Percutaneous injuries and transmission of pathogens in the workplace can be prevented. Safety-engineered devices are part of the solution, but the other, larger part of the equation is a safety-minded healthcare worker. The large number of injuries that still occur even with the widespread use of safety-engineered devices is proof that caution and preventative measures still need to be taken when working with and around needles and sharps.
This course is designed for nurses in all practice settings.
The purpose of this course is to encourage awareness of all types of sharps exposures and improve adherence to injury-prevention strategies.
Upon completion of this course, you should be able to:
- Outline the epidemiology of needlestick and sharps injuries.
- Discuss the immediate treatment and reporting of occupational needlestick/sharps injuries.
- Describe postexposure prophylaxis regimens for HIV and hepatitis B.
- Evaluate key components of a percutaneous injury prevention program.
Carol Shenold, RN, ICP, graduated from St. Paul’s Nursing School, Dallas, Texas, achieving her diploma in nursing. Over the past thirty years she has worked in hospital nursing in various states in the areas of obstetrics, orthopedics, intensive care, surgery and general medicine.
Mrs. Shenold served as the Continuum of Care Manager for Vencor Oklahoma City, coordinating quality review, utilization review, Case Management, Infection Control, and Quality Management. During that time, the hospital achieved Accreditation with Commendation with the Joint Commission, with a score of 100.
Mrs. Shenold was previously the Infection Control Nurse for Deaconess Hospital, a 300-bed acute care facility in Oklahoma City. She is an active member of the Association for Professionals in Infection Control and Epidemiology (APIC). She worked for the Oklahoma Foundation for Medical Quality for six years.
Contributing faculty, Carol Shenold, RN, ICP, has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Mary Franks, MSN, APRN, FNP-C
The division planner has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Sarah Campbell
The Director of Development and Academic Affairs has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
The purpose of NetCE is to provide challenging curricula to assist healthcare professionals to raise their levels of expertise while fulfilling their continuing education requirements, thereby improving the quality of healthcare.
Our contributing faculty members have taken care to ensure that the information and recommendations are accurate and compatible with the standards generally accepted at the time of publication. The publisher disclaims any liability, loss or damage incurred as a consequence, directly or indirectly, of the use and application of any of the contents. Participants are cautioned about the potential risk of using limited knowledge when integrating new techniques into practice.
It is the policy of NetCE not to accept commercial support. Furthermore, commercial interests are prohibited from distributing or providing access to this activity to learners.
Supported browsers for Windows include Microsoft Internet Explorer 9.0 and up, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Supported browsers for Macintosh include Safari, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Other operating systems and browsers that include complete implementations of ECMAScript edition 3 and CSS 2.0 may work, but are not supported. Supported browsers must utilize the TLS encryption protocol v1.1 or v1.2 in order to connect to pages that require a secured HTTPS connection. TLS v1.0 is not supported.
The role of implicit biases on healthcare outcomes has become a concern, as there is some evidence that implicit biases contribute to health disparities, professionals' attitudes toward and interactions with patients, quality of care, diagnoses, and treatment decisions. This may produce differences in help-seeking, diagnoses, and ultimately treatments and interventions. Implicit biases may also unwittingly produce professional behaviors, attitudes, and interactions that reduce patients' trust and comfort with their provider, leading to earlier termination of visits and/or reduced adherence and follow-up. Disadvantaged groups are marginalized in the healthcare system and vulnerable on multiple levels; health professionals' implicit biases can further exacerbate these existing disadvantages.
Interventions or strategies designed to reduce implicit bias may be categorized as change-based or control-based. Change-based interventions focus on reducing or changing cognitive associations underlying implicit biases. These interventions might include challenging stereotypes. Conversely, control-based interventions involve reducing the effects of the implicit bias on the individual's behaviors. These strategies include increasing awareness of biased thoughts and responses. The two types of interventions are not mutually exclusive and may be used synergistically.
#31023: Sharps Safety and Needlestick Prevention
Sharps safety and needlestick prevention are critical safety issues for healthcare workers. Since 1991, when the Occupational Safety and Health Administration (OSHA) first issued its Bloodborne Pathogens Standard, the emphasis of regulatory and legislative action to reduce the number of injuries and occupational disease exposures has been on enacting a hierarchy of work-control measures. The federal Needlestick Safety and Prevention Act (signed into law in 2000) authorized OSHA's revision of the Bloodborne Pathogens Standard to more explicitly require the use of safety-engineered sharp devices [30]. However, even with safety-engineered devices and other products aimed at needlestick and sharps injury reduction, healthcare systems continue to deal with bloodborne pathogen exposures from percutaneous injuries [1]. Awareness of the problem, and knowledge about devices and procedures that help prevent needlestick and sharps accidents, is therefore the key to injury prevention. Education of all staff that might come into contact with blood, needles, and sharps is crucial to any successful disease transmission reduction effort [1]. Other strategies to prevent infection include immunization against hepatitis B virus (HBV) and postexposure prophylaxis (PEP) after possible exposures to human immunodeficiency virus (HIV) and HBV. Insight into the scope of the problem and the devices and procedures responsible for injury can help nurses remain aware of the dangers associated with them.
