The burden of musculoskeletal diseases in the United States is substantial. The complexity of osteoarthritis creates a challenge for diagnosis and management. Inadequate education and training in musculoskeletal diseases has left many primary care providers--often the first ones to evaluate individuals with signs and symptoms of osteoarthritis--feeling ill-equipped to manage the disease. Continuing education can help fill this substantial educational gap. This course will provide an overview of the prevalence of osteoarthritis in various demographic groups in the United States. What is known regarding the etiology and pathogenesis of the disease will be explored, including known risk factors. The diagnostic criteria for osteoarthritis at various anatomic sites will be provided, including the role of the functional assessment. Finally, evidence-based guidelines for the management of osteoarthritis will be discussed, and treatments for which evidence is lacking will be identified.
This course is designed for physicians, physician assistants, nurses, and other healthcare professionals involved in the care of patients with osteoarthritis.
The high prevalence of osteoarthritis and its substantial burden at both the individual and healthcare system levels demands sound knowledge and clinical skills in diagnosing and managing the disease. The purpose of this course is to provide healthcare professionals with the information necessary to adequately assess osteoarthritis symptoms, treat osteoarthritis patients based on evidence-based guidelines, and appropriately refer to specialists.
Upon completion of this course, you should be able to:
- Discuss the prevalence of osteoarthritis in the context of demographic variables.
- Describe what is known about the etiology and pathogenesis of osteoarthritis.
- List the risk factors for the development of osteoarthritis.
- Identify the diagnostic criteria for osteoarthritis at various anatomic sites.
- Describe the roles of radiography and patient-related factors in the diagnosis of osteoarthritis.
- Recommend lifestyle changes and education strategies that should be incorporated into the osteoarthritis treatment plan.
- Apply evidence-based guidelines for the appropriate use of oral and topical analgesics to manage osteoarthritis symptoms.
- Analyze the appropriateness of intra-articular medications for the treatment of osteoarthritis.
- Discuss alternative therapies that lack evidence to support their routine use in the management of osteoarthritis.
- Identify operative procedures used to manage osteoarthritis.
Lori L. Alexander, MTPW, ELS, MWC, is President of Editorial Rx, Inc., which provides medical writing and editing services on a wide variety of clinical topics and in a range of media. A medical writer and editor for more than 30 years, Ms. Alexander has written for both professional and lay audiences, with a focus on continuing education materials, medical meeting coverage, and educational resources for patients. She is the Editor Emeritus of the American Medical Writers Association (AMWA) Journal, the peer-review journal representing the largest association of medical communicators in the United States. Ms. Alexander earned a Master’s degree in technical and professional writing, with a concentration in medical writing, at Northeastern University, Boston. She has also earned certification as a life sciences editor and as a medical writer.
Contributing faculty, Lori L. Alexander, MTPW, ELS, MWC, has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
John M. Leonard, MD
Jane C. Norman, RN, MSN, CNE, PhD
The division planners have disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Sarah Campbell
The Director of Development and Academic Affairs has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
The purpose of NetCE is to provide challenging curricula to assist healthcare professionals to raise their levels of expertise while fulfilling their continuing education requirements, thereby improving the quality of healthcare.
Our contributing faculty members have taken care to ensure that the information and recommendations are accurate and compatible with the standards generally accepted at the time of publication. The publisher disclaims any liability, loss or damage incurred as a consequence, directly or indirectly, of the use and application of any of the contents. Participants are cautioned about the potential risk of using limited knowledge when integrating new techniques into practice.
It is the policy of NetCE not to accept commercial support. Furthermore, commercial interests are prohibited from distributing or providing access to this activity to learners.
Supported browsers for Windows include Microsoft Internet Explorer 9.0 and up, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Supported browsers for Macintosh include Safari, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Other operating systems and browsers that include complete implementations of ECMAScript edition 3 and CSS 2.0 may work, but are not supported. Supported browsers must utilize the TLS encryption protocol v1.1 or v1.2 in order to connect to pages that require a secured HTTPS connection. TLS v1.0 is not supported.
The role of implicit biases on healthcare outcomes has become a concern, as there is some evidence that implicit biases contribute to health disparities, professionals' attitudes toward and interactions with patients, quality of care, diagnoses, and treatment decisions. This may produce differences in help-seeking, diagnoses, and ultimately treatments and interventions. Implicit biases may also unwittingly produce professional behaviors, attitudes, and interactions that reduce patients' trust and comfort with their provider, leading to earlier termination of visits and/or reduced adherence and follow-up. Disadvantaged groups are marginalized in the healthcare system and vulnerable on multiple levels; health professionals' implicit biases can further exacerbate these existing disadvantages.
Interventions or strategies designed to reduce implicit bias may be categorized as change-based or control-based. Change-based interventions focus on reducing or changing cognitive associations underlying implicit biases. These interventions might include challenging stereotypes. Conversely, control-based interventions involve reducing the effects of the implicit bias on the individual's behaviors. These strategies include increasing awareness of biased thoughts and responses. The two types of interventions are not mutually exclusive and may be used synergistically.
#94954: Osteoarthritis
Many conditions comprise musculoskeletal diseases, but osteoarthritis is by far the most common joint disorder, particularly osteoarthritis of the knee. The disease exacts a high cost in terms of pain and decreased function. Osteoarthritis is a leading cause of activity limitation and absenteeism among working-age adults and is associated with a significant decline in function among older individuals. The toll of osteoarthritis on the healthcare system is also great, with high rates of physician office visits and hospitalizations, and the burden of the disease is expected to increase.
Osteoarthritis is a complex disease. Its etiology is not completely understood, and its risk factors and clinical and radiographic presentation vary according to the joint site. This complexity creates a challenge for diagnosis and management. Although diagnostic criteria exist, diagnosis can be difficult for a variety of reasons, most notably, a low sensitivity of radiographs in detecting early osteoarthritic changes and the lack of correlation between radiographic evidence of disease and symptoms. As no curative therapy for osteoarthritis is currently available, management is focused on decreasing pain and increasing function. The great range in treatment options has made it difficult to determine which ones are most effective; more than 50 treatment modalities have been addressed in 23 guidelines for the management of knee and hip osteoarthritis alone. Adding to the challenge of selecting appropriate therapy is evolving evidence on the efficacy of specific options; systematic reviews, meta-analyses, and randomized controlled clinical trials have demonstrated that many commonly used treatment options for osteoarthritis offer limited or no benefit. This course addresses osteoarthritis of the most commonly involved joints (knee, hip, and hand), providing important details on risk factors, diagnosis, and the most current evidence-based recommendations for treatment.
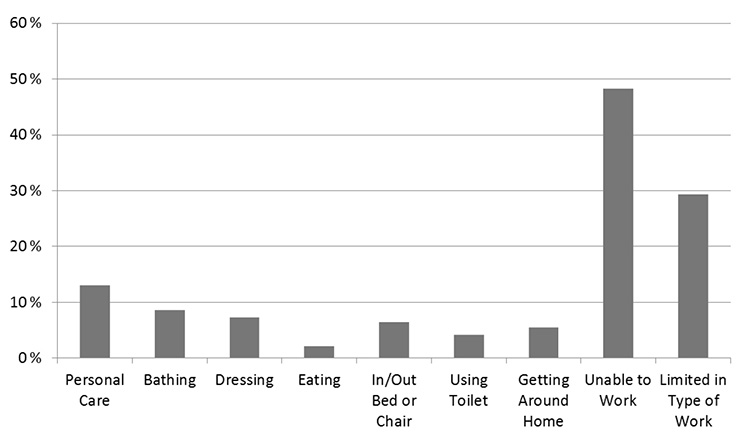
Arthritis and musculoskeletal diseases were, and continue to be, the leading cause of activity limitation across all age groups in the United States (Figure 1) [1,2,3]. Approximately 55.4 million adults in the United States have diagnosed arthritis [4]. Osteoarthritis is by far the most common type of arthritis and is one of the leading chronic diseases in the United States, affecting an estimated 30.8 million adults and nearly 50% of people by 85 years of age [4,5,6,7]. In addition, the prevalence of the condition is rapidly increasing; from 1997 to 2009, the prevalence increased 95% overall and 151% among individuals 45 to 64 years of age and continues to increase concurrent with the aging population and obesity epidemic [4,8]. By 2040, it is projected that 78 million individuals (26% of the United States population) will have diagnosed arthritis [6]. It is the leading cause of chronic disability in individuals older than 70 years [9]. This exponential rise is unique to osteoarthritis, as there have not been similar increases in the prevalence of other types of joint diseases [4,8].
Osteoarthritis exacts a cost in terms of pain, limited mobility, and decreased function among a wide range of individuals. Among working-age individuals, arthritis is a leading cause of activity limitation and absenteeism [7,10]. For the older population, osteoarthritis is associated with a significant decline in function and causes a higher rate of disability than any other chronic condition, including cardiovascular disease [11,12].
The toll of osteoarthritis on the healthcare system is also high. Arthritis (all types) is a leading reason for physician office visits, and hospitalizations for osteoarthritis increased nearly 70% between 2007 and 2018 [8,13]. It has been noted that the increase in hospitalizations is primarily related to higher rates of joint replacement; specifically, a significant increase in knee and hip replacement surgery [1,14]. An estimated 704,000 hospitalizations in 2012 were due to osteoarthritis-related knee replacement surgery (compared with 416,000 in 2004), and an estimated 296,000 hospitalizations were for osteoarthritis-related first-time hip replacement in 2012 (compared with 172,000 in 2003) [15]. Osteoarthritis is also a substantial economic burden; according to the Medical Expenditure Panel Survey for the years 1996–2005, osteoarthritis raised aggregate annual medical care expenditures by $185.5 billion ($149.4 billion in insurer expenditures and $36.1 billion in out-of-pocket expenditures) [16,17]. Data from the Healthcare Cost and Utilization Project (HCUP) indicate that osteoarthritis was the second most expensive condition billed to Medicare ($11.3 billion) and first most expensive billed to private insurance ($4.6 billion) in 2017 [18]. For total knee arthroplasty alone, Medicare was billed $3.5 billion, the program's largest expenditure for a single procedure [4]. Between 2008 and 2011, earning losses due to osteoarthritis cost an estimated $80 billion per year. A 2012 study showed that osteoarthritis was the most frequent cause of work loss, affecting more than 20 million individuals and costing the U.S. economy more than $100 billion annually [4].The burden of osteoarthritis is expected to increase as the population grows older and lives longer, especially given the high rate of obesity [1,19].
The high prevalence of osteoarthritis and its substantial burden at both the individual and healthcare system levels demand that clinicians have sound knowledge and clinical skills in diagnosing and managing the disease. However, several studies have shown that medical education in musculoskeletal disorders is inadequate, and competency examinations and surveys have shown that medical students and residents lack the necessary knowledge and clinical confidence in this field [20,21,22,23,24]. As a result, the Association of American Medical Schools has made recommendations for improving the undergraduate medical school curriculum on musculoskeletal diseases [25]. Inadequate education and training in musculoskeletal diseases has left many primary care physicians—often the first ones to evaluate individuals with signs and symptoms of osteoarthritis—feeling ill-equipped to manage the disease [22,26,27]. This course is designed to help fill this substantial educational gap by providing an overview of the prevalence and natural history of osteoarthritis, details on risk factors for the disease, and a discussion of the evidence base for a wide range of medical treatment options. Because surgical treatment options are not within the purview of primary care physicians, these options will be addressed briefly. The primary focus of this course is osteoarthritis of the knee, hip, and hand, as disease at these joints has the greatest clinical impact and is associated with the greatest public health burden [1,19]. In addition, most of the literature on osteoarthritis focuses on these joints. Osteoarthritis of other joints—primarily the shoulder, elbow, and ankle—is discussed as appropriate.
As noted, osteoarthritis develops most frequently in the knee, hip, and hand. Although pain in the lower back and the neck are the most frequently occurring musculoskeletal conditions and are the leading cause of functional limitation and work absences, the etiology of back and neck pain is often unclear, with many cases involving muscles and ligaments rather than osteoarthritic changes [5,28,29].
Osteoarthritis is classified as primary or secondary. The cause of primary osteoarthritis is idiopathic; no abnormality is the cause of changes in the joint [9]. Secondary osteoarthritis is the result of a known cause, most often trauma/injury or systemic diseases. Secondary osteoarthritis is most often found in the shoulder, elbow, and ankle and is more likely to become clinically apparent at a younger age than primary osteoarthritis [9,30,31,32]. A population-based study showed that secondary osteoarthritis related to trauma accounts for approximately 12% of the overall prevalence of symptomatic osteoarthritis of the knee, hip, or ankle [33]. Injuries sustained in sports activities comprise a large portion of post-traumatic osteoarthritis [34]. A wide variety of systemic diseases have been identified as frequent causes of secondary osteoarthritis; these conditions include metabolic diseases, endocrine disorders, bone dysplasias, and crystal deposition diseases (Table 1) [9,35].
SYSTEMIC CONDITIONS ASSOCIATED WITH SECONDARY OSTEOARTHRITIS
| Disease | Joint Affected |
|---|---|
| Metabolic Diseases | |
| Hemochromatosis | Knee, hip, ankle |
| Gaucher disease | Knee, hip |
| Hemoglobinopathies (e.g., sickle cell disease and thalassemia) | Knee, hip |
| Wilson disease (hepatolenticular degeneration) | Knee, hip |
| Ochronosis | Knee, hip |
| Ehlers-Danlos syndrome (and other joint hypermobility) | Knee, hip |
| Avascular necrosis | Hip, ankle |
| Endocrine Diseases | |
| Acromegaly | Knee, hip |
| Hypothyroidism (severe stages) | Knee, hip |
| Hyperparathyroidism | Knee, hip |
| Bone Dysplasias | |
| Multiple epiphyseal dysplasia | Knee, hip |
| Spondyloepiphyseal dysplasia | Knee, hip |
| Progressive hereditary arthro-ophthalmopathy (Stickler syndrome) | Knee, hip |
| Osteo-onychodystrophy (nail-patella syndrome) | Knee, hip |
| Epiphyses-related conditions | Knee, hip |
| Osteochondritis dissecans | Elbow, ankle |
| Calcium Crystal Deposition Diseases | |
| Calcium pyrophosphate deposition disease | Knee, hip, MCP joint (especially middle and index fingers) |
| Apatite crystal deposition disease | Knee, hip |
| Gout | Hip |
| Other Systemic Diseases | |
| Neuropathic arthropathy (Charcot joints) | Knee, hip |
| Paget disease (osteitis deformans) | Knee, hip |
| Osteopetrosis | Knee, hip |
| Chondrocalcinosis | Hip |
| MCP = metacarpophalangeal. | |
Research has shown that the symptoms of osteoarthritis do not correlate well with its radiographic evidence [19,39,40,41]. According to a systematic literature review, radiographic evidence of osteoarthritis is found in 15% to 76% of individuals with pain, and 15% to 81% of individuals with radiographic evidence of disease have pain [39]. An estimated 40% of individuals with structural changes on radiographs are asymptomatic [39,40]. In addition, many individuals have joint-related symptoms and no radiographic evidence [5,9]. As a result of this discordance, the disease is defined as either radiographic (evidence on imaging studies) or symptomatic (frequent pain in a joint plus radiographic evidence of osteoarthritis in that joint) [42]. Total joint replacement is used as a surrogate measure of symptomatic end-stage osteoarthritis, as the procedure is the option chosen when nonoperative measures have failed to manage pain and improve function and mobility.
Some large-scale, population-based studies have been used to determine the prevalence of osteoarthritis overall and within demographic subgroups (by age, gender, and race/ethnicity) and according to joint site. Among the most-often cited sources are the Framingham Osteoarthritis Study and the Johnston County Osteoarthritis Project. The Framingham Osteoarthritis Study involved a cohort of approximately 2,400 adults (26 years of age and older) from the Framingham Heart Study, and osteoarthritis of the knee and hand were evaluated [43,44]. The Johnston County Osteoarthritis Project was designed to compare the prevalence of knee and hip osteoarthritis in approximately 3,000 White and Black men and women (45 years of age and older) in a rural county in North Carolina [45,46].
In addition, information on the prevalence of osteoarthritis has been gathered through several national surveys, such as the National Health Interview Survey (NHIS), the National Health and Nutrition Examination Survey (NHANES), the National Hospital Discharge Survey, and the Ambulatory Care Survey. The NHIS is conducted among a cross-section of adults (18 years of age and older) each year. NHANES involves a nationally representative sample of about 5,000 persons each year who are interviewed and physically examined. The National Hospital Discharge Survey and the Ambulatory Care Survey capture the number of specific diagnoses for inpatient stays and outpatient visits, respectively.
Determining the prevalence of osteoarthritis is challenging for several reasons. First, few epidemiologic data are available for specific types of arthritis or joint-specific osteoarthritis, and the questions in surveys such as NHIS and NHANES refer to a single category of arthritis. Given that osteoarthritis has been shown to represent an overwhelming proportion of all types of arthritis, it seems reasonable to expect that osteoarthritis would account for most of the data gathered in a broad "arthritis" category [5]. In addition, although survey questions specifically refer to "doctor-diagnosed" arthritis, survey data have limitations, as they represent self-reports of the disease. Further complicating the situation are the differences across studies in how osteoarthritis is defined—radiographic or symptomatic—and in how radiographic changes are defined—mild or moderate/severe. Also problematic is the lack of correlation between radiographic evidence of osteoarthritis and symptoms and the high number of individuals who do not seek medical care for joint-related symptoms.
Several studies point to a high—and increasing—prevalence of arthritis. Data from the 2013–2015 NHIS showed a prevalence of doctor-diagnosed arthritis of 22.7% in adults, a rate similar to the 21% reported in a later analysis of combined data from the 2002, 2003, and 2006 NHIS [6,47,48]. These rates represent a substantial increase over previous decades; according to the 1971–1975 NHANES (NHANES I), the prevalence of osteoarthritis was approximately 12% among adults [49]. In the NHIS, the prevalence of arthritis varied substantially with age, ranging from 13.8% for those 18 to 44 years of age to 42.7% for those 65 years of age and older [50].
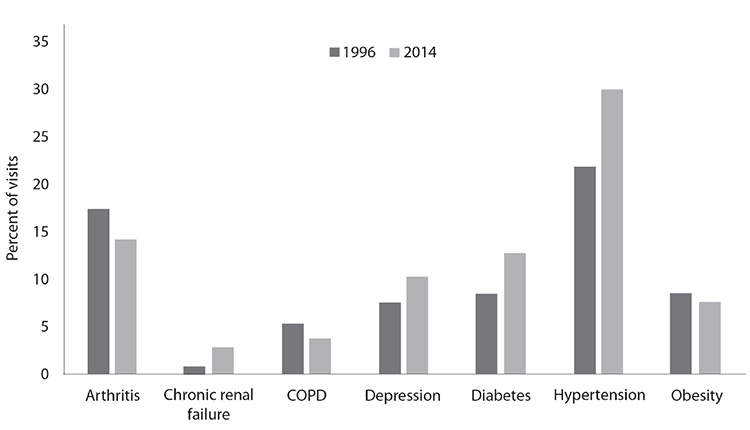
Data on hospitalizations indicate an increase in the prevalence of arthritis. The number of hospital stays with a principal diagnosis of arthritis increased from 921,000 in 2009 to 1.1 million in 2018 [8,51]. Osteoarthritis moved from the sixth leading principal diagnosis in 1990 to the second leading diagnosis in 2018 [1,51,52]. Although the number of physician office visits for arthritis decreased slightly from 1996 to 2014, arthritis was the third-leading chronic condition diagnosis for visits in 2018, accounting for 11.5% of all adult (18 years of age and older) visits (Figure 2) [53].
Data show that the prevalence of arthritis (and osteoarthritis specifically) can differ substantially according to age, gender, and race/ethnicity.
The prevalence of all types of arthritis increases with age. According to a CDC analysis of data from the 2016–2018 NHIS, the prevalence was 7.1% for individuals 18 to 44 years of age, 30.5% for individuals 45 to 64 years of age, and 50.4% for individuals 65 years of age and older [50].
The prevalence of osteoarthritis, specifically, also increases according to age, with the highest prevalence among those 65 years of age and older [50]. (The lower rate of hospitalization for osteoarthritis among individuals 85 years of age and older is more a reflection of lower rates of arthroplasty than of actual frequency of osteoarthritis.) The increases in osteoarthritis over time follow the same age-related pattern. Between 1997 and 2009, the prevalence of osteoarthritis increased 151% among individuals 45 to 64 years of age and 58% among individuals 65 to 84 years of age [8]. Between 2009 and 2013, the prevalence of osteoarthritis increased 42% among individuals 45 to 64 years of age and 25% among individuals 65 to 84 years of age [8,54].
The increased prevalence of radiographic and symptomatic osteoarthritis among older individuals is found across all joints. In the Nurses' Health Study, the risk of hip replacement for women 70 years of age or older was nine times greater than for women younger than 55 years of age [54]. Similarly, in the NHANES III, the prevalence of radiographic knee osteoarthritis increased with age, from a low of 17.7% for the 60 to 64-year age-group to 26.0% for the 80 years and older age-group [55]. The prevalence of hand osteoarthritis also increases significantly with age, and a review of the literature (1950–2009) demonstrated that the prevalence can reach 80% in the older population [56,57].
