Sexual dysfunction is distressing for female patients and their partners and can have a profoundly negative impact on patient quality of life and self-image. However, providers often find discussion of patient's sexual concerns difficult, due in part to a lack of knowledge, skills, and confidence in their ability to initiate discussion and assess and treat sexual dysfunction. To compound this, there are few approved options for the treatment of female sexual dysfunction. This course will outline appropriate approaches to the assessment, diagnosis, and treatment of female sexual dysfunction in order to enhance patients-provider communication and improve patient outcomes and well-being.
This course is designed for healthcare and mental health providers involved in the assessment and/or treatment of female sexual dysfunction.
Particularly in women, sexual dysfunction is often under-recognized and untreated, and many patients are reluctant to discuss sexual concerns with healthcare and mental health providers. The purpose of this course is to provide healthcare professionals with the information necessary to identify and appropriately treat female sexual dysfunction.
Upon completion of this course, you should be able to:
- Define various female sexual disorders.
- Identify instruments used in the assessment and diagnosis of sexual problems in women.
- Outline the epidemiology of female sexual dysfunction.
- Discuss the etiology of and risk factors for various forms of female sexual dysfunction.
- Describe aspects of the diagnostic workup for female sexual dysfunction.
- Evaluate treatment options for a variety of female sexual disorders.
Mark Rose, BS, MA, LP, is a licensed psychologist in the State of Minnesota with a private consulting practice and a medical research analyst with a biomedical communications firm. Earlier healthcare technology assessment work led to medical device and pharmaceutical sector experience in new product development involving cancer ablative devices and pain therapeutics. Along with substantial experience in addiction research, Mr. Rose has contributed to the authorship of numerous papers on CNS, oncology, and other medical disorders. He is the lead author of papers published in peer-reviewed addiction, psychiatry, and pain medicine journals and has written books on prescription opioids and alcoholism published by the Hazelden Foundation. He also serves as an Expert Advisor and Expert Witness to law firms that represent disability claimants or criminal defendants on cases related to chronic pain, psychiatric/substance use disorders, and acute pharmacologic/toxicologic effects. Mr. Rose is on the Board of Directors of the Minneapolis-based International Institute of Anti-Aging Medicine and is a member of several professional organizations.
Contributing faculty, Mark Rose, BS, MA, LP, has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
John M. Leonard, MD
Jane C. Norman, RN, MSN, CNE, PhD
Alice Yick Flanagan, PhD, MSW
The division planners have disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Sarah Campbell
The Director of Development and Academic Affairs has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
The purpose of NetCE is to provide challenging curricula to assist healthcare professionals to raise their levels of expertise while fulfilling their continuing education requirements, thereby improving the quality of healthcare.
Our contributing faculty members have taken care to ensure that the information and recommendations are accurate and compatible with the standards generally accepted at the time of publication. The publisher disclaims any liability, loss or damage incurred as a consequence, directly or indirectly, of the use and application of any of the contents. Participants are cautioned about the potential risk of using limited knowledge when integrating new techniques into practice.
It is the policy of NetCE not to accept commercial support. Furthermore, commercial interests are prohibited from distributing or providing access to this activity to learners.
Supported browsers for Windows include Microsoft Internet Explorer 9.0 and up, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Supported browsers for Macintosh include Safari, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Other operating systems and browsers that include complete implementations of ECMAScript edition 3 and CSS 2.0 may work, but are not supported. Supported browsers must utilize the TLS encryption protocol v1.1 or v1.2 in order to connect to pages that require a secured HTTPS connection. TLS v1.0 is not supported.
The role of implicit biases on healthcare outcomes has become a concern, as there is some evidence that implicit biases contribute to health disparities, professionals' attitudes toward and interactions with patients, quality of care, diagnoses, and treatment decisions. This may produce differences in help-seeking, diagnoses, and ultimately treatments and interventions. Implicit biases may also unwittingly produce professional behaviors, attitudes, and interactions that reduce patients' trust and comfort with their provider, leading to earlier termination of visits and/or reduced adherence and follow-up. Disadvantaged groups are marginalized in the healthcare system and vulnerable on multiple levels; health professionals' implicit biases can further exacerbate these existing disadvantages.
Interventions or strategies designed to reduce implicit bias may be categorized as change-based or control-based. Change-based interventions focus on reducing or changing cognitive associations underlying implicit biases. These interventions might include challenging stereotypes. Conversely, control-based interventions involve reducing the effects of the implicit bias on the individual's behaviors. These strategies include increasing awareness of biased thoughts and responses. The two types of interventions are not mutually exclusive and may be used synergistically.
#93032: Female Sexual Dysfunction
Sexual dysfunction is a highly prevalent condition. Among U.S. adults 18 to 59 years of age, an estimated 31% of men and 43% of women have sexual function concerns. Sexual problems are most prevalent in older men and young women [1,2,3]. Female sexual dysfunction broadly includes sexual pain and/or diminished or loss of sexual desire/interest, arousal, or orgasm, and diagnosis requires patient distress in addition to impairment [4].
Sexual dysfunction can adversely and profoundly impact patients' quality of life and self-image. Particularly in women, sexual dysfunction is often under-recognized and untreated, and many patients are reluctant to discuss sexual concerns with primary care providers [5,6].
In contrast to male sexual dysfunction, female sexual dysfunction has, until recently, received sparse study and characterization [7]. Before the 1990s, sexual dysfunction was assumed to arise from psychologic problems and was treated by psychologic intervention. The 1998 introduction and success of sildenafil (Viagra) helped shift research and clinical focus to a biomedical paradigm. However, current understanding of sexual dysfunction incorporates a broader, more complex framework. Sexual function is influenced by a complex interaction of physiologic, sociocultural, and psychologic factors. Their relative contribution to sexual dysfunction across patients varies broadly, and physiologic contribution to sexual dysfunction is decidedly more prominent in men. Nonetheless, all female patients presenting with sexual complaints should receive diagnosis and treatment based on assessment of five key biopsychosocial domains [4,8,9]:
Medical factors
Cultural or religious factors
Individual vulnerability factors
Relationship factors
Partner factors
Almost all patients require a biopsychosocial treatment approach. Even strictly physiologic sexual dysfunction can result in patient demoralization, loss of confidence, relationship problems, or sex avoidance, all of which are effectively addressed by psychologic treatment. Several medications studied for female sexual dysfunction show only modest benefit, but adding an educational or psychologic intervention can robustly increase efficacy and patient response. Combining medication and psychosocial intervention is more effective than either one alone in most female sexual dysfunction [10,11].
The concept of sexual health has also evolved from a narrow focus on the prevention of sexually transmitted infections and unplanned pregnancy, to one that encompasses broader elements of reproductive health and sexual function. Sexual health is now understood as the ability to have pleasurable and safe sexual experiences free from coercion, with sexual health and sexual relationships strongly inter-related to general health and fundamental to individual, family, and social life in all cultures throughout the adult lifespan [12]. Clinicians require familiarity with knowledge advances; comprehension of the diverse etiologies, risk factors, and management strategies in specific female sexual dysfunction; and skills development to initiate and address this important aspect of wellness and quality of life [6,13].