Before federal legislation (the Needlestick Safety and Prevention Act of 2000) required the widespread use of needlestick and sharps prevention devices, a large surveillance study found that approximately 385,000 needlestick and sharps injuries occurred each year in U.S. hospitals alone, and as many as 50% to 60% of these were unreported [5,6]. Although some studies have shown a modest reduction in the number of percutaneous injuries with the use of safety-engineered devices, the evidence quality is poor and there is significant bias [7,8]. The number of injuries still occurring is alarming. In 2024, there were 26.83 percutaneous injuries in non-teaching hospitals per 100 average daily census (i.e., 100 occupied beds) and 39.14 per 100 average daily census in teaching hospitals [10]. The CDC conservatively estimates there continue to be 385,000 needlestick and sharps injuries each year in U.S. hospitals [9].
One report found that about 20% of needlestick injuries occurred with a safety-engineered needle, mainly while the safety feature was not activated [10]. A majority of injuries occur during the safety device's use (53.8%) and after use but before disposal (20.1%); there are also a significant number of injuries associated with disposal (13.4%). International Safety Center data from 2024 confirm that 29.8% of the time an injury occurs when the safety feature on a safety-engineered device is only partially activated, but that number falls to 6.3% when the safety device is fully activated [10].
The most common devices associated with injury are disposable syringes (30%), suture needles (20%), scalpel blades (8%), winged steel needles (12%), intravenous catheter stylets (5%), and phlebotomy needles (3%) [1]. Specific tasks that cause the highest percentage of injuries include intramuscular or subcutaneous injection (21.1%), suturing (25.6%), cutting (6.7%), and venous blood sampling (8%) [10]. Approximately 30% of injuries happen to workers who are not the primary user of the device [14].
Nurses, physicians, and surgical attendants have the highest rates (respectively) of exposure to blood/bodily fluids and needlesticks and sharps injuries [10]. Nurses sustain the greatest number of injuries per year, primarily because they comprise the largest proportion of hospital staff [1]. Studies have found that nurses working at hospitals with low nurse staffing or high patient loads incur more needlesticks than nurses working at well-staffed or low patient-volume hospitals [2,3]. Additionally, working in a facility with "low perceived nurse manager leadership" has been associated with increased risk of needlestick injuries.
Needlestick and sharps injuries also occur in many healthcare workplaces outside of hospitals [1]. Although most needlestick and sharps injuries occur in inpatient units (39%) and operating rooms (25%), nursing homes, clinics, emergency departments, and private homes and offices are the settings of thousands of percutaneous exposures. These settings may lack the implementation of injury prevention programs and measures that large, well-funded hospitals employ. The American Nurses Association reports that, when all work settings are factored, healthcare workers incur between 600,000 and 800,000 needlestick and sharps injuries per year in the United States, or nearly 2,000 per day [14].
The most alarming potential exposures are to HBV, hepatitis C virus (HCV), and HIV, with several other pathogens (e.g., herpes viruses, malaria, Mycobacterium tuberculosis) identified as concerning, but less often transmitted during patient care [1,4]. Because of under-reporting, it is difficult to assess the true prevalence of transmitted infections from percutaneous exposures in the workplace, but the risk of becoming infected subsequent to exposure is well documented. According to the CDC, the risk of transmission from an infected patient to a healthcare worker following a needlestick or sharps injury is 6% to 30% for HBV (in unvaccinated workers), 2% to 4% for HCV, and 0.3% for HIV [4]. These figures are solely for known percutaneous exposure to infected blood. The likelihood of transmission though percutaneous exposure to other bodily fluids is lower, because blood contains the greatest amount of infectious virus particle titers of all bodily fluids. In contrast, exposure to blood splash on mucous membranes or skin presents a lower risk of infection (less than 0.1% for HIV); no documented cases of HIV infection from a few drops of blood on intact skin have been reported [4,21]. Deep injuries, injuries from a needle that was in the source patient's artery or vein, or injuries from devices visibly contaminated with blood are most likely to result in seroconversion [15]. High source-patient viral load increases the risk.
