization schedule to take advantage of the opportunity to decrease both the burden of disease and the risk of complications. In 2018, the recombinant zoster vaccine (RZV) was added as the preferred vaccine, and in 2020, the ZVL vaccine was discontinued [19]. RZV has better proven efficacy in preventing herpes zoster compared with ZVL, and breakthrough cases are associated with less severe herpes zoster-related pain and less interference on activities of daily living [56].
Although treatment for herpes zoster is available, it does not always fully alleviate symptoms [63]. In addition, the potential effectiveness of treatment initiated more than 72 hours after rash onset has not been established. When PHN occurs, treatments often have limited effectiveness, and tolerance in older patients may be poor. In a large clinical trial comparing RZV to placebo, the incidence of herpes zoster was reduced by 97.2% in vaccinated patients, and pain associated with shingles was substantially reduced [51]. The overall efficacy of RZV against the incidence of of PHN (defined as persistent pain for 90 days) was 91.2%.
Of note, the zoster vaccine is recommended whether or not the patient has had a prior episode of shingles [19,63]. Patients who previously received the ZVL vaccine should be revaccinated with RZV [19,117]. Unlike the ZVL vaccine, RZV can be used in patients who have received the varicella vaccine and in those who are immunocompromised [117].
Recommendation for Children: PCV13 is recommended at 2, 4, 6, and 12 to 15 months of age. (PPSV23 is also recommended for certain risk groups at 2 years of age or older, with a single revaccination after 2 years.)
Recommendation for Adults: Pneumococcal vaccination (1 dose of PCV15, PCV20, or PCV21) is recommended for all individuals 50 years or older who have not previously received a dose, whose previous vaccination history is unknown, or who previously received PCV7. If PCV15 is used, administer 1 dose PPSV23 at least one year after the PCV15 dose.
The pneumococcal conjugate vaccine recommended for routine use in healthy children, PCV13, covers 13 serotypes of Streptococcus pneumoniae. The use of this vaccine has led to a significant decline in IPD, from 98.7 cases per 100,000 children younger than 5 years of age in 1997–1999, to less than one case per 100,000 by 2007 and continuing to 2015 [2,52]. Rates of all-cause pneumonia in children younger than 2 years of age have also declined, by about 35% between 1997 and 2006 with use of a vaccine covering seven serotypes [53]. Most of this decline occurred shortly after the vaccine became available.
However, the rates of non-PCV type IPD had been rising, and overall rates of IPD plateaued between 2002 and 2005 [52]. This prompted the development of the 13-valent pneumococcal conjugate vaccine, licensed in 2010. PCV13 includes coverage for six additional serotypes, which are responsible for a large proportion of remaining IPD [54]. Invasive pneumococcal disease caused by the 13 serotypes covered by PCV13 decreased from 91 cases per 100,000 people in 1998 to 0.56 case per 100,000 people in 2021 [26].
Recommendation for Infants: Within one week of birth, RSV immunization (one dose nirsevimab) should be administered to infants born in October through March whose mothers did not receive RSV vaccine, who received the vaccine less than 14 days prior to delivery, or whose RSV vaccination status is unknown. For infants born between April and September whose mothers fit these criteria, one dose nirsevimab should be administered shortly before the start of RSV season.
Recommendation for Adults: One dose RSV vaccine is recommended for all pregnant patients at 32 to 36 weeks' gestation from September through January in most of the continental United States, regardless of previous RSV infection. Based on shared clinical decision-making, one dose RSV vaccine may be administered to patients 60 to 74 years of age. One dose RSV vaccine is recommended for all patients 75 years of age and older with no evidence of immunity.
Starting in 2024, the immunization schedule includes recommendations for use of the RSV vaccines. Two RSV vaccines are available in the United States: Arexvy and Abrysvo. The strongest recommendation is for the use of RSV vaccination during pregnancy (32 weeks and 0 days through 36 weeks and 6 days gestation from September through January, in most of the continental United States) to prevent RSV lower respiratory tract infection in infants. Abrysvo is the only RSV recommended for use during pregnancy. All infants born to mothers who received RSV vaccine at least 14 days prior to delivery generally do not require immunization. However, infants born to mothers who did not receive the vaccine or whose vaccine status is unknown should receive nirsevimab immunization. In addition, infants with prolonged birth hospitalization discharged October through March should be immunized shortly before or promptly after discharge [121]. The 2025 immunization schedule clarified that additional doses of RSV vaccine are not recommended in subsequent pregnancies [19].
Vaccination with a single RSV vaccine dose has demonstrated moderate-to-high efficacy in preventing symptomatic RSV-associated lower respiratory tract disease among adults 60 years of age or older. In 2024, the ACIP added a recommendation for RSV vaccination for older adults based on shared clinical decision-making and generally for all patients 75 years of age and older [123]. Persons 60 years of age and older who are most likely to benefit from vaccination include those with chronic medical conditions (e.g., lung diseases, cardiovascular diseases, neurologic or neuromuscular conditions, kidney disorders, liver disorders, hematologic disorders, diabetes, and moderate or severe immune compromise); those who are considered to be frail; those of advanced age; those who reside in nursing homes or other long-term care facilities; and those with other underlying medical conditions or factors that a healthcare provider determines might increase the risk of severe respiratory disease [122].
In general, the timing of RSV vaccination is based on the seasonal patterns of RSV disease transmission. Providers in jurisdictions with RSV seasonality that differs from most of the continental United States (e.g., Alaska, tropical climates) should follow guidance from public health authorities or regional medical centers on timing of administration based on local RSV seasonality [12,19].
Confusion about contraindications can lead to undervaccination or, occasionally, to serious adverse events if contraindicated vaccines are given. There are a few general safety considerations that apply to all vaccines. There are also several situations in which healthcare professionals may hesitate to administer vaccines, when in fact most could be given with a high degree of safety.
As a general rule, a serious allergic reaction to a prior dose or a severe allergy to any vaccine component is a contraindication to the use of any vaccine; however, mild or moderate allergy to a vaccine component is not considered to be a contraindication [55]. In most cases, vaccination should be deferred in the setting of moderate or severe acute illness.
On the other hand, vaccination is generally not contraindicated in the following situations [55]:
Mild acute illness, with or without low-grade fever, or recovering from illness
Lack of previous physical examination in well-appearing person
Current use of antimicrobial therapy (except certain antivirals with VAR and zoster)
Premature birth (except HepB in certain circumstances)
Recent exposure to infectious disease
History of non-vaccine allergy
Current use of allergen extract immunotherapy
History of Guillain-Barré syndrome (GBS)
The prescribing information for VAR does note a small possibility of transmission of vaccine virus to healthy susceptible contacts (including pregnant women if they are susceptible to varicella) and recommends weighing this small risk against the risk of acquiring and transmitting natural varicella virus [57].
The following details about specific contraindications and cautions are based primarily on recommendations from the CDC. The CDC reports and current prescribing information should always be consulted.
The ingredients, contraindications, and precautions for any vaccine should be reviewed before administering it to a patient with known allergies or a history of a severe reaction to a previous dose or to any vaccine ingredient. However, clinicians can be well served by recalling many of the potential hypersensitivities. Table 6 is based on a list of contraindications and cautions as recommended by the CDC, which provides recommendations when anaphylactic allergy is present [55]. (A fully definitive list is beyond the scope of this course. For a comprehensive list, visit https://www.vaccinesafety.edu/components.)