Female sexual dysfunction is an umbrella term encompassing a range of common disorders, including hypoactive sexual desire, reduced subjective and/or physical genital arousal (e.g., poor sensation or lubrication, vasocongestion), sexual pain, and inability to achieve orgasm and/or satisfaction. Female sexual dysfunction is multidimensional, and different female sexual dysfunction conditions often co-occur. Emotional and contextual factors significantly influence the physiologic components of sexual response and behavior, and as noted, many patients benefit from a tailored multimodal approach [14].
The concept of "normal" female sexual function is misleading because definition and objective measurement are absent. This leaves the description of "normal" to statistical and/or cultural norms, even though normal function may vary between women and within the same woman over her lifetime. The first model to describe healthy sexual response came from Masters and Johnson in 1966. They proposed a four-stage (excitement, plateau, orgasm, resolution) linear model based on laboratory measurements of physiologic change in men and women. Kaplan and Leif modified this model to incorporate desire in order to capture the psychologic, emotional, and cognitive components of sexual response. Their revised linear model described three phases: desire, excitement, and orgasm [8].
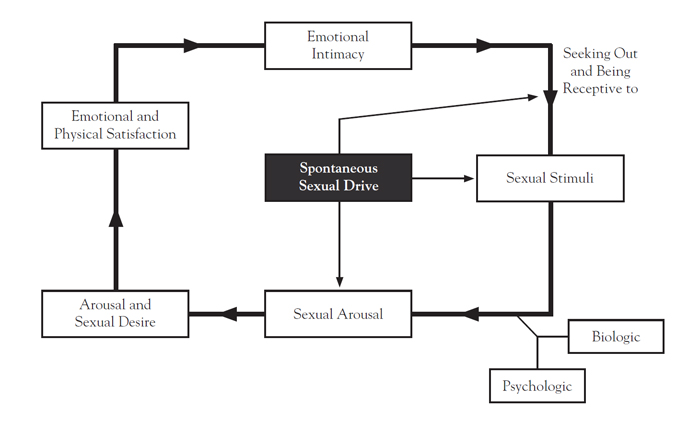
Building on observations that female sexual response often does not follow a linear trajectory, Basson introduced an intimacy-based circular model to help explain the multifactorial nature of female sexual response (Figure 1) [15,16]. Her model acknowledged the interconnection between emotional intimacy, sexual stimuli, psychologic factors, and relationship satisfaction that determines sexual response. Also introduced were the concepts of sexual neutrality and responsive desire, which states that instead of sexual motivation by spontaneous desire, women may experience sexual desire from arousal in the context of a loving relationship [8].
The multifactorial model and additional research suggest that female sexual response is not uniformly linear, the distinction between desire and arousal phases is likely artificial, and desire and arousal are difficult to separate because normal desire can include a responsive component. These findings shaped the revision of female sexual dysfunction by the American Psychiatric Association in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) [4,15]. Low sexual desire, termed hypoactive sexual desire disorder (HSDD) in the DSM-IV-TR, was merged with female sexual arousal disorder to form the diagnostic entity of female sexual interest/arousal disorder (FSIAD) in the DSM-5 [4,17,18]. This remains the case in the text revision of the DSM-5 released in 2022 [4].
To increase objectivity and precision and avoid overdiagnosis of transient sexual problems, diagnosis requires at least a six-month duration and more precise severity criteria. With loss of desire, the experience of personal distress replaces partner or relationship as the primary focality of stress [4,8]. The requirement for diagnosis of marked personal distress from female sexual dysfunction was made because many more women experience low sexual desire/arousal or orgasmic difficulties than are distressed by them. While orgasmic difficulties and low desire/arousal are less prevalent among premenopausal than postmenopausal women, premenopausal women are more like to be distressed by these issues [19].
With female orgasm, sexual desire at the outset of a wanted sexual experience is not necessary for orgasm to occur. Sexual or nonsexual erotic stimulation may result in orgasms, and despite sexual satisfaction, orgasms may not occur or may occur in multiples [20]. In light of the highly prevalent female experience of moderate-to-high levels of sexual satisfaction despite an absence of sexual desire between sexual encounters, the Endocrine Society now considers the incentives/motivations model of human sexual response to more accurately reflect sexual experience. This model states that desire for sex per se is just one of many reasons or incentives for sex [21,22].
For a definitive diagnosis, all disorders of female sexual function require that symptoms persist for at least six months [18]. The disorder must not be better explained by a nonsexual mental disorder or a consequence of severe relationship distress or other significant stressors and must not be attributed to the effects of a substance, medication, or other medical condition.
According to the DSM-5-TR, FSIAD is defined as lack of or significantly reduced sexual interest or arousal as manifested by at least three of the following [4]:
Absent/reduced interest in sexual activity
Absent/reduced sexual or erotic thoughts or fantasies
No or reduced initiation of sexual activity and unreceptive to partner's attempts to initiate
Absent/reduced sexual excitement or pleasure during sexual activity in all/almost all (75% to 100%) sexual encounters
Absent/reduced sexual interest or arousal in response to any internal or external sexual or erotic cues (written, verbal, or visual)
Absent/reduced genital or nongenital sensations during sexual activity in all/almost all (75% to 100%) sexual encounters
Furthermore, FSIAD may be described as primary, if the woman has never experienced sufficient arousal (even with sufficient desire and sexual stimulation), or secondary, if the woman has been sufficiently aroused in the past but currently experiences decreased arousal [4]. If the problem occurs in all sexual situations, it is generalized; if the problem occurs only in some sexual situations, it is considered situational.
The DSM-5-TR criteria for the diagnosis of female orgasmic disorder requires that the woman experience markedly less intense orgasms or marked delay in, marked infrequency of, or absence of orgasm in all or almost all (75% to 100%) sexual activity [4]. The severity of female orgasmic disorder is specified as mild, moderate, or severe on the basis of the level of distress the patient exhibits over the symptoms.
Genitopelvic pain or penetration disorder is characterized by persistent or recurrent difficulties with one or more of the following [4]:
Vaginal penetration during intercourse
Marked vulvovaginal or pelvic pain during intercourse or penetration attempts
Marked fear or anxiety about vulvovaginal or pelvic pain in anticipation of, during, or because of vaginal penetration
Marked tensing or tightening of pelvic floor muscles during attempted vaginal penetration
Vulvodynia
Vulvar pain generally falls into one of two categories: vulvodynia or vestibulodynia. Vulvodynia is pain with no visible abnormalities [19]. However, vulvar pain may also be secondary to underlying conditions. Vestibulodynia (also referred to as provoked vestibulodynia) is a subtype of vulvodynia whereby pain is provoked by any direct contact with the vulvar vestibule. The pain arising from provoked vestibulodynia is characterized as sharp and burning. Provoked vestibulodynia is classified as primary or secondary according to pain onset [19]. Primary provoked vestibulodynia is diagnosed when pain has been present since the first attempt to insert anything, including a tampon or a penis, into the vagina. When the pain gradually appears following a period of painless sexual activities (or examination/tampon insertion in sexually inactive women), this is considered secondary provoked vestibulodynia.