Although the number of needlestick and sharps injuries remains high, the CDC documented only 11 cases of HBV transmission and 11 cases of HCV transmission in healthcare workers in 2017, and there has been only one confirmed case of occupational transmission of HIV in a healthcare worker since 1999 [18,19]. The CDC credits the effectiveness of more widespread and earlier treatment to reduce patient viral loads, combined with prevention strategies such as postexposure management and prophylaxis as well as improved technologies and training to reduce sharps injuries and other exposures, with the reduction in occupational transmission of HIV to healthcare workers. This is likely the case for HBV and HCV as well. Vaccination of at-risk healthcare workers, along with the use of Universal Precautions and other OSHA-required measures, has drastically reduced transmission of HBV; in 1983, there were approximately 17,000 occupational HBV transmission infections in healthcare workers [1,20].
The first step after a needlestick or sharps injury is to gently wash the exposed area with soap and water without scrubbing; skin washes and topical antiseptics (e.g., 2% to 4% chlorhexidine) have not been found to reduce rates of disease transmission but can be used [4,12,15,25]. Allowing the wound to bleed freely is recommended, but expressing or sucking the wound is contraindicated, as are caustic agents (e.g., bleach) or the injection of antiseptics or disinfectants into the wound. Drying the area and using a sterile dressing or bandage to cover the wound is the final step. Deep scalpel or other sharps injuries should receive treatment as needed.
After receiving first aid, the incident should be immediately reported to a supervisor, and the source of the exposure should be documented, if possible [4,12]. Data from 2024 indicated that the source patient was identified in 89.6% of cases [10]. Reporting a percutaneous injury is crucial to ensure timely and proper counseling and prophylaxis, but barriers to reporting have been identified. Reluctance to report exposures to supervisors might be due to fear of being criticized or reprimanded, concerns about confidentiality, or the belief that reporting might take too much time [25]. However, it is a vital component of any safety program. Reporting allows supervisors and administrators to make informed decisions regarding staffing levels, workload, and safety equipment and policy changes, which can lead to a reduction of injuries in the future [1]. Reporting and documentation also help ensure access to compensation payment and that all health expenditures are billed to workers' compensation and not to the nurse's health insurance.
Employers must follow all workplace, state, and federal requirements for documenting and reporting workplace sharps injuries and exposures to blood, bodily fluids, or tissue [11]. This includes adherence to the OSHA requirements. Exposure information should be included in the report and recorded in the exposed worker's confidential medical record [4]. The medical record and report should be made available to a healthcare provider who can provide counseling and perform all medical evaluations, procedures, and treatments (including PEP, when indicated) in accordance with the recommendations of the U.S. Public Health Service. The report and medical record should contain [1]:
Unique identification number for the incident
Date and time of the injury
Details of the procedure being performed, including where and how the exposure occurred, whether the exposure involved a sharp device, the type of device, whether there was visible blood on the device, and how and when during its handling the exposure occurred
Details of the exposure, including the type and amount of fluid or material and the severity of the exposure. For a percutaneous injury, details would include the depth of the wound, the gauge of the needle, and whether fluid was injected.
Details about the exposure source, including whether the patient was infected with HIV, HCV, and/or HBV and his or her hepatitis B e antigen (HBeAg) and, if the source was infected with HIV, the stage of disease, history of antiretroviral therapy, and viral load, if known. If this information is not known from the medical record, then the source patient should be asked to obtain serologic testing for HBV, HCV, and HIV.
Details about the exposed person (e.g., HBV vaccination and vaccine-response status)
Details about counseling, post-exposure management, and follow-up
If the source patient can be located, his or her consent is required to obtain serologic testing for potentially infectious diseases, including HBV, HCV, and HIV [15]. PEP should be started within hours of exposure [12].
As noted, transmission of infectious diseases due to occupational exposure of healthcare workers has occurred in needlestick accidents and blood splashes to the mucous membranes [4]. In the case of HIV, needlestick is the most likely route. Thousands of healthcare personnel who were so exposed have been studied, and only 58 cases of well-documented infection have been reported in the United States. Of these cases, 24 were nurses and all except one occurred before 1999 [19]. The risk of infection through this route is low, and every effort should be made to decrease the exposure rate. Educational efforts, implementation of engineering controls in needled and sharp-edged medical devices, the use of hard plastic needle disposal units where these devices are most frequently used, and the development of procedural details to avoid blood and bodily fluid contact have greatly reduced the exposure rate. Healthcare personnel should apply Universal Precautions, as discussed in the OSHA Bloodborne Pathogens standard regulations, to all activities to avoid contact with human fluids.
The following recommendations apply to situations in which healthcare professionals have had exposure to a source person with HIV or where information suggests that there is likelihood that the source person is HIV-infected. Because most occupational HIV exposures do not result in the transmission of HIV, potential toxicity should be carefully considered when prescribing PEP. The update focused on tolerability, side effects, toxicity, safety in pregnancy and lactation, pill burden, and frequency of dosing to maximize adherence to a PEP regimen [17]. When possible, these recommendations should be implemented in consultation with persons having expertise in antiretroviral therapy and HIV transmission, due to the complexity of selecting appropriate treatment.