HYPERSENSITIVIES AND VACCINE RECOMMENDATIONS
| Hypersensitivity | Vaccine | CDC Recommendation | |||||
|---|---|---|---|---|---|---|---|
| Yeast |
| Do not use | |||||
| Latex | Rotavirus (RV1), MenB | Check packaging to see if latex is used and for guidance | |||||
| Gelatin |
| Use extreme caution if administering | |||||
| Neomycin |
| Do not use | |||||
| Streptomycin | IPV | Do not use | |||||
| Polymyxin B |
| Do not use | |||||
| Thimerosal | Some brands/formulations, including certain DTaP, influenza (IIV), Td, DT | Check package insert |
Immunodeficiency creates a potentially confusing situation regarding vaccination, because there are different degrees and causes of immune suppression. In general, the CDC recommends that MMR, varicella, and LAIV, which contain live virus, should not be used [55]. The prescribing information for LAIV notes that administration to immunocompromised patients requires careful weighing of benefits and risks [22]. If the patient is healthy but there is a close contact who is severely immunosuppressed and requires care in a protective environment, IIV4 is preferred over LAIV [55].
VAR also contains live virus. According to the CDC, it is contraindicated in patients with cellular immunodeficiencies but may be used in patients with impaired humoral immunity [55]. The prescribing information, however, includes hypogammaglobulinemic and dysgammaglobulinemic states as contraindications [57]. If a first-degree relative has congenital or hereditary immunodeficiency, VAR should not be given unless the patient's own immune competence has been verified [57,59]. For such patients, the prescribing information for MMR notes that it, too, should also be deferred until immune competence is confirmed [60]. According to the prescribing information for VAR, because there may be rare transmission of the vaccine virus between recipients and susceptible contacts, recipients should try to avoid contact with susceptible, high-risk contacts for up to six weeks [57]. This includes immunocompromised persons and pregnant women if they are susceptible to chickenpox. (If contact is unavoidable, vaccination risk should be weighed against the risk of acquiring and transmitting natural varicella virus.)
Unlike the ZVL vaccine, which was contraindicated in most immunodeficient individuals, RZV is considered safe and is recommended for patients with immunodeficiency. According to the ACIP, RZV should be administered to adults 19 years of age or older who are or will be at increased risk for herpes zoster due to immunodeficiency or immunosuppression caused by known disease or therapy [58,117].
The safety and efficacy of the rotavirus vaccines have not been established in patients who are immunosuppressed. In such patients, the ACIP recommendation is to consult with an infectious disease specialist or immunologist before giving the vaccine [40]. In phase 3 studies of RV5, viral shedding was observed as long as 15 days after a dose, raising concerns about use in patients with immunosuppressed contacts [61]. However, the actual risk of transmission is unknown. RV1 can also be shed after a dose, with shedding tending to peak at about seven days [62]. Again, the risk of transmission is not known.
Many vaccines may be less immunogenic in patients who are immunosuppressed. Potential effectiveness, as well as timing in patients taking immunosuppressive therapy, should be considered.
A few of the routine vaccines for healthy persons are contraindicated in pregnancy. MMR and VAR should not be used, and the CDC recommends against the use of LAIV [55]. The zoster vaccine should also be delayed, although the ACIP makes no recommendation for use during pregnancy [58]. For many other vaccines, safety during pregnancy is unknown. For example, there is little safety data on MCV and HPV vaccines when used in pregnant women, although caution is indicated with HPV [32,42,48,55]. If Td or Tdap is to be given, administration during the second or third trimester is preferred. For many vaccines without good pregnancy data, providers are encouraged to report any exposure to the vaccine in a pregnant woman to the manufacturer's pregnancy registry; details are provided in the prescribing information.
In general, prescribing information should be consulted for recommendations regarding individual vaccines and pregnancy, and risks and benefits reviewed with the patient as necessary.
While a positive purified protein derivative (PPD) test on its own is not generally a contraindication to vaccination, some vaccines should not be used in the presence of active, untreated tuberculosis. In such cases, MMR should not be given, due to a theoretical risk of exacerbating the disease [55].
Some vaccines have been associated with Guillain-Barré syndrome (GBS), although it is often unclear whether the vaccines actually cause this syndrome [55]. This section will summarize contraindications of routine vaccines for healthy children and adults with a history of GBS; more information about certain vaccines and GBS is included in the section on vaccine safety.
DTaP, Tdap, and Td all require caution if GBS occurred in a patient within six weeks after a previous dose of a vaccine containing tetanus toxoid [21,23,64,65]. Similarly, IIV/RIV requires caution if GBS occurred within six weeks of a prior influenza vaccination, and the CDC suggests considering not vaccinating such patients if they are not at high risk of influenza complications [21,64]. The prescribing information for LAIV recommends caution in any patient with a history of GBS, and the ACIP has identified history of GBS after an influenza vaccination as a contraindication [21,22]. The actual risks with these or other vaccines are not known, and providers should weigh the potential risk of vaccinating against the patient's risk of serious illness.
There are several other concerns or cautions with specific vaccines. Although it is not possible to list every issue here, a few of the specific contraindications will be discussed.
Some studies have suggested a small increase in the risk of intussusception following rotavirus vaccination [38,66]. In patients with a history of intussusception, benefits and risks should be weighed on an individual basis.
Both DTaP and Tdap are contraindicated if encephalopathy occurred within seven days of a prior dose of a vaccine with pertussis components [23,25,55]. This is based on a possible link between DTP and encephalopathy and evidence suggesting an association between acellular pertussis vaccines and encephalopathy in Japan (about one attributable case per 10 million doses). Canadian surveillance data from 1993 to 2002, on the other hand, did not find a link between whole-cell or acellular pertussis vaccines and acute encephalopathy cases. Contraindications and precautions listed in the prescribing information for vaccines with pertussis components also include the presence of unstable or evolving neurologic disorders, and package inserts and the ACIP recommendations should be reviewed for details [67,68,69,70,71,72]. The CDC recommends that decisions about DTaP in children with proven or suspected neurologic conditions be decided on an individual basis [55].
With DTaP, caution should also be observed if reactions after a prior dose included events such as high fever, collapse or shock-like state, or persistent/inconsolable crying lasting three hours or more within two days of prior dose, or seizure within three days [23,25,55]. However, according to the ACIP recommendations, such reactions following DTP or DTaP should not be considered contraindications to use of Tdap or Td in adolescents and adults.
Of note, the prescribing information for some, but not all, tetanus toxoid-containing vaccines does caution against use in patients who have had neurologic reactions following a previous dose of Td or of tetanus toxoid.
History of an Arthus reaction is another consideration with tetanus toxoid-containing or diphtheria toxoid-containing vaccines [23,25,55]. An Arthus reaction is a local vasculitis that is associated with an immune reaction. Although it is an uncommonly reported event after vaccination, it can occur with vaccines containing tetanus or diphtheria toxoid. Signs include swelling, induration, edema, and hemorrhage, and there may be local necrosis. Pain is severe. The CDC recommends that, in a patient who experienced an Arthus reaction after a prior dose of tetanus toxoid- or diphtheria toxoid-containing vaccine, providers should consider deferring doses of DTaP, Tdap, or Td for at least 10 years [55]. If the reaction was to a vaccine with diphtheria toxoid but not tetanus toxoid, and more than 10 years have elapsed since tetanus vaccination, the patient can be evaluated for serum antitetanus level to determine if tetanus protection is needed before vaccination is considered.