Data on the age at the time of diagnosis of osteoarthritis at other joints are limited. However, studies have indicated a younger age at the time of clinical presentation of elbow osteoarthritis (approximately 50 years) and ankle osteoarthritis (43 to 58 years) [32,58].
The overall prevalence of arthritis (all types) has consistently been higher among women than men [50]. According to a CDC analysis of NHIS data from 2016–2018, the prevalence of arthritis was approximately 24.2% for women compared with 18.5% for men [50]. With respect to osteoarthritis specifically, women accounted for approximately 59% of hospitalizations for osteoarthritis in 2013, a proportion that has been essentially the same since 1997 [59,60]. One exception to this female predominance relates to age; within the population of individuals younger than 50 years of age, osteoarthritis is more common in men, a difference that has been attributed to a higher rate of osteoarthritis secondary to joint injury [61]. Because osteoarthritis is overall more prevalent in women and women use healthcare resources to a greater degree than men, the economic burden of osteoarthritis is disproportionately high among women. The total expenditures related to osteoarthritis among women account for nearly two-thirds of the increased cost, or $118 billion [17].
Studies have also provided information regarding gender differences in the prevalence of osteoarthritis according to the affected joint. These studies have shown that symptomatic knee, hip, and hand osteoarthritis are more prevalent among women than among men, with the greatest difference related to knee osteoarthritis (Table 2) [45,46,56,62]. Again, there is one exception to female predominance: osteoarthritis of the elbow, which has a male-to-female ratio of approximately 4:1 [58]. This gender difference is likely due to the predominance of elbow osteoarthritis among individuals who have an occupation involving strenuous manual labor [58]. Information on gender differences in osteoarthritis at other joint sites is lacking.
COMPARISON OF JOINT-SPECIFIC OSTEOARTHRITIS IN MEN AND WOMENa
| Joint | Radiographic Osteoarthritisb | Symptomatic Osteoarthritis | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall | Women | Men | Overall | Women | Men | |||
| Knee | 0.9% | 1.2% | 0.4% | 12.1% | 13.6% | 10.0% | ||
| Hip | 2.5% | 2.5% | 2.6% | 9.7% | 11.1% | 8.3% | ||
| Hand | 7.3% | 9.5% | 4.8% | 8.0% | 8.9% | 6.7% | ||
| ||||||||
Knee
Osteoarthritis of the knee is estimated to account for 83% of the total number of osteoarthritis cases [2]. Using data from NHANES III, Dillon et al. found that symptomatic radiographic knee osteoarthritis did not differ by gender but that the prevalence of asymptomatic radiographic osteoarthritis was greater among women (42% vs. 31%) [62]. In addition, there were significantly more moderate-to-severe osteoarthritic changes among women (13% vs. 17%) [62]. In the Johnston County Osteoarthritis Project, symptoms, radiographic knee osteoarthritis (mild and moderate-to-severe), and symptomatic knee osteoarthritis were all more prevalent among women than men [45]. Data from the Global Burden of Disease Study 2010 found that the prevalence of knee osteoarthritis in women is nearly twice that of men worldwide [63]. Results of a 2020 study indicate that the worldwide prevalence of knee osteoarthritis in women is more than two-thirds higher than that of men [64].
Hip
In the Johnston County Osteoarthritis study, hip symptoms, mild radiographic osteoarthritis, and symptomatic osteoarthritis were more prevalent among women than men. However, the prevalence of moderate-to-severe radiographic osteoarthritis was similar (2.6% for men vs. 2.5% for women) [46].
Hand
The data on gender differences for osteoarthritis of the hand have been conflicting. Of 1,041 men and women (71 to 100 years of age), the prevalence of symptomatic hand osteoarthritis was twice as high among women in the Framingham Osteoarthritis Study (26% vs. 13%), but NHANES III data showed that the prevalence of symptomatic hand osteoarthritis was similar among men and women [44,56]. The difference may be due to the older age of individuals in the Framingham study, as the prevalence of hand osteoarthritis increases significantly with age [56]. A review of the literature (1950–2009) supports a gender difference in the prevalence of osteoarthritis of the hand [57].
Data from 2016–2018 NHIS showed a higher prevalence of arthritis (all types) in the non-Hispanic White population (23.2%) compared with the non-Hispanic Black (21.8%), Hispanic (16.4%), and Asian/Pacific Islander populations (12.2%) [50]. In contrast, the prevalence was higher for the American Indian/Alaska Native population (26.8%) [50].
Knee
Studies have consistently shown that osteoarthritis of the knee is more prevalent in the Black population than the White population. Multivariable analysis of data from NHANES III showed significantly higher odds of radiographic knee osteoarthritis (Kellgren-Lawrence grade 2 or higher) among non-Hispanic Black participants (52%) compared with White (36%) or Mexican American (38%) participants [62,65]. Although the findings of the Johnston County Osteoarthritis Project also demonstrated that knee-related symptoms, radiographic knee osteoarthritis (mild), and symptomatic knee osteoarthritis were all more prevalent among Black individuals than White individuals, the difference was slight. However, the prevalence of moderate-to-severe radiographic osteoarthritis was significantly greater for both men and women in the Black population (11% vs. 5% for Black vs. White men and 16% vs. 8% for Black vs. White women) [45]. A study of more than 1,000 premenopausal and perimenopausal women demonstrated that early osteoarthritis changes were more prevalent in Black women than White women (23% vs. 9%) [66]. The prevalence of knee osteoarthritis has also been found to be higher in the Chinese population than in the White population [67].
Hip
In the Johnston County Osteoarthritis Project, the greatest racial/ethnic difference was found for mild radiographic hip osteoarthritis among men (23.8% vs. 33.2% for White vs. Black men) [46]. There were also racial differences among men and women for symptomatic hip osteoarthritis (7.6% vs. 11.7% for White vs. Black men, and 10.8% vs. 12.2% for White vs. Black women) [46]. Among women, the prevalence of moderate-to-severe radiographic hip osteoarthritis was higher for the Black population (2.3% vs. 3.5%) [46]. A subsequent study indicated that the radiographic features and patterns of hip osteoarthritis differed according to race and gender, which suggests that anatomic and/or development variations in the joint may contribute to differences [68]. Hip osteoarthritis has been found to be less prevalent among Chinese individuals than among White individuals [67].
Hand
In a study of more than 1,000 younger women (premenopausal and perimenopausal), the prevalence of hand osteoarthritis was higher among Black women (26%) than among White women (19%), and the specific hand joints affected differed between the two groups [66]. However, NHANES III data indicated that symptomatic hand osteoarthritis occurred less frequently among non-Hispanic Black individuals than White individuals [56]. Research has also indicated that hand osteoarthritis is less common in the Chinese population than in the White population [67,68].
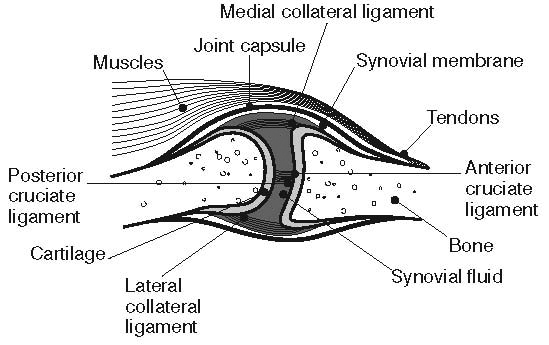
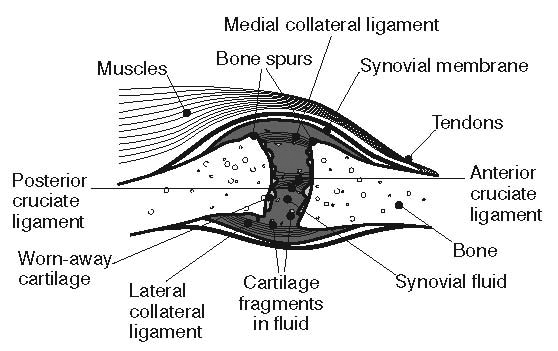
Historically, osteoarthritis has been considered to be a disease of articular cartilage, but research has indicated that the condition involves the entire joint organ [9,69,70]. The loss of articular cartilage has been thought to be the primary change, but a combination of cellular changes and biomechanical stresses causes several secondary changes, including subchondral bone remodeling; the formation of osteophytes; the development of bone marrow lesions; changes in the synovium, joint capsule, ligaments, and periarticular muscles; and meniscal tears and extrusion (Figure 3 and Figure 4) [19,71,72,73,74]. These changes lead to structural and functional changes in the joint, causing pain, disability, and psychologic distress [70].
Normal adult articular cartilage is made up of extracellular matrix (approximately 98% to 99%) and chondrocytes (1% to 2%) [75]. The chondrocytes secrete enzymes and cytokines that help regulate the normal cycle of degradation and repair of articular cartilage by inhibiting the production of proteoglycans and collagen, the two major components of the extracellular matrix [75]. Damage to the extracellular matrix interferes with its ability to bind or exclude water, resulting in edema and subsequent softening of the cartilage and expansion of the matrix, which makes the matrix vulnerable to further injury and breakdown of its components [9,76,77,78].
Among the enzymes stimulated by chondrocytes are matrix metalloproteinases (e.g., collagenase, stromelysin, and gelatinase) and other proteinases (e.g., cathepsin and tissue plasminogen activator). Interleukin-1 (IL-1) is the cytokine that has been identified as playing an important role in promoting the synthesis of degradative enzymes, and tumor necrosis factor-alpha and IL-6 have been found to work synergistically with IL-1. Inhibitors of these enzymes and cytokines, such as tissue inhibitor of metalloproteinase (TIMP) and plasminogen activator inhibitor-7 (PAI-7), help stimulate a repair process by keeping degradation in check. In addition, polypeptides, such as insulin-like growth factor-1 (IGF-1) and transforming growth factor-beta (TGF-beta), stimulate chondrocytes to synthesize proteoglycans. When chondrocyte function is lost, the balance between degradation and repair is lost, resulting in damage to the articular cartilage [79].
There are some indications that the early structural changes of osteoarthritis (such as bone marrow lesions and cartilage defects) may be reversible, especially among younger individuals [72,74]. However, it is difficult to detect early changes, given the high percentage of individuals who are asymptomatic during the early development of osteoarthritis [70]. Still, the potential reversibility sets up early changes as a target for disease-modifying interventions, and research is being directed in this area.
As damage occurs to the articular cartilage, fragments of cartilage may break off and enter into the joint capsule, where they can damage the synovial lining of the joint and interfere with proper joint function. Continued erosion of cartilage results in narrowing of the joint space, with the potential for bone-to-bone contact. Eburnation, or the formation of a new articulating surface from subchondral bone, may occur. Bone remodeling may also occur in the subchondral bone, which may cause overgrowth of bone at the edges of the joint. These osteophytes usually develop in the nonweight-bearing area of a joint. In osteoarthritis of the distal interphalangeal joints, these osteophytes are dorsolateral swellings referred to as Heberden's nodes [36].
The lack of clarity about the etiology of osteoarthritis is further complicated by the terminology used to refer to the disease. The term "osteoarthritis" implies an inflammatory process, but inflammation is not a hallmark characteristic of the disease; if inflammation is involved, it is usually mild and affects only the synovium and periarticular tissues [79]. Alternative terms that have been suggested include "osteoarthrosis" and "degenerative joint disease," but neither term is completely satisfactory. The former is vague, and although the latter term is more accurate, it implies a process that naturally occurs with aging, and many differences between the osteoarthritic joint and the aging joint have been identified (Table 3) [9,72].
DIFFERENCES BETWEEN OSTEOARTHRITIC JOINTS AND AGING JOINTS
| Feature | Osteoarthritic Joint | Aging Joint |
|---|---|---|
| Fibrillation in cartilage | Primarily weight-bearing joints | Nonweight-bearing joints |
| Cartilage mass | Hypertrophy, erosion | No change |
| Water content of cartilage | Edema (early stage) | No change or dehydration |
| Cell activity | Increased activity and proliferation | Reduced |
| Synovium | Mild focal superficial inflammation | Atrophy |
| Bone changes | Subchondral bone remodeling | Osteopenia |
The definition and natural history of osteoarthritis continues to evolve as research provides new information. Some researchers have now posited that an inflammatory process is present during the early development of osteoarthritis, with a suggestion that osteoarthritis has a biochemical and inflammatory profile similar to that of metabolic syndrome [74,80]. Another study providing evidence of a different natural history of osteoarthritis indicated that structural changes precede articular damage. In that study, the results of magnetic resonance imaging (MRI) of healthy knees and knees with early osteoarthritis suggested that such changes as subchondral bone expansion, bone marrow lesions, and meniscal tears and extrusion lead to defects in the articular cartilage, which may or may not subsequently result in loss of articular cartilage and radiographic evidence of osteoarthritis [74].
The cause of osteoarthritis-related pain is not well understood. Because articular cartilage is aneural and avascular, degradation of cartilage, a primary characteristic of osteoarthritis, is not likely to be the direct source of pain, stiffness, or other typical symptoms [70]. The probable sources of pain, therefore, are other tissues in the joint structure that are richly innervated, such as the subchondral bone, periosteum, periarticular ligaments, periarticular muscle, synovium, and joint capsule [9,70]. Pain is most likely generated by several factors, and the predominant source of pain has been unclear, as the severity of osteoarthritis on radiographs does not correspond to the degree of pain [70]. However, the improved imaging of the joint provided by MRI has allowed researchers to explore the source of osteoarthritis-related pain, and studies have shown that bone marrow lesions, synovitis/effusion, subarticular bone attrition, osteophytes in the patellofemoral compartment, and meniscal tears are strongly associated with severity of pain in knee osteoarthritis [9,41,81,82,83]. The evidence has been strongest for bone marrow lesions and synovitis, and the association is greater for pain on weight-bearing (compared with nonweight-bearing) joints [83]. Psychologic and social factors also play an important role in osteoarthritis-related pain [9,70].
There is substantial heterogeneity in osteoarthritis across anatomic sites with regard to risk factors, clinical features, and outcomes, which has drawn some researchers to conclude that osteoarthritis of different joints are distinct clinical entities [84,85]. Some examples to support the concept of distinct disease entities include [31,32,36,86]:
Primary osteoarthritis of the knee is more common than secondary osteoarthritis, but primary osteoarthritis of the ankle is rare, with the disease at that joint occurring more often after trauma (e.g., fracture or ligamentous injury).
Overweight/obesity has been identified as the most common risk factor with knee osteoarthritis, but mechanical overuse is the primary predisposing factor for hand osteoarthritis.
Erosion of articular cartilage and narrowing of the joint space are hallmark characteristics of knee and hip osteoarthritis, but articular cartilage is relatively preserved. There is no joint space narrowing in primary osteoarthritis of the elbow.
Osteoarthritis of more than one joint may be a distinct disease in which a genetic predisposition plays a more important role than biomechanical factors [84].
The risk factors for osteoarthritis include several modifiable as well as nonmodifiable factors (Table 4) [9,30,32,36,37,38,57,87,88,89]. Secondary osteoarthritis can also develop as a result of a systemic disease, as noted earlier [70]. Some of the same risk factors for the development of osteoarthritis are also factors that have been noted to increase the risk of disease progression.
RISK FACTORS FOR OSTEOARTHRITIS
| Risk Factor | Joint | ||
|---|---|---|---|
| Nonmodifiable | |||
| Age | Knee, hip, hand | ||
| Gender | Knee, hip, hand (women); elbow, cervical spine (men) | ||
| Race/ethnicity | Knee, hip, hand | ||
| Genetic predisposition | Knee, hip, hand | ||
| Modifiable | |||
| Overweight/Obesity | Knee, hip, hand, shoulder | ||
| Previous trauma, joint injury | Ankle, glenohumeral joint, knee, hip, hand, wrist | ||
| High-impact sports | Knee, hip | ||
| Occupational activities |
| ||
| Other | |||
| Muscle weakness | Knee | ||
| Malalignment | Knee, hip, ankle | ||
| Bone density (high) | Knee, hip, hand | ||
| Vitamin C and D deficiency | Knee, hip | ||
| Estrogen deficiency | Knee, hip | ||
| Developmental deformities | Hip, glenohumeral joint, ankle | ||
| Joint laxity | Knee, hip, hand | ||
| Repeated episodes of gout or septic arthritis, or infection | Knee, hip, glenohumeral joint (infection) | ||
As discussed, age, gender, and race/ethnicity influence the development of osteoarthritis at many joint sites. Genetic predisposition is another nonmodifiable risk factor. Among the modifiable risk factors, the greatest contributor to development of the disease is overweight/obesity. Previous trauma/joint injury and specific sporting or occupational activities are other important risk factors. The potential contribution of many other factors is still being explored.
Studies have indicated that there may be a genetic factor to the development of osteoarthritis, and the familial risk factor for osteoarthritis of the knee, hip, and hand has ranged from 27% to 60% [35,57,84]. It is thought that most genes related to osteoarthritis affect the development of the disease at any joint but that specific genes may also be involved at specific joints [35,84]. Over the past several years, a candidate gene study and several genome-wide association studies have collectively established 15 loci associated with knee or hip osteoarthritis that have been replicated with genome-wide significance, providing further evidence of joint-specific effects in osteoarthritis [19,84,85,90,91,92,93,94]. In 2019, researchers performed a genome-wide association study with more than 77,000 participants and identified 64 loci, 52 of them being novel. Of these 64 loci, therapeutics are currently available or in clinical trials for 10 of the effector genes, making them a future prospect for effective treatment of osteoporosis [95]. Despite the increased reports of potential risk loci for osteoarthritis, some research indicates that epigenetic changes may have a role in the pathogenesis of osteoarthritis [96].
Clinical studies have long demonstrated that the risk of osteoarthritis is higher for individuals who are overweight or obese, and obesity has been referred to as the most important modifiable risk factor for severe osteoarthritis of the knee and, to a lesser extent, of the hips [9,97,98,99]. In a meta-analysis, those who were obese or overweight were nearly three times as likely to report osteoarthritis of the knee [100]. Overweight as a risk factor is thought to be related to the increased load on weight-bearing joints; however, some studies have indicated an association between obesity and osteoarthritis of the hand and shoulder, which suggests factors other than joint overload [30,36,57]. Factors that have been proposed are a metabolic intermediary (such as diabetes or lipid abnormalities) or an increased production of humoral factors (produced by excess adipose tissue), which alters the metabolism of articular cartilage [9,101].
The data on osteoarthritis and overweight have been more consistent for osteoarthritis of the knee than for disease at other joint sites, and most studies have indicated that overweight/obesity is a greater risk factor for women [38,84,87,97,101,102,103,104,105]. In the Framingham Osteoarthritis Study, there was more than a 50% decrease in the risk among women who had a loss of approximately 11 pounds or a decrease in body mass index (BMI) of 2 or more [97]. Weight gain was also associated with an increased risk for osteoarthritis, but the difference was not significant [97]. In a population-based case-control study in England (525 men and women [45 years of age and older] with primary knee osteoarthritis and 525 matched controls), the risk of osteoarthritis increased progressively with higher BMI; compared with a BMI of 24.0–24.9, the risk was 0.1 for a BMI of less than 20 and 13.6 for a BMI of 36 or greater [99].
The results of a large, prospective population-based cohort study (28,449 subjects; 17,203 women and 11,246 men) in Sweden indicated that all measures of overweight (BMI, waist circumference, waist-hip ratio, and percentage body fat) were significantly associated with a higher incidence of osteoarthritis of the knee in both men and women [103]. Across studies, the relative risk of osteoarthritis of the knee and hip has been 2 to 10 times higher for the BMI in the top quartile compared with BMI in the lowest quartile, with the risk typically higher for knee osteoarthritis than hip osteoarthritis and for women compared with men [54,103,106,107,108]. Among men, the risk for knee and hip osteoarthritis has increased with a higher BMI, even within the normal range [109]. In addition, the risk for osteoarthritis of the hip has been greater for individuals who had a high BMI beginning at a younger age [54,106].
There is no evidence that routine, moderate exercise or leisure recreational activity increases the risk of osteoarthritis of the knee or hip [54,84]. In a systematic review of 72 studies, a high level of physical activity was not a risk factor for osteoarthritis of the knee or hip, provided that the activity did not cause pain in the joint or predispose to trauma [110]. However, the risk for osteoarthritis appears to be associated with increasing intensity and/or duration of the activities, and there is moderate-to-strong evidence of an increased risk of osteoarthritis of the knee and hip with high-intensity, high-impact sports activities, especially when individuals are involved in such activities before the age of 50 years [87,110,111]. The risk of osteoarthritis of the hip and knee also has been found to be greater among individuals who participate at an elite level in sports that involve high joint loads. Overall, the risk associated with high-intensity sports is not as great as that associated with overweight or trauma [110]. With respect to other joints, the risk of osteoarthritis of the elbow has been increased after weight-lifting and throwing activities, the risk of osteoarthritis of the shoulder has been increased in association with overhead sports activities, and the risk of osteoarthritis of the spine has been higher after participation in wrestling, gymnastics, tennis, and weight-lifting [30,31,34].
Many researchers have theorized that injury is a stronger risk factor than sports participation itself, especially when participation continues after injury to a joint or cartilage [35,110]. One systematic review evaluated studies that included injury, sport/physical activity, overweight/obesity, and/or occupational activity as risk factors; outcomes included osteoarthritis of the hip, knee, and/or ankle [112]. Joint injury, obesity, and occupational activity were all associated with an increased risk of osteoarthritis of the knee and hip, with joint injury identified as a significant risk factor for both knee and hip osteoarthritis. Meniscal tears and injury to a cruciate ligament have been shown to be risk factors for osteoarthritis of the knee, chronic rotator cuff tear is a risk factor for osteoarthritis of the glenohumeral joint, and injury to the ankle ligaments increases the risk for osteoarthritis of the ankle in the long-term (more than 25 years) [30,34,112,113,114].