The preferred regimen for PEP following exposure to HIV is a basic regimen that should be appropriate for most HIV exposures: integrase strand transfer inhibitors plus two nucleoside reverse transcriptase inhibitors. This consists of bictegravir/emtricitabine/tenofovir alafenamide (Biktarvy) or dolutegravir plus tenofovir and emtricitabine or lamivudine [17]. This preparation is available as a starter packet that should be stocked at every healthcare facility where exposure to HIV is possible. PEP regimens should be started as soon as possible after occupational exposure to HIV, and they should be continued for a four-week duration. As noted, the regimen has been selected for its tolerability and safety profile. There are several alternative regimens that may be selected due to individual patient concerns.
Healthcare professionals with occupational exposure to HIV should receive follow-up counseling, postexposure testing, and medical evaluation regardless of whether they receive PEP. The 2025 guideline highlights the importance of follow-up within 72 hours to allow the initial shock to fade and to provide greater opportunity for full understanding of the risks and benefits of PEP; confirmation testing to ensure the necessity of PEP; monitoring for adverse reactions and side effects; and treating comorbidities and altering the regimen [17]. If PEP is used, drug-toxicity monitoring should be performed at baseline and again two weeks after starting PEP. Clinical judgment, based on medical conditions that may have existed pre-exposure and/or as a result of the regimen, should determine the scope of testing. If the source patient is found to be HIV negative, PEP should be discontinued immediately [17]. For the complete recommendations, visit the CDC website at https://stacks.cdc.gov/view/cdc/183609.
Recommendations for HBV postexposure management include initiation of the hepatitis B vaccine series to any susceptible, unvaccinated person who sustains an occupational blood or bodily fluid exposure. PEP with hepatitis B immune globulin (HBIG) and/or hepatitis B vaccine series should be considered for occupational exposures after evaluation of the hepatitis B surface antigen status of the source as well as the vaccination and vaccine-response status of the exposed person (Table 1) [25,33]. Neither pregnancy nor lactation is a contraindication to receiving the hepatitis B vaccine.
POSTEXPOSURE MANAGEMENT OF HEPATITIS B, BY VACCINATION AND RESPONSE STATUS
| Healthcare Personnel (HCP) Status | Postexposure Testing | Postexposure Prophylaxis | Postvaccination Serologic Testingb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Source Patient (HBsAg) | HCP Testing (Anti-HBs) | HBIGa | Vaccination | ||||||||
| Documented responderc after complete series (three or more doses) | No action needed | ||||||||||
| Documented nonresponderd after six doses | Positive/unknown | — | HBIG x2 separated by one month | — | No | ||||||
| Negative | No action needed | ||||||||||
| Response unknown after three doses | Positive/unknown | <10 mIU/mLe | HBIG x1 | Initiate revaccination | Yes | ||||||
| Negative | <10 mIU/mL | None | |||||||||
| Any result | ≥10 mIU/mL | No action needed | |||||||||
| Unvaccinated/incompletely vaccinated or vaccine refuser | Positive/unknown | — | HBIG x1 | Complete vaccination | Yes | ||||||
| Negative | — | None | Complete vaccination | Yes | |||||||
| |||||||||||
The HBV vaccine is effective in preventing hepatitis B infection, likely for the lifetime of an individual who completes the series and has an adequate immune response [20]. Although HBV surface antigen antibody (anti-HBs) concentrations decline rapidly in the first year and then slowly taper thereafter, almost all individuals remain protected indefinitely due to preservation of immune memory. Studies have proven that HBV infections are prevented for at least 20 years after vaccination, even when individuals have undetectable levels of anti-HBs [20]. Vaccination may be the single most important HBV prevention measure, as evidenced by the significant decline in occupational transmission rates after widespread healthcare worker vaccination campaigns [4,20].
The Advisory Committee on Immunization Practices (ACIP) recommends universal hepatitis B vaccination for all adults who have not completed the vaccine series working in settings in which a high percentage of patients are at risk for HBV infection [20]. This includes healthcare and other professionals (e.g., laboratory workers) working in sexually transmitted infection/HIV testing and treatment facilities, drug-abuse treatment and prevention settings, healthcare settings targeting services to intravenous drug users, healthcare settings targeting services to men who have sex with men, and correctional facilities [20]. The ACIP emphasizes that the risk to professionals not included in this recommendation is similar to that of the general population. However, the CDC recommendations differ, advising instead that healthcare workers who have a reasonable expectation of being exposed to blood on the job should be offered the hepatitis B vaccine [23]. Given that universal hepatitis A/B vaccination for newborns and children is recommended and the risks of vaccination are nearly nonexistent, the ACIP distinction between nurses working with low- or high-risk groups seems irrelevant [25].