Certain vaccines contain diphtheria or tetanus components, although they are indicated for prevention of other diseases. For example, MCV and PCV contain a diphtheria component (but no tetanus toxoid) and therefore should be avoided in patients with hypersensitivity to diphtheria toxoid [73,74]. In MCV, Neisseria meningitides capsular proteins are conjugated to diphtheria toxoid protein. In PCV, capsular antigens of Streptococcus pneumoniae are conjugated to diphtheria CRM197 protein. Certain Hib vaccines contain a Haemophilus influenzae capsular polysaccharide bound to a tetanus toxoid [75]. As always, vaccine components should be reviewed in patients who have known hypersensitivities or have had serious reactions to prior vaccinations.
The ACIP recommends that LAIV not be used in patients with asthma or other conditions predisposing to flu complications [12,21]. In most cases, IIV or another type can be used instead. LAIV should also be avoided in children and adolescents who are receiving aspirin or salicylate therapy. Acute respiratory illness with nasal congestion, which could interfere with delivery of the vaccine, is a reason to consider delaying the use of this vaccine until the congestion has decreased. Children younger than 5 years of age who have recent or recurrent wheezing should not receive LAIV [12,21].
According to the prescribing information, PPSV should be deferred in patients with febrile respiratory illness or other active infection, unless the benefit of vaccinating at that time outweighs the risk [76]. Some providers revaccinate with PPSV every five years. However, revaccination is not recommended in most healthy patients [77]. Most adults will need one lifetime dose. A second dose should be given to patients who are 65 years of age and older if they were previously vaccinated with PPSV prior to 65 years of age and if more than five years have passed [19]. Children and adults at very high risk of serious pneumococcal disease or who are likely to have a rapid decline in antibody levels (such as those with anatomic or functional asplenia or who are immunocompromised) should also receive a second dose at least five years after the first [19].
Vaccine safety is initially established through clinical trials, and benefits must be shown to outweigh any risks before a new vaccine can be approved. However, the trial populations are not necessarily large enough to ensure that all possible adverse events are observed. Postmarketing surveillance provides additional safety information.
In the United States, vaccine safety is monitored through three major systems. The Vaccine Adverse Event Reporting System (VAERS) invites voluntary reporting [80]. VAERS receives approximately 30,000 reports annually, with most reports coming from vaccine manufacturers and healthcare providers. Approximately 20% of reports relate to storage and handling of vaccines, and about 85% to 90% of the reports relating to vaccine reactions describe mild side effects such as fever, arm soreness, and crying or mild irritability. Reporting forms are available at the VAERS website, https://vaers.hhs.gov. This type of surveillance is a useful way to collect information about possible adverse events, particularly uncommon events. However, with no control group, it is often difficult to be certain whether reported events are truly related to vaccination. Researchers often compare reported events to background rates of disease, but because reporting is voluntary (referred to as passive reporting), it is not possible to know the true number of events. VAERS therefore serves primarily as an "early warning system," alerting the CDC to potential problems that require further investigation.
The Vaccine Safety Datalink (VSD) is a collaborative project, partnering the CDC with nine large managed-care organizations [81]. Each managed-care organization tracks and reports data about vaccinations given, medical outcomes, and patient demographics. The VSD project is designed to allow planned safety studies and rapid investigations of concerns raised by patterns in VAERS data or other sources.
The Clinical Immunization Safety Assessment (CISA) Project is a network of vaccine safety experts from the CDC's Immunization Safety Office, seven medical research centers, and other partners [82]. Researchers at these centers evaluate and investigate questions about health risks that may be associated with immunization.
Safety information about several specific vaccines is discussed below, with an emphasis on issues that have been in the news and may thus be on patients' or parents' minds.
Although measles was considered effectively eliminated in the United States in 2000, resurgence in the disease and regional outbreaks have resulted from suboptimal vaccination rates. In 2014, there were 667 cases of measles in the United States, more than 10 times the number of cases in 2000; another even larger spike occurred in 2019 (1,282 cases in 31 states) [6]. A large outbreak in 2014–2015 was linked to unvaccinated children visiting Disneyland, the source patient probably being infected overseas (likely the Philippines) [6]. The decrease in vaccine coverage is in part attributed to the false belief that the MMR vaccine may cause autism. Based on multiple studies, experts generally agree that there is no evidence for a link between the MMR vaccine and autism, and it is important that clinicians address these misconceptions with patients. In 2004, the Institute of Medicine (IOM) reported that "the body of epidemiological evidence favors rejection of a causal relationship between the MMR vaccine and autism" [83]. The American Academy of Pediatrics has also concluded that the evidence does not support such a connection. In addition, autism is not thought to be immune-mediated, and there is no clear mechanism by which MMR would cause this disorder [84].
Research on the topic includes a Canadian study involving 27,749 children born between 1987 and 1998 [85]. This study found no association between rates of pervasive developmental disorder and either one or two doses of the MMR vaccine. In a 2015 retrospective cohort study of 95,727 children, MMR vaccine receipt was not found to predict autism diagnosis, even among children with older siblings with an autism spectrum disorder [78]. A study of 657,461 children born in Denmark between 1999 and 2010 found no increased risk of autism in those who received the MMR vaccine, including in special subgroups (e.g., autism risk factors, other childhood vaccinations) [115].
Some of the concern about MMR and autism is based on a study in the late 1990s that found measles virus ribonucleic acid (RNA) in the gastrointestinal tissue of children with gastrointestinal problems and autism. However, a case-control study designed to explore this issue further found no association between autism and persistent measles virus RNA in the gastrointestinal tract, or between autism and MMR exposure [86].
Another study used polymerase chain reaction to detect measles virus nucleic acids in the peripheral blood mononuclear cells of children with autism spectrum disorder [87]. This study found no evidence of measles virus persistence in affected children.
Some of the concerns about autism involve the use of thimerosal, a mercury-containing preservative. The IOM has concluded that, as with concerns about MMR, the evidence favors rejecting the idea of a causal relationship between thimerosal-containing vaccines and autism [83]. In addition, the same study that looked at MMR and autism in a large cohort of Canadian children also looked for any relationship between ethylmercury exposure and autism and failed to find a connection [85]. Exposure levels were comparable to levels in the United States during the 1990s. Another study, which examined the incidence of autism in California children before and after thimerosal was removed from childhood vaccines, found no decrease in autism following the change [88].
Most vaccines for children 6 years of age or younger that had contained thimerosal either no longer contain this preservative or contain only trace amounts—small enough that the FDA considers them "preservative free" [89]. IIV vaccines are now largely in this category, as "preservative-free" preparations of IIV are widely available. For the 2024–2025 season, "most" of IIV vaccines are thimerosal-free or thimerosal-reduced formulations [118].
Some parents worry that receiving multiple vaccines at a single visit is hard on a child's immune system or that it will weaken the child's immune defenses. However, there is no evidence that giving multiple vaccinations at a single visit weakens the immune system [84]. In addition, although more childhood vaccines are given than in the past, the immunologic load has actually decreased due to advances in vaccine technology [84].