The prevalence of osteoarthritis has been shown to be higher among individuals in occupations involving repetitive tasks that place a high load on a joint and cause fatigue in the muscles that protect the joint, although the precise nature of the biomechanical stresses that lead to osteoarthritis are unclear [84,87,110,115]. Occupations that have been associated with high rates of osteoarthritis are manual labor/construction work (knee, hip, elbow, and shoulder), farming (hip), and housekeeping/housecleaning and clothing industry (hand) [30,31,36,57,62,84,115,116,117]. Specific occupational actions/activities that have been identified as risk factors for osteoarthritis of the hip or knee include heavy lifting (55 pounds or more), kneeling, squatting, walking more than 2 miles per day, climbing, jumping, and unnatural body positions [110,115]. Occupations associated with increased risk of osteoarthritis of the hip in men include working in agriculture (including fishery, forestry, and food production), which doubles the risk. Construction, metal working, and sales as well as exposure to whole-body vibration (e.g., while driving vehicles) has been shown to increase the risk by approximately 50% to 60% [118]. Obese workers with such exposures are at additional risk of osteoarthritis of the knee [115]. Some studies have indicated that occupational workload is a more significant factor for osteoarthritis of the knee than for osteoarthritis of the hip, but little research has been conducted among female workers [119,120]. One nationwide register-based follow-up study that included women found that construction, farming, and healthcare work (compared to office work) increases the risk of osteoarthritis of the hip and knee in both men and women, with farmers having the highest risk of osteoarthritis of the hip and construction and healthcare workers having the highest risk of osteoarthritis of the knee. The risk estimates were generally higher for men, with an exception for construction work, in which the risk estimates of osteoarthritis of the knee were similar or slightly higher for women [120].
One systematic review (25 studies) found moderate evidence for a relationship between kneeling, heavy lifting, and knee osteoarthritis; a limited number of studies indicated that the association was stronger for the combination of kneeling/squatting and heavy lifting than for kneeling/squatting or heavy lifting alone [121]. Two studies examined the interaction of obesity with kneeling/squatting and lifting [122,123]. In both studies, squatting/kneeling and high BMI carried independent risk of knee osteoarthritis, but their combination raised the risk 5- to 15-fold. In addition, limited data indicated a relationship between climbing stairs or ladders and an increased risk for knee osteoarthritis [121]. Although most studies of occupational risk for osteoarthritis have been conducted with men, some have shown similar results among women [35].
Muscle weakness as a risk factor has been primarily studied in the setting of knee osteoarthritis. Weakness of the quadriceps muscle has been found frequently among individuals with knee osteoarthritis, but it was thought to be the result of atrophy that developed as the individual tried to minimize pain in the joint [84,124]. However, studies have indicated that weakness of this muscle may actually be a risk factor, with the weak muscle unable to appropriately distribute load across the knee joint and maintain joint stability [125,126]. Such dysfunction may actually precede and expedite cartilage deterioration [127].
In individuals with osteoarthritis of the knee, quadriceps strength is an important determinant of physical function [128]. Reduced strength of the quadriceps muscle as a risk factor has been found to be more common among women, especially in relation to higher body weight, and to be related to symptomatic osteoarthritis and not radiographic evidence of osteoarthritis [125,126,129,130,131]. Weakness of the hamstring muscle has not been found to increase the risk of osteoarthritis of the knee. However, individuals with osteoarthritis of the knee have well-documented hamstrings strength deficits [126,129,132,133,134].
Several other risk factors have been identified as potential contributors to the development of osteoarthritis. Among these are malalignment, bone density, vitamin C and D deficiency, and estrogen deficiency. Additional research is needed to determine the effect of these factors on the development of disease.
Poor bone alignment resulting from developmental abnormalities or injury changes the load distribution on a joint [84]. The resultant increase in compressive loading in an area of the joint can increase the risk of osteoarthritis [84]. For example, genu varum (bow-leggedness) and genu valgum ("knock-kneed") have been shown to increase the risk of osteoarthritis at the medial and lateral compartment of the knee, respectively [135,136]. However, study results have varied.
One evaluation of 110 knees with tibiofemoral osteoarthritis and 356 random control knees demonstrated that knee alignment was not associated with either radiographic tibial osteoarthritis or medial tibiofemoral osteoarthritis, and the authors suggested that malalignment was a marker of disease severity rather than a risk factor [137]. An observational, longitudinal study of the Multicenter Osteoarthritis Study cohort found that varus but not valgus alignment increased the risk of incident tibiofemoral osteoarthritis, and that both varus and valgus alignment increased the risk of disease progression in arthritic knees [138]. A third study of malalignment included 881 subjects from the Multicenter Osteoarthritis Study and 1,358 subjects from the Osteoarthritis Initiative study. The researchers found that all strata of malalignment increased the risk of progression of radiographic knee osteoarthritis and incidence as well as the risk of lateral cartilage damage [139]. Forefoot varus malalignment has been found to be related to a higher rate of hip osteoarthritis and hindfoot malalignment with a higher rate of ankle osteoarthritis [32,140].
Bone density is related to osteoarthritis, with a high bone mineral density found in association with an increased prevalence of knee, hip, and hand osteoarthritis [35,36,84,141,142,143,144]. Higher bone mineral density has also been reported in association with osteoarthritis of the spine [145,146]. The reason for the relationship is not clear, and some inconsistencies and areas of controversy remain [142]. Shared genetic factors and lifetime exposure to estrogen (exogenous and endogenous) have been suggested [35,142,147,148].
Deficiency of vitamin C or D has been targeted as a potential contributor to osteoarthritis because of its role in antioxidation or bone metabolism, respectively [84]. The literature on the role of vitamin deficiency in osteoarthritis is limited, but the findings of some early studies have indicated that low levels of vitamin C and D may be associated with early osteoarthritic changes [35,74]. For example, in the Framingham Osteoarthritis Study, the risk of radiographic osteoarthritis of the knee and knee pain were substantially lower among individuals in the highest tertile of vitamin C intake [149]. A study of the effect of dietary antioxidants, including vitamin C, found a significant positive association between dietary vitamin C intake and radiographic knee osteoarthritis [150]. Ascorbic acid has also been found to provide protection for human chondrocytes against oxidative stress that can lead to osteoarthritis and cartilage aging [151]. Vitamin D deficiency appears to be related to progression of osteoarthritis rather than initial development; this may be because the lack of vitamin D impairs the bone response to osteoarthritic changes [84]. Low levels of vitamin D were not related to the prevalence of osteoarthritis in the Framingham Osteoarthritis Study, but the risk for progression was three times higher for individuals in the lowest tertile of vitamin D level than for individuals in the highest tertile [152]. However, later studies found that vitamin D supplementation does not reduce knee pain or progression of osteoarthritis of the knee, though there may be an association between a low level of vitamin D and an increased risk of both new-onset hip osteoarthritis and its progression [153,154,155,156,157]. One study suggests that vitamin D deficiency exacerbates pain and dysfunction and results in a poorer quality of life in patients with knee osteoarthritis [158]. However, a subsequent study found no association between serum vitamin D concentration and knee pain in patients with osteoarthritis [159].
There is increasing evidence that estrogens fulfill an important role in maintaining the homeostasis of articular tissues and of the joint itself and that they may also have a protective role against the development of osteoarthritis [160]. The dramatic rise in the prevalence of osteoarthritis among postmenopausal women, which is associated with the presence of estrogen receptors in joint tissues, suggests a link between osteoarthritis and loss of ovarian function [161,162,163,164,165]. Numerous clinical studies have shown that osteoarthritis is related to estrogen levels, with a greater prevalence in women than men and a clear increase in women at menopause [161,162,166,167,168]. Additional research will help shed light on the role that estrogen deficiency plays in the mechanisms of menopause-induced osteoarthritis [160].
The diagnosis of osteoarthritis at most joints is made primarily on the basis of clinical findings, with imaging studies and laboratory tests more useful for ruling out other diagnoses rather than for confirming the diagnosis of osteoarthritis [40,79,169]. Although radiographic findings are considered to be diagnostic criteria for osteoarthritis, radiographs are not usually part of the initial diagnostic evaluation for several reasons. The primary reasons are the lack of evidence of early osteoarthritic changes on radiographs and the poor correlation between symptoms and radiographic evidence of osteoarthritis [19,39,40,41]. Thus, the absence of radiographic evidence of osteoarthritis in the presence of joint-related symptoms should not exclude the diagnosis of osteoarthritis.
However, radiographs are often included in the diagnostic evaluation and are essential to the diagnosis of osteoarthritis at some joints, such as the shoulder, elbow, and ankle [30,32,58]. Radiographic evidence of osteoarthritis is most commonly graded according to the Kellgren-Lawrence system, which uses a scale of 0 to 4 [65]:
0: No radiographic evidence of osteoarthritis
1: Possible small osteophytes and joint space narrowing, both of which are of doubtful clinical significance
2: Definite osteophytes and normal joint space (or possible narrowing)
3: Multiple moderate osteophytes, definite narrowing of the joint space, some sclerosis, possibility of deformity of the bone contour
4: Large osteophytes, severe narrowing of the joint space, severe sclerosis, definite deformity of the bone contour
Similarly, no abnormal laboratory findings are associated with osteoarthritis, but again, blood tests can help rule out other diseases or conditions [9,40]. For example, an erythrocyte sedimentation rate and/or rheumatoid factor titer can help determine a diagnosis of rheumatoid arthritis, and a complete blood count can be used to help rule out infection [30,79].
The differential diagnosis of osteoarthritis varies according to the anatomic site as well as such patient-related factors as age, gender, and history (Table 5) [30,32,38,40,170,171,172]. In general, the differential diagnosis includes infection, traumatic injuries, bursitis, other types of arthritis, and overuse syndromes [40]. In addition, clinicians should consider secondary osteoarthritis in patients who have metabolic bone disorders, endocrine diseases, and other systemic conditions, as described earlier [40]. Ancillary testing should be done for patients who have joint pain at night, who have progressive joint pain, or who have a strong family history of inflammatory arthritis [79]. Many features on clinical evaluation and imaging studies are characteristic of osteoarthritis, and some features differ according to joint site (Table 6) [30,31,38,58,171].
JOINT-SPECIFIC DIFFERENTIAL DIAGNOSIS FOR OSTEOARTHRITIS
| Joint | Potential Diagnoses | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Knee |
| |||||||||||
| Hip |
| |||||||||||
| Hand |
| |||||||||||
| Shoulder |
| |||||||||||
| Elbow |
| |||||||||||
| Ankle |
| |||||||||||
| MCPJ = metacarpophalangeal joint; PIPJ = proximal interphalangeal joint. | ||||||||||||
TYPICAL CHARACTERISTICS OF OSTEOARTHRITIS BY JOINT SITE
| Joint | Clinical Characteristics | Findings on Imaging Studies | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Knee |
|
| ||||||||||
| Hip |
|
| ||||||||||
| Hand |
|
| ||||||||||
| Glenohumeral Joint |
|
| ||||||||||
| Elbow |
|
| ||||||||||
| Ankle | History of trauma/injury to the joint |
|
The American College of Rheumatology (ACR) developed classification criteria for knee, hip, and hand osteoarthritis, and these have been widely accepted [171,174,175,176,177]. More recently, the European League Against Rheumatism (EULAR) has established evidence-based recommendations for diagnosis of the knee and hand [173,178]. Evidence-based criteria for classification of osteoarthritis at other joints are not available.
Regardless of the affected joint, pain is the most common presenting feature of osteoarthritis. Because many individuals with joint pain do not seek medical care specifically for the pain, clinicians should ask their patients about joint-related symptoms at all routine office visits and other healthcare encounters [179,180].
When obtaining a history, questions should focus on the nature of joint-related symptoms, patients' self-reports of limitations in function or activities, and information related to established risk factors for osteoarthritis. The following questions can help elicit important information needed for a diagnosis:
Do you have any joints that hurt? If so, how long have they been bothering you?
When does the pain occur? After certain physical activities? At rest?
Do you have relief of pain if you rest?
Does the pain bother you at night? Does pain wake you up at night?
Are your joints stiff when you wake up in the morning? If so, how long does the stiffness last?
Do the joints that hurt ever lock up or give out on you?
Do you have a family history of osteoarthritis or rheumatoid arthritis?
What types of recreational activities or sports do you participate in? If you play sports, do you do so for leisure or competitively?
What is your occupation? Are there tasks or activities that are part of your job that bother any joints?
Have you ever had an injury to a joint?
Are there daily activities or other tasks that you cannot do because of pain or other symptoms in any joint?
When considering patients' self-reports of pain and function, clinicians should understand that these self-reports can differ according to gender and race/ethnicity [48,181,182]. Self-reports of work or activity limitations or severe pain have been significantly more common among Black, Hispanic, and mixed-race individuals than among White individuals with osteoarthritis; the rate of self-reports for Asian/Pacific Islander and Alaska Native/American Indian populations have been similar to those for the White population [48]. Among participants in the Johnston County Osteoarthritis Project, total scores on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and scores on the pain and function subscales were significantly worse for Black individuals than for White individuals with knee osteoarthritis. The total WOMAC scores were similar for the two racial groups among individuals who had only hip osteoarthritis or hip and knee osteoarthritis [182]. The researchers hypothesized that high BMI and frequent depressive symptoms in the Black population may have contributed to the racial/ethnic differences.
Obtaining an accurate history necessitates effective patient-physician communication, which is challenging given the high number of people with inadequate language proficiency and/or health literacy [183,184]. Clinicians should ensure that patients understand history-related questions and should seek the help of a professional translator if necessary. (A more comprehensive discussion of patient-physician communication and literacy appears later in this course.)
The physical examination should include [9,19]:
Assessment of body weight and BMI
Palpation of joints for pain and/or tenderness
Evaluation of joints for signs of swelling, enlargement, or deformity
Determination of crepitus during joint movement
Range of motion in the joint
Determination of muscle strength and ligament stability
Additional evaluation may be necessary according to the joint causing symptoms.
The primary symptom of osteoarthritis of the knee is pain, especially with weight-bearing exercise or activity, that improves with rest. Stiffness in the joint occurs in the morning, lasting 30 minutes or less, and may occur after periods of inactivity [185].
Individuals with osteoarthritis of the knee usually have tenderness on joint palpation, osseous enlargement, crepitus on motion, and/or limitation of joint motion [185]. Inflammation is not typically present; when present, it is mild and usually localized to the joint [185].
Radiographs of the knee are not routinely needed for a diagnosis of knee osteoarthritis. The characteristic findings of osteoarthritis on radiographs include osteophytes and joint space narrowing. Changes in the structure of the knee joint have been found more frequently on MRI than on plain radiographs, and the use of MRI in diagnosis may become more common [74]. MRI may also be helpful in ruling out other causes of knee pain with radiographic findings similar to those of osteoarthritis, such as osteochondritis dissecans and avascular necrosis [19].
The ACR developed the classification criteria for osteoarthritis of the knee with three "trees" designed to enable diagnosis based on only the clinical findings (history and physical examination), a combination of clinical and radiographic findings, or a combination of clinical and laboratory findings (Table 7) [174,177]. The criteria for clinical and radiographic findings has the best reported sensitivity/specificity (91%/86%), compared with that for clinical and laboratory findings (92%/75%) and clinical findings only (95%/69%) [174].
AMERICAN COLLEGE OF RHEUMATOLOGY CLASSIFICATION CRITERIA FOR OSTEOARTHRITIS OF THE KNEE
| Based on Clinical Findings Only | ||||||||||
| ||||||||||
| Based on Clinical and Radiographic Findings | ||||||||||
| ||||||||||
| Based on Clinical and Laboratory Findings | ||||||||||
|
According to the EULAR guidelines on the diagnosis of knee osteoarthritis, a diagnosis can be made with 99% confidence when three symptoms and three signs are present [173]:
Persistent knee pain
Limited morning stiffness
Reduced function
Crepitus
Restricted movement
Osseous enlargement
The clinical presentation of hip osteoarthritis is similar to that of knee osteoarthritis, with pain being the most common symptom driving individuals to seek medical care [177,186]. Pain related to hip osteoarthritis is an ache—most often diffuse—that is usually felt during use of the joint and relieved by rest. Pain is typically gradual, variable, or intermittent; the joint may feel stiff after a period of inactivity [177,186]. The loss of function or mobility is usually related to the degree of pain.
The strongest sign of hip osteoarthritis on physical examination is pain that is exacerbated by internal or external rotation of the hip with the knee in full extension [38,177]. Other signs include crepitus and gait abnormalities (resulting from alterations in walking to avoid pain) [186]. Deformity and instability are late signs of severe osteoarthritis, but they are uncommon [186]. Both hips should be examined if osteoarthritis is suspected, as the disease occurs bilaterally in approximately 20% of individuals [38].
The ACR criteria for classification enable diagnosis of osteoarthritis of the hip on the basis of the clinical presentation and either laboratory or radiographic findings. According to this set of criteria, which has a reported sensitivity/specificity of 89%/91%, diagnosis requires patient-reported pain in the hip and at least two of the following three signs [175,177]:
Erythrocyte sedimentation rate (Westergren) of less than 20 mm/hour
Radiographic evidence of femoral or acetabular osteophytes
Radiographic evidence of joint space narrowing (superior, axial, and/or medial)
Osteoarthritis of the hand is characterized by pain with use, which affects one or a few joints at any one time, and mild stiffness in the morning and/or after a period of inactivity [178]. The severity of osteoarthritis-related pain varies, and the pain may be intermittent. The joints most often affected are the distal and proximal interphalangeal joints and the base of the thumb [176,177,178]. Individuals who have evidence of osteoarthritis at several joints in the hand are at increased risk for generalized osteoarthritis, and clinicians should evaluate such patients as appropriate [178].
Osteoarthritis of the hand may be associated with substantial limitations in function, and the clinician should ask the patient whether he or she has difficulty with such tasks as dressing, eating, writing, handling or fingering small objects, and carrying or lifting 10 pounds [44,56]. Several validated questionnaires are available to assess function of the hand, and the choice of questionnaire depends primarily on the clinical question [171]. Individuals with symptomatic osteoarthritis of the hand also may have reduced maximal grip strength [44,56].
The ACR criteria for classification of osteoarthritis of the hand enable diagnosis on the basis of only clinical findings [176,177]. They consist of pain, aching, or stiffness in the hand and at least three of the following features:
Hard tissue enlargement of at least 2 of 10 selected joints
Hard tissue enlargement of at least two distal interphalangeal joints
Fewer than three swollen metacarpophalangeal joints
Deformity of at least 1 of 10 selected joints
The 10 selected joints are the second and third distal interphalangeal, the second and third proximal interphalangeal, and the first carpometacarpal joints of both hands [177]. This set of criteria yields a sensitivity/specificity of 94%/87% [176]. The evidence-based recommendations for the diagnosis of hand osteoarthritis developed by EULAR support the ACR's criteria of only clinical findings, stating that a confident clinical diagnosis can be made in adults older than 40 years of age on the basis of the described clinical findings [171].
Hard tissue enlargements on the distal interphalangeal joints (Heberden and Bouchard nodes) are the clinical finding that is most characteristic of osteoarthritis of the hand [56,176,177]. Although radiographic findings are not an established diagnostic criterion, evidence of osteophytes is the only unique radiographic criterion for a diagnosis [176]. Other classic radiographic findings include joint space narrowing, subchondral bone sclerosis, or subchondral cysts [171,176]. The diagnosis of hand osteoarthritis does not require blood tests, but such tests may be helpful in excluding coexisting disease or in identifying an inflammatory arthritis [171].
Pain related to osteoarthritis of the shoulder is typically progressive, related to activity, deep in the joint, and often localized posteriorly [30]. Pain is usually present at rest and interferes with sleep, with nocturnal pain becoming more common as the disease progresses. More advanced disease is also associated with stiffness that limits function.
Younger patients with shoulder pain should be asked about previous trauma, dislocation, or surgery for shoulder instability, as all have been related to the development of osteoarthritis [30]. In the early stages of disease, the findings of the physical examination may be unremarkable. Some signs indicative of osteoarthritis are painful crepitus, enlargement of the joint, tenderness at the joint line, and joint effusion. The range of motion is usually decreased, especially in external rotation and abduction. In advanced stages of disease, grinding may be audible or palpable when mechanical stress is placed on the shoulder. Signs that are not indicative of shoulder osteoarthritis are lack of pain on palpation or passive range of motion (e.g., bursitis, rotator cuff disease, or biceps tenderness) and loss of passive or active range of motion (e.g., calcific tendinitis or idiopathic adhesive capsulitis) [187].
Unlike the case with osteoarthritis at other sites, imaging studies are essential for the diagnosis of osteoarthritis of the shoulder [30]. Signs of early disease include slight narrowing of the joint space, small osteophytes, subchondral sclerosis, cysts, and eburnation or advanced loss of articular cartilage. Narrowing of the joint space can be best detected with either an axillary view or an anteroposterior view, with the arm held in 45 degrees of abduction [188]. MRI can demonstrate wearing of articular cartilage, and computed tomography arthrograms can be used to localize articular defects [30].