Vaginismus
Vaginismus is the involuntary contraction of pelvic musculature surrounding the outer third of the vagina. The incidence of vaginismus is unknown, and the ranges quoted in the literature vary widely. Vaginismus may be lifelong (primary), acquired (secondary), complete, partial, and/or situational [19].
Deep Dyspareunia
Deep dyspareunia is pain associated with deep penetration that may be primary (lifelong) or secondary [19]. The pain may be present every time penetration is attempted, sporadic, or cyclical in nature. In some patients, it is restricted to vigorous intercourse or to positions facilitating deeper penetration. Diagnosis is generally made based on patient history and physical examination [23].
Genitourinary Syndrome of Menopause
Genitourinary syndrome of menopause is the term now used in place of vaginal atrophy and vulvovaginal atrophy. Introduced in 2014 by the International Society for the Study of Women's Sexual Health and the North American Menopause Society, genitourinary syndrome of menopause is a medically more accurate, all-encompassing, and publicly acceptable term than its predecessor. It is defined as a constellation of symptoms and signs associated with age-related decreases in estrogen and other sex steroids, including changes to the labia majora/minora, clitoris, vestibule/introitus, vagina, urethra, and bladder. Symptoms of genitourinary syndrome of menopause must be bothersome and not be better explained by another diagnosis and include genital dryness, burning, and irritation; sexual symptoms of lack of lubrication, discomfort or pain, and impaired function; and urinary symptoms of urgency, dysuria, and recurrent urinary tract infections. Women may present with some or all of these signs and symptoms [24]. In a survey of 3,046 women with genitourinary syndrome of menopause, 85% of partnered women reported some loss of intimacy, 59% reported diminished enjoyment of sex, and 47% reported interference with their relationship [25]. In addition, 29% reported sleep disruption and 27% described a negative effect on their general quality of life. Only 7% stated their provider initiated a discussion about genitourinary syndrome of menopause.
The Female Sexual Function Index (FSFI) is the criterion standard instrument in assessing female sexual dysfunction and treatment response in clinical trials and can also be used in clinical practice. The FSFI consists of 19 questions with a severity scale, rated by the patient or subject. Sexual function is assessed across six domains: desire, arousal, lubrication, orgasm, satisfaction, and pain. The total FSFI score is the sum of all six domain scores. Higher scores indicate better sexual function, with a highest possible total score of 36. Scores of 26.55 or less are considered female sexual dysfunction in premenopausal and postmenopausal women [26,27].
The Decreased Sexual Desire Screener (DSDS) is a validated, five-question, self-administered screening instrument. It is used in clinical practice to help practitioners identify generalized acquired hypoactive sexual desire in premenopausal and postmenopausal women. This screener is a time-efficient, pragmatic adjunct to the patient history and physical examination in the diagnostic workup of hypoactive sexual desire [8].
The Female Sexual Distress Scale (FSDS) assesses sexual dysfunction and associated distress [28]. The revised FSDS consists of 13 items the patient is asked to score on a scale of 0 (never) to 4 (always). The items focus on patients' experiences of anger, embarrassment, shame, guilt, and unhappiness with sex and their sexual performance in order to assess the level of distress experienced as a result of sexual problems.
The lifetime prevalence of any female sexual dysfunction is 40% [1,2]. In one study, roughly 40% to 45% of adult women had at least one manifest sexual dysfunction (somewhat often, often, nearly always, or always), although distress over the dysfunction was not measured [29]. At any time, around 10% of women experience low or absent sexual desire, making it the most common female sexual dysfunction, followed by orgasmic impairment [4].
The Prevalence of Female Sexual Problems Associated with Distress and Determinates of Treatment Seeking (PRESIDE) survey of 31,581 U.S. women 18 years of age and older found low desire and distress in 8.9% of women 18 to 44 years of age, 12.3% of those 45 to 64 years of age, and 7.4% in women older than 65 years of age, indicating a peak during middle age [1]. A cross-sectional study of 2,207 U.S. women 30 to 70 years of age found an overall 8.3% prevalence of HSDD [30]. A multi-national Global Study of Sexual Attitudes and Behaviors (GSSAB) study of women 40 to 80 years of age found decreased libido in 26% to 43% [3]. Other studies have shown similar trends [8].
The reported prevalence of female orgasmic problems ranges from 8% to 72%, with approximately 10% of women never experiencing orgasm in their lifetime [4]. Although these rates vary by age, culture, symptom duration, and severity, the true prevalence is probably not captured because associated distress is usually not assessed. The PRESIDE study found a female orgasmic disorder prevalence of 21% [1]. The GSSAB study reported an inability to achieve orgasm in 18% to 41% of participants [3]. Studies of women with female orgasmic disorder tend to show a high prevalence of comorbid FSIAD, suggesting that clinicians often ignore the criterion of orgasm absence following normal sexual excitement.
Provoked vestibulodynia is a highly prevalent cause of dyspareunia in younger women [19]. The estimated prevalence of vestibulodynia is 12% in the general population and 15% among gynecologic clinic patients [31,32]. A U.S. community survey of chronic (≥12 months) vulvar pain found a 9.9% prevalence, with 45% of those affected reporting adverse impact on their sexual functioning [33].
In general, female sexual dysfunction is the result of a combination of medical, sociocultural, individual vulnerability, and relationship/partner factors [4]. The multifactorial nature of FSIAD and female orgasmic disorder is underscored by the finding that a majority of premenopausal women identified non-physiologic factors, such as stress, fatigue, dissatisfaction with physical appearance, and other sexual difficulties, as contributory to decreased desire [34].
A variety of systemic and localized diseases or conditions may contribute to female sexual dysfunction through direct or indirect mechanisms (Table 1) [8,35]. Medical etiologic and risk factors include existing medical conditions and the effects of drugs or medications. Automatic sexual responses are largely governed by the autonomic nervous system, intact nerve mediation, vascular function, and hormonal factors. Disruptions with any of these functions can impair genital arousal. Many prescribed and recreational/street substances can induce female sexual dysfunction, and chronic tobacco or alcohol use may affect sexual function through vascular or neurologic damage [6].