Parents and physicians who remember the withdrawal of the original rotavirus vaccine may worry about a risk of intussusception. Each of the current rotavirus vaccines has been tested in large safety studies.
Safety testing for RV5 included the Rotavirus Efficacy and Safety Trial, involving more than 68,000 infants [90]. However, postlicensure data from the Mini-Sentinel program for 2004–2011 indicate a slightly increased risk of intussusception after the first dose (but not after subsequent doses) [38]. Prelicensure clinical trials did raise the possibility of Kawasaki disease as an uncommon adverse event, with five cases seen in infants who received the vaccine and one case in a child who received placebo (a non-significant difference) [40]. There have been a few cases reported since licensure, but these are not thought to exceed the background rate [91].
Original studies with RV1 involved more than 63,000 infants [39]. Again, no association with intussusception was observed. Since then, a major study in the United States did note a possibly increased risk of intussusception [38]. According to the CDC, there is a 1 in 20,000 to 1 in 100,000 risk of intussusception from either rotavirus vaccine [92]. Composite safety data have shown numerically higher cases of Kawasaki disease with the vaccine than with placebo, but again this was not a statistically significant difference [62].
GBS was associated with a swine flu vaccine in 1976, with an estimated 1 case per 100,000 people vaccinated [21]. Some observational studies since then have found a small increase in GBS cases associated with influenza vaccination, while others have found no link. Whether there is an association between current influenza vaccines and GBS is not known. According to the CDC, based on studies in prior seasons, if an association does exist the risk would likely be low (i.e., one case per 1 million people vaccinated). The IOM conducted a thorough scientific review of this issue in 2003 and concluded that people who received the 1976 swine influenza vaccine had an increased risk for developing GBS. Scientists have multiple theories regarding why this increased risk may have occurred, but the exact reason for this association remains unknown [93].
As of early 2008, there had been 26 confirmed case reports of GBS within six weeks of vaccination with MenACWY-D [94]. This is likely similar to the background rate, and causality has not been established. However, the CDC and the FDA have noted that the timing in relation to vaccination was reason to pursue the question further and to gather more information. Two large studies were conducted to determine if MenACWY-D was the cause of GBS in pre-teens and teenagers, but no link was found among 21 million vaccinated individuals [94,119]. The other MenACWY vaccines are also not associated with GBS.
Providers are asked to report any cases of GBS that coincide with vaccination to VAERS. Providers are also asked to report all GBS cases to their state health departments, in accordance with local guidelines. More complete data collection will help to clarify whether GBS is a concern with these vaccines.
Clinical trials and the post-licensure monitoring data of three HPV vaccines (two discontinued and one in current use) show that they are safe [107]. Since the licensure of the HPV vaccines, both the CDC and the FDA have monitored HPV vaccine safety through VAERS, VSD, and CISA systems. It should be noted that most of the available data is from the quadrivalent Gardasil formulation, which is no longer available in the United States. A 2009 CDC/FDA report found that the most common adverse events reported to VAERS following vaccination with Gardasil were fainting, swelling at the injection site, headache, and nausea. Seven percent were considered serious. However, no common pattern for serious events has emerged, making it difficult to form theories about causality. GBS was reported but did not appear to occur at a rate above background levels. Blood clots were reported in a small number of patients, most of whom had pre-existing risk factors (e.g., smoking, obesity, use of oral contraceptives). Over the first three years of its use, more than 28 million doses of Gardasil 9 were administered, and 7,244 adverse events were reported to VAERS, of which 3% (217 events) were classified as serious [107].
VSD surveillance examined adverse events associated with administration of Gardasil (e.g., GBS, stroke, venous thromboembolism) and found no statistically significant increased risk for any of these adverse events [79]. Ongoing safety studies for HPV include review of serious individual reports to VAERS; VAERS data reviews by the FDA; review of two years of safety data on Gardasil used in boys and men; research on venous thromboembolism following HPV vaccination; and continued consultation with CISA [107].
Because of postmarketing reports, the prescribing information for the HPV vaccines includes a warning that syncope, sometimes associated with seizure-like activity, has been reported following vaccination [107]. Patients should be observed for 15 minutes following injection.
Barriers to on-time vaccination among children and adolescents can be traced to many different issues, including parental concerns, the need for multiple visits, cultural differences, and financial constraints. Some parents are uncomfortable with the idea of multiple shots given at a single visit, and some have safety concerns that lead them to forgo certain vaccinations for their children or refuse immunization entirely. In some cases, parents are simply unaware of their children's preventive care needs.
In the last decade, news reports and social media misinformation have increased parents' concerns about vaccine safety and have led some parents to reconsider the value of immunization. Although certain vaccinations are required for school attendance, parents can usually opt out for religious reasons. Some states allow "philosophical" objections as well, creating room for parents who feel uneasy about childhood vaccinations to avoid them. In places where requirements are stricter, some parents are choosing to home school their children rather than accept vaccination [96].
Healthcare providers can have an influence when parents are concerned or confused about vaccines. For example, in one survey, 28% of parents had some level of uncertainly about vaccines [97]. For those who ultimately decided to allow timely vaccination, assurances or information provided by a healthcare provider were important reasons for the decision.
When explaining vaccine recommendations or vaccine safety, the provider should take into account the parents' level of health literacy, any language or reading literacy barriers, and social and cultural expectations. For example, for some parents, written material may not be sufficient due either to a low level of literacy or to a desire to discuss the information with the physician directly.
Because patient education is such a vital aspect of vaccine promotion, it is each practitioner's responsibility to ensure that information and instructions are explained in such a way that allows for patient understanding. When there is an obvious disconnect in the communication process between the practitioner and patient due to the patient's lack of proficiency in the English language, an interpreter is required.
In this multicultural landscape, interpreters are a valuable resource to help bridge the communication and cultural gap between clients/patients and practitioners. Interpreters are more than passive agents who translate and transmit information back and forth from party to party. When they are enlisted and treated as part of the interdisciplinary clinical team, they serve as cultural brokers, who ultimately enhance the clinical encounter.
Many parents are upset by the idea of multiple shots on a single visit, feeling that their children will be too frightened or upset. Some parents request that certain shots be delayed, and some providers have devised alternative immunization schedules that spread injections out over time. However, there is evidence that delaying vaccinations to reduce the number of injections can lead to undervaccination. When doses are deferred, immunization coverage at both 1 and 2 years of age declines [98]. Future visits may be missed or delayed, and children may be left vulnerable to vaccine-preventable illnesses.
One way to help reduce the number of injections is to make use of combination formulations, which allow for multiple vaccines in one shot [99]. In addition to the familiar MMR and DTaP, Tdap, and Td vaccines, available combination products include [12,19]:
HepA and HepB (adults only)
DTaP and IPV
DTaP, IPV, and HepB
DTaP, IPV, and Hib
DTaP, IPV, Hib, and HepB
MMR and varicella
MenACWY and MenB
Some of these products include premixed components, while others involve components that must be combined by the healthcare provider according to instructions. Except for products that are designed to be used in this manner, individual vaccines should not be combined in a single syringe.
In their 2025 General Recommendations on Immunization, the ACIP recommended the use of combination vaccines whenever possible to reduce the number of injections and improve coverage [100]. The 2025 immunization schedule includes these formulations as an option when any component of the combination is indicated, other components are not contraindicated, and the combination vaccine is FDA approved for that dose of the series [12].