A blood panel can help identify infection. An erythrocyte sedimentation rate greater than 45 mm/hour may indicate rheumatoid arthritis, an underlying malignancy, or chronic infection. These blood tests are sensitive but not specific in determining causes of shoulder pain [189].
Individuals with osteoarthritis of the elbow typically have pain, stiffness, and weakness in the joint [31]. Later stage disease is associated with pain when carrying a heavy object at the side of the body with the elbow in extension. The history is important when evaluating symptoms related to the elbow because of the strong relationship between trauma or occupation with osteoarthritis, especially in individuals who are younger than 40 years of age [58]. Primary osteoarthritis of the elbow is often associated with osteoarthritis at another joint site, especially the second and third metacarpophalangeal joints, the knee, and the hip, and those joints should be evaluated as appropriate [190].
Range of motion should be examined in flexion-extension and pronation-supination. Most patients will have pain at the endpoints of range of motion rather than at other points throughout the arc of motion. Crepitus can usually be heard during range of motion.
As with osteoarthritis of the shoulder, osteoarthritis of the elbow can be diagnosed with standard radiographs, and anteroposterior and lateral projections are best [31,58]. A distinction of primary elbow osteoarthritis is preservation of the joint space, even when disease is at an advanced stage [31,58]. Other radiographic characteristics of primary osteoarthritis are an anterior and medial osteophyte (involving the coronoid process) and a posteromedial osteophyte (olecranon process). The location and size of osteophytes can be determined by computed tomography (CT) with three-dimensional reconstructions [58]. It may be difficult to detect loose bodies on plain radiographs [58].
A history of ankle fracture or ligamentous injury is a hallmark feature of osteoarthritis of the ankle [32]. Diagnostic evaluation includes radiographs of the ankles made with the patient standing. MRI is also recommended, as it can provide evidence of osteonecrosis as well as indicate the amount of involvement, the extent of bone loss, and the size of subchondral cysts [32].
There is currently no curative therapy for osteoarthritis, and treatments to alter or arrest the disease process are few and mostly ineffective [19]. However, researchers are actively attempting to improve these medications to make them more effective, and, as of 2019, several novel drugs for blocking inflammation using antibiodies and pathway inhibitors are in various phases of clinical trials [191,192]. Current management is focused on decreasing pain and increasing function [193,194]. Several treatment approaches have been used for osteoarthritis and subsequently included in practice guidelines. The range in options has made it difficult for clinicians to determine which ones are most effective; more than 50 treatment modalities have been addressed in 23 guidelines for the management of knee and hip osteoarthritis alone [193]. These guidelines have been established by professional organizations in the United States, such as the ACR, the American Academy of Orthopaedic Surgeons (AAOS), and the American Geriatrics Society (AGS); and in Europe, such as EULAR, the Osteoarthritis Research Society International (OARSI), and the National Institute for Health and Clinical Excellence (NICE). The guidelines have addressed osteoarthritis in general, osteoarthritis at specific joints (primarily the knee and hip), and exercise programs (Table 8) [171,185,193,194,195,196,197,198,199]. In addition, the Agency for Healthcare Research and Quality (AHRQ) has commissioned research for comparative effectiveness studies and evidence reports related to osteoarthritis [200,201,202].
CLINICAL PRACTICE GUIDELINES FOR THE DIAGNOSIS AND MANAGEMENT OF OSTEOARTHRITIS
| Knee | ||||||||||
| ||||||||||
| Hip | ||||||||||
| ||||||||||
| Hand | ||||||||||
| ||||||||||
| Shoulder | ||||||||||
| Izquierdo R, Voloshin I, Edwards S, et al. Treatment of glenohumeral osteoarthritis. J Am Acad Orthop Surg. 2010;18(6):375-382. | ||||||||||
| Other | ||||||||||
|
Despite the availability of these guidelines, gaps in evidence-based recommendations exist. There are currently no evidence-based guidelines on the management of osteoarthritis of the elbow, ankle, or spine; there is only one (European) guideline on management of osteoarthritis of the hand [171]. Most of the treatment options in use are not based on clinical studies of these specific areas but are instead extracted from evidence obtained from clinical studies of other limb joints [203]. Adding to the challenge of selecting appropriate therapy is evolving evidence on the efficacy of specific options; systematic reviews, meta-analyses, and randomized controlled clinical trials have demonstrated that many commonly used treatment options for osteoarthritis offer no or limited benefit.
As clinicians on the frontline of care, primary care providers and nurses are typically the first to see individuals with symptoms indicative of osteoarthritis. Primary care providers can coordinate the management of osteoarthritis, and a multidisciplinary approach is best. The ACR and the Association of Rheumatology Health Professionals (a division of the ACR) support such an approach, noting that the healthcare team may include a rheumatologist, primary physician, nurse, nurse practitioner, physician assistant, physical therapist, occupational therapist, physiatrist, psychiatrist, psychologist, orthopedic surgeon, social worker, registered dietician, vocational counselor, and others [204]. A primary care physician should consider referral to a rheumatologist in the following situations [19]:
Atypical signs and symptoms (e.g., pain at night, prolonged stiffness in the morning, involvement of multiple joints)
Overall evaluation to address needs for nonpharmacologic treatment
Lack of response to standard treatment
Need for operative procedures (arthroscopy and arthroplasty)
The optimal management of osteoarthritis encompasses both nonpharmacologic and pharmacologic measures, beginning with basic modalities and following a so-called pyramid approach as the disease progresses or symptoms do not respond [205]. Several factors should be considered when selecting treatment modalities, including risk factors (e.g., age, comorbidity, overweight/obesity), the level of pain and functional limitations, signs of inflammation, and degree of structural damage [206].
Many treatment options are associated with benefits and risks, and the clinician should discuss the benefits and risks with patients and support their participation in the decision-making process [207,208]. Patient preferences are an important consideration when choosing treatment options and establishing treatment goals, and the ACR advocates care that addresses treatment goals that are meaningful to the individual patient [204]. Decision aids can help enhance patients' knowledge of treatment options, improve patients' participation in their care, and produce realistic expectations of outcomes [208]. Decision aids for osteoarthritis have been developed in a variety of media (e.g., print, online, video) and are available online (https://decisionaid.ohri.ca) [208].
The pain and disability associated with osteoarthritis often has a substantial psychologic and social effect. It is important to discuss these aspects with patients and to address psychologic issues, especially depression, in order for treatment measures to be effective [88].
Several nonpharmacologic treatment options have been found to be effective in managing osteoarthritis (Table 9).
EVIDENCE-BASED NONPHARMACOLOGIC OPTIONS FOR THE MANAGEMENT OF OSTEOARTHRITIS
| Intervention | Joint | ||||
|---|---|---|---|---|---|
| Patient education and self-management | All joints | ||||
| Weight loss/maintenance of optimum weight | Knee, hip | ||||
| Regular exercise | Knee, hip | ||||
| Knee, hip, hand, elbow | ||||
| Braces, orthotics, walking aids | Knee, hip, ankle, hand |
Education and self-management, through lifestyle modifications are universally recognized as the core of treatment in clinical guidelines [193]. This recommendation is based on research showing that education helps patients become more involved in their care, leading to improved outcomes [207]. The AHRQ notes that an effective partnership is the key to the effective management of osteoarthritis; the healthcare professional's role in this partnership is to [207]:
Encourage patients to change their behavior to improve symptoms or slow disease progression
Promote the proper use of medications
Instruct patients on how to interpret and report symptoms accurately
Support patients' efforts to maintain normal activities
Help patients adjust to new social and economic circumstances and cope with emotional consequences
Clinicians should emphasize to patients that adhering to the management program will alleviate their symptoms, improve their function, and enhance their quality of life. Education should be tailored to address individual needs. For example, patients who participate in sports should be advised to avoid sports with direct contact and high impact and to wear protective equipment to prevent injury [84]. Similarly, for patients in occupations with high risk for osteoarthritis, clinicians should discuss the importance of avoiding high-risk tasks. Education and training in ergonomic principles, pacing of activity, and use of assistive devices should be offered to patients with hand osteoarthritis [171]. It is also essential to encourage patients with osteoarthritis of the glenohumeral joint or the elbow to modify activities that led to the development of the disease [31,58]. Periodic contact during follow-up can help promote self-management [193].
Clinicians should also encourage patients to participate in formal self-management programs in the community or online and to use reliable educational resources, such as the Arthritis Foundation (https://www.arthritis.org) [172].
When educating a patient about osteoarthritis and its management, it is essential to ensure that he or she understands the treatment plan and his or her role in self-management. However, according to the National Assessment of Health Literacy, 14% of individuals in the United States have "below basic" health literacy, which means they lack the ability to understand health information and make informed health decisions [183,209]. Data from 2017 indicate that this figure is 19% [210]. Understanding the problem of health literacy is especially important for clinicians managing osteoarthritis, as low health literacy is more common among older individuals, the population most affected by the disease [211]. Health literacy also varies widely according to race/ethnicity, and level of education, and clinicians are often unaware of the literacy level of their patients [210,211,212]. Predictors of limited health literacy are poor self-rated reading ability, low level of education, male gender, and non-White race [212,213]. Ensuring that patients understand health information is essential, as limited health literacy has been associated with poor health outcomes [214].
Several instruments are available to test patients' literacy level, and they vary in the amount of time needed to administer and reliability in identifying low literacy. A review of several instruments demonstrated that the two most accurate tools for identifying literacy are the Rapid Estimate of Adult Literacy in Medicine (REALM) and the shortened version of the Test of Functional Health Literacy in Adults (S-TOFHLA) [211]. REALM takes 3 minutes to administer, whereas S-TOFHLA takes 7 to 12 minutes to administer [211]. More rapid testing is available in the form of the Newest Vital Sign (NVS), an instrument named to promote the assessment of health literacy as part of the overall routine patient evaluation [183,215]. The NVS takes fewer than three minutes to administer, has correlated well with more extensive literacy tests, and has performed moderately well at identifying limited literacy [211,212]. Two questions have also been found to perform moderately well in identifying patients with inadequate or marginal literacy: "How confident are you in filling out medical forms by yourself?" and "How often do you have someone help you read health information?" [211].
Compounding health literacy are language and cultural barriers, which have the potential for far-reaching effect, given the growing percentages of racial/ethnic populations. According to the U.S. Census Bureau, more than 66 million Americans speak a language other than English in the home, with approximately 25.3 million of them (8.2% of the population) speaking English less than "very well" [216]. It has been suggested that when patients are first evaluated, they should be asked what language is spoken at home and if they speak English "very well" [217]. In addition, patients should also be asked what language they prefer for their medical care information, as some patients prefer their native language even though they have said they can understand and discuss symptoms in English [217]. Many studies have demonstrated that the lack of an interpreter for patients with limited English proficiency compromises the quality of care and that the use of professional interpreters improves communication (errors and comprehension), utilization, clinical outcomes, and patient satisfaction with care.
"Ad hoc" interpreters (e.g., family members, friends, and bilingual staff members) are often used instead of professional interpreters for a variety of reasons, including convenience and cost. However, clinicians should check with their state's health officials about the use of ad hoc interpreters, as several states have laws about who can interpret medical information for a patient [218]. Even when allowed by law, the use of a patient's family member or friend as an interpreter should be avoided, as the patient may not be as forthcoming with information and the family member or friend may not remain objective [218]. Children should especially be avoided as interpreters, as their understanding of medical language is limited and they may filter information to protect their parents or other adult family members [218]. Individuals with limited English language skills have actually indicated a preference for professional interpreters rather than family members [219].
Most important, perhaps, is the fact that clinical consequences are more likely with ad hoc interpreters than with professional interpreters [220]. A systematic review of the literature showed that the use of professional interpreters facilitates a broader understanding and leads to better clinical care than the use of ad hoc interpreters, and many studies have demonstrated that the lack of an interpreter for patients with limited English proficiency compromises the quality of care and that the use of professional interpreters improves communication (errors and comprehension), utilization, clinical outcomes, and patient satisfaction with care [221,222].
Clinicians should adapt their discussions and educational resources to a patient's identified health literacy level and degree of language proficiency. The use of plain language (free of medical jargon), asking patients to repeat pertinent information, regularly assessing recall and comprehension, and using translated educational materials can all help ensure that patients better understand their disease and its management, ultimately leading to higher quality care.
Given the strong correlation between overweight/obesity (defined as a BMI greater than 25) and osteoarthritis of the knee and hip, weight reduction and maintenance of a healthy weight are central to guidelines on the management of osteoarthritis at these sites [185,198,206,223,224]. A systematic review showed that a moderate weight-loss program (0.25% of body weight per week) can reduce pain and physical disability for individuals with osteoarthritis of the knee [225]. In its 2021 guideline for the treatment of osteoarthritis of the knee, the AAOS recommends weight reduction, specifically, achieving and/or maintaining a BMI ≤25 [198].
The recommended approach to weight loss is through dietary modifications and an exercise program [224]. The Arthritis, Diet, and Activity Promotion Trial (ADAPT), which involved 316 overweight or obese adults with knee osteoarthritis, demonstrated that an 18-month program of modest weight loss and modest exercise provided the most benefit (compared with a diet-only or exercise-only program) [226]. Individuals in the diet-plus-exercise group had significant improvements in self-reported physical function, six-minute walk distance, stair-climb time, and knee pain. The 2019 ACR guideline specifies weight reduction counseling [185].
Regular, moderate exercise can help maintain overall health, muscle strength, and range of motion of the joint. In addition, several physical therapy strategies can help improve function and relieve pain.
Regular Exercise
Some patients may fear that regular exercise will exacerbate pain, but a review of the literature has shown that moderate exercise does not increase the risk for progression of osteoarthritis, provided that care is taken to avoid injury [110,227]. The goal of an exercise program is to control pain, increase flexibility, and improve muscle strength and endurance [228]. The exercise program should be individualized to the patient, with consideration given to the patient's age, comorbidities, and mobility [229]. Guidelines suggest that exercise should be prescribed for all patients with osteoarthritis, regardless of age, severity of pain and disability, and comorbidity [199]. The American Geriatric Society notes that absolute contraindications to an exercise program include uncontrolled arrhythmias, third-degree heart block, changes on recent electrocardiography, unstable angina, acute myocardial infarction, and acute congestive heart failure [228]. Relative contraindications include cardiomyopathy, valvular heart disease, poorly controlled blood pressure, and uncontrolled metabolic disease [228].
Promoting exercise as part of an overall positive lifestyle change can increase the effectiveness of the program [229]. Supervised group exercise and home-based programs have been shown to be equally effective, allowing patients to select the type of program they prefer [229]. The Ottawa Panel recommends that significant weight-loss occur before starting weight-bearing exercise (particularly for obese patients) in order to maintain joint integrity and to avoid joint disease and dysfunction [224].
Low-impact aerobic exercise, such as walking, bicycling, swimming, or water aerobics, has shown to offer substantial benefit in terms of improved physical function, reduction of pain and disability, and enhanced perceived quality of life in individuals with knee osteoarthritis, especially overweight/obese individuals [198,227,230,231]. Although there is little evidence that exercise is of benefit to individuals with hip osteoarthritis, one systematic review found that land-based (as opposed to water-based) therapeutic exercise programs can reduce pain and improve physical function [229,231]. Adherence is the primary predictor of long-term outcome from exercise for the management of knee or hip osteoarthritis; because of this, clinicians should encourage patients to engage in exercises they enjoy, as this can enhance the likelihood of long-term adherence. Long-term monitoring, frequent contact during follow-up, and/or involving family members in the program may also enhance adherence [193,229,232].
In 2000, the ACR guidelines for the management of knee and hip osteoarthritis included a recommendation for aerobic exercise [71]. A year later, the AGS published evidence-based recommendations for exercise as part of managing osteoarthritis in older individuals. (These recommendations were not joint-specific.) In 2005, a European multidisciplinary expert panel developed evidence-based recommendations for exercise to manage osteoarthritis of the knee or hip [229]. The AAOS incorporated these previous recommendations into its 2008 guideline for the treatment of knee osteoarthritis, noting that patients should be encouraged to participate in low-impact aerobic fitness exercises (level of evidence: I, A) and quadriceps strengthening (level of evidence: II, B) [232]. Subsequent reviews of the literature have supported that exercise reduces pain and improves physical function [227,233]. The 2021 AAOS guideline on the treatment of knee osteoarthritis strongly recommends the implementation of a variety of physical therapy and exercise modalities, including low-impact aerobic exercise, aquatic exercise, strength training, self-management programs, and neuromuscular education [198]. Exercise recommendations should be consistent with national guidelines. The 2019 ACR Technical Expert Panel recommends land- or water-based aerobic exercise for all patients (depending on patient preference) except those who are extremely overweight or aerobically deconditioned; for these patients, water-based exercise is recommended until conditioning (i.e., improved aerobic capacity) is achieved [185].
Physical Therapy Strategies
Substantial improvement in symptoms related to osteoarthritis of the knee has been achieved through several physical therapy strategies, including range-of-motion (flexibility) exercises, muscle stretching, and soft tissue mobilization [172]. A combination of physical therapy (to the knee as well as to the lumbar spine, hip, and ankle, as required) and a standardized exercise program provided more benefit than placebo (subtherapeutic ultrasound to the knee) in a small randomized study (83 patients) of the management of knee osteoarthritis [234]. Patients treated with the combination therapy had clinically and statistically significant improvements in WOMAC score and six-minute walk distance, whereas no improvements were found in the placebo group. The benefits were sustained at one year, and fewer patients in the treatment group had undergone knee arthroplasty (5% vs. 20%) at that time. Another small randomized study compared home-based physical therapy with clinically based physical therapy [235]. The 134 participants with knee osteoarthritis were randomly assigned to a clinic treatment group or a home exercise group. The clinic treatment group received supervised exercise and individualized manual therapy; they also completed a four-week home exercise program. The home exercise group completed the four-week home exercise program, with reinforcement at a clinic visit two weeks later. Both groups showed clinically and statistically significant improvements in six-minute walk distances and WOMAC scores at four weeks and eight weeks. By four weeks, WOMAC scores had improved by 52% in the clinic treatment group and by 26% in the home exercise group. Average six-minute walk distances had improved about 10% in both groups. At one year, both groups were substantially and about equally improved over baseline measurements. Subjects in the clinic treatment group were less likely to be taking medications for their arthritis and were more satisfied with the overall outcome of their rehabilitative treatment compared with subjects in the home exercise group [235].
The AGS guidelines recommend flexibility exercises, strengthening exercises, and endurance exercises, along with heat modalities, for older patients with all types of osteoarthritis. Range-of-motion (flexibility) exercises can help decrease stiffness, increase joint mobility, and prevent soft-tissue contractures [228]. Static stretching can improve range of motion. Exercises that combine flexibility and resistance training (e.g., yoga, tai chi) have significant therapeutic benefit for knee osteoarthritis [185,198]. The goal of strengthening exercises is to increase the strength of the muscles that support the affected joint [228]. Exercises to strengthen the quadriceps muscles have led to improvements in pain and function for individuals with knee osteoarthritis [172]. In addition, studies suggest that strengthening the quadriceps muscles may help delay progression of knee and hip osteoarthritis [229]. In general, patients should begin a strengthening exercise regimen with isometric exercises and advance to isotonic resistance exercises as tolerated [228]. Isometric, isotonic, and isokinetic training have similar long-term benefits [198]. In general, it appears that exercise is beneficial, but the mode of exercise may not matter as much as engaging in any exercise program [198].
The findings of a systematic review suggest that therapeutic ultrasound may help reduce pain and increase function for patients with osteoarthritis of the knee [236]. However, the quality of the evidence is low, which left the authors of this and another review uncertain about the magnitude of the effects of the treatment modality [236,237]. The AAOS guideline on the treatment of knee osteoarthritis refrains from making a recommendation for or against therapeutic ultrasound; however, the authors of the guideline reviewed several studies showing evidence of its benefit, particularly when combined with various forms of exercise [198].
With regard to other joints, EULAR guidelines recommend an exercise regimen that involves range-of-motion and strengthening exercises for all individuals with osteoarthritis of the hand [178]. The guidelines also recommend local application of heat, especially before exercise; heat can be applied with a hot pack or paraffin wax [178]. Thermal agents/modalities in combination with exercise are also endorsed by the ACR [185].
The AAOS found inconclusive evidence for physical therapy as an effective treatment option for osteoarthritis of the glenohumeral joint and is unable to recommend for or against physical therapy as part of initial treatment of the condition [197]. Similarly, a supervised physical therapy program is not routinely a treatment approach for osteoarthritis of the ankle [32]. Physical therapy should begin in the early stages of osteoarthritis of the elbow (mild pain and loss of less than 15 degrees of motion) [31]. Strategies may include gentle range-of-motion exercises to maintain mobility and strength [31].
Braces
Although valgus or varus bracing can theoretically relieve pain and improve function by shifting joint load away from the medial or lateral compartment of the knee, respectively, the AAOS found inconclusive evidence of the efficacy of these types of braces in terms of relieving pain or improving function or quality of life [198]. Braces can provide significant pain relief and improved function to patients who have unicompartmental knee osteoarthritis. Braces also can provide a subjective feeling of more normal tibiofemoral kinematics. There is a theoretical benefit of increased confidence in the knee during activities by providing a sense of security to the knee. Apart from some skin irritation or discomfort, there are almost no harms in trialing a brace [198].