DISEASES OR CONDITIONS THAT MAY CONTRIBUTE TO FEMALE SEXUAL DYSFUNCTION
| Category | Conditions |
|---|---|
| Cardiovascular | Hypertension, coronary artery disease |
| Endocrine | Diabetes, thyroid disorders, hyperprolactinemia, adrenal disorders, hypopituitarism |
| Gastroenterologic | Hepatic dysfunction, inflammatory bowel disease, irritable bowel syndrome |
| Autoimmune/arthritic | Systemic lupus erythematosus, arthritis |
| Infection | Systemic infections, sexually transmitted infections (hepatitis B/C, syphilis) |
| Malignancy | Breast cancer and treatment (negatively impacting body image and/or disrupting endocrine pathways), colon cancer |
| Neurologic | Epilepsy, multiple sclerosis, stroke and trauma, degenerative diseases, Parkinson disease, dementias, hypothalamic disorders, fibromyalgia |
| Vascular | Peripheral vascular disease, coronary artery disease |
| Diseases or conditions involving the genitals or proximal organ systems | |
| Dermatologic (vaginal region) | Dermatitis, herpes simplex, psoriasis, lichen sclerosis, carcinoma |
| Musculoskeletal | Mechanical low back pain, spinal stenosis, hip fracture, pelvic floor muscle spasm |
| Neurologic | Nerve entrapment syndromes, chronic pain disorders |
| Urologic | Recurrent bacterial cystitis, interstitial cystitis, bladder cancer, renal dysfunction |
| Gynecologic | Vaginitis, vestibulodynia (e.g., vaginismus, vestibulitis), vulvodynia, pelvic floor dysfunction, endometriosis, premature ovarian failure, menopausal atrophy, ovarian masses, uterine fibroids, prolapse, gynecologic malignancies |
Medical conditions affecting the autonomic nervous and vascular systems are known risk factors for diminished sexual arousal, including diabetes, multiple sclerosis, and spinal cord injuries. Other medical conditions (or their treatment) that can indirectly affect desire and arousal include urinary tract infections, recurrent vaginal infections, and pelvic or genital surgery or radiation therapy. Anti-estrogenic treatment for hormone-sensitive breast cancer is also a risk factor [6].
A thorough diagnostic/differential workup is essential to detect contributory or causal medical conditions. Some cases of female sexual dysfunction originate from etiologies that, if detected, can be treated and female sexual dysfunction resolved.
Hyperthyroidism
Hyperthyroidism, which is relatively common in women, can lead to sexual dysfunction. Compared to age-matched healthy female controls, women with hyperthyroidism show statistically and clinically lower scores in FSFI desire, arousal, lubrication, orgasm, satisfaction, and pain domains, and significantly higher scores on the Beck Depression Inventory (BDI) [36]. Female sexual dysfunction in hyperthyroidism is associated with higher depressive levels, increased sex hormone-binding globulin (SHBG), and decreased free testosterone levels [36].
Iron-Deficiency Anemia
In women of reproductive age, iron-deficiency anemia may cause anxiety, a major risk factor for female sexual dysfunction. The association of iron-deficiency anemia with female sexual dysfunction was evaluated in 207 women (mean age: 33.6 years) before and after iron-deficiency anemia treatment. Pre- and post-treatment comparisons found significant differences in hemoglobin, hematocrit, serum iron, and serum iron-binding capacity. Anxiety scores decreased and FSFI scores significantly increased after iron-deficiency anemia treatments. Excessive anxiety and female sexual dysfunction may be present in women of reproductive age with anemia, both reversible with effective iron-deficiency anemia treatment [37].
The acute effects of alcohol or drugs can intensify or impair sexual desire, response, and orgasm in women, while heavy or chronic use of most substances induces sexual dysfunction.
Cannabis
In study from 1982, female cannabis users reported increased pleasure (76%) and improved orgasm quality (30%) with acute drug use. Cannabis was thought to increase disinhibition and erotic fantasies [38]. A 2020 survey of 452 female regular cannabis users (30 to 49 years of age) found that increasingly higher levels of cannabis use were correlated with increased frequency of intercourse and fewer reports of sexual dysfunction [39]. A 2021 study in slightly younger adults (average age: 29.9 years) also found that frequent cannabis users have better overall sexual function [146]. The authors noted the results of several other studies with similar findings, including increased desire, arousal, orgasm, and satisfaction in cannabis users, but also one study in which, despite overall improvements in sexual satisfaction, desire, orgasm, and sexual pain with cannabis use before sex, no increase (or decrease) in vaginal lubrication was noted [146,147].
Cannabis use is thought to reduce the anxiety and shame often associated with sexual activity [146]. Additionally, cannabis interacts directly with the human endocannabinoid system, which is known to regulate sexual function; higher levels of endocannabinoids are associated with increased arousal [39].
Alcohol
Alcohol acts acutely to disinhibit behavior and may increase sexual receptivity or initiation [19]. However, chronic alcohol use may lead to loss of desire, decreased arousal, and anorgasmia. Inhibition of hypothalamic-pituitary-adrenal (HPA) axis function decreases estradiol levels, which may interfere with vaginal lubrication [19].
Opioids
The rush of euphoria and the subsequent period of relaxation induced by heroin and other opioids may reduce interest in sex. The acute heroin effect has been termed "pharmacogenic orgasm" [40]. Women who frequently use opioids or develop opioid abuse/addiction show a high prevalence of decreased libido, interest, arousal, and orgasm [40].
Stimulants
Methamphetamine and cocaine acutely reduce sexual inhibition, enhance well-being and excitement, and increase the likelihood of high-risk sexual behavior [19]. Long-term use and withdrawal of methamphetamine has been linked to sexual dysfunction in men, but the impact on female sexual desire and performance is unclear [41]. Chronic cocaine use results in high prevalence of female sexual dysfunction and negative effects on sex life [42].
A mixed methods study explored the relationship between sexual behavior and methamphetamine use in a community-based, racially diverse sample of 322 women 40 years of age and older [43]. Mean days of past month methamphetamine use was 18. Most women (91%) used other illicit drugs (most commonly crack cocaine) in addition to methamphetamine. The women reported being frequently high on methamphetamine while having sex (median: 80%). The findings suggest a strong relationship between methamphetamine use and sexual desire and protracted sexual encounters. Sexual pleasure was described as a key benefit of methamphetamine use.
Nicotine
Cigarette smoking is an established sexual dysfunction risk factor in men and women, though the link is stronger in men [44]. In one study of healthy nonsmoking women (average age: 20 years), nicotine reduced physiologic genital arousal by an average of 30% in 60% of the participants without effecting self-rated sexual arousal or mood. The authors conclude that nicotine may be the primary cigarette constituent that disrupts genital hemodynamics and triggers a biochemical and vascular cascade, impairing normal female sexual arousal response [45].
Among the prescribed drugs with known potential to impair sexual functioning, antidepressants are perhaps the most widely prescribed [46]. Among antidepressants, selective serotonin reuptake inhibitors (SSRIs) and serotonergic/noradrenergic reuptake inhibitors (SNRIs) show the highest rates of sexual dysfunction, including impaired sexual motivation, desire, arousal, and orgasm affecting men and women.
In both men and women, antidepressant-induced sexual side effects largely result from increased serotonin (5-HT) neurotransmission via reuptake blockade of serotonin transporters. Antidepressants that primarily increase dopamine and norepinephrine neurotransmission produce markedly fewer sexual side effects. SSRI/SNRI-induced sexual side effects are likely mediated by inhibitory actions on dopamine signaling in sex brain circuits and can be decreased by simultaneously increasing norepinephrine and dopamine neurotransmission but not by increasing norepinephrine alone. A meta-analysis of sexual side effect rates with SSRIs and venlafaxine reported that orgasm and desire dysfunction are more common in men, while arousal dysfunction is more common in women [47].