Since 1991, newborns have typically been vaccinated against HBV before hospital discharge or soon thereafter, and many other individuals were vaccinated as children, so many new nurses are already immune [24]. This is important because many needlestick injuries and blood exposures occur during nurse schooling. Additionally, many nursing programs now require proof of HBV vaccination or hepatitis B antibody titer. The recommendations are therefore more pertinent to older individuals who refused vaccination or who are employed at facilities that do not require vaccination.
Hepatitis B vaccine is available as a single-antigen formulation (Recombivax HB, Engerix-B, Heplisav-B, and PreHevbrio) or as a combination hepatitis A/B vaccine (Twinrix, Pediarix, Vaxelis) [25,31,16]. Primary vaccination of adults (and PEP) usually consists of three doses of 10 mcg or 20 mcg recombinant HBV surface anitgen (HBsAg) protein administered intramuscularly into the deltoid muscle at 0, 1, and 6 months. Healthcare workers at risk for hepatitis A should receive the combination vaccine for pre-exposure prophylaxis [25]. Postvaccination serologic testing for anti-HBs is recommended one to two months after the last vaccine dose for individuals at risk for or following an occupational percutaneous injury or exposure to blood, bodily fluids, or tissues. Anti-HBs levels ≥10 mIU/mL are considered an adequate immune response [25]. Individuals with an inadequate response after completion of the initial series (i.e., anti-HBs <10 mIU/mL) should be given a booster dose, which has been found to elicit adequate response in 48% of weak responders. Those who maintain anti-HBs levels <10 mIU/mL after a single booster dose should be given two additional doses; approximately 42% of these individuals will develop protective surface antigen levels. Cumulatively, 69% of initial nonresponders will achieve anti-HBs levels of ≥10 mIU/mL after three-dose revaccination [25].
There is no PEP for HCV, and immune globulin and antiviral agents (e.g., interferon with or without ribavirin) are not recommended. In cases of possible exposure, the HCV status of the source and the exposed person should be determined. For healthcare professionals exposed to an HCV-positive source, follow-up HCV testing should be performed to determine if infection develops [22].
Healthcare professionals exposed to hepatitis viruses should refrain from donating blood, plasma, organs, tissue, or semen [22]. When based only on exposure to HBV- or HCV-positive blood, modifications to an exposed healthcare professional's patient-care responsibilities are not necessary. Acutely infected healthcare professionals should be evaluated according to current guidelines; healthcare professionals chronically infected with HBV or HCV should follow all recommended infection control practices [22,25].
Since the implementation of Universal Precautions in healthcare settings (formalized during the HIV/AIDS epidemic in the mid-1980s), the incidence of blood and bodily fluid exposure to the skin and mucous membranes has decreased significantly [1,29,32]. Use of personal protective equipment and work-practice controls are the cornerstone of Universal Precautions. At its core, Universal Precautions are based on the principle that all blood and other bodily fluids should be treated as if they are infectious [1]. But no matter how effective protective equipment is at preventing surface contact, needles and other sharps easily penetrate most barrier precautions. Although safety-engineered devices and controls have lowered the occurrence of many types of injuries, percutaneous injuries and exposures continue to occur [1]. Understanding how and when percutaneous injuries occur is key to needlestick and sharps injury prevention. One analysis by the CDC found that needlestick injuries most commonly occurred at the following points in patient care [1]:
Manipulating a needle in the patient (41%)
After use and before disposal of a device (40%)
During or after disposal (15%)
Before working with needles and sharps, it is important to ensure that lighting in the room allows for clear visualization of procedures. If corrective lenses are needed, they should be worn. Be aware of potential distractions (e.g., opening doors, people walking behind) before working, and assess the patient's potential for being combative or uncooperative. Discuss the procedure with the patient, with an emphasis on avoiding any sudden movements. Know where sharps disposal containers are located, and plan ahead for safe recapping, safety-feature activation, and disposal.
Recapping needles is associated with a significant percentage of needlestick injuries. A one-handed recapping technique should be used, rather than using two hands to recap needles, if a safety-engineered method or device is not available. The one-handed, passive technique is often called the "scoop" technique and consists of the needle cap being removed and placed on its side on a surface, ready to have the used needle inserted, lifted (scooped up) off the surface, allowed to fully cover the needle, then pressed firmly back onto the assembly with the other hand before discarding in a rigid sharps container. There are also needle cap holder devices that can be used to assist with one-handed recapping [1].
Nearly every type of needle or sharp instrument now has a safety-engineered version; listing all of the various manufacturers' patented variations is beyond the scope of this course. Because regulations may only specify that a safety-engineered device, rather than a brand or type, should be used, it is typically up to employers to decide which specific devices will be provided in the workplace. No matter the devices used, thorough education in the use of each device (new to the employer or employee) is vital [1].