With regard to other joints, a thumb splint may be helpful for people with osteoarthritis of the thumb base, and a brace has been suggested as part of conservative management of osteoarthritis of the ankle. However, these recommendations are based on expert opinion only [32,178].
Orthotics
It has been proposed that lateral and medial wedges may help relieve the symptoms of medial and lateral compartment osteoarthritis of the knee, respectively, by reducing joint load. However, studies have not provided evidence that wedges alone improve osteoarthritis-related symptoms [172,198,238]. The ACR guidelines suggest that patients may benefit from the use of wedged insoles to correct abnormal biomechanics related to varus deformity of the knee, but the AAOS recommends that lateral heel wedges not be prescribed for patients with symptomatic medial compartmental osteoarthritis of the knee [185,198].
Walking Aids
The ACR recommends use of a cane on the contralateral side to help decrease pain and improve function for patients who have persistent pain related to knee or hip osteoarthritis [172,185].
Footwear
Clinicians should also advise patients with hip or knee osteoarthritis about appropriate footwear; the optimum shoe may be one that is flat or has a low heel and that is flexible (rather than stabilizing) [238]. Foot orthoses to correct varus malalignment of the forefoot may help reduce pain in the hip among individuals with osteoarthritis of that joint [140]. Modifications to footwear may be helpful for people with osteoarthritis of the ankle [32].
Other
The ACR recommends patellar taping for the short-term relief of pain and improvement of function among individuals with symptomatic osteoarthritis of the knee [185]. Although the AAOS has previously endorsed taping, the 2021 update does not address this approach [198,232].
Transcutaneous electrical nerve stimulation (TENS) has been used as part of management of knee osteoarthritis, but the data on its effectiveness are conflicting. One review of the literature (systematic reviews published between 2000 and 2007) demonstrated evidence of moderate quality that TENS reduces pain [233]. The authors of a subsequent review (up to 2008) reported that they could not confirm the benefit of TENS for the relief of pain, noting that the review was inconclusive because of the inclusion of small trials of questionable quality [239]. The ACR recommends the use of electrical stimulation only for patients with severe pain who are candidates for total knee arthroplasty but who are unwilling or unable to undergo the procedure (i.e., contraindication due to comorbidities/medication) [185]. The AAOS is unable to recommend for or against any form of electrotherapy [198].
The available literature demonstrates that acupuncture provides minimal, short-term relief of pain related to knee osteoarthritis [233,240,241]. Acupuncture was considered to be a therapy "under investigation" at the time of publication of the 2000 ACR guidelines for the management of osteoarthritis of the knee and hip [71]. The 2019 ACR expert panel recommends the use of acupuncture only for patients with severe pain who are candidates for total knee arthroplasty but who are unwilling or unable to undergo the procedure [185]. The AAOS applied a limited strength recommendation for the use of acupuncture as an adjunctive therapy for pain relief in patients with symptomatic osteoarthritis of the knee. This is a downgraded recommendation because of inconsistent evidence and a lack of internal consistency with recommendations of equal supporting evidence [198]. No recommendations have been made regarding the use of acupuncture as part of the treatment of osteoarthritis at other joint sites.
No drugs have been found to effectively alter the disease process or the structural properties of the joint; therefore, the goal of pharmacologic therapies is to relieve pain. Oral analgesics form the basis of pharmacologic management, and other effective pharmacologic options, depending on the joint, include topical analgesics, viscosupplementation, and intra-articular corticosteroids (Table 10).
EVIDENCE-BASED PHARMACOLOGIC OPTIONS FOR THE MANAGEMENT OF OSTEOARTHRITISa
| Pharmacologic Approach | Notes | |||
|---|---|---|---|---|
| Oral analgesics | Insufficient evidence to recommend for osteoarthritis of the shoulder | |||
| Acetaminophen | Up to 4 g/day | |||
| Nonselective nonsteroidal anti-inflammatory drugs (NSAIDs) (e.g., naproxen, ibuprofen) | A gastroprotective agent (proton-pump inhibitor) should be prescribed for patients at high risk for gastrointestinal complications | |||
| Cyclooxygenase-2 (COX-2) selective NSAIDs | Some agents associated with an increased risk of myocardial infarction | |||
| Tramadol | Considered separately from opioid analgesics due to modulatory effect on serotonin and norepinephrine levels | |||
| Opioid analgesics | No recommendation for or against use for osteoarthritis of the hip, knee, or shoulder. Should not be used for osteoarthritis of the hand. Weak opioids may be used for pain refractory to other pharmacologic agents. | |||
| Topical analgesics (e.g., NSAIDs, capsaicin) | Insufficient evidence to recommend for osteoarthritis of the shoulder | |||
| Intra-articular corticosteroids |
| |||
| Viscosupplementation (hyaluronan) |
| |||
| aNo evidence-based guidelines are available for osteoarthritis of the elbow or ankle. | ||||
Educating patients about their pharmacologic treatment plan is crucial. A questionnaire designed to assess patients' knowledge of osteoarthritis and its management demonstrated a substantial lack of knowledge about analgesics [242]. Fewer than one-third of the patients knew that they could take analgesics prophylactically, and 70% did not know that analgesics should be taken when pain starts to build. In addition, approximately one-third did not know that nonsteroidal anti-inflammatory drugs (NSAIDs) should be taken with food or following a meal [242]. Another small study showed that patients with multiple coexisting conditions are dissatisfied with the complex medication regimen required for comorbidities [243]. Patients in this study were unclear on how to take analgesics on an "as needed" basis, pointing to the need for clearer guidance from clinicians and other healthcare professionals [243].
Because of the wide range of pain relievers available, the challenge is to select an agent that will provide optimum relief with minimum adverse events. The oral pain relievers used for osteoarthritis include acetaminophen, nonselective NSAIDs, cyclooxygenase-2 (COX-2) selective NSAIDs, opioids, and tramadol. The 2021 AAOS guideline specifies NSAIDs (oral or topical) as first-line pharmacologic treatments for symptomatic osteoarthritis of the knee. The guideline does not recommend tramadol, citing a significant increase of adverse events and lack of efficacy at improving pain or function for treatment of osteoarthritis of the knee [198]. The 2019 ACR guideline recommends NSAIDs (including COX-2-selective agents) and tramadol as first-line treatment for hand osteoarthritis, and acetaminophen, NSAIDs, and tramadol for knee and hip osteoarthritis [185].
Some guidelines recommended acetaminophen as the initial analgesic for the management of mild-to-moderate pain related to osteoarthritis, but this recommendation has since been shown to be questionable [193]. A comparative effectiveness study conducted by the AHRQ found good evidence that acetaminophen is modestly inferior in efficacy compared with NSAIDs but has a lower risk of gastrointestinal complications [244]. An update to this study found that no currently available analgesic offered a clear overall advantage compared with the others [200]. Its original findings on acetaminophen remained the same, with the addition that acetaminophen poses a higher risk of liver injury [200]. Other research has shown that NSAIDs are more effective than acetaminophen for relieving osteoarthritis-related pain, especially moderate-to-severe pain [245]. The 2021 AAOS guideline provides a strong recommendation for oral acetaminophen to improve pain and function in the treatment of knee osteoarthritis when not contraindicated [198]. The working group noted that when oral acetaminophen was compared to NSAIDs, the use of oral NSAIDs provided a significant reduction in pain and improved function. As a result, providers may consider using oral NSAIDs instead of acetaminophen when a contraindication to oral NSAIDs does not exist [198]. NSAIDs should be prescribed at the lowest effective dose, and their long-term use should be avoided [193]. A COX-2 selective agent or an NSAID with a prescription for a gastroprotective agent (such as a proton-pump inhibitor) may be used for patients who have an increased risk for gastrointestinal complications [193].
There is good evidence that nonselective NSAIDs and COX-2-selective NSAIDs have comparable efficacy and that COX-2-selective agents are comparable to each other [200,246]. Although COX-2-selective agents have better tolerability in general compared with NSAIDs, there is considerable variability across individual drugs in terms of protection against serious gastrointestinal events [246]. In addition, some COX-2 selective NSAIDs have been associated with an increased risk of myocardial infarction, and these drugs should be used with caution in patients with cardiovascular risk factors [200,246].
Studies have found that opioids were more effective overall than control interventions with respect to pain relief and improved function, but the beneficial effects were small to moderate and were outweighed by a substantial increase in the risk of adverse events [247,248]. The authors of the review concluded that opioids should not be used routinely for individuals with osteoarthritis, even for severe pain. Some guidelines suggest the use of weak narcotics or opioids for pain that has been refractory to other pharmacologic agents; however, the guidelines note that strong opioids should be used sparingly [193]. The 2021 AAOS guideline on the treatment of knee osteoarthritis emphasizes the importance of removal of oral narcotics from the medications prescribed due to the rise of the opioid epidemic in the United States [198]. The ACR guidelines conditionally recommend against using opioid analgesics for osteoarthritis of the hand [185].
In reviewing the literature for its guidelines on the treatment of osteoarthritis of the glenohumeral joint, the AAOS was not able to find sufficient evidence to support several pharmacologic treatments, including acetaminophen, NSAIDs, opioids, or narcotics. As a result, the AAOS states it is unable to recommend for or against the use of any of these options for the initial treatment of patients with osteoarthritis of this joint [197].
Moderate-quality evidence indicates that compared with placebo, tramadol alone or in combination with acetaminophen probably has no important benefit on mean pain or function in people with osteoarthritis, although slightly more people in the tramadol group report an important improvement (defined as 20% or more). Moderate-quality evidence shows that adverse events probably cause substantially more participants to stop taking tramadol. The increase in serious adverse events with tramadol is less certain, due to the small number of events [249].
There is good evidence that topical NSAIDs have efficacy comparable to oral NSAIDs, although most trials have involved knee osteoarthritis only, and head-to-head trials have not been large enough to evaluate the comparative risk of serious cardiovascular events and gastrointestinal effects [200]. There is also good evidence that topical NSAIDs are safer than oral NSAIDs, but a systematic literature review showed that systemic adverse events have occurred in a substantial proportion of older adults treated with topical NSAIDs [250]. Capsaicin has also been effective in relieving osteoarthritis-related pain, and some guidelines have suggested the use of this topical agent as an alternative treatment or an adjunct to treatment with oral analgesics [193]. The ACR guideline recommends against the use of topical capsaicin for hand osteoarthritis and for topical NSAIDs for hand and knee osteoarthritis [185]. The AHRQ comparative review found that topical capsaicin was superior to placebo but associated with increased local adverse events and withdrawals due to adverse events [200].
As is the case for oral analgesics, the AAOS was not able to find sufficient evidence to support the use of topical analgesics for the treatment of glenohumeral joint osteoarthritis and is unable to recommend for or against the use of these agents for the initial treatment of patients with osteoarthritis of that joint [197].
Most of the evidence regarding the efficacy of intra-articular injection of long-acting corticosteroids comes from the literature on osteoarthritis of the knee, and many experts have called for more research on this treatment approach at the hip and other joints [35,223]. In general, this treatment option is used for moderate-to-severe pain in a joint that has not responded to nonpharmacologic measures or to oral analgesics. Pain relief is thought to be related to the anti-inflammatory effects of the corticosteroid [251,252].
Certain guidelines conditionally recommend intra-articular injection of corticosteroids into the knee or hip, especially after aspiration of fluid in patients who have signs of local inflammation with joint effusion [9,185,206]. For example, the ACR recommends this therapy for knee and hip osteoarthritis if the patient does not have satisfactory response to acetaminophen and topical NSAIDs and if there is a contraindication to oral NSAIDs. The AAOS provides a moderate recommendation for the use of intra-articular corticosteroid therapies. Extended release agents are recommended over immediate release to improve patient outcomes [198].]. Although the approach is otherwise widely recommended, it is acknowledged that intra-articular corticosteroids provide short-term relief only [35,253,254]. A meta-analysis of 28 trials (1,973 patients) of knee osteoarthritis showed a benefit of pain relief for two to four weeks, with no benefit in terms of functional improvement and no benefit in either pain or function beyond four weeks [253]. An update to the meta-analysis, which included 27 trials (1,767 patients), found that the overall quality of the evidence did not clearly support a benefit of intra-articular corticosteroid use after one to six weeks [254]. Despite the short-term benefit found in most studies, clinical experience has shown longer relief in many patients [35]. Because of the potential side effects of intra-articular injections, which include long-term damage to joint cartilage, flare after injection, and infection, most physicians do not recommend more than three to four injections per joint per year [9,35]. Intra-articular injection is more technically difficult in the hip joint than in the knee, and radiographic or ultrasonographic guidance has been suggested, although there are no comparative data to provide evidence that accuracy is increased with such guidance [9,223].
Recommendations for the use of intra-articular corticosteroids at other joints are based primarily on expert opinion, as randomized controlled trials are lacking or have included small numbers of patients. EULAR guidelines for osteoarthritis of the hand note that intra-articular corticosteroids are effective for painful flares of osteoarthritis, especially of the trapeziometacarpal joint [178]. As with data on the hip and knee, intra-articular injections have provided benefit for up to four weeks [9,178].
Although intra-articular corticosteroids are often used in clinical practice to treat shoulder pain of all etiologies, the AAOS concluded that there was insufficient evidence to support the use of this approach for the treatment of osteoarthritis of the glenohumeral joint [197]. Intra-articular corticosteroids are also options for refractory pain in individuals with osteoarthritis of the elbow or ankle, although data are lacking to support the approach [31,32,58].
Endogenous hyaluronan (also known as hyaluronic acid) is a primary component of the extracellular matrix of synovial membrane and tissue and articular cartilage, as well as the synovial fluid [255]. It provides viscoelasticity and lubrication to the joint and helps to maintain tissue hydration. The use of exogenous hyaluronan to treat osteoarthritis—known as viscosupplementation—began in the 1960s; several formulations of viscosupplements are now available, each produced by different manufacturers with different molecular weights. Data on comparison of high-molecular-weight and low-molecular-weight hyaluronic acid have been conflicting, with some studies indicating that high-molecular-weight hyaluronic acid is more effective, whereas other analyses have shown that the efficacy is similar [256,257]. Research reviewed by the AAOS panel suggests that high-molecular-weight hyaluronic acid is more effective than low-molecular-weight [198]. The AAOS guideline does not recommend hyaluronic acid for routine use in the treatment of symptomatic knee osteoarthritis [198]. How hyaluronan and similar products alleviate osteoarthritis-related symptoms is not entirely clear, but its action is thought to be related to its anti-inflammatory, anabolic, and chondroprotective properties [71,258].
It is difficult to determine the efficacy of hyaluronan because research evidence is confounded by different molecular weights of hyaluronan preparations, different dosing schedules, and poor trial design, and the level of evidence across studies has been low [223,255,256,259]. Most of the evidence available is related to osteoarthritis of the knee, with limited data available on use of the treatment for osteoarthritis of the hip, hand, or shoulder.
Since the publication of the 2000 ACR guidelines, certain studies and analyses have supported the efficacy of hyaluronan/hylan derivatives for relieving pain and improving function in patients with symptomatic osteoarthritis of the knee (compared with placebo), with the greatest benefit found in conjunction with less severe pain and disability at 5 to 13 weeks after injection [206,256,260,261]. However, researchers have noted that the effect size is small compared with placebo and that the effect may be overestimated as a result of publication bias [255,256,262]. When compared with NSAIDs, hyaluronan takes longer to relieve knee symptoms; additionally, the dosing schedule necessitates more office visits than intra-articular corticosteroids, creating inconvenience and increasing costs [206,223]. Uncontrolled and small studies of hyaluronic acid for hip osteoarthritis have shown pain reduction after treatment, but intra-articular corticosteroids were more effective in one small study [223,259,263].
In its 2019 recommendations, the ACR conditionally recommends against using intra-articular therapies for hand osteoarthritis [185]. This recommendation is based largely on the absence of evidence from randomized controlled trials to support the benefits as well as the potential for harm from such therapy [185].
In its 2021 guideline on the treatment of osteoarthritis of the knee, the AAOS notes that it cannot recommend the use of intra-articular hyaluronic acid for individuals with symptomatic disease [198]. The rationale for this recommendation is based on a lack of efficacy, not potential harm. Treatment with hyaluronan has been reported to be well tolerated, with a low incidence of adverse events [259,260,264]. Among the potential adverse events are transient pain (mild to moderate) at the injection site and increases in joint pain and/or swelling [71]. The NICE guidelines, revised in 2014, also note that intra-articular injections of hyaluronan cannot be recommended for the treatment of osteoarthritis [199,255].
Evidence of benefit of hyaluronan for osteoarthritis of other joints is limited. A small study (56 patients) showed that a single course of three injections of intra-articular sodium hyaluronate relieved pain and improved joint function in patients with osteoarthritis of the carpometacarpal joint of the thumb. Although the effects were achieved more slowly than treatment with triamcinolone, the duration of benefit was longer (up to six months) [265]. In another small study (16 men), intra-articular sodium hyaluronate (administered once weekly for five weeks) improved scores for pain (primarily at rest) related to osteoarthritis of the trapeziometacarpal joint [178].
The AAOS recommends viscosupplementation as an option for patients with glenohumeral joint osteoarthritis but notes that the level of evidence for the recommendation is weak [197]. A case series of 18 patients with post-traumatic osteoarthritis of the elbow demonstrated short-term pain relief and very limited improvement in function, and the authors concluded that viscosupplementation was not suitable for the condition [266]. Descriptions of suggested treatment options for osteoarthritis of the ankle have not included hyaluronan, although a review of seven studies (275 patients) published between 2006 and 2008 suggested that viscosupplementation may be of benefit for osteoarthritis at that joint [267].
In the United States, glucosamine and chondroitin are heavily marketed as dietary supplements that promote "joint health" and relieve the symptoms of osteoarthritis of the knee and hip. Glucosamine and chondroitin are both made in the body; glucosamine is an amino sugar that is thought to enhance the formation and repair of cartilage, and chondroitin is a carbohydrate found in cartilage that is thought to promote water retention and elasticity and to inhibit the enzymes that degrade cartilage. More than 20 products contain glucosamine alone, chondroitin alone, or a combination of the two, and contamination or mislabeling has been found for some products [268].
Data on the efficacy of glucosamine and chondroitin are available primarily for osteoarthritis of the knee, with limited data on its effectiveness for osteoarthritis of the hip; no studies have been done to evaluate the use of these supplements for osteoarthritis at other joint sites. Several early systematic reviews failed to show a benefit of glucosamine and/or chondroitin in terms of pain, stiffness, and function when compared with placebo [223,269]. These findings were supported by the results of the Glucosamine/Chondroitin Arthritis Intervention Trial (GAIT), a randomized study involving 1,583 patients with symptomatic knee osteoarthritis that has provided the best evidence to date on these supplements [201,270]. The results demonstrated that glucosamine and chondroitin sulfate, alone or in combination, did not reduce pain more effectively than placebo [270]. A multicenter study done as part of GAIT showed that the combination of glucosamine and chondroitin sulfate did not alter progression of knee osteoarthritis, with no clinically important difference in the loss of joint space width compared with placebo [271]. A report on the two-year results from GAIT noted that there were no significant differences in pain among groups treated with glucosamine, chondroitin sulfate, a combination of the two supplements, or a placebo [272].
A systematic review evaluated the benefit and harm of chondroitin compared with placebo or a comparable oral medication (e.g., NSAIDs, analgesics, opioids, glucosamine) [273]. The review included 43 randomized controlled trials, including 4,962 participants treated with chondroitin and 4,148 participants given placebo or another control. The majority of trials were in osteoarthritis of the knee, with few in the hip or hand, and the length of the trials varied from one month to three years. In studies of less than six months in length, participants treated with chondroitin achieved significantly better pain scores than those given placebo (absolute risk difference: 10% lower); the risk difference for pain was 9% lower in studies longer than six months. A 20% reduction in knee pain was achieved by 53 of 100 participants in the chondroitin group versus 47 of 100 in the placebo group. Differences in the composite of pain, function, and disability favored chondroitin compared with placebo in studies of less than six months. Chondroitin was associated with significantly lower odds of serious adverse events compared with placebo. Chondroitin alone or in combination with glucosamine or another supplement is associated with a significant reduction in pain compared with placebo or an active control; no significant differences in the numbers of adverse events were reported. As stated, the authors found that most of the randomized trials included were of low quality; overall, the benefit of chondroitin was small to moderate [273]. Other analyses and results of randomized controlled trials have indicated that glucosamine and/or chondroitin sulfate have no or modest benefit in terms of pain, function, or structural alterations [274,275,276].
In its guidelines on the management of osteoarthritis of the hand, knee, and hip, the ACR deems the evidence on glucosamine and chondroitin to be inconclusive and conditionally recommends against their use [185]. The 2021 AAOS guideline on the management of knee osteoarthritis suggests that glucosamine and/or chondroitin may be helpful in reducing pain and improving function for patients with mild-to-moderate knee osteoarthritis; however, the evidence is inconsistent and limited, and additional research clarifying the efficacy of each supplement is needed [198]. With regard to osteoarthritis of the glenohumeral joint, the AAOS is not able to recommend for or against the use of glucosamine and/or chondroitin [197].