Many other prescribed medications and classes are associated with female sexual dysfunction side effects. A partial list is shown in Table 2[8].
MEDICATIONS ASSOCIATED WITH REDUCED FEMALE SEXUAL DESIRE, AROUSAL AND/OR ORGASM
| Drug Class | Specific Drug Category or Agent | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Anticonvulsants |
| ||||||||
| Cardiovascular and antihypertensive agents |
| ||||||||
| Hormonal medications |
| ||||||||
| Analgesics |
| ||||||||
| Others |
|
The assumption that statin use leads to reduced sex hormone biosynthesis in women, possibly resulting in menstrual irregularities, menopausal disorders, infertility, and low libido, is based on studies that failed to evaluate the effects of statins on gonadal-sexual function in women. A 2015 comparison of 2,890 statin users with 2,890 nonusers (average age: 58 years) found statin use lacked significant association with menstrual disorders, menopausal disorders, infertility, or ovarian/sexual dysfunction [48]. This study questions previous associations of statin use with female sexual dysfunction.
Aging can affect all aspects of female sexual function, and the greatest prevalence of low desire/arousal and distress occurs in women 45 to 64 years of age. Intensity of sexual desire may decline due to neuroendocrine changes, such as declining testosterone, changes in neurochemistry, and indirect changes from loss of estrogen. Genital sensation may change, with arousal and orgasm requiring stronger and longer stimulation. Low estrogen levels can lead to vulvovaginal atrophy and dyspareunia associated with decreased desire. These factors, and the unique psychosocial factors present during this life phase, influence sexual function during the menopausal transition [8,49,50]. The Nurses' Health Study II included 68,131 women 48 to 68 years of age. Sexual activity and dysfunction symptoms were assessed using the FSFI index. Of the participants, 73% were sexually active; 50% of these women reported symptoms of sexual dysfunction. Symptoms were less common among unpartnered (42%) than partnered women (51%). A positive association between menopause and sexual dysfunction was greater among unpartnered than partnered women [51].
Smoking, alcohol consumption, and obesity are associated with higher rates of sexual dysfunction, and stresses such as a difficult work environment, long work hours, and a lack of privacy may negatively impact relationship well-being and sexual functioning. Patients may fail to connect life stressors with sexual dysfunction, so this area should be thoroughly assessed during the patient evaluation. Responses vary, however, as many women with busy and sometimes stressful lives maintain satisfying sexual relationships [19].
Cultural or religious factors with potential negative impact on female sexual function include inhibitions or conflicted attitudes toward sexuality. Cultural, social, and religious values or mores can negatively influence female sexual function and sexual desire in particular, especially in women raised in highly restrictive cultures or religions. Beliefs such as "good" or "virtuous" women should not enjoy or initiate sex or that men always exploit women (or vice versa) may lead to negative feelings or thoughts during sexual activity. Negative attitudes from childhood may persist to affect women emotionally, even when no longer held as adults [19,52,53].
A study of risk factors for impaired ability to experience orgasm during sexual activity found that lower levels of education and sex education during childhood and/or adolescence were significantly associated with female orgasmic disorder. Anxiety and low sexual desire were found to predict orgasm problems, while masturbation and completion of high school increased the likelihood of reaching orgasm during sexual activity [54].
Individual vulnerability factors that increase the risk for sexual dysfunction in women include a history of sexual or emotional abuse, current psychiatric conditions (e.g., depression), and life stressors (e.g., job loss).
Sexual abuse is associated with lower physiologic sexual arousal responses and higher rates of FSIAD. The mechanism is unclear, but biologic factors, (e.g., HPA axis dysregulation) and psychologic factors (e.g., post-traumatic stress disorder [PTSD]) may be contributory. However, not all women with sexual abuse history develop sexual dysfunction, and women who have been sexually abused may develop sexual problems not necessarily related to the abuse but to medical issues such as chronic fatigue syndrome, irritable bowel syndrome, or fibromyalgia [55]. Chronic pelvic pain was found by one study to be strongest predictor of sexual abuse history in women seeking medical attention for pelvic floor disorders [56]. Other types of childhood abuse may affect sexual arousal in adulthood. Women with major depressive disorder or PTSD resulting from abuse should be treated for those disorders before, or along with, the disorder of sexual function [6].
In a comparison of women with and without past childhood sexual abuse, increased genital arousal was reported as pleasant and satisfying by 70% without childhood sexual abuse history and 29% with childhood sexual abuse history [57]. Women with a history of childhood sexual abuse were more likely to experience shame and anxiety with their sexual responses. High sexual functioning levels did not correlate with lack of sexually related distress in women with childhood sexual abuse history. Treatments aimed at improving sexual functioning may be less effective in these patients, as worsening of distress is likely. Patients' views of their own sexual functioning should be addressed before implementing treatment to improve sexual function. Cognitive and evaluative processes may be more important than sexual functioning in determining levels of sexual distress in women with childhood sexual abuse history, but this does not apply to every patient; previous research found childhood sexual abuse history in female college students was unrelated to negative sexual outcomes in function and satisfaction [57,58].
Psychologic factors significantly influence sexual desire and arousal and may override biologic factors. Depression is strongly associated with sexual difficulties and dissatisfaction. The effects of depression on mood, energy, interests, and self-esteem can greatly impair sexual interest and satisfaction, especially in younger patients. Other adverse effects on female sexual function can develop from depression, such as an inability to attain adequate vaginal lubrication or orgasm. Also reported with depression are distressing hypersexuality symptoms [59,60].
Studies have found higher levels of anxiety in women with sexual problems and, conversely, higher rates of sexual dysfunction in women with anxiety. Anxiety during sexual activity is a contributor to sexual dysfunction. This anxiety can originate from negative attitudes resulting from negative or traumatic past experiences, such as childhood sexual abuse or sexual victimization experienced as an adult [61,62]. Sexual anxiety may result from body image and self-esteem issues or from fear of failure to respond sexually or enjoy the sexual activity. Anxiety can cause sexual dysfunction through cognitive distraction or the intrusion of non-erotic thoughts or feelings (e.g., fear of not getting aroused or that her partner finds her unattractive). Focus on these non-erotic thoughts is described as "spectatoring," or monitoring and judging one's appearance and behavior during sexual activity. Spectatoring interferes with sexual response and is linked to poorer sexual functioning [63,64,65]. Negative body image includes concerns and negative assessment of one's sexual attractiveness, weight, and physical condition, and each significantly predicts poor sexual satisfaction after controlling for sexual functioning status. The adverse effect of negative body image on sexual satisfaction results from the higher frequency of appearance-based distracting thoughts during sexual activity [66].
Significant life stress can also lead women to experience low sexual desire or difficulty with arousal. Excessive anger, sadness, personality disorders, histrionic personality, and low/fragile self-regulation and self-esteem can all impair sexual response and negatively affect sexual function [6,67].