The Vaccines for Children (VFC) program is designed to help overcome cost as a barrier to childhood vaccination. All of the ACIP-recommended vaccines are available for children enrolled in Medicaid, with VFC covering children through 18 years of age [101]. Children who have no health insurance coverage, children who are underinsured, and children who are American Indian or Alaska Native are also eligible for vaccines through VFC.
"Underinsured" children are those who have private health insurance coverage that does not include vaccines, that covers only certain vaccines, or that has a cap on the amount to be paid for vaccinations [101]. In each case, VFC will cover vaccines that the insurance does not. These children must visit a Federally Qualified Health Center (FQHC) or Rural Health Clinic (RHC) to receive the covered vaccines. An FHQC is a center with a special government designation to provide care to an underserved population. A typical FQHC would be a community health center in an underserved area. An RHC is a specially certified clinic in an underserved area or one where there is a recognized shortage of healthcare professionals [101]. All other children may receive vaccines from any enrolled VFC Program provider; most physicians, clinics, hospitals, public health clinics, community health clinics, and some schools are VFC Program providers.
Although the vaccines are free and patients cannot be charged for them, providers participating in VFC may charge an administrative fee to cover other costs [101]. These fees are established by the states. Healthcare providers can learn more about VFC, including how to become a VFC provider, at the Vaccines for Children Program website, https://www.cdc.gov/vaccines/programs/vfc/index.html.
Reminding parents to bring their children in for vaccinations is a proven way to increase coverage and is recommended in standards developed by the National Vaccine Advisory Committee and supported by other organizations [102,103]. Reminders need not take up extensive staff time. Mailed reminders have been shown to increase child vaccination rates and so have telephone calls, which may be computer-generated to save work by the office staff [104,105,106]. Outreach should be more intensive for families at high risk of missing appointments [102].
Setting up a system of reminders for the physician who is responsible for prescribing the vaccinations can also be helpful. Charts can be flagged, or a computerized database can be used. The National Vaccine Advisory Committee also recommends conducting chart audits to review how well the practice is meeting immunization needs and to look for areas for improvement [102].
Barriers to adult vaccination are similar to those impacting children and adolescents. These include: cultural differences, lack of information about what vaccinations are needed and when, lack of physician recommendation, unawareness that the protection they received as children for some diseases decreases over time, unawareness of vaccines received in childhood, lack of insurance, and mismanagement of time/priorities during office visits.
Lack of awareness is a primary reason that adults miss recommended vaccinations. It is common for adults to report that no healthcare provider had recommended a given vaccination, and so they did not know it was needed. There may also be cultural differences in how adults approach vaccination or in how services are provided. According to 2021 surveillance data, racial/ethnic disparities exist for all seven vaccines the CDC is tracking [7]. The gap is most marked for Black adults, whose vaccination rate averaged 18% lower than their White counterparts with respect to seasonal influenza, tetanus (with pertussis), pneumococcal, herpes zoster, and hepatitis B [7].
"Missed opportunities," visits during which a patient was eligible for a vaccination but did not receive it, are common for adults. Reasons include constraints on time during office visits, a focus on acute care needs instead of prevention, and a lack of standing orders or an office reminder system that could prompt staff to offer the recommended vaccines [109,110].
There is evidence that when physicians recommend preventive services, patients are interested in receiving them. For example, 95.1% of patients in a national survey stated that they would accept the herpes zoster vaccination if their doctor recommended it [111]. Standards provided by the National Vaccine Advisory Committee, in cooperation with more than 60 organizations, offer evidence-based methods to help reduce missed opportunities for adults [110]. Providers should assess the vaccination status of all new patients and review vaccination status annually. Pneumococcal vaccination status should be reviewed when patients present for influenza vaccination.
Standing orders for vaccination should be used, based on evidence that they improve adult vaccination coverage in many different settings [110]. Reminder systems for staff can also improve vaccination rates. In one review of studies, use of physician reminder systems, such as chart notations, stickers, and patient lists, improved coverage by a median of 22% [112]. Assessing a practice's success at vaccinating patients who are eligible and reporting the results to staff can also help to improve coverage [110].
Telephone calls, mailed reminders, and texts/electronic reminders can help raise vaccination coverage among adults as well as among children [110]. Reminders can specify that patients are due or overdue for vaccinations, or they can invite patients to contact the provider's office to see which vaccinations they need. As with children, adults who are likely to miss appointments or fail to comply with recommendations may need particularly intensive follow-up.
Staying up-to-date, working with patients to maximize vaccination coverage, and monitoring and improving day-to-day practice can all help to improve vaccination rates. However, keeping up with changes to the child, adolescent, and adult immunization schedules can be challenging. Annual schedules often change from year to year and include both major changes and subtle ones. Mid-year announcements from the CDC and the ACIP require clinicians to be alert to new information and to make adjustments to practice. To help clinicians check for updates, verify information about vaccines, and locate answers to common clinical questions, the CDC provides a Vaccines and Immunizations website, as does the Immunization Action Coalition. Healthcare professionals should consider every healthcare visit as an opportunity to assess vaccination status and administer vaccines when needed. This will improve rates across the life spectrum, from infancy to elderly.
| Centers for Disease Control and Prevention Vaccines and Immunizations |
| https://www.cdc.gov/vaccines |
| Immunization Action Coalition |
| https://www.immunize.org |
| Vaccine Adverse Event Reporting System (VAERS) |
| https://vaers.hhs.gov |
| Vaccines for Children Program (VFC) |
| https://www.cdc.gov/vaccines/programs/vfc/index.html |
1. Centers for Disease Control and Prevention. Viral Hepatitis Surveillance—United States, 2022. Available at https://www.cdc.gov/hepatitis/statistics/2022surveillance/index.htm. Last accessed February 17, 2025.
2. Centers for Disease Control and Prevention. Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction—eight states, 1998–2005. MMWR. 2008;57(6):144-148.
3. Lexau CA, Lynfield R, Danila R, et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294(16):2043-2051.
4. Hill HA, Yankey D, Elam-Evans LD, Singleton JA, Sterrett N. Vaccination coverage by age 24 months among children born in 2018 and 2019—National Immunization Survey-Child, United States, 2019–2021. MMWR. 2023;72(2):33-38..
5. Zhao Z, Smith PJ. Trends in vaccination coverage disparities among children, United States, 2001–2010. Vaccine. 2013;31(19):2324-2327.
6. Centers for Disease Control and Prevention. Measles Cases and Outbreaks. Available at https://www.cdc.gov/measles/data-research/index.html. Last accessed February 17, 2025.
7. Lu PJ, Hung MC, Srivastav A, et al. Surveillance of vaccination coverage among adult populations—United States, 2018. MMWR. 2021;70(SS3):1-26.
8. Schaffner W. Update on vaccine-preventable diseases: are adults in your community adequately protected? J Fam Pract. 2008;57 (4 suppl):S1-S11.
9. O'Leary ST. Immunization Schedule Updated for 2022. Available at https://publications.aap.org/aapnews/news/19567. Last accessed February 24, 2025.
10. Hall CB. The recommended childhood immunization schedule of the United States. Pediatrics. 1995;95(1):135-137.
11. Centers for Disease Control and Prevention. Past Immunization Schedules. Available at https://www.cdc.gov/vaccines/schedules/resource-library/index.html. Last accessed February 24, 2025.