In a systematic review undertaken to evaluate the effectiveness of 22 herbal medicinal products, there was some evidence of pain relief with topical capsaicin, avocado-soybean unsaponifiables, and SKI306X (a Chinese herbal mixture). However, none of the 22 products had proof of effectiveness beyond doubt [277]. According to a review of studies involving antioxidant and anti-inflammatory supplements, the following cannot be recommended for the treatment of osteoarthritis: vitamin E (alone); a combination of vitamins A, C, and E; ginger; turmeric; omega-3 fatty acids; or Zyflamend (an extract of 10 different herbs) [278]. Additional clinical trials are needed before alternative supplements can be recommended.
Operative treatment for osteoarthritis should be delayed until all possible nonoperative options have been exhausted [19]. In general, the indications for operative treatment are debilitating pain and major limitations in function and activities of daily living [19,185].
In an effort to delay total knee or hip replacement, many have recommended arthroscopic lavage and debridement, but several studies, systematic reviews, and meta-analyses have shown that there is no evidence to support the efficacy of this approach for treatment of osteoarthritis of the knee [279,280,281,282]. In addition, comparisons between the use of intra-articular corticosteroids and joint lavage showed no differences between the two treatments with respect to efficacy or safety [198,253,254]. Arthroscopic lavage and debridement may be useful for removing unstable tissues (such as loose bodies, meniscal tears, or loose cartilage) that are causing mechanical symptoms [19,279].
In its guideline on the management of knee osteoarthritis, the AAOS recommends against performing arthroscopy with debridement or lavage in patients with a primary diagnosis of symptomatic osteoarthritis of the knee [198]. This recommendation does not apply to patients with meniscal tear, loose body, or other mechanical derangement, with concomitant diagnosis of knee osteoarthritis [198]. It is suggested that clinical judgment, along with patient preference, should guide the consideration for meniscectomy. The AAOS found insufficient evidence on arthroscopic treatment of the glenohumeral joint and is therefore unable to recommend for or against the procedure [197].
Experts have described satisfactory outcomes after arthroscopic debridement of the elbow [31,283]. The ideal candidate for the procedure is younger than 60 years of age, is active, and has impingement pain at the extremes of the range of motion but not at the midpoint of the arc of motion or at rest [31,58]. Compared with open debridement, the arthroscopic procedure is associated with decreased intraoperative bleeding and less postoperative pain. The procedure is technically demanding but is safe when performed by an experienced surgeon familiar with the technique [31].
Debridement (through arthroscopy or arthrotomy) of the ankle has relieved pain, decreased swelling and stiffness, and improved the activity level in more than half of patients [32]. Improvement is most likely when debridement is done to remove osteophytes, smooth unstable chondral surfaces, and remove loose bodies [32].
The 2021 AAOS guideline on the treatment of knee osteoarthritis includes a limited recommendation for valgus-producing proximal tibial osteotomy [198]. Despite the lack of a true randomized controlled trial comparing high tibial osteotomy to nonoperative management, the studies reviewed by the AAOS workgroup all agree with the premise that pain is reduced by the procedure [198].
Total arthroplasty (joint replacement) is considered when all other options have failed. Indications for the procedure are severe symptomatic disease (chronic pain and disability) [19]. The procedure has led to high rates of good-to-excellent results when done at the knee and hip and is cost-effective compared with nonoperative management [19,38].
Knee Arthroplasty
In 2018, an estimated 715,203 total knee arthroplasties were completed in the United States [284]. According to the National Institutes of Health, the success of total knee arthroplasty in most patients is strongly supported by more than 20 years of follow-up data, with significant improvement in pain, joint function, and quality of life in 90% of patients [285]. However, some patients will experience prosthesis failure, and risk factors for failure include male gender, age younger than 55 years at the time of surgery, obesity, and the presence of comorbidities. In terms of factors related to the surgeon, greater procedure volume (both of the surgeon and the facility), prosthesis choice, and surgical technique (e.g., proper alignment of the prosthesis) all contribute to better patient outcomes [285]. It is important to note that both knee and hip arthroplasty are associated with a high risk of deep vein thrombosis (DVT) and pulmonary embolism compared with other surgeries. Without prophylaxis, DVT will develop in most patients [286]. Therefore, prophylactic treatment, usually with either low-molecular-weight heparin or warfarin, is recommended for patients undergoing one of these procedures, unless contraindications are present.
Because there are anatomic differences in joint structure and size between men and women, a gender-specific knee prosthesis was designed specifically for women [287]. Researchers believed that the better fit would lead to improvements in recovery and outcomes for women who had total knee arthroplasty. In one study, 85 women who received a standard joint in one knee and the gender-specific joint in the other knee were followed up for two years after the surgery [287]. Patient satisfaction, range of motion while lying, and WOMAC scores were similar for both prostheses. The researchers did note that the standard prostheses appeared to fit at the distal part of the femur better than the gender-specific type; furthermore, the small size of the gender-specific prosthesis exposed more bone and resulted in more bleeding immediately after surgery. Although the study concluded that there were no benefits to the use of gender-specific prostheses in women undergoing total knee arthroplasty, research evaluating long-term effects is necessary.
Postoperative rehabilitation is a necessary component of recovery after operative treatment of osteoarthritis and requires cooperation of the entire multidisciplinary team. In the case of total knee arthroplasty, patients should be guided on a postoperative exercise and rehabilitation plan that focuses on obtaining an acceptable level of joint function, range of motion, and quality of life (e.g., ability to perform activities of daily living unassisted). In some cases, a continuous passive motion device may be used. This device has been suggested as a means to obtain greater range of motion more quickly after surgery [288]. While this may be the case, no long-term benefits (e.g., ultimate range of motion) have been definitively proven, and evidence on the short-term effects are conflicting [286,288,289]. It is not a recommendation of the AAOS or the ACR at this time.
In general, the institution of a structured exercise plan, guided by the physician and physical therapist, will assist patients in regaining range of motion and return to performing daily activities. A daily physical therapy program after total knee arthroplasty should continue for four to six weeks, at which point the patient's needs will be reassessed. According to one study, the greatest improvements in lower-extremity functional status after total knee arthroplasty were demonstrated in the first 12 weeks, with little improvement noted after 26 weeks [290]. By the end of physical therapy, the patient should be able to perform activities of daily living and progress to ambulating on flat surfaces and stairs. Strengthening and stretching exercises focusing on the hamstrings and quadriceps should be incorporated into the program.
There is some debate regarding the importance of supervised outpatient physical therapy compared with exercise programs carried out in the patient's home. One meta-analysis of 10 randomized controlled trials found that supervised physical therapy provided no benefits for patients who were younger at the time of surgery and had few or no comorbidities [291]. However, the researchers noted that there is a lack of evidence regarding the use of outpatient physical therapy for older patients with comorbidities and those who have undergone more complicated surgeries.
One study that included older patients (60 to 79 years of age) was designed to determine the functional differences between the effects of supervised physiotherapy with a standardized home exercise program following total knee arthroplasty [292]. All patients were evaluated for joint range of motion, pain, functional status, overall quality of life, and depressive symptoms. Postoperative assessment showed a significant clinical improvement in both groups, and the authors found no significant difference between the groups in range of motion and functional status.
Hip Arthroplasty
Total hip arthroplasty is also relatively common, with 599,494 procedures completed in 2018 [293]. This procedure is recommended for the treatment of osteoarthritis in older patients for whom nonsurgical interventions have been ineffective. Some data suggest that the benefit of arthroplasty of the hip is greater when done earlier in the course of disease [38]. According to one study, female gender, the presence of comorbidities, contralateral hip osteoarthritis, back pain, and poor preintervention health or mental health status were predictors of poorer outcomes and lesser improvements in quality-of-life measures after total hip arthroplasty [294].
Although steps are taken to prevent it, leg length can be altered as a result of total hip arthroplasty. It is important for the leg on the operative side to be measured and, if there is a discrepancy, corrected with the use of orthotics.
As with knee arthroplasty, individuals who have undergone total hip arthroplasty require a physical therapy and exercise regimen that will allow them to obtain the optimal level of joint function and flexibility. The goals of therapy are the same as those described for total knee arthroplasty. One consideration is when to initiate physical therapy in order to gain the most improvement, particularly considering that improvements seem to plateau after 12 to 26 weeks. In a study of 593 patients who had total hip arthroplasty (performed by six different surgeons using different surgical techniques), 191 began physical therapy on the day of surgery and the remaining 402 patients began physical therapy on postoperative day 1 [295]. The length of stay was significantly shorter for the patients who had early physical therapy (2.16 days compared with 3.38 days).
Another consideration is the type of postoperative exercise and rehabilitation program recommended. One study compared a conventional rehabilitation program with the use of early maximal strength training in patients who had received a total hip replacement [296]. Individuals in the treatment group performed leg press and abduction with the operated leg five times a week for four weeks in addition to the conventional program (supervised physical therapy three to five times per week for four weeks). The researchers found that those who included maximal strength training in their postsurgical physical therapy had a significantly larger increase in muscular strength and a trend toward a better work efficiency than those in the conventional therapy group.
Other Joints
Other joint replacement procedures are not done as widely and are not associated with the same success as knee and hip arthroplasty. The AAOS guideline on the treatment of osteoarthritis of the glenohumeral joint includes a weak recommendation for total shoulder arthroplasty and hemiarthroplasty as options, with a moderate recommendation for total arthroplasty over hemiarthroplasty [197].
The use of total elbow arthroplasty is limited by the high risk for instability and loosening and is rarely used to treat primary osteoarthritis [31,58]. When performed in younger patients, long-term success of the procedure has been limited because of high functional demands [31]. As a result, total replacement should be reserved for patients older than 65 years of age who are willing to accept low levels of activity [31,58].
The complex anatomic and biomechanical features of the ankle joint have challenged the use of joint replacement [32]. New designs of prostheses have led to good-to-excellent outcomes postoperatively, but complications have included osteomyelitis and osteolysis. In addition, only short-term data are available.
An estimated 30.8 million adults have osteoarthritis, making it the most common joint disorder, and this number is expected to rise as the population grows older and lives longer. The disease is a leading cause of activity limitation and absenteeism among working-age adults and is associated with a significant decline in function among older individuals. The etiology of osteoarthritis is complex and not completely understood; some experts have theorized that osteoarthritis represents distinct disease entities according to the joint site, as the risk factors and clinical presentation vary across joints. This variation, along with a lack of correlation between symptoms and radiographic evidence, has created challenges in diagnosing osteoarthritis. In addition, clinicians must consider a wide range of differential diagnoses when evaluating a patient with joint pain. Diagnostic criteria have been well-established for osteoarthritis of the most common joints (knee, hip, and hand), and evidence-based recommendations for diagnosis of the knee and hand have been published. The clinical presentation and history remain the most important components of diagnosis for osteoarthritis at most joint sites. No curative therapy is available for osteoarthritis, and management is thus focused on decreasing pain and increasing function. Evolving evidence has shown that many commonly used treatment options for osteoarthritis offer no or limited benefit. Healthcare professionals must be familiar with the available evidence-based guidelines for the management of osteoarthritis (knee, hip, hand, and shoulder) and discuss appropriate options with their patients. A shared decision-making process and a multidisciplinary approach are keys to successful management.
1. United States Bone and Joint Initiative. The Burden of Musculoskeletal Diseases in the United States. 3rd ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2014.
2. American Academy of Orthopaedic Surgeons. BJD Measures Global Burden of Disease. Available at https://www.aaos.org/AAOSNow/2013/Feb/clinical/clinical7/?ssopc=1. Last accessed September 7, 2022.
3. The Burden of Musculoskeletal Diseases in the United States. Activity Limitation Due to Select Medical Conditions. Available at https://www.boneandjointburden.org/fourth-edition/ic0/activity-limitation-due-select-medical-conditions. Last accessed September 7, 2022.
4. Arthritis Foundation. Arthritis by the Numbers: Book of Trusted Facts and Figures. Available at https://www.arthritis.org/getmedia/e1256607-fa87-4593-aa8a-8db4f291072a/2019-abtn-final-march-2019.pdf. Last accessed September 7, 2022.
5. Lawrence R, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35.
6. Centers for Disease Control and Prevention. Arthritis-Related Statistics. Available at https://www.cdc.gov/arthritis/data_statistics/arthritis-related-stats.htm. Last accessed September 7, 2022.
7. U.S. Bone and Joint Initiative. Fact Sheet: By the Numbers, Arthritis and Other Rheumatic Conditions (AORC). Available at https://www.boneandjointburden.org/docs/By%20The%20Numbers%20-%20Arthritis_4E_Nov%202018%20%282%29.pdf. Last accessed September 7, 2022.
8. Agency for Healthcare Research and Quality. HCUP Facts and Figures, 2009. Available at https://www.hcup-us.ahrq.gov/reports/factsandfigures/2009/pdfs/FF_report_2009.pdf. Last accessed September 7, 2022.
9. Lozada CJ. Osteoarthritis. Available at https://emedicine.medscape.com/article/330487-overview. Last accessed September 7, 2022.
10. Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Osteoarthritis and absenteeism costs: evidence from U.S. National Survey Data.J Occup Environ Med. 2010;52(3):263-268.
11. Jinks C, Jordan K, Croft P. Osteoarthritis as a public health problem: the impact of developing knee pain on physical function in adults living in the community: (KNEST 3). Rheumatology. 2007;46(5):877-881.
12. Guccione A, Felson DR, Anderson JJ, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84(3):351-358.
13. Healthcare Cost and Utilization Project. Most Common Diagnoses for Inpatient Stays: 2018. Available at https://www.hcup-us.ahrq.gov/faststats/NationalDiagnosesServlet. Last accessed September 7, 2022.
14. Agency for Healthcare Research and Quality. Agency News and Notes [archive]. Hospitalizations For Osteoarthritis Are Rising Sharply. Available at https://archive.ahrq.gov/research/nov08/1108RA33.htm. Last accessed September 7, 2022.
15. Agency for Healthcare Research and Quality. HCUP Projections: Mobility/Orthopedic Procedures 2003 to 2012. Available at https://www.hcup-us.ahrq.gov/reports/projections/2012-03.pdf. Last accessed September 7, 2022.
17. Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Insurer and out-of-pocket costs of osteoarthritis in the U.S.: evidence from national survey data. Arthritis Rheum. 2009;60(12):3546-3553.
18. Agency for Healthcare Research and Quality. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2017. Available at https://www.hcup-us.ahrq.gov/reports/statbriefs/sb261-Most-Expensive-Hospital-Conditions-2017.jsp. Last accessed September 7, 2022.
20. Freedman K, Bernstein J. The adequacy of medical school education in musculoskeletal medicine. J Bone Joint Surg Am. 1998;80(10):1421-1427.
21. Akesson K, Dreinhöfer KE, Woolf AD. Improved education in musculoskeletal conditions is necessary for all doctors. Bull World Health Organ. 2003;81(9):677-683.
22. Matzkin E, Smith EL, Freccero D, Richardson AB. Adequacy of education in musculoskeletal medicine. J Bone Joint Surg Am. 2005;87(2):310-314.
23. Schmale G. More evidence of educational inadequacies in musculoskeletal medicine. Clin Orthop Relat Res. 2005;(437):251-259.
24. Day C, Yeh AC, Franko O, Ramirez M, Krupat E. Musculoskeletal medicine: an assessment of the attitudes and knowledge of medical students at Harvard Medical School. Acad Med. 2007;82(5):452-457.
25. Association of American Medical Colleges. Report VII. Contemporary Issues in Medicine: Musculoskeletal Medicine Education. Medical School Objectives Project. Washington, DC: Association of American Medical Colleges; 2005.
26. Lynch J, Schmale GA, Schaad DC, Leopold SS. Important demographic variables impact the musculoskeletal knowledge and confidence of academic primary care physicians. J Bone Joint Surg Am. 2006;88(7):1589-1595.
27. Petrella R, Davis P. Improving management of musculoskeletal disorders in primary care: the Joint Adventures Program. Clin Rheumatol. 2007;26(7):1061-1066.
28. Sayre E, Li LC, Kopec JA, Esdaile JM, Bar S, Cibere J. The effect of disease site (knee, hip, hand, foot, lower back or neck) on employment reduction due to osteoarthritis. PLoS. 2010;5(5):e10470.
29. Kalichman L, Li L, Kim DH, et al. Facet joint osteoarthritis and low back pain in the community-based population. Spine (Phila Pa 1976). 2008;33(23):2560-2565.
30. Millett P, Gobezie R, Boykin RE. Shoulder osteoarthritis: diagnosis and management. Am Fam Physician. 2008;78(5):605-611, 612.
31. Cheung EV, Adams R, Morrey BF. Primary osteoarthritis of the elbow: current treatment options. J Am Acad Orthop Surg. 2008;16(2):77-87.
32. Chou LB, Coughlin MT, Hansen S Jr, et al. Osteoarthritis of the ankle: the role of arthroplasty. J Am Acad Orthop Surg. 2008;16(5):249-259.
33. Brown T, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20(10):739-744.
34. Maffulli N, Longo UG, Gougoulias N, Caine D, Denaro V. Sports injuries: a review of outcomes. Br Med Bull. 2011;97:47-80.
35. National Health Service UK. Overview: Osteoarthritis. Available at https://www.nhs.uk/conditions/osteoarthritis. Last accessed September 7, 2022.
36. Feydy A, Pluot E, Guerini H, Drape J-L. Osteoarthritis of the wrist and hand, and spine. Radiol Clin N Am. 2009;47:723-759.
37. Valderrabano V, Horisberger M, Russell I, Dougall H, Hintermann B. Etiology of ankle osteoarthritis. Clin Orthop Relat Res. 2009;467(7):1800-1806.
39. Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskeletal Disord. 2008;9:116.
40. Altman R, Kuritzky L, Ruoff G. Improving long-term management of osteoarthritis: strategies for primary care physicians. J Fam Pract. 2009;58(2 Suppl):S17-S24.
41. Kornaat PR, Bloem JL, Ceulemans RYT, et al. Osteoarthritis of the knee: association between clinical features and MR imaging findings. Radiology. 2006;239(3):811-817.
42. Centers for Disease Control and Prevention. Osteoarthritis (OA). Available at https://www.cdc.gov/arthritis/basics/osteoarthritis.htm. Last accessed September 7, 2022.
43. Kim C, Linsenmeyer KD, Vlad S, et al. Prevalence of radiographic and symptomatic hip osteoarthritis in an urban U.S. community: the Framingham Osteoarthritis Study. Arthritis Rheumatol. 2014;66(11):3013-3017.
44. Zhang Y, Niu J, Kelly-Hayes M, Chaisson CE, Aliabadi P, Felson DT. Prevalence of symptomatic hand osteoarthritis and its impact on functional status among the elderly: the Framingham Study. Am J Epidemiol. 2002;156(11):1021-1027.
45. Jordan J, Helmick CG, Renner JB, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007;34(1):172-180.
46. Jordan J, Helmick CG, Renner JB, et al. Prevalence of hip symptoms and radiographic and symptomatic hip osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2009;36(4):809-815.
47. Hootman JM, Helmick CG, Barbour KE, Theis KA, Boring MA. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitation among U.S. adults, 2015–2040. Arthritis Rheumatol. 2016;68(7):1582-1587.
48. Bolen J, Schieb L, Hootman JM, et al. Differences in the prevalence and impact of arthritis among racial/ethnic groups in the United States, National Health Interview Survey, 2002, 2003, and 2006. Prev Chronic Dis. 2010;7(3):A64.
49. Cunningham L, Kelsey JL. Epidemiology of musculoskeletal impairments and associated disability. Am J Public Health. 1984;74(6):574-579.
50. Theis KA, Murphy LB, Guglielmo D, et al. Prevalence of arthritis and arthritis-attributable activity limitation—United States, 2016-2018. MMWR. 2021;70(40):1401-1407.
51. McDermott KW, Roemer M. Most Frequent Principal Diagnoses for Inpatient Stays in U.S. Hospitals, 2018. HCUP Statistical Brief #277. Rockville, MD: Agency for Healthcare Research and Quality; 2021.
52. Pfuntner A, Wier LM, Stocks C. Most Frequent Conditions in U.S. Hospitals, 2011. HCUP Statistical Brief #162. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
53. National Center for Health Statistics. Ambulatory Health Care Data. 2018 NAMCS Summary Tables. Available at https://www.cdc.gov/nchs/ahcd/web_tables.htm. Last accessed September 7, 2022.
54. Karlson E, Mandl LA, Aweh GN, Sangha O, Liang MH, Grodstein F. Total hip replacement due to osteoarthritis: the importance of age, obesity, and other modifiable risk factors. Am J Med. 2003;114(2):93-98.
55. Reichmann WM, Katz JN, Kessler CL, Jordan JM, Losina E. Determinants of self-reported health status in a population-based sample of persons with radiographic knee osteoarthritis. Arthritis Rheum. 2009;61(8):1046-1053.
56. Dillon C, Hirsch R, Rasch EK, Gu Q. Symptomatic hand osteoarthritis in the United States: prevalence and functional impairment estimates from the third U.S. National Health and Nutrition Examination Survey, 1991–1994. Am J Phys Med Rehabil. 2007;86(1):12-21.
57. Kalichman L, Hernandez-Molina G. Hand osteoarthritis: an epidemiological perspective. Semin Arthritis Rheum. 2010;39(6):465-476.
58. Gramstad G, Galatz LM. Management of elbow osteoarthritis. J Bone Joint Surg Am. 2006;88:421-430.
59. The Burden of Musculoskeletal Diseases in the United States. Arthritis. Available at https://www.boneandjointburden.org/fourth-edition/iii0/arthritis. Last accessed September 7, 2022.