Problems with partner erectile dysfunction or premature ejaculation, communication problems, differing levels of desire for sexual activity, or partner violence can affect female sexual function and increase risk of female sexual dysfunction [6]. Non-sexual relationship aspects, such as frequent arguments, anger and resentment toward the partner, lack of trust, poor communication, and lack of quality couple time, can interfere with the feeling of closeness and intimacy important to quality lovemaking and ability to respond sexually [19,68,69]. External stressors, such as financial hardship, career-related pressures, and familial obligations, can contribute to decreased sexual desire [8].
Deficient or false information concerning normal sexual functioning can promote beliefs that are antagonistic to sexual function, interfering with sexual response. Some women with arousal problems are unaware of the location or role of their clitoris in sexual arousal and orgasm. Older women may be anxious and have diminished sexual response from believing myths related to "normal" sexual behavior with aging. Women with erroneous beliefs may place unrealistic expectations on self (e.g., "Normal women always orgasm from vaginal penetration") or partners (e.g., "Not getting instantly erect means you are no longer attracted to me") [19].
Female sexual dysfunction may stem from an insufficiently stimulating sexual routine involving limited sensual (as opposed to genital) fondling and caressing, limited or ineffective genital caressing and stimulation, or a predictable and mundane sexual routine. Poor communication about sexual preferences and desires between partners is the usual cause, due to the woman lacking assertiveness, expecting her partner to know her needs without instruction, or unawareness of her sexual preferences [19,70,71].
Common etiologies of dyspareunia include genitourinary syndrome of menopause, dermatologic diseases, infections (e.g., genital herpes, candidiasis), and visible or non-visible lesions [19].
Genitourinary syndrome of menopause affects up to 45% of postmenopausal women [72]. Independent of dyspareunia, female sexual dysfunction can develop in women with genitourinary syndrome of menopause from recurrent urinary tract infections (and resultant interruptions of daily functioning), reduced libido, and impaired mood and social interactions from nocturia-induced sleep loss. Urinary urgency and concerns over possible incontinence can render sex unpleasant due to fear of odor, embarrassment, shame, or loss of self-esteem. Women may develop anxiety or depression over symptoms they associate with aging and age-related bodily changes [73]. The loss of vulvovaginal tissue elasticity, vaginal wall thinning, and decreased vaginal lubrication with vaginal atrophy increase the risks of micro-tears, genital lesions, and sexually transmitted infections during intercourse [73].
Female sexual dysfunction is a prevalent consequence of vestibulodynia. Of 161 women (average age: 36 years) presenting to a vulvovaginal specialty clinic with chronic vaginal complaints, 53% experienced female sexual dysfunction in the previous month, a prevalence double the population rate [74].
The etiology of provoked vestibulodynia is not fully understood but likely involves multiple factors, including congenital disorders, genetic and immunologic factors, hormonal factors (including oral contraceptive use), central neuropathic pain, nociceptor proliferation, and myofascial hypertonicity [75]. Most reject psychosexual dysfunction as the primary causation, although healthcare providers recognize the frequent association between psychologic and sexual distress with chronic sexual pain in women [19]. A large study of genital pain in sexually active college students found women with any sexual pain differed from those with no pain by higher rates of sexual dysfunction, but that women with high versus low sexual pain were distinguished primarily by vaginal lubrication [76].
Combined Hormonal Contraceptive-Induced Vestibulodynia
The use of combined estrogen/progestin contraception significantly increases the risk of vestibulodynia. Combined hormonal contraception inhibits luteinizing hormone, which decreases ovarian testosterone production. The synthetic estrogen and progestin components of combined hormonal contraception are metabolized in the liver, and this increases hepatic SHBG production. Decreased ovarian production of testosterone and increased SHBG diminish circulating bioavailable (free) testosterone levels [77,78]. Combined hormonal contraception also alters morphologic patterns and hormone receptors in vestibular mucosa and lower vulvar vestibule pain threshold [79]. Relative to earlier options, newer progestins in combined hormonal contraception produce significantly decreased free testosterone [80]. Earlier research failed to link combined hormonal contraception use with loss of libido and vestibular pain, but these studies did not examine contraception type or duration. Other studies of vulvar vestibule hormone receptor expression did not find differences between patients with vestibulodynia and pain-free volunteers, but failed to control for combined hormonal contraception use [80,81,82].
Vaginismus may be precipitated by painful intercourse, painful pelvic examination, sexual assault, pelvic inflammatory disease, gynecologic surgery, urogenital atrophy, vulvar dermatologic conditions, or childbirth. Generalized anxiety levels are usually elevated in patients with vaginismus, suggesting that vaginismus and anxiety disorders share common predisposing factors [83]. Maintaining factors should be considered, and continuation of intercourse despite pain can promote anticipatory muscle spasm [19].
Deep dyspareunia is often caused by endometriosis, interstitial cystitis, adnexal pathology, retroverted uterus, shortened vagina, pelvic floor hypertonicity, over-vigorous penetration, post-surgical vault or intraperitoneal scarring, uterine prolapse, excessive penile length, pelvic congestion syndrome, ovarian cysts, or constipation [19].
Relevant to patients with dyspareunia, especially in those with genitourinary syndrome of menopause, is the role of estrogen as dominant regulator of vaginal physiology. Estrogen-receptor density is highest in the vagina, with decreasing density across the external genitalia to the skin. The density of androgen receptors is the reverse, with low levels in the vagina and higher levels in the external genitalia [84]. Increasing evidence supports topical androgen safety for some women with genitourinary syndrome of menopause, and this differential receptor density may help explain topical androgen efficacy in women lacking topical estrogen response.
Perception of the clinician as uncomfortable, disinterested, or reluctant hinders patient communication about sexual concerns [8]. Conversely, acknowledging to patients that their sexual function concerns are valid makes them feel heard and is therapeutic even when the issue cannot be immediately resolved. When uncovering an issue, healthcare professionals and patients can jointly decide on management. Treatment is generally initiated by a physician or referred to a specialist, although an interested and prepared primary care provider can address many sexual dysfunction problems without referral [19].
The same general approach to assessment and diagnostic workup is used regardless of specific sexual complaint. A full assessment investigates biopsychosocial factors that may be involved in predisposing, precipitating, and/or maintaining the sexual dysfunction [85,86]. It is best performed by obtaining a medical, sexologic, psychologic, and relationship history; conducting a physical examination and appropriate laboratory testing; differential diagnosis; and evaluation of distress severity [6]. Treatment planning follows the comprehensive biopsychosocial diagnosis, and interventions are tailored to the patient [85,86].
In this multicultural landscape, interpreters are a valuable resource to help bridge the communication and cultural gap between clients/patients and practitioners. Discussions of sexuality can be sensitive, and removing possible language barriers using professional interpreters is recommended for patients for whom English is not their first language. Interpreters are more than passive agents who translate and transmit information back and forth from party to party. When they are enlisted and treated as part of the interdisciplinary clinical team, they serve as cultural brokers who ultimately enhance the clinical encounter.