12. Issa AN, Wodi AP, Moser CA, Cineas S. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger —United States, 2025. MMWR. 2025;74:26-29.
13. U.S. Food and Drug Administration. Bexcero: Highlights of Prescribing Information. Available at https://www.fda.gov/media/90996/download?attachment. Last accessed February 25, 2025.
14. National Cancer Institute. HPV Vaccination. Available at https://progressreport.cancer.gov/prevention/hpv_immunization#jump-links-field-data-source. Last accessed February 17, 2025.
15. Centers for Disease Control and Prevention. Notice to readers: recommended adult immunization schedule—United States, 2002–2003. MMWR. 2002;51(40):904-908.
16. Centers for Disease Control and Prevention. Cancers Linked with HPV Each Year. Available at https://www.cdc.gov/cancer/hpv/cases.html. Last accessed February 21, 2025.
17. Markowitz LE, Liu G, Hariri S, Steinau M, Dunne EF, Unger ER. Prevalence of HPV after introduction of the vaccination program in the United States. Pediatrics. 2016;137(3):e1-e9.
18. Advisory Committee on Immunization Practices. Use of serogroup B meningococcal vaccines in adolescents and young adults: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR. 2015;64(41):1171-1176.
19. Wodi AP, Issa AN, Moser CA, Cineas S. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older—United States, 2025. MMWR. 2025;74(2):30-33.
20. Grohskopf LA, Ferdinands JM, Blanton LH, Broder KR, Loehr J. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2024–25 influenza season. MMWR. 2024;73(RR5):1-25.
21. Fiore AE, Shay DK, Broder K, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR. 2008;57(RR7):1-60.
22. U.S. Food and Drug Administration. FluMist Influenza Vaccine Live, Intranasal. Available at https://www.fda.gov/media/160349/download. Last accessed February 21, 2025.
23. Broder KR, Cortese MM, Iskander JK, et al. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2006;55(RR3):1-34.
24. Centers for Disease Control and Prevention. 2022 Final Pertussis Surveillance Report. Available at https://www.cdc.gov/pertussis/media/pdfs/2024/06/pertuss-surv-report-2022_final-508.pdf. Last accessed February 21, 2025.
25. Kretsinger K, Broder KR, Cortese MM, et al. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2006;55(RR17):1-33.
26. Centers for Disease Control and Prevention. Pneumococcal Disease Surveillance and Trends. Available at https://www.cdc.gov/pneumococcal/php/surveillance. Last accessed February 24, 2025.
27. Government of the District of Columbia. School Health Requirements. Available at https://dcps.dc.gov/page/school-health-requirements. Last accessed February 21, 2025.
28. American Cancer Society. Key Statistics for Cervical Cancer. Available at https://www.cancer.org/cancer/types/cervical-cancer/about/key-statistics.html. Last accessed February 21, 2025.
29. Centers for Disease Control and Prevention. Vaccines and Preventable Diseases: Who Should NOT Get Vaccinated with These Vaccines? Available at https://www.cdc.gov/vaccines/vpd/should-not-vacc.html. Last accessed February 24, 2025.
30. U.S. Food and Drug Administration. Vaccines, Blood and Biologics: Cervarix. Available at https://www.fda.gov/vaccines-blood-biologics/vaccines/cervarix. Last accessed February 24, 2025.
31. U.S. Food and Drug Administration. Vaccines, Blood and Biologics: Gardasil. Available at https://www.fda.gov/vaccines-blood-biologics/vaccines/gardasil. Last accessed February 24, 2025.
32. Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2014;63(RR5):1-30.
33. Manhart LE, Holmes KK, Koutsky LA, et al. Human papillomavirus infection among sexually active young women in the United States: implications for developing a vaccination strategy. Sex Transm Dis. 2006;33(8):502-508.
34. Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the U.S. JAMA. 2007;297(8):813-819.
35. Brown DR, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naïve women aged 16–26 years. J Infect Dis. 2009;199(7):926-935.
36. Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301-314.
37. Merck. Highlights of Prescribing Information: Gardasil. Available at https://www.merck.com/product/usa/pi_circulars/g/gardasil/gardasil_pi.pdf. Last accessed February 24, 2025.
38. Yih WK, Lieu TA, Kulldorff M, et al. Intussusception risk after rotavirus vaccination in U.S. infants. N Engl J Med. 2014;370(6):503-512.
39. Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354(1):11-22.
40. Cortese MM, Parashar UD. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2009;58(RR2):1-25.
41. Centers for Disease Control and Prevention. About Rotavirus. Available at https://www.cdc.gov/rotavirus/index.html. Last accessed February 24, 2025.
42. Bilukha OO, Rosenstein N. Prevention and control of meningococcal disease. MMWR. 2005;54(RR7):1-21.
43. Centers for Disease Control and Prevention. Clinical Overview of Meningococcal Disease. Available at https://www.cdc.gov/meningococcal/clinical-info.html. Last accessed February 24, 2025.
44. Fishbein DB, Broder KR, Markowitz L, Messonnier N. New, and some not-so-new, vaccines for adolescents and diseases they prevent. Pediatrics. 2008;121(suppl 1):S5-S14.
45. Centers for Disease Control and Prevention. Licensure of a meningococcal conjugate vaccine (Menveo) and guidance for use—Advisory Committee on Immunization Practices, 2010. MMWR. 2010;59(09);273.
46. U.S. Food and Drug Administration. First Vaccine Approved by FDA to Prevent Serogroup B Meningococcal Disease. Available at https://www.fda.gov/news-events/press-announcements/first-vaccine-approved-fda-prevent-serogroup-b-meningococcal-disease. Last accessed February 24, 2025.
47. Centers for Disease Control and Prevention. Notice to readers: revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11–18 years with meningococcal conjugate vaccine. MMWR. 2007;56(31):794-795.
48. Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2006;55(RR7):1-23.
49. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR. 2018;67(3):103-108.
50. Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland M, Sy LS. A population-based study of the incidence and complications of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82(11):1341-1349.
51. Harbecke R, Cohen JI, Oxman MN. Herpes zoster vaccines. J Infect Dis. 2021;224(12 Suppl 2):S429-S442.
52. Centers for Disease Control and Prevention. Manual for the Surveillance of Vaccine-Preventable Diseases. 5th ed. Atlanta, GA: Centers for Disease Control and Prevention; 2012.
53. Centers for Disease Control and Prevention. Pneumonia hospitalizations among young children before and after introduction of pneumococcal conjugate vaccine—United States, 1997–2006. MMWR. 2009;58(1):1-4.
54. Centers for Disease Control and Prevention. Licensure of 13-valent pneumococcal conjugate vaccine for adults aged 50 years and older. MMWR. 2012;61(21):394-395.
55. Centers for Disease Control and Prevention. Contraindications and Precautions. Available at https://www.cdc.gov/vaccines/hcp/imz-best-practices/contraindications-precautions.html?CDC_AAref_Val=https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/contraindications.html. Last accessed February 21, 2025.
56. Curran D, Oostvogels L, Heineman T, et al. Quality of life impact of a recombinant zoster vaccine in adults ≥50 years of age. J Gerontol A Biol Sci Med Sci. 2019;74(8):1231-1238.