60. The Burden of Musculoskeletal Diseases in the United States. Healthcare Utilization. Available at https://www.boneandjointburden.org/fourth-edition/iiia20/healthcare-utilization. Last accessed September 7, 2022.
62. Dillon C, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991. J Rheumatol. 2006;33(11):2271-2279.
63. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990– a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163-2196.
64. Cui A, Li H, Wang D, Zhong J, Chen Y, Lu H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. eClin Med. 2020;29:100587.
65. Kellgren J, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494-501.
66. Sowers M, Lachance L, Hochberg M, Jamadar D. Radiographically defined osteoarthritis of the hand and knee in young and middle-aged African American and Caucasian women. Osteoarthritis Cartilage. 2000;8(2):69-77.
67. Allen K. Racial and ethnic disparities in osteoarthritis phenotypes. Curr Opin Rheumatol. 2010;22(5):528-532.
68. Nelson A, Braga L, Renner JB, et al. Characterization of individual radiographic features of hip osteoarthritis in African American and White women and men: the Johnston County Osteoarthritis Project. Arthritis Care Res (Hoboken). 2010;62(2):190-197.
70. Hunter DJ. Imaging insights on the epidemiology and pathophysiology of osteoarthritis. Rheum Dis Clin N Am. 2009;35(4):447-463.
71. American College of Rheumatology. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43(9):1905-1915.
72. Pollard TCB, Gwilym SE, Carr AJ. The assessment of early osteoarthritis. J Bone Joint Surg Br. 2008;90-B(4):411-421.
73. Dequeker J, Luyten FP. The history of osteoarthritis-osteoarthrosis. Ann Rheum Dis. 2008;67(1):5-10.
74. Ding C, Jones G, Wluka AE, Cicuttini F. What can we learn about osteoarthritis by studying a healthy person against a person with early onset of disease? Curr Opin Rheumatol. 2010;22(5):520-527.
75. Dequeker J, Dieppe PA. Disorders of bone cartilage and connective tissue. In: Klippel J, Dieppe PA (eds). Rheumatology. 2nd ed. London: Mosby; 1998.
76. Dijkgraaf L, de Bont LG, Boering G, Liem RS. The structure, biochemistry, and metabolism of osteoarthritic cartilage: a review of the literature. J Oral Maxillofac Surg. 1995;53:1182-1192.
77. Pritzker K. Pathology of osteoarthritis. In: Brandt K, Doherty M, Lohmander LS (eds). Osteoarthritis. 2nd ed. Oxford: Oxford University Press; 2003: 49-58.
78. Sandell L. Modern molecular analysis of a traditional disease: progression in osteoarthritis. Arthritis Rheum. 2007;56:2474-2477.
79. Hinton R, Moody RL, Davis AW, Thomas SF. Osteoarthritis: diagnosis and therapeutic considerations. Am Fam Physician. 2002;65(5):841-848.
80. Katz J, Agrawal S, Velasquez M. Getting to the heart of the matter: osteoarthritis takes its place as part of the metabolic syndrome. Curr Opin Rheumatol. 2010;22(5):512-519.
81. Torres L, Dunlop DD, Peterfy C, et al. The relationship between specific tissue lesions and pain severity in persons with knee osteoarthritis. Osteoarthritis Cartilage. 2006;14(10):1033-1040.
82. Felson D, Niu J, Guermazi A, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56(9):2986-2992.
83. Lo G, McAlindon TE, Niu J, et al. Bone marrow lesions and joint effusion are strongly and independently associated with weight-bearing pain in knee osteoarthritis: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2009;17(12):1562-1569.
84. Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635-646.
85. Peach C, Carr AJ, Laughlin J. Recent advances in the genetic investigation of osteoarthritis. Trends Mol Med. 2005;11:186-191.
86. Saltzman C, Salamon ML, Blanchard GM, et al. Epidemiology of ankle arthritis: report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44-46.
87. Bierma-Zeinstra S, Koes BW. Risk factors and prognostic factors of hip and knee osteoarthritis. Nat Clin Pract Rheumatol. 2007;3(2):78-85.
88. Altman R. Early management of osteoarthritis. Am J Manag Care. 2010;16(Suppl Management):S41-S47.
90. Panoutsopoulou K, Zeggini E. Advances in osteoarthritis genetics. J Med Genet. 2013;50(11):715-724.
91. Rodriguez-Fontenla C, Calaza M, Evangelou E, et al. Assessment of osteoarthritis candidate genes in a meta-analysis of nine genome-wide association studies. Arthritis Rheumatol. 2014;66(4):940-949.
92. Ramos YFM, den Hollander W, Bovee JV, et al. Genes involved in the osteoarthritis process identified through genome-wide expression analysis in articular cartilage; the RAAK Study. PLoS One. 2014;9(7):e103056.
93. arcOGEN Consortium, arcOGEN Collaborators, Zeggini E, et al. Identification of new susceptibility loci for osteoarthritis (arcOGEN): a genome-wide association study. Lancet. 2012;380(9844):815-823.
94. Valdes AM, Styrkarsdottir U, Doherty M, et al. Large scale replication study of the association between HLA and II/BTNL2 variants and osteoarthritis of the knee in European-descent populations. PLoS One. 2011;6(8):e23371.
95. Tachmazidou I, Hatzikotoulas K, Southam L, et al. Identification of new therapeutic targets for osteoarthritis through genome-wide analyses of UK Biobank data. Nature Genetics. 2019;51:230-236.
96. Cai Z, Long T, Zhao Y, Lin R, Wang Y. Epigenetic regulation in knee osteoarthritis. Front Genet. 2022;13:942982.
97. Felson D, Zhang Y, Anthony JM, Naimark A, Anderson JJ. Weight loss reduces the risk for symptomatic osteoarthritis in women. The Framingham Study. Ann Intern Med. 1992;116:535-539.
98. Felson D, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum. 1998;41(8):1343-1355.
99. Coggon D, Reading I, Croft P, McLaren M, Barrett D, Cooper C. Knee osteoarthritis and obesity. Int J Obes Relat Metab Disord. 2001;25(5):622-627.
100. Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthr Cartil. 2010;18(1):24-33.
101. Plotnikoff R, Karunamuni N, Lytwyak E, et al. Osteoarthritis prevalence and modifiable factors: a population study. BMC Public Health. 2015;1195.
102. Felson D, Zhang Y, Hannan MT, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 1997;40(4):728-733.
103. Lohmander LS, Gerhardsson M, Rollof J, Nilsson PM, Engström G. Incidence of severe knee and hip osteoarthritis in relation to different measures of body mass: a population-based prospective cohort study. Ann Rheum Dis. 2009;68(4):490-496.
104. Chaganti RK, Lane N. Risk factors for incident osteoarthritis of the hip and knee. Curr Rev Musculoskelet Med. 2011;4(3):99-104.
105. Maleki-Fischbach M, Jordan JM. Sex differences in magnetic resonance imaging-based biomarkers and in those of joint metabolism. Arthritis Res Ther. 2010;12(4):1-8.
106. Flugsrud G, Nordsletten L, Espehaug B, Havelin LI, Engeland A, Meyer HE. The impact of body mass index on later total hip arthroplasty for primary osteoarthritis: a cohort study in 1.2 million persons. Arthritis Rheum. 2006;54(3):802-807.
107. Liu B, Balkwill A, Banks E, Cooper C, Green J, Bersal V. Relationship of height, weight and body mass index to the risk of hip and knee replacements in middle-aged women. Rheumatology. 2007;46(5):861-867.
108. Wang Y, Simpson JA, Wluka AE, et al. Relationship between body adiposity measures and risk of primary knee and hip replacement for osteoarthritis: a prospective cohort study. Arthritis Res Ther. 2009;11(2):R31.
109. Jarvholm B, Lewold S, Malchau H, Vingard E. Age, body weight, smoking habits and the risk of severe osteoarthritis in the hip and knee in men. Eur J Epidemiol. 2005;20(6):537-542.
110. Vignon E, Valat JP, Rossignol M, et al. Osteoarthritis of the knee and hip and activity: a systematic international review and synthesis (OASIS). Joint Bone Spine. 2006;73(4):442-455.
111. Johnsen MB, Hellevik AI, Baste V, et al. Leisure time physical activity and the risk of hip or knee replacement due to primary osteoarthritis: a population based cohort study (The HUNT Study). BMC Musculoskelet Disord. 2016;17:86.
112. Richmond SA, Fukuchi RK, Ezzat A, Schneider K, Schneider G, Emery CA. Are joint injury, sport activity, physical activity, obesity, or occupational activities predictors for osteoarthritis? A systematic review. J Orthop Sports Phys Ther. 2013;43(8):515-519.
113. Roos H, Laurén M, Adalberth T, Roos EM, Jonsson K, Lohmander LS. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum. 1998;41:687-693.
114. Englund M, Roos EM, Lohmander LS. Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis: a sixteen-year follow-up of meniscectomy with matched controls. Arthritis Rheum. 2003;48:2178-2187.
115. Palmer KT. Occupational activities and osteoarthritis of the knee. Br Med Bull. 2012;102(1):147-170.
116. Rossignol M, Leclerc A, Hilliquin P, et al. Primary osteoarthritis and occupations: a national cross sectional survey of 10,412 symptomatic patients. Occup Environ Med. 2003;60(11):882-886.
117. Rossignol M, Leclerc A, Allaert FA, et al. Primary osteoarthritis of the hip, knee, and hand in relation to occupational exposure. Occup Environ Med. 2005;62(11):772-777.
118. Unverzagt S, Bolm-Audorff U, Frese T, et al. Influence of physically demanding occupations on the development of osteoarthritis of the hip: a systematic review. J Occup Med Toxicol. 2022;17(1):18.
119. Jarvholm B, From C, Lewold S, Malchau H, Vingard E. Incidence of surgically treated osteoarthritis in the hip and knee in male construction workers. Occup Environ Med. 2008;65(4):275-278.
120. Andersen S. Thygesen LC, Davidsen M, Helweg-Larsen K. Cumulative years in occupation and the risk of hip or knee osteoarthritis in men and women. Occup Environ Med. 2012;69(5):325-330.
121. Jensen LK. Knee osteoarthritis: influence of work involving heavy lifting, kneeling, climbing stairs or ladders, or kneeling/squatting combined with heavy lifting. Occup Environ Med. 2008;65(2):72-89.
122. Coggon D, Croft P, Kellingray S, Barrett D, McLaren M, Cooper C. Occupational physical activities and osteoarthritis of the knee. Arthritis Rheum. 2000;43(7):1443-1449.
123. Vrezas I, Elsner G, Bolm-Audorff U, Abolmaali N, Seidler A. Case-control study of knee osteoarthritis and lifestyle factors considering their interaction with physical workload. Int Arch Occup Environ Health. 2010;83(3):291-300.
124. Hurley M, Scott DL, Rees J, Newham DJ. Sensorimotor changes and functional performance in patients with knee osteoarthritis. Ann Rheum Dis. 1997;56:641-648.
125. Slemenda C, Brandt KD, Hellman DK, et al. Quadriceps weakness and osteoarthritis of the knee. Ann Intern Med. 1997;127(2):97-104.
126. Slemenda C, Heilman DK, Brandt KD, et al. Reduced quadriceps strength relative to body weight: a risk factor for knee osteoarthritis in women? Arthritis Rheum. 1998;41(11):1951-1959.
127. Bennell KL, Hunt MA, Wrigley TV, Lim BW, Hinman RS. Role of muscle in the genesis and management of knee osteoarthritis. Rheum Dis Clin North Am. 2008;34(3):731-754.
128. Liikavainio T, Lyytinen T, Tyrväinen E, Sipiläa S, Arokoski JP. Physical function and properties of quadriceps femoris muscle in men with knee osteoarthritis. Arch Phys Med Rehabil. 2008;89(11):2185-2194.
129. Segal N, Torner JC, Felson D, et al. Effect of thigh strength on incident radiographic and symptomatic knee osteoarthritis in a longitudinal cohort. Arthritis Rheum. 2009;61(9):1210-1217.
130. Segal NA, Glass NA. Is quadriceps muscle weakness a risk factor for incident or progressive knee osteoarthritis? Phys Sports Med. 2011;39(4):44-50.
131. Segal NA, Glass NA, Felson DT, et al. Effect of quadriceps strength and proprioception on risk for knee osteoarthritis. Med Sci Sports Exerc. 2010;42(11):2081-2088.
132. Alnahdi AH, Zeni JA, Snyder-Mackler L. Muscle impairments in patients with knee osteoarthritis. Sports Health. 2012;4(4):284-292.
133. Diracoglu D, Baskent A, Yagci I, Ozcakar L, Aydin R. Isokenitic strength measurements in early knee osteoarthritis. Acta Rheumatol Port. 2009;34(1):72-77.
134. Emrani A, Bagheri H, Hadian MR, Jabal-Ameli M, Olyaei GR, Talebian S. Isokinetic strength and functional status in knee osteoarthritis. J Phys Ther Sci. 2006;18(2):107-114.
135. Tetsworth K, Paley D. Malalignment and degenerative arthropathy. Orthop Clin North Am. 1994;25:367-377.
136. Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001;286:188-195.
137. Hunter D, Niu J, Felson DT, et al. Knee alignment does not predict incident osteoarthritis: the Framingham Osteoarthritis Study. Arthritis Rheum. 2007;56(4):1212-1218.
138. Sharma L, Song J, Dunlop D, et al. Varus and valgus alignment and incident and progress knee osteoarthritis. Ann Rheum Dis. 2010;69(11):1940-1945.
139. Felson DT, Niu J, Gross KD, et al. Valgus malalignment is a risk factor for lateral knee osteoarthritis incidence and progression: findings from the Multicenter Osteoarthritis Study and the Osteoarthritis Initiative. Arthritis Rheum. 2013;62(2):355-362.
140. Gross K, Niu J, Zhang YQ, et al. Varus foot alignment and hip conditions in older adults. Arthritis Rheum. 2007;56(9):2993-2998.
141. Zhang Y, Hannan MT, Chaisson CE, et al. Bone mineral density and risk of incident and progressive radiographic knee osteoarthritis in women: the Framingham Study. J Rheumatol. 2000;27(4):1032-1037.
142. Hardcastle SA, Dieppe P, Gregson CL, Smith GD, Tobias JH. Osteoarthritis and bone mineral density: are strong bones bad for joints? Bonekey Reports. 2015;624(4):1-8.
143. Chaganti RK, Parimi N, Lang T, et al. Bone mineral density and prevalent osteoarthritis of the hip in older men for the Osteoporotic Fractures in Men (MrOS) Study Group. Osteoporos Int. 2010;21(8):1307-1316.
144. Nevitt MC, Zhang Y, Javaid MK, Neogi T, Curtis JR, Niu J et al. High systemic bone mineral density increases the risk of incident knee OA and joint space narrowing, but not radiographic progression of existing knee OA: the MOST study. Ann RheumDis. 2010;69(1):163-168.
145. Hart DJ, Mootoosamy I, Doyle DV, Spector TD. The relationship between osteoarthritis and osteoporosis in the general population: the Chingford Study. Ann Rheum Dis. 1994;53(1):158-162.
146. Peel NF, Barrington NA, Blumsohn A, Colwell A, Hannon R, Eastell R. Bone mineral density and bone turnover in spinal osteoarthrosis. Ann Rheum Dis. 1995;54(11):867-871.
147. Hardcastle SA, Dieppe P, Gregson CL, Arden NK, Spector TD, Hart DJ, Edwards MH et al. Osteophytes, enthesophyte and high bone mass; a bone-forming triad with relevance for osteoarthritis? Arthritis Rheum. 2014;66(9):2429-2439.
148. Gregson C, Leo PJ, Clark GR, et al. A GWAS in an extreme high bone mass population shows excess signal from genes associated with BMD in the normal population. Bone Abstracts. 2013;1:31.
149. McAlindon T, Jacques P, Zhang Y, et al. Do antioxidant micronutrients protect against the development and progression of knee osteoarthritis? Arthritis Rheum. 1996;39(4):648-656.
150. Li H, Zeng C, Wei J, et al. Associations between dietary antioxidants intake and radiographic knee osteoarthritis. Clin Rheumatol. 2016;35(6):1585-1592.
151. Chang Z, Huo L, Li P, Wu Y, Zhang P. Ascorbic acid provides protection for human chondrocytes against oxidative stress. Mol Med Rep. 2015;12(5):7086-7092.
152. McAlindon T, Felson DT, Zhang Y. Relation of dietary intake and serum levels of vitamin D to progression of osteoarthritis of the knee among participants in the Framingham Study. Ann Intern Med. 1996;125:353-359.
153. McAlindon T, LaValley M, Schneider E, et al. Effect of vitamin D supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis: a randomized controlled trial. JAMA. 2013;309(2):155-162.
154. Jin X, Jones G, Cicuttini F, et al. Effect of vitamin D supplementation on tibial cartilage volume and knee pain among patients with symptomatic knee osteoarthritis: a randomized clinical trial. JAMA. 2016;315(10):1005-1013.
155. Lane N, Gore LR, Cummings SR. Serum vitamin D levels and incident changes of radiographic hip osteoarthritis: a longitudinal study. Study of Osteoporotic Fractures Research Group. Arthritis Rheum. 1999;42:854-860.
156. Chaganti R, Parimi N, Cawthon P, Dam TL, Nevitt MC, Lane NE. Association of 25-hydroxyvitamin D with prevalent osteoarthritis of the hip in elderly men: the Osteoporotic Fractures in Men Study. Arthritis Rheum. 2010;62(2):511-514.
157. Laslett LL, Quinn S, Burgess JR, et al. Moderate vitamin D deficiency is associated with changes in knee and hip pain in older adults: a 5-year longitudinal study. Ann Rheum Dis. 2014;73(4):697-703.
158. Alkan G, Akgol G. Do vitamin D levels affect the clinical prognoses of patients with knee osteoarthritis? J Back Musculoskelet Rehabil. 2017;30(4):897-901.
159. Cakar M, Ayanoglu S, Cauuk H, Seyran M, Dedeoglu SS, Gurbuz H. Association between vitain D concentrations and knee pain in patients with osteoarthritis. Peer J. 2018;6:e4670.
160. Roman-Blas JA, Castañeda S, Largo R, Herrero-Beaumont G. Osteoarthritis associated with estrogen deficiency. Arthritis Res Ther. 2009;11(5):241.
161. Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13:769-781.
162. Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778-799.
163. Braidman IP, Hainey L, Batra G, Selby PL, Saunders PT, Hoyland JA. Localization of estrogen receptor beta protein expression in adult human bone. J Bone Miner Res. 2001;16:214-220.
164. Dietrich W, Haitel A, Holzer G, Huber JC, Kolbus A, Tschugguel W. Estrogen receptor-beta is the predominant estrogen receptor subtype in normal human synovia. J Soc Gynecol Investig. 2006;13:512-517.
165. Sapir-Koren R, Livshits G. Postmenopausal osteoporosis in rheumatoid arthritis: the estrogen deficiency-immune mechanisms link. Bone. 2017;103:102-115.
166. Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman BN, Aliabadi P, Levy D. The incidence and natural history of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1995;38:1500-1505.
167. Punzi L, Ramonda R, Sfriso P. Erosive osteoarthritis. Best Pract Res Clin Rheumatol. 2004;18:739-758.
168. Sowers MR, McConnell D, Jannausch M, Buyuktur AG, Hochberg M, Jamadar DA. Estradiol and its metabolites and their association with knee osteoarthritis. Arthritis Rheum. 2006;54:2481-2487.
169. Cibere J. Do we need radiographs to diagnose osteoarthritis? Best Pract Res Clin. 2006;20:27-38.
171. Kloppenburg M, Kroon FPB, Blanco FJ, et al. 2018 update of the EULAR recommendations for the management of hand osteoarthritis. Ann Rheum Dis. 2019;78:16-24.
172. Hunter D, Lo GH. The management of osteoarthritis: an overview and call to appropriate conservative treatment. Rheum Dis Clin N Am. 2008;34:689-712.
173. Zhang W, Doherty M, Peat G, et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis. 2010;69(3):483-489.
174. Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039-1049.
175. Altman R, Alarcon G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991;34(5):505-514.
176. Altman R, Alarcon G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum. 1990;33:1601-1610.
177. Johns Hopkins Arthritis Center. ACR Diagnostic Guidelines. Available at https://www.hopkinsarthritis.org/physician-corner/education/arthritis-education-diagnostic-guidelines. Last accessed September 7, 2022.
178. Zhang W, Doherty M, Leeb BF, et al. EULAR evidence based recommendations for the management of hand osteoarthritis: report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT).Ann Rheum Dis. 2007;66(3):377-388.
179. Bedson J, Mottram S, Thomas E, Peat G. Knee pain and osteoarthritis in the general population: what influences patients to consult? Fam Pract. 2007;24(5):443-453.
180. Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001;60:91-97.
181. Burns R, Graney MJ, Lummus AC, Nichols LO, Martindale-Adams J. Differences of self-reported osteoarthritis disability and race.J Natl Med Assoc. 2007;99(9):1046-1051.
182. Allen K, Helmick CG, Schwartz TA, DeVellis RF, Renner JB, Jordan JM. Racial differences in self-reported pain and function among individuals with radiographic hip and knee osteoarthritis: the Johnston County Osteoarthritis Project. Osteoarthritis Cartilage. 2009;17(9):1132-1136.