A complete medical history can identify contributions to female sexual dysfunction from medical or psychiatric disorders, medications, and oral contraceptives. The following areas should be addressed [6,8]:
Menstrual irregularities indicating hormonal disorders such as hyperpro-lactinemia or hypothyroidism
Menstrual cycle, menopause, pregnancy, or breastfeeding
Somatic problems
Diseases known to cause lubrication problems (e.g., diabetes, recurrent vaginal infections)
Sexually transmitted infections
Urinary incontinence
Iatrogenic causes (e.g., pelvic surgery, radiation therapy, medications)
When appropriate and safe for the patient, a sexual history is helped with input from the woman's partner. The sexual history should be conducted in a culturally sensitive manner, with consideration of patient background, lifestyle, and status of the partner relationship, and should include direct questions about sexual behavior and safe sex practices [85].
A detailed history of arousal and orgasm problems and the conditions under which they occur is important. Inquire about the patient's ability to become mentally sexually excited, awareness of a genital response during sexual stimulation (such as tingling or lubrication), presence of vaginal dryness or dyspareunia, whether she receives adequate sexual stimulation, presence of sexual problems with pain or desire, and extent of distress by the problem [6].
If present, ask the patient about vulvar pain history, chronicity, characteristics, and aggravating and relieving factors [87,88]. Patients with chronic vulvar pain often experience intense feelings of frustration, guilt, anger, and helplessness, all of which significantly impact sex life. A history of multiple medical provider contacts and interventions are common in these cases.
With vaginismus, accurate diagnosis is usually made by patient history and physical exam findings. Pain with intercourse is the most common presenting symptom, described as burning, tearing, or the sensation of a cut in the vagina or of sandpaper rubbing the vagina. The pain can lead to inability to have penetrative intercourse; actual muscle contraction can prevent intromission [19,83,89].
Evaluate cognitive and affective factors that may influence female sexual dysfunction by inquiring regarding thoughts before, during, and after the sexual experience, which can include the patient feeling distracted, sexually substandard, or unsafe. Clarify her emotions by asking whether she feels sadness, guilt, or anger during sexual activity, and assess for depression, PTSD, and anxiety disorders [6].
Assess relationship factors by inquiry of partner sexual dysfunction and relational problems, such as lack of attraction or conflicts. Patients should also be assessed for a history of unwanted or coerced sexual experience and physical or emotional abuse during childhood or adolescence, including whether past experiences affect current self-image [6]. With lifelong or generalized orgasmic disorder, inquiry should explore negative attitudes toward sex and whether this originates from childhood sexual experiences or unresolved feelings associated with previous abuse or assault [19].
A gynecologic exam is always recommended for patients with sexual complaints and should focus on changes or abnormalities in vulvar anatomy, signs of inflammation, skin color and quality, appearance of vaginal mucosa (estrogenized and moistened or atrophic), signs of myogenic or referred pain or associated urogenital and rectal pain, inspection of pelvic floor trophism, muscular tone and strength, scarring, determination of pH, and sampling and culture of discharge if infection is suspected [6].
Many women with sexual pain have normal findings, but the physical exam may uncover underlying contributors by presence of vulvovaginal atrophy (dyspareunia), genital sensory changes (vulvodynia or neuropathy), pelvic floor muscle contraction (vaginismus), and/or pelvic floor prolapse [8]. With presenting complaints of chronic vulvar pain, the physical examination may focus on whether pain symptoms are provoked by vaginal penetration [90].
Vaginismus is difficult to delineate from provoked vestibulodynia, and they often co-occur. Pelvic examination may be very difficult but, if tolerated, enables vaginismus diagnosis as follows [19,83,89]:
First degree: Perineal and levator spasm relieved with reassurance
Second degree: Perineal spasm maintained throughout pelvic examination
Third degree: Levator spasm and elevation of buttocks
Fourth degree: Levator and perineal spasm, elevation of buttocks, adduction, and retreat
The laboratory workup should include:
Complete blood count (CBC)
Chemistry panel
Hormone panel
Vitamin B12 and folate levels
Use CBC, serum chemistry panel (electrolytes, blood urea nitrogen, creatinine, glucose, and liver function), and vitamin B12/folate findings to rule out systemic disorder (e.g., nutrient deficiency, renal, liver, or inflammatory disease) or peripheral neuropathy [91]. An informative hormone panel should include [21,91]:
Thyroid test (thyroid-stimulating hormone [TSH] and free T4) to rule out hyper- or hypothyroidism
Estradiol to rule out estrogen deficiency
Follicle-stimulating hormone and luteinizing hormone (cutoff correlate with sexual dysfunction)
Prolactin to rule out hyperprolactinemia
Testosterone (total and free), only with testosterone therapy monitoring
Consider lipid profiles in patients at risk for metabolic or cardiovascular disease and urinalysis for infection and glucosuria.
Most laboratory tests can be performed in the primary care setting. Specialized assessments requiring referral are seldom needed and include vaginal pH and local vascular function assessment using photoplethysmography and vagina thermal clearance [92].
The diagnosis of female sexual dysfunction requires that another disorder or the effects of a substance are not present to better account for symptom complaints. When this criterion is not met, secondary sexual disorder is diagnosed.
In addition to the biopsychosocial contributors to or causations of female sexual dysfunction discussed previously, additional factors to assess when provoked vestibulodynia is suspected may include vulvar dermatosis, vulvovaginal infections, atrophy, and pudendal nerve entrapment [19].
For patients with orgasmic dysfunction, differential diagnosis will include endocrine disorders (e.g., hypothyroidism, Cushing syndrome, Addison disease, hypopituitarism, hyperprolactinemia, low estrogen and androgen levels) and medical conditions affecting pelvic blood and nerve supply (e.g., hypertension, multiple sclerosis, Parkinson disease, diabetic neuropathy, spinal cord injury) [93,94,95,96].
When a patient presents with chronic vulvar pain, the physical exam determines the differential diagnosis [19]. If there are no visible lesions, differential diagnosis includes essential vestibulovulvodynia, vaginismus, interstitial cystitis, fibromyalgia, Sjögren syndrome, constipation, depression, anxiety, and adverse life events. For patients with visible lesions (consistent or intermittent), possible underlying causes of vulvar pain include herpes, Candida infection, atrophic vaginitis, dermatologic conditions, allergic/irritant dermatitis, Behçet disease and other genital ulcer diseases, vulvar intraepithelial neoplasia or carcinoma, obstetrical scarring, gynecologic surgery, and female genital cutting/mutilation.
Abuse, serious psychiatric issues, or unresolved physical or sexual trauma identified during assessment or therapy requires prompt specialist referral, as these issues require stabilization or resolution before the sexual dysfunction can be treated. Women with lifelong symptoms, psychiatric comorbidities, or ongoing intrapersonal, interpersonal, or sociocultural issues that impact sexual function can be especially challenging to treat in the primary care setting, and specialist referral should be considered. Establishing a network of healthcare providers trained in sexual health and medicine is helpful [8].