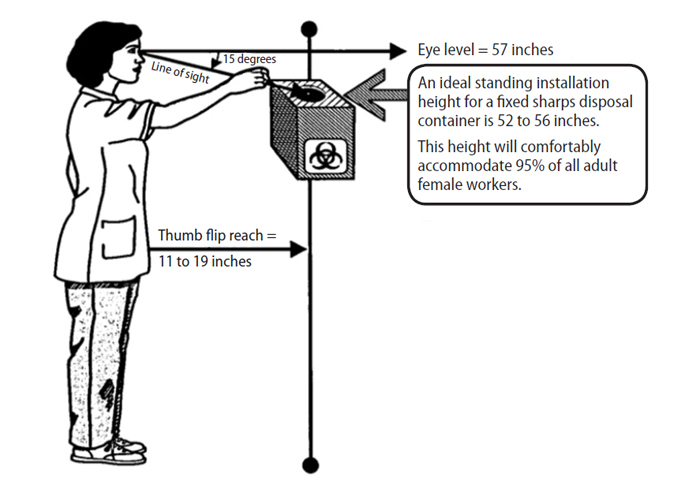
Sharps containers are an integral part of percutaneous injury prevention [1]. Needles and sharps should always be disposed of immediately after use so the potential for accidents is minimized. Mandating the use of rigid, puncture-proof sharps containers was an important regulatory aspect of the original 1991 OSHA Bloodborne Pathogens Standard and remains so to this day [28]. An aspect of sharps container use that is often overlooked is the ideal location for placement. There should be unobstructed access to the container so the worker can easily place the used device into the opening without having to make any awkward movements [28]. The top opening of the container should be visible to the user. This is important, not only because workers need to know where the opening is, but because they need to be able to see the fill status of the container and if there are any potential hazards where they are reaching. Fingers should never enter the sharps container [1].
Most nurses are women, and a sharps container for a standing workstation placed between 52–56 inches from the floor will accommodate 95% of female nurses (Figure 1) [27,28]. For seated workstations, the preferred height is 38–42 inches. Taller healthcare practitioners will also be able to comfortably and safely use containers placed at these heights.
Home healthcare workers should have an approved sharps container available to them and should plan to use the container to dispose of needles and other sharps as needed [26]. The National Institute for Occupational Safety and Health (NIOSH) has published work-control guidance for home healthcare workers regarding sharps safety that is applicable to many work settings. According to this guideline, individuals are at risk for needlestick or sharps injuries when [26]:
Handling needles that must be taken apart or manipulated after use (e.g., recapping, activating the safety feature)
Disposing of needles attached to tubing
Manipulating the needle in the patient
Recapping a needle
Using needles or glass equipment to transfer bodily fluid between containers
Failing to dispose of used needles in puncture-resistant sharps containers
Lacking proper workstations for procedures using sharps
Working too quickly
Bumping into a needle, a sharp, or another worker while either person is holding a sharp
Operating rooms are a setting in which a large percentage of injuries take place. In surgical settings, the CDC has identified work-control practices that can prevent or lessen the incidence of percutaneous injuries, including [1]:
Using instruments, rather than fingers, to grasp needles, retract tissue, and load/unload needles and scalpels
Giving verbal announcements when passing sharps
Avoiding hand-to-hand passage of sharp instruments by using a basin or neutral zone
Using alternative cutting methods such as blunt electrocautery and laser devices when appropriate
Substituting endoscopic surgery for open surgery when possible
Using round-tipped scalpel blades instead of pointed sharp-tipped blades
Double gloving
Needles or sharps should not be passed from one worker to another; the primary user should recap or activate the safety feature and dispose of the needle as soon as the procedure is completed [1]. When needles or sharps must be passed, use of a tray, basin, or neutral zone is recommended. It is also important to announce when moving a needle or putting a needle into the neutral zone [1]. Always maintain eye contact with the device to avoid accidentally injuring another worker. Although every needle should be disposed in a rigid sharps container, workers should remember to never place their hands into an area they cannot clearly see, such as in a garbage can or into bed linens, in case a needle was left there by accident. When an improperly disposed device is encountered, it should be handled carefully using a gloved hand opposite the sharp end or with a mechanical instrument [1]. If a sharps container is overfilled, tongs should be used to move some of the devices to a new container. If sharps are sticking out of the sides of a container, notify safety personnel [1]. Filled sharps containers should be stored in a secure area for final disposal.
Another way to reduce percutaneous injuries is by substituting a risky procedure for a safer procedure [26]. One example is administering a tablet, capsule, lozenge, or patch instead of an intravenous (IV) or intramuscular drug whenever possible. IV delivery systems that do not use or do not permit needle access have significantly reduced injuries associated with IVs [1]. Needleless sutures can significantly reduce injuries associated with suturing.