183. Committee on Health Literacy Board on Neuroscience and Behavioral Health. Health Literacy. A Prescription to End Confusion. Washington, DC: The National Academies Press; 2004.
184. Kirsch I, Jungeblut A, Jenkins L, Kolstad A. Adult Literacy in America: A First Look at the Results of the National Adult Literacy Survey (NALS). Washington, DC: National Center for Education Statistics, U.S. Department of Education; 1993.
185. Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020;72(2):220-233.
186. The New York City Department of Health and Mental Hygiene. Preventing, diagnosing, and managing osteoarthritis of the hip and knee. City Health Information. 2012;31(2):9-16.
187. Gomoll A, Katz JN, Warner JJ, Millett PJ. Rotator cuff disorders: recognition and management among patients with shoulder pain. Arthritis Rheum. 2004;50(12):3751-3761.
188. Warner J, Iannotti JP, Flatow EL (eds). Complex and Revision Problems in Shoulder Surgery. 2nd ed. Philadelphia, PA: Lippincott, Williams, and Wilkins; 2005.
189. Li SF, Henderson J, Dickman E, Darzynkiewicz R. Laboratory tests in adults with monoarticular arthritis: can they rule out a septic joint? Acad Emerg Med. 2004;11(3):276-280.
191. Ashraf S, Radhi M, Gowler P, et al. The polyadenylation inhibitor cordycepin reduces pain, inflammation and joint pathology in rodent models of osteoarthritis. Scientific Reports. 2019;9: 4696.
192. Global Healthy Living Foundation. New Medications for Osteoarthritis. Available at https://creakyjoints.org/education/osteoarthritis/new-drugs. Last accessed September 7, 2022.
193. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis. Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137-162.
194. Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis. Part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18(4):476-499.
195. Hochberg M, Altman RD, Brandt KD, et al. Guidelines for the medical management of osteoarthritis. Part I: osteoarthritis of the hip. Arthritis Rheum. 1995;38(11):1535-1540.
196. Hochberg M, Altman RD, Brandt KD, et al. Guidelines for the medical management of osteoarthritis. Part II: osteoarthritis of the knee. Arthritis Rheum. 1995;38(11):1541-1546.
197. Journal of the American Academy of Orthopaedic Surgeons. Treatment of Glenohumeral Osteoarthritis. Available at https://journals.lww.com/jaaos/Fulltext/2010/06000/Treatment_of_Glenohumeral_Osteoarthritis.10.aspx. Last accessed September 7, 2022.
198. American Academy of Orthopaedic Surgeons. Management of Osteoarthritis (OA) of the Knee (Non-Arthroplasty). Available at https://www.aaos.org/globalassets/quality-and-practice-resources/osteoarthritis-of-the-knee/oak3cpg.pdf. Last accessed September 7, 2022.
199. National Clinical Guideline Centre. Osteoarthritis. Care and Management in Adults. London: National Institute for Health and Care Excellence (NICE); 2014.
200. Agency for Healthcare Research and Quality. Comparative Effectiveness Review No. 38: Analgesics for Osteoarthritis: An Update of the 2006 Comparative Effectiveness Review. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
201. Samson D, Grant MD, Ratko TA, Bonnell CJ, Ziegler KM, Aronson N. Treatment of Primary and Secondary Osteoarthritis of the Knee. Evidence Report/Technology Assessment No. 157. Prepared by Blue Cross and Blue Shield Association Technology Evaluation Center Evidence-based Practice Center under Contract No. 290-02-0026. Rockville, MD: Agency for Healthcare Research and Quality; 2007.
202. Agency for Healthcare Research and Quality. Total Knee Replacement. Evidence Report/Technology Assessment No. 86. Rockville, MD: Agency for Healthcare Research and Quality; 2003.
203. Tejero S, Prada-Chamorro E, González-Martin D, et al. Conservative treatment of ankle osteoarthritis. J Clin Med. 2021;10(19):4561.
204. American College of Rheumatology. Position Statement on Interdisciplinary Care for Patients with Rheumatic and Musculoskeletal Diseases, 2015. Available at https://www.rheumatology.org/Portals/0/Files/Multidisciplinary%20Care%20for%20Patients%20with%20Rheumatic%20and%20Musculoskeletal%20Disease.pdf. Last accessed September 7, 2022.
205. Langworthy M, Saad A, Langworthy NM. Conservative treatment modalities and outcomes for osteoarthritis: the concomitant pyramid of treatment. Phys Sports Med. 2010;38(2):133-145.
206. Jordan K, Arden NK, Doherty M, et al. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2003;62(12):1145-1155.
207. Agency for Healthcare Policy and Research. Managing Osteoarthritis: Helping the Elderly Maintain Function and Mobility. Research in Action, Issue 4. AHRQ Publication No. 02-0023. Rockville, MD: Agency for Health Care Policy and Research; 2002.
208. Stacey D, Hawker G, Dervin G, et al. Improving shared decision making in osteoarthritis. BMJ. 2008;336(7650):954-955.
209. Kutner M, Greenberg E, JinY, Paulsen C. The Health Literacy of America's Adults: Results From the 2003 National Assessment of Adult Literacy (NCES 2006-483). Washington, DC: National Center for Education Statistics; 2006.
210. Program for the International Assessment of Adult Competencies (PIACC). Highlights of PIACC 2017 U.S. Results. Available at https://nces.ed.gov/surveys/piaac/national_results.asp. Last accessed September 7, 2022.
211. Powers B, Trinh JV, Bosworth HB. Can this patient read and understand written health information? JAMA. 2010;304(1):76-84.
212. Shah L, West P, Bremmeyr K, Savoy-Moore RT. Health literacy instrument in family medicine: the "newest vital sign" ease of use and correlates. J Am Board Fam Med. 2010;23(2):195-203.
213. Jeppesen K, Coyle JD, Miser WF. Screening questions to predict limited health literacy: a cross-sectional study of patients with diabetes mellitus. Ann Fam Med. 2009;7(1):24-31.
214. Berkman N, Sheridan SL, Donahue KE, et al. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155(2):97-107.
215. Weiss B, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the Newest Vital Sign. Ann Fam Med. 2005;3:514-522.
216. U.S. Census Bureau. American Community Survey. DP02: Selected Social Characteristics in the United States: 2020: ACS5-Year Estimates Data Profiles. Available at https://data.census.gov/cedsci/table?tid=ACSDP5Y2020.DP02. Last accessed September 7, 2022.
217. Karliner L, Napoles-Springer AM, Schillinger D, Bibbins-Domingo K, Pérez-Stable EJ. Identification of limited English proficient patients in clinical care. J Gen Intern Med. 2008;23(10):1555-1560.
218. Sevilla Matir J, Willis DR. Using bilingual staff members as interpreters. Fam Pract Manage. 2004;11(7):34-36.
219. Ngo-Metzger Q, Massagli MP, Clarridge BR, et al. Linguistic and cultural barriers to care. Perspectives of Chinese and Vietnamese immigrants. J Gen Intern Med. 2003;18(1):44-52.
220. Flores G. Language barriers to health care in the United States. N Engl J Med. 2006;355(3):229-231.
221. Flores G. The impact of medical interpreter services on the quality of health care: a systematic review. Med Care Res Rev. 2005;62(3):255-299.
222. Karliner L, Jacobs EA, Chen AH, Mutha S. Do professional interpreters improve clinical care for patients with limited English proficiency? A systematic review of the literature. Health Serv Res. 2007;42(2):727-754.
223. Zhang W, Doherty M, Arden N, et al. EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2005;64(5):669-681.
224. Brosseau L, Wells GA, Tugwell P, et al. Ottawa Panel evidence-based clinical practice guidelines for the management of osteoarthritis in adults who are obese or overweight. Phys Ther. 2011;91(6):843-861.
225. Christensen R, Bartels EM, Astrup A, Bliddal H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2007;66(4):433-439.
226. Messier S, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the arthritis, diet, and activity promotion trial. Arthritis Rheum. 2004;50(5):1501-1510.
227. Bosomworth N. Exercise and knee osteoarthritis: benefit or hazard? Can Fam Physician. 2009;55(9):871-878.
228. American Geriatrics Society Panel on Exercise and Osteoarthritis. Exercise prescription for older adults with osteoarthritis pain: consensus practice recommendations. J Am Geriatr Soc. 2001;49(6):808-823.
229. Roddy E, Zhang W, Doherty M, et al. Evidence-based recommendations for the role of exercise in the management of osteoarthritis of the hip or knee-the MOVE consensus. Rheumatology. 2005;44(1):67-73.
230. Cadmus L, Patrick MB, Maciejewski ML, Topolski T, Belza B, Patrick DL. Community-based aquatic exercise and quality of life in persons with osteoarthritis. Med Sci Sports Exerc. 2010;42(1):8-15.
231. Fransen M, McConnell S, Harmer AR, Van der Esch M, Simic M, Bennell KL. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;(1):CD004376.
232. Richmond J, Hunter D, Irrgang J, et al. Treatment of osteoarthritis of the knee (nonarthroplasty). J Am Acad Orthop Surg. 2009;17(9):591-600.
233. Jamtvedt G, Dahm KT, Christie A, et al. Physical therapy interventions for patients with osteoarthritis of the knee: an overview of systematic reviews. Phys Ther. 2008;88(1):123-136.
234. Deyle G, Henderson NE, Matekel RL, et al. Effectiveness of manual physical therapy and exercise in osteoarthritis of the knee: a randomized controlled trial. Ann Intern Med. 2000;132(3):173-181.
235. Deyle GD, Allison SC, Matekel RL, et al. Physical therapy treatment effectiveness for osteoarthritis of the knee: a randomized comparison of supervised clinical exercise and manual therapy procedures versus a home exercise program. Phys Ther. 2005;85(12):1301-1317.
236. Rutjes A, Nuesch E, Sterchi R, Juni P. Therapeutic ultrasound for osteoarthritis of the knee or hip. Cochrane Database Syst Rev. 2010;(1):CD003132.
237. Dantas LO, Osani MC, Bannuru RR. Therapeutic ultrasound for knee osteoarthritis: a systematic review and meta-analysis with grade quality assessment. Braz J Phys Ther. 2021; 25(6):688-697.
238. Hinman R, Bennell KL. Advances in insoles and shoes for knee osteoarthritis. Curr Opin Rheumatol. 2009;21(2):164-170.
239. Rutjes A, Nuesch E, Sterchi R, et al. Transcutaneous electrostimulation for osteoarthritis of the knee. Cochrane Database Syst Rev. 2009;(4):CD002823.
240. Manheimer E, Linde K, Lao L, Bouter LM, Berman BM. Meta-analysis: acupuncture for osteoarthritis of the knee. Ann Intern Med. 2007;146(12):868-877.
241. Williamson L, Wyatt MR, Yein K, Melton JTK. Severe knee osteoarthritis: a randomized controlled trial of acupuncture, physiotherapy (supervised exercise) and standard management for patients awaiting knee replacement. Rheumatology. 2007;46(9):1445-1449.
242. Hill J, Bird H. Patient knowledge and misconceptions of osteoarthritis assessed by a validated self-completed knowledge questionnaire (PKQ-OA). Rheumatology. 2007;46(5):796-800.
243. Manias E, Claydon-Platt K, McColl GJ, Bucknall TK, Brand CA. Managing complex medication regimens: perspectives of consumers with osteoarthritis and healthcare professionals. Ann Pharmacother. 2007;41(5):764-771.
244. Chou R, Helfand M, Peterson K, Dana T, Roberts C. Comparative Effectiveness and Safety of Analgesics for Osteoarthritis [archived]. Available at https://effectivehealthcare.ahrq.gov/products/osteoarthritis-pain-2006/research. Last accessed September 7, 2022.
245. Towheed T, Maxwell L, Judd MG, Catton M, Hochberg MC, Wells G. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2006;(1):CD004257.
246. Chen Y, Jobanputra P, Barton P, et al. Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib a and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation. Health Technol Assess. 2008;12(11):1-278.
247. Nuesch E, Rutjes AW, Husni E, Welch V, Juni P. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database Syst Rev. 2009;(4):CD003115.
248. da Costa BR, Nüesch E, Kasteler R, et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database Syst Rev. 2014;17(9):CD003115.
249. April KT, Bisaillon J, Welch V, et al. Tramadol for osteoarthritis. Cochrane Database Syst Rev. 2019;(5):CD005522.
250. Makris U, Kohler MJ, Fraenkel L. Adverse effects of topical nonsteroidal anti-inflammatory drugs in older adults with osteoarthritis: a systematic literature review. J Rheumatol. 2010;37(6):1236-1243.
251. Neustadt DH. Intra-articular therapy. In: Moskowitz RW, Howell DS, Altman RD, eds. Osteoarthritis. 3rd ed. Philadelphia, PA: W.B. Saunders Co; 2001: 393-409.
252. Lineker SC, Bell MJ, Boyle J, et al. Implementing arthritis clinical practice guidelines in primary care. Med Teach. 2009;31(3):230-237.
253. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005328.
254. Jüni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015;22(10):CD005328.
255. National Collaborating Centre for Chronic Conditions. Osteoarthritis: National Clinical Guideline for Care and Management in Adults. London: Royal College of Physicians; 2008.
256. Lo G, LaValley M, McAlindon T, Felson DT. Intra-articular hyaluronic acid in treatment of knee osteoarthritis: a meta-analysis. JAMA. 2003;290(23):3115-3121.
257. Reichenbach S, Blank S, Rutjes AW, et al. Hylan versus hyaluronic acid for osteoarthritis of the knee: a systematic review and meta-analysis. Arthritis Rheum. 2007;57(8):1410-1418.
258. Strauss E, Hart JA, Miller MD, Altman RD, Rosen JE. Hyaluronic acid viscosupplementation and osteoarthritis: current uses and future directions. Am J Sports Med. 2009;37(8):1636-1644.
259. van den Bekerom M, Lamme B, Sermon A, Mulier M. What is the evidence for viscosupplementation in the treatment of patients with hip osteoarthritis? Systematic review of the literature. Arch Orthop Trauma Surg. 2008;128(8):815-823.
260. Brzusek D, Petron D. Treating knee osteoarthritis with intra-articular hyaluronans. Curr Med Res Opin. 2008;24(12):3307-3322.
261. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005321.
262. Richardson C, Plaas A, Block JA. Intra-articular hyaluronan therapy for symptomatic knee osteoarthritis. Rheum Dis Clin North Am. 2019;45(3):434-451.
263. Qvistgaard E, Christensen R, Torp-Pedersen S, Bliddal H. Intra-articular treatment of hip osteoarthritis: a randomized trial of hyaluronic acid, corticosteroid, and isotonic saline. Osteoarthritis Cartilage. 2006;14(2):163-170.
264. Reid MC. Viscosupplementation for osteoarthritis: a primer for primary care physicians. Adv Ther. 2013;30(11):967-986.
265. Fuchs S, Monikes R, Wohlmeiner A, Heyse T. Intra-articular hyaluronic acid compared with corticoid injections for the treatment of rhizarthrosis. Osteoarthritis Cartilage. 2006;14(1):82-88.
266. van Brakel R, Eygendaal D. Intra-articular injection of hyaluronic acid is not effective for the treatment of post-traumatic osteoarthritis of the elbow. Arthroscopy. 2006;22(11):1199-1203.
267. Migliore A, Giovannangeli F, Bizzi E, et al. Viscosupplementation in the management of ankle osteoarthritis: a review. Arch Orthop Trauma Surg. 2011;131(1):139-147.
268. Consumer Lab. Product Review: Glucosamine, Chondroitin, MSM, and Boswellia Supplements for Joint Health. Available at https://www.consumerlab.com/reviews/Joint_Supplements_Glucosamine_Chondroitin_MSM_Review/jointsupplements/. Last accessed September 7, 2022.
269. Towheed T, Maxwell L, Anastassiades TP, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2005;(2):CD002946.
270. Clegg D, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis.N Engl J Med. 2006;354(8):795-808.
271. Sawitzke A, Shi H, Finco MF, et al. The effect of glucosamine and/or chondroitin sulfate on the progression of knee osteoarthritis: a report from the glucosamine/chondroitin arthritis intervention trial. Arthritis Rheum. 2008;58(10):3183-3191.
272. Sawitzke A, Shi H, Finco MF, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69(8):1459-1464.
273. Singh JA, Noorbaloochi S, MacDonald R, Maxwell LJ. Chondroitin for osteoarthritis. Cochrane Database Syst Rev. 2015;(1):CD005614.
274. Reichenbach S, Sterchi R, Scherer M, et al. Meta-analysis: chondroitin for osteoarthritis of the knee or hip. Ann Intern Med. 2007;146(8):580-590.
275. Rozendaal RM, Koes BW, van Osch GJVM, et al. Effect of glucosamine sulfate on hip osteoarthritis: a randomized trial. Ann Intern Med. 2008;148(4):268-277.
276. Black C, Clar C, Henderson R, et al. The clinical effectiveness of glucosamine and chondroitin supplements in slowing or arresting progression of osteoarthritis of the knee: a systematic review and economic evaluation. Health Technol Assess. 2009;13(52):1-148.
277. Cameron M, Gagnier JJ, Little CV, Parsons TJ, Blumle A, Chrubasik S. Evidence of effectiveness of herbal medicinal products in the treatment of arthritis. Part I: osteoarthritis. Phytother Res. 2009;23(11):1497-1515.
278. Rosenbaum C, O'Mathuna DP, Chavez M, Shields K. Antioxidants and anti-inflammatory dietary supplements for osteoarthritis and rheumatoid arthritis. Altern Ther Health Med. 2010;16(2):32-40.
279. Siparsky P, Ryzewicz M, Peterson B, Bartz R. Arthroscopic treatment of osteoarthritis of the knee: are there any evidence-based indications? Clin Orthop Relat Res. 2007;455:107-112.
280. Laupattarakasem W, Laopaiboon M, Laupattarakasem P, Sumananont C. Arthroscopic debridement for knee osteoarthritis. Cochrane Database Syst Rev. 2008;(1):CD005118.
281. Avouac J, Vicaut E, Bardin T, Richette P. Efficacy of joint lavage in knee osteoarthritis: meta-analysis of randomized controlled studies. Rheumatology. 2010;49(2):334-340.
282. Kirkley A, Birmingham TB, Litchfield RB, et al. A randomized trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2008;359(11):1097-1197.
284. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project: User Support. 2018 U.S. National Inpatient Stays (Knee Arthroplasty). Available at https://hcup-us.ahrq.gov/faststats/NationalProceduresServlet. Last accessed September 7, 2022.
285. National Institutes of Health. Consensus Development Panel on Total Knee Replacement. Consensus Statement on Total Knee Replacement: Final Statement. Rockville, MD: U.S. Department of Health and Human Services; 2004.
286. Rasul AT Jr, Wright J. Total Joint Replacement Rehabilitation. Available at https://emedicine.medscape.com/article/320061-overview. Last accessed September 7, 2022.
287. Kim Y-H, Choi Y, Kim J-S. Comparison of a standard and a gender-specific posterior cruciate-substituting high-flexion knee prosthesis: a prospective, randomized, short-term outcome study. J Bone Joint Surg. 2010;92:1911-1920.
288. Ververeli PA, Sutton DC, Hearn SL, Booth RE, Hozack WJ, Rothman RR. Continuous passive motion after total knee arthroplasty: analysis of cost and benefits. Clin Orthop Relat Res. 1995;(321):208-215.
289. Bennett LA; Brearley SC; Hart JA; Bailey MJ. A comparison of 2 continuous passive motion protocols after total knee arthroplasty: a controlled and randomized study. J Arthroplasty. 2005;20(2):225-233.
290. Kennedy DM, Stratford PW, Riddle DL, Hanna SE, Gollish JD. Assessing recovery and establishing prognosis following total knee arthroplasty. Phys Ther. 2008;88(1):22-32.
291. Coppola SM, Collins SM. Is physical therapy more beneficial than unsupervised home exercise in treatment of postsurgical knee disorders? A systematic review. Knee. 2009;16(3):171-175.
292. Buker N, Akkaya S, Akkaya N, et al. Comparison of effects of supervised physiotherapy and a standardized home program on functional status in patients with total knee arthroplasty: a prospective study. J Phys Ther Sci. 2014;26(10):1531-1536.
293. Agency for Healthcare Research and Quality. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project: User Support. 2018 U.S. National Inpatient Stays: Hip Arthroplasty. Available at https://hcup-us.ahrq.gov/faststats/NationalProceduresServlet. Last accessed September 7, 2022.
294. Quintana JM, Escobar A, Aguirre U, Lafuente I, Arenaza JC. Predictors of health-related quality-of-life change after total hip arthroplasty. Clin Orthop Relat Res. 2009;467(11):2886-2894.
1. American Academy of Orthopaedic Surgeons. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on Treatment of Osteoarthritis of the Knee. 3rd ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2021. Available at https://www.aaos.org/globalassets/quality-and-practice-resources/osteoarthritis-of-the-knee/oak3cpg.pdf. Last accessed September 22, 2022.
2. Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020;72(2):220-233. Available at https://www.rheumatology.org/Portals/0/Files/Osteoarthritis-Guideline-Early-View-2019.pdf. Last accessed September 22, 2022.
3. National Clinical Guideline Centre. Osteoarthritis: Care and Management in Adults. London: National Institute for Health and Care Excellence; 2020. Available at https://www.nice.org.uk/guidance/cg177. Last accessed September 22, 2022.
Mention of commercial products does not indicate endorsement.