57. Merck. Varivax (Varicella Virus Vaccine Live). Available at https://www.merck.com/product/usa/pi_circulars/v/varivax/varivax_pi.pdf. Last accessed February 24, 2025.
58. Anderson TC, Masters NB, Guo A, et al. Use of recombinant zoster vaccine in immunocompromised adults aged ≥19 years: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR. 2022;71(7):80-84.
59. Marin M, Guris D, Chaves SS, Schmid S, Seward JF. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2007;56(RR4):1-40.
60. Merck. MMR-II (Measles, Mumps, and Rubella Virus Vaccine Live). Available at https://www.merck.com/product/usa/pi_circulars/m/mmr_ii/mmr_ii_pi.pdf. Last accessed February 24, 2025.
61. Merck. Highlights of Prescribing Information: RotaTeq (Rotavirus Vaccine, Live, Oral, Pentavalent). Available at https://www.merck.com/product/usa/pi_circulars/r/rotateq/rotateq_pi.pdf. Last accessed February 24, 2025.
62. GlaxoSmithKline. Highlights of Prescribing Information: Rotarix (Rotavirus Vaccine, Live, Oral). Available at https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Rotarix/pdf/ROTARIX-PI-PIL.PDF. Last accessed February 24, 2025.
63. Hales CM, Harpaz R, Ortega-Sanchez I, Bialek SR. Update on recommendations for use of herpes zoster vaccine. MMWR. 2014;63(33):729-731.
64. LexiDrug. Available at http://online.lexi.com. Last accessed February 24, 2025.
65. GlaxoSmithKline. Highlights of Prescribing Information: Hiberix (Haemophilus b Conjugate Vaccine [Tetanus Toxoid Conjugate]). Available at https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Hiberix/pdf/HIBERIX.PDF. Last accessed February 24, 2025.
66. Centers for Disease Control and Prevention. Rotavirus Vaccine Safety. Available at https://www.cdc.gov/vaccine-safety/vaccines/rotavirus.html. Last accessed February 24, 2025.
67. Sanofi Pasteur. Daptacel. Available at https://www.fda.gov/vaccines-blood-biologics/vaccines/daptacel. Last accessed February 24, 2025.
68. Geier DA, Geier MR. An evaluation of serious neurological disorders following immunization: a comparison of whole-cell pertussis and acellular pertussis vaccines. Brain Dev. 2004;26(5):296-300.
69. Sanofi Pasteur. Highlights of Prescribing Information: Daptacel (Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine Adsorbed). Available at https://www.fda.gov/media/74035/download. Last accessed February 24, 2025.
70. U.S. Food and Drug Administration. Infanrix. Available at https://www.fda.gov/vaccines-blood-biologics/vaccines/infanrix. Last accessed February 24, 2025.
71. GlaxoSmithKline. Highlights of Prescribing Information: Boostrix (Tetanus Toxoid, Reduced Diptheria Toxoid, and Acellular Pertussis Vaccine, Adsorbed). Available at https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Boostrix/pdf/BOOSTRIX.PDF. Last accessed February 24, 2025.
72. Sanofi Pasteur. Highlights of Prescribing Information: Adacel (Tetanus Toxoid, Reduced Diptheria Toxoid, and Acellular Pertuss is Vaccine Adsorbed). Available at https://www.fda.gov/media/119862/download. Last accessed February 24, 2025.
73. U.S. Food and Drug Administration. Highlights of Prescribing Information: Menactra Meningococcal [groups A, C, Y and W-135] Polysaccharide Diphtheria Toxoid Conjugate Vaccine. Available at https://www.fda.gov/media/75619/download. Last accessed February 24, 2025.
74. Wyeth Pharmaceuticals, Inc. Highlights of Prescribing Information: Prevnar 13 (Pneumococcal 13-Valent Conjugate Vaccine [Diphtheria CRM197 Protein]). Available at https://labeling.pfizer.com/showlabeling.aspx?id=501. Last accessed February 24, 2025.
75. Sanofi Pasteur. Haemophilus B Conjugate Vaccine (Tetanus Toxoid Conjugate) ActHIB. Available at https://www.fda.gov/media/74395/download. Last accessed February 24, 2025.
76. Merck. Highlights of Prescribing Information: Pneumovax 23 (Pneumococcal Vaccine Polyvalent). Available at https://www.merck.com/product/usa/pi_circulars/p/pneumovax_23/pneumovax_pi.pdf. Last accessed February 24, 2025.
77. Centers for Disease Control and Prevention. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR. 2010;59(34):1102-1106.
78. Jain A, Marshall J, Buikema A, Bancroft T, Kelly JP, Newschaffer CJ. Autism occurrence by MMR vaccine status among U.S. children with older siblings with and without autism. JAMA. 2015;313(15):1534-1540.
79. Stokley S, Jeyarajah J, Yankey D, et al. Human papillomavirus vaccination coverage among adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014—United States. MMWR. 2015;63(29);620-624.
80. U.S. Department of Health and Human Services. Vaccine Adverse Event Reporting System (VAERS). Available at https://vaers.hhs.gov. Last accessed February 24, 2025.
81. Centers for Disease Control and Prevention. Vaccine Safety Datalink (VSD) Project. Available at https://www.cdc.gov/vaccine-safety-systems/vsd/. Last accessed February 24, 2025.
82. Centers for Disease Control and Prevention. Clinical Immunization Safety Assessment (CISA) Project. Available at https://www.cdc.gov/vaccine-safety-systems/hcp/cisa. Last accessed February 24, 2025.
83. Immunization Safety Review Committee. Immunization Safety Review: Vaccines and Autism. Washington, DC: National Academies Press; 2004.
84. Gerber JS, Offit PA. Vaccines and autism: a tale of shifting hypotheses. Clin Infect Dis. 2009;48(4):456-461.
85. Fombonne E, Zakarian R, Bennett A, Meng L, McLean-Heywood D. Pervasive developmental disorders in Montreal, Quebec, Canada: prevalence and links with immunizations. Pediatrics. 2006;118(1):e139-e150.
86. Hornig M, Briese T, Buie T, et al. Lack of association between measles virus vaccine and autism with enteropathy: a case-control study. PLoS One. 2008;3(9):e3140.
87. D'Souza Y, Fombonne E, Ward BJ. No evidence of persisting measles virus in peripheral blood mononuclear cells from children with autism spectrum disorder. Pediatrics. 2006;118(4):1664-1675.
88. Schechter R, Grether JK. Continuing increases in autism reported to California's developmental services system: mercury in retrograde. Arch Gen Psychiatry. 2008;65(1):19-24.
89. U.S. Food and Drug Administration. Thimerosal in Vaccines. Available at https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/thimerosal-and-vaccines. Last accessed February 24, 2025.
90. Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine.N Engl J Med. 2006;354(1):23-33.
91. Hua W, Izurieta HS, Slade B, et al. Kawasaki disease after vaccination: reports to the Vaccine Adverse Event Reporting System, 1990–2007. Pediatr Infect Dis J. 2009;2(11):943-947.
92. Centers for Disease Control and Prevention. Questions & Answers About Intussusception and Rotavirus Vaccine. Available at https://www.cdc.gov/vaccines/vpd/rotavirus/about-intussusception.html. Last accessed February 24, 2025.
93. Centers for Disease Control and Prevention. Guillain-Barré Syndrome and Flu Vaccine. Available at https://www.cdc.gov/flu/vaccine-safety/guillainbarre.html. Last accessed May 24, 2024.