Willingness of healthcare workers to change behaviors that can lead to injury is influenced by a variety of factors. It has been shown that nurses are most willing to make changes to their set behaviors when they understand that they are at risk of injury and that the risk is substantial [1]. It is also important to know the change is worth the extra effort and the new techniques, devices, and practices will make a difference to personal safety. The CDC has identified factors that can inhibit the acceptance of safety practices, including [1,14]:
Perceived conflict of interest between providing optimal patient care and protecting oneself from exposure (e.g., failure to associate workplace safety with improved patient care)
Belief that precautions are not warranted in some specific situations (despite the lesson of Universal Precautions that every procedure and patient should be considered infectious)
Increased job demands that cause work to be hurried
Perceived poor safety climate in the workplace
Failure to anticipate the potential for exposure
Risk-taking personality
Unfortunately, there is no ideal model for learning guaranteed to be the most effective for teaching injury prevention given the diversity of personality types in the workplace [1]. Nurse managers and administrators should identify deficits among their workforce and maintain a commitment to educating and protecting their employees. The burden falls on workers to recognize their own areas for improvement (and to report any injuries they incur) and on supervisors and healthcare systems to respond with effective risk reduction strategies.
For the most part, percutaneous injuries and transmission of pathogens in the workplace can be prevented. Safety-engineered devices are part of the solution, but the other, larger part of the equation is a safety-minded healthcare worker. The large number of injuries that continue to occur despite the widespread use of safety-engineered devices is proof that caution and preventative measures are still needed when working with and around needles and sharps. The most effective measure in preventing needlestick injury is to administer medications via another route, such as substituting an oral medication for an intramuscular or intravenous preparation. But in many instances, needles must be used. Selecting a device that has a proven track record for safety and educating staff on proper use, safety-feature activation, and disposal is the next best measure.
When injuries do occur, it is important that they are reported so appropriate prophylaxis and counseling can be received. Reporting also ensures that workers compensation insurance is accessed and that assessments of workplace safety can include injury and device data. Lastly, it is important that all healthcare workers that may have exposure receive PEP for HIV and HBV, when appropriate. Following these steps can help prevent percutaneous injuries and workplace disease transmission.
1. Centers for Disease Control and Prevention. Workbook for Designing, Implementing, and Evaluating a Sharps Injury Prevention Program. Available at https://www.cdc.gov/infection-control/media/pdfs/sharps-safety-workbook-2008-p.pdf. Last accessed November 4, 2025.
2. Clarke SP, Sloane DM, Aiken LH. Effects of hospital staffing and organizational climate on needlestick injuries to nurses. Am J Public Health. 2002;92(7):1115-1119.
3. Clarke SP, Rockett JL, Sloane DM, Aiken LH. Organizational climate, staffing, and safety equipment as predictors of needlestick injuries and near-misses in hospital nurses. Am J Infect Control. 2002;30(4):207-216.
4. Centers for Disease Control and Prevention. Bloodborne Infectious Disease Risk Factors. Available at https://www.cdc.gov/niosh/healthcare/risk-factors/bloodborne-infectious-diseases.html. Last accessed November 12, 2025.
5. Panlilio AL, Orelien JG, Srivastava PU, et al. Estimate of the annual number of percutaneous injuries among hospital-based healthcare workers in the United States, 1997–1998. Infect Control Hosp Epidemiol. 2004;25(7):556-562.
6. Mannocci A, De Carli G, Di Bari V, et al. How much do needlestick injuries cost? A systematic review of the economic evaluations of needlestick and sharps injuries among healthcare personnel. Infect Control Hosp Epidemiol. 2016;37(6):635-646.
7. Lavoie MC, Verbeek JH, Pahwa M. Devices for preventing percutaneous exposure injuries caused by needles in healthcare personnel. Cochrane Database Syst Rev. 2014;(3):CD009740.
8. Ballout RA, Diab B, Harb AC, Tarabay R, Khamassi S, Akl EA. Use of safety-engineered devices by healthcare workers for intravenous and/or phlebotomy procedures in healthcare settings: a systematic review and meta-analysis. BMC Health Serv Res. 2016;16:458.
9. Centers for Disease Control and Prevention. Sharps Safety for Healthcare Settings. Available at https://www.cdc.gov/infection-control/hcp/sharps-safety/. Last accessed November 5 2025.
10. International Safety Center. EPINet Report for Needlestick and Sharp Object Injuries. Available at https://aoec.org/wp-content/uploads/Official-2024-NeedleSummary-06-25.pdf. Last accessed November 6, 2025.
11. Occupational Safety and Health Administration. Bloodborne Pathogens and Needlestick Prevention. Available at https://www.osha.gov/bloodborne-pathogens/standards. Last accessed December 2, 2025.