94. Centers for Disease Control and Prevention. Meningococcal Vaccine Safety. Available at https://www.cdc.gov/vaccine-safety/vaccines/meningococcal.html. Last accessed February 24, 2025.
95. Centers for Disease Control and Prevention. Sexually Transmitted Infections Prevalence, Incidence, and Cost Estimates in the United States. Available at https://www.cdc.gov/sti/php/communication-resources/prevalence-incidence-and-cost-estimates.html?CDC_AAref_Val=https://www.cdc.gov/std/statistics/prevalence-2020-at-a-glance.htm. Last accessed February 21, 2025.
96. Joyner C. Parents Homeschool to Avoid Vaccinating Their Kids. Available at http://usatoday30.usatoday.com/news/health/2008-10-21-home-school-vaccinate_N.htm. Last accessed February 24, 2025.
97. Gust DA, Darling N, Kennedy A, Schwartz B. Parents with doubts about vaccines: which vaccines and reasons why. Pediatrics. 2008;122(4):718-725.
98. Meyerhoff AS, Jacobs RJ. Do too many shots due lead to missed vaccination opportunities? Does it matter? Prev Med. 2005;41(2):540-544.
99. Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. 14th ed. Atlanta, GA: Centers for Disease Control and Prevention; 2021.
100. Kroger AT, Duchin J, Vázquez M. General Best Practices for Immunization. Available at https://www.cdc.gov/vaccines/hcp/imz-best-practices/. Last accessed February 24, 2025.
101. Centers for Disease Control and Prevention. About the Vaccines for Children (VFC) Program. Available at https://www.cdc.gov/vaccines-for-children/about/index.html. Last accessed February 24, 2025.
102. National Vaccine Advisory Committee. Standards for child and adolescent immunization practices. Pediatrics. 2003;112(4):958-963.
103. Adetunji Y, Macklin D, Patel R, Kinsinger L. American College of Preventive Medicine practice policy statement: childhood immunizations. Am J Prev Med. 2003;25(2)169-175.
104. Lieu TA, Capra AM, Makol J, Black SB, Shinefield HR. Effectiveness and cost-effectiveness of letters, automated telephone messages or both for underimmunized children in a health maintenance organization. Pediatrics. 1998;101(4):E3.
105. Irigoyen MM, Findley S, Earle B, Stambaugh K, Vaughan R. Impact of appointment reminders on vaccination coverage at an urban clinic. Pediatrics. 2000;106(4):919-923.
106. Dini EF, Linkins RW, Sigafoos J. The impact of computer-generated messages on childhood immunization coverage. Am J Prev Med. 2000;18(2):132-139.
107. Centers for Disease Control and Prevention. Questions About HPV Vaccine Safety. Available at https://www.cdc.gov/vaccine-safety/vaccines/hpv.html. Last accessed February 24, 2025.
108. Petrosky E, Bocchini JA Jr, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the Advisory Committee on Immunization Practices. MMWR. 2015;64(11):300-304.
109. Lu PJ, Euler GL, Jumaan AO, Harpaz R. Herpes zoster vaccination among adults aged 60 years or older in the United States: uptake of the first new vaccine to target seniors. Vaccine. 2009;27(6):882-887.
110. Poland GA, Shefer AM, McCauley M, et al. Standards for adult immunization practices. Am J Prev Med. 2003;25(2):144-150.
111. Lu PJ, Euler GL, Harpaz R. Herpes zoster vaccination among adults aged 60 years and older, in the U.S., 2008. Am J Prev Med. 2011;40(2):e1-e6.
112. Willis BC, Ndiaye SM, Hopkins DP, Shefer A. Improving influenza, pneumococcal polysaccharide, and hepatitis B vaccination coverage among adults aged <65 years at high risk: a report on recommendations of the Task Force on Community Preventive Services. MMWR. 2005;54(RR5):1-11.
113. U.S. Food and Drug Administration. FDA Approves Expanded Use of Gardasil 9 to Include Individuals 27 through 45 Years Old. Available at https://www.fda.gov/news-events/press-announcements/fda-approves-expanded-use-gardasil-9-include-individuals-27-through-45-years-old. Last accessed February 24, 2025.
114. Office of Disease Prevention and Health Promotion. Increase the proportion of adolescents who get recommended doses of the HPV vaccine—IID-08. Available at https://health.gov/healthypeople/objectives-and-data/browse-objectives/vaccination/increase-proportion-adolescents-who-get-recommended-doses-hpv-vaccine-iid-08. Last accessed February 17, 2025.
115. Hviid A, Hansen JV, Frisch M, Melbye M. Measles, mumps, rubella vaccination and autism: a nationwide cohort study. Ann Intern Med. 2019;170(8):513-520.
116. Schwartz KL, Kwong JC, Deeks SL, et al. Effectiveness of pertussis vaccination and duration of immunity. CMAJ. 2016;188(16):E399-E406.
117. Anderson TC, Masters NB, Guo A, et al. Use of recombinant zoster vaccine in immunocompromised adults aged ≥19 years: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR. 2022;71(3):80-84.
118. Centers for Disease Control and Prevention. Seasonal Influenza Vaccine Supply: Frequently Asked Questions. Available at https://www.cdc.gov/flu/hcp/vaccine-supply/vaxadmin.html. Last accessed February 24, 2025.
119. Yih WK, Weintraub E, Kulldorff M. No risk of Guillain-Barré syndrome found after meningococcal conjugate vaccination in two large cohort studies. Pharmacoepidemiol Drug Saf. 2012;21(12):1359-1360.
120. Collins JP, Crowe SJ, Ortega-Sanchez IR, et al. Use of the Pfizer pentavalent meningococcal vaccine among persons aged ≥10 years: recommendations of the Advisory Committee on Immunization Practices―United States, 2023. MMWR. 2024;73(15):345-350.
121. Fleming-Dutra KE, Jones JM, Roper LE, et al. Use of the Pfizer respiratory syncytial virus vaccine during pregnancy for the prevention of respiratory syncytial virus-associated lower respiratory tract disease in infants: recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR. 2023;72(41):1115-1122.
122. Melgar M, Britton A, Roper LE, et al. Use of respiratory syncytial virus vaccines in older adults: recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR. 2023;72(29):793-801.
123. Centers for Disease Control and Prevention. CDC Updates RSV Vaccination Recommendation for Adults. Available at https://www.cdc.gov/media/releases/2024/s-0626-vaccination-adults.html. Last accessed February 24, 2025.
124. Texas Department of State Health Services. Measles Outbreak – Feb. 25, 2025. Available at https://www.dshs.texas.gov/news-alerts/measles-outbreak-feb-25-2025. Last accessed February 26, 2025.
1. Advisory Committee on Immunization Practices. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger—United States, 2025. MMWR. 2025;74(2):26-29. Available at https://www.cdc.gov/mmwr/volumes/74/wr/mm7402a2.htm. Last accessed February 27, 2025.
2. Advisory Committee on Immunization Practices. Recommended immunization schedule for adults aged 19 years and older—United States, 2025. MMWR. 2025;74(2):30-33. Available at https://www.cdc.gov/mmwr/volumes/74/wr/mm7402a3.htm. Last accessed February 27, 2025.
Mention of commercial products does not indicate endorsement.