12. Centers for Disease Control and Prevention. Stop Sticks Campaign. Available at https://archive.cdc.gov/#/details?url=https://www.cdc.gov/nora/councils/hcsa/stopsticks/whattodo.html. Last accessed November 25, 2025.
13. Centers for Disease Control and Prevention. Interim Statement Regarding Potential Fetal Harm from Exposure to Dolutegravir: Implications for HIV Post-exposure Prophylaxis (PEP). Available at https://stacks.cdc.gov/view/cdc/80420. Last accessed December 4, 2025.
14. American Nurses Association. Fact Sheet: Safe Needles Save Lives. Available at https://www.nursingworld.org/globalassets/docs/ana/snsl-fact-sheet_final110110.pdf. Last accessed November 12, 2025.
15. Zehnder NG. What Should I Do If I Get a Needlestick? Available at https://www.the-hospitalist.org/hospitalist/article/124195/what-should-i-do-if-i-get-needlestick. Last accessed December 4, 2025.
16. Centers for Disease Control and Prevention. Hepatitis B Vaccine Administration. Available at https://www.cdc.gov/hepatitis-b/hcp/vaccine-administration/index.html. Last accessed December 4, 2025.
17. Kofman AD, Struble KA, Heneine W, et al. 2025 US Public Health Service Guidelines for the Management of Occupational Exposures to Human Immunodeficiency Virus and Recommendations for Post-exposure Prophylaxis in Healthcare Settings. Available at https://stacks.cdc.gov/view/cdc/183609. Last accessed December 4, 2025.
18. Centers for Disease Control and Prevention. Surveillance for Viral Hepatitis. Available at https://archive.cdc.gov/www_cdc_gov/hepatitis/statistics/2017surveillance/index.htm. https://www.cdc.gov/hepatitis/statistics/2020surveillance/index.htm. Last accessed November 14, 2025.
19. Joyce MP, Kuhar D, Brooks JT. Notes from the field: occupationally acquired HIV infection among health care workers—United States, 1985–2013. MMWR. 2015;63(53):1245-1246.
20. Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR. 2006;55(RR16):1-33.
21. Centers for Disease Control. HIV Occupational Transmission. Available at https://www.cdc.gov/hiv/causes/occupational-transmission.html. Last accessed November 12, 2025.
22. Centers for Disease Control and Prevention. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HBV, HCB, and HIV and recommendations for postexposure prophylaxis. MMWR. 2001;50(RR11):1-42.
23. Centers for Disease Control and Prevention. Preventing Hepatitis in Healthcare Settings. Available at https://www.who.int/europe/activities/preventing-hepatitis-in-health-care-settings. Last accessed November 25, 2025.
24. Centers for Disease Control and Prevention. Achievements in public health: hepatitis B vaccination—United States, 1982–2002. MMWR. 2002;51(25):549-552, 563.
25. Schillie S, Murphy TV, Sawyer M, et al. CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR. 2013;62(RR10):1-19.
26. National Institute for Occupational Safety and Health. How to Prevent Needlestick and Sharps Injuries. Available at https://www.cdc.gov/niosh/docs/2012-123/pdfs/2012-123.pdf. Last accessed November 25, 2025.
27. American Nurses Association. Sharps Injury Prevention. Available at https://www.nursingworld.org/practice-policy/work-environment/health-safety/safe-needles/. Last accessed November 25, 2025.
28. Centers for Disease Control and Prevention. Selecting, Evaluating, and Using Sharps Disposal Containers. Available at https://www.cdc.gov/niosh/docs/97-111/default.html. Last accessed December 4, 2025.
29. Wilburn SQ, Eijkemans G. Preventing needlestick injuries among healthcare workers: a WHO-ICN collaboration. Int J Occup Environ Health. 2004;10(4):451-456.
30. Centers for Disease Control and Prevention. FDA, NIOSH & OSHA joint safety communication: Blunt-tip surgical suture needles reduce needlestick injuries and the risk of subsequent bloodborne pathogen transmission to surgical personnel. Available at https://stacks.cdc.gov/view/cdc/21956#tabs-2. Last accessed November 5, 2025.
31. Weng MK, Doshani M, Khan MA, et al. Universal hepatitis B vaccination in adults aged 19–59 years: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR. 2022;71(13):477-483.
32. International Safety Center. Moving the Sharps Safety in Healthcare Agenda Forward in the United States: 2020 Consensus Statement and Call to Action. Available at https://www.facs.org/media/mf2czh1p/consensus-statement-on-sharps_2020.pdf. Last accessed December 4, 2025.
1. Schillie S, Murphy TV, Sawyer M, et al. CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR. 2013;62(RR10):1-19. Available at https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6210a1.htm. Last accessed December 8, 2025.
Mention of commercial products does not indicate endorsement.