A general approach for managing female sexual dysfunction and associated conditions involves the use of a biopsychosocial approach that combines medical assessment and treatment; patient education, counseling, or therapy to address partner and relationship issues; and patient information on community resources [19,97]. Even with effective medical treatment, many women require counseling and support for optimal treatment response. A time-limited, problem-focused approach, termed the 20-Minute Hour, is sufficient to effectively assess and manage sexual concerns without disruption to the office schedule. In addition, the PLISSIT (permission, limited information, specific suggestions, intensive therapy) approach can assist in determining the appropriate level of intervention. When appropriate and safe, providers should involve the woman's partner during assessment and treatment of sexual dysfunction; outcomes are often improved by partner involvement.
Sexual dysfunction treatment that includes a psychologic component is broadly endorsed. Pharmacotherapy cannot address important psychosocial factors of performance anxiety, poor self-confidence, partner sexual dysfunction, relationship conflict or poor communication, sexual factors in the relationship (e.g., sexual scripts, sexual satisfaction), and contextual factors (e.g., life stressors) [10]. Even when sexual dysfunction is primarily physiologic, virtually all patients experience negative psychologic and interpersonal effects. These include interpersonal conflict, depression, performance anxiety, and avoidance of sex. If unaddressed, these will interfere with the efficacy of medical therapies [98,99].
Clinicians often observe that patient change with positive medication response evokes change in the partner, such as women experiencing increased sexual desire when a partner is emotionally supportive. In these cases, psychosocial intervention is critical [11].
Psychotherapy should be initiated before medication in patients with severe depression, substance abuse disorders, or in abusive/chaotic relationships, as medication has little benefit in these contexts. Medical therapy alone is appropriate in couples in which the sexual dysfunction has a clear medical precipitant, couples in a high-quality relationship, or patients with few psychologic concerns [11].
Providers should identify and dispel myths about sex that can negatively influence sexual behavior and contribute to or maintain female sexual dysfunction; the value of basic sex education for this goal should not be overlooked. Many women lack knowledge of basic sexual and reproductive anatomy and physiology. Education should address the diverse range of "normal" sexual function and distorted sexual beliefs or misinformation propagated by popular media and society [8].
Lifestyle and behavior change should be emphasized to all patients with sexual dysfunction to help optimize sexual functioning. Simple suggestions can be highly beneficial. Remind patients that changing lifestyle to health-promoting behaviors through diet, exercise, smoking cessation, and stress reduction can improve physical well-being and self-esteem, which may improve physiologic and psychologic aspects of sexual desire and response [8].
Some women with desire/arousal concerns can be given suggestions on circumventing boredom and routine, such as planning romantic encounters or incorporating erotica, in order to foster an environment that optimizes sexual desire. Encourage patients to improve partner intimacy through shared activities, date nights, and effective communication [8].
Sex therapy and CBT are the major psychologic treatment approaches supported in the empirical literature. Traditional sex therapy aims to improve an individual's or couple's erotic experiences while reducing anxiety and self-consciousness about sexual activity [8]. Cognitive-behavioral sex therapy includes emphasis on modifying thought patterns or beliefs that interfere with intimacy and sexual pleasure [100]. CBT may improve orgasmic ability and satisfaction by diminishing sex-associated anxiety and cognitive distortions in female orgasmic dysfunction [20].
For psychologic therapy of female sexual dysfunction, a meta-analysis found greatest improvement in symptom severity and sexual satisfaction in FSIAD and female orgasmic disorder and modest benefit in vaginismus. Strongest evidence was in sexual skills training and CBT for FSIAD; sexual skills training for female orgasmic disorder; and CBT for vaginismus. Group therapy improved symptom severity and sexual satisfaction, and individual or couples therapy improved symptom severity but not satisfaction [101]. Psychotherapy also benefits patients with acquired or situational low desire when treatment addresses initiating or maintaining circumstances or behaviors [102]. Traditional sex therapy and/or CBT can benefit arousal problems by increasing awareness of genital responses and subjective arousal [6].
For female orgasmic dysfunction, behavioral exercises involving directed masturbation are empirically valid and effective in women with lifelong, generalized anorgasmia and beneficial in acquired anorgasmia for women averse to touching their genitalia. Women too uncomfortable to share intimate details or techniques they need for satisfaction with their partner should be considered for sex therapy referral [20].
The advent of phosphodiesterase type 5 (PDE5) inhibitors prompted an increase in published pharmacotherapy trials in female sexual dysfunction. Of 20 random controlled trials of testosterone for FSIAD, 10% were published during 1988–1998, and 90% published after 1998, the year sildenafil was introduced [103].
Despite the heightened focus on finding a drug therapy for female sexual dysfunction analogous to the agents available for erectile dysfunction, only two medications have received U.S. Food and Drug Administration (FDA) approval for the treatment of HSDD in premenopausal women [104]. This is not from gender inequality in sexual medicine, but from the need for balancing benefits and risks to provide effective and safe treatments to women of any age [14]. The first approved agents was flibanserin, a serotonin 5-HT1A agonist/5-HT2A antagonist, developed for the treatment of FSIAD. Sprout Pharma re-submitted a drug approval application for flibanserin to the FDA for the third time in February 2015, with two previous FDA rejections over safety and efficacy concerns [105]. It finally gained approval in August 2015 [104].
In 2019, the FDA approved bremelanotide for the treatment of HSDD in premenopausal women with no known other cause of low sexual desire [106]. This agent is a melanocortin receptor agonist, but the mechanism by which it improves sexual desire and related distress is unknown. In two randomized controlled trials involving 1,247 women, 25% of those treated with bremelanotide had an increase of 1.2 or more in their sexual desire score (range of 1.2 [low end] to 6.0 [high end]) compared with about 17% of those who took placebo [106]. In addition to flibanserin and bremelanotide, several agents are used off-label and others are being developed or evaluated in clinical trials.
Symptomatic genitourinary syndrome of menopause commonly presents with symptoms of vaginal dryness, irritation of the vulva, burning, dysuria, dyspareunia, and vaginal discharge, potentially severe enough to interfere with the ability to have pain-free sexual activity. Dyspareunia is strongly associated with female sexual dysfunction in postmenopausal women, and decreased genital arousal and vulvar pain can occur as a consequence of genitourinary syndrome of menopause. Clitoral atrophy and phimosis of the prepuce may lead to dyspareunia, which decreases interest in sex or causes avoidance of sexual activity. Most cases of symptomatic genitourinary syndrome of menopause respond to treatment, and a stepped approach can be applied in mild-to-moderate cases beginning with conservative approaches and, if needed, progressing to estrogen, ospemifene, and testosterone or dehydroepiandrosterone (DHEA) [84].
First-line therapy for mild-to-moderate genitourinary syndrome of menopause and dyspareunia involves nonhormonal vaginal lubricants used with intercourse/vaginal sexual activity, long-acting vaginal moisturizers, and regular sexual activity [84]. If improvement is not noted with these approaches, hormone or other pharmacotherapy may be initiated.
Estrogen replacement has long been used in the treatment of symptomatic genitourinary syndrome of menopause. Topical estrogen therapy applied to the vagina is now preferred over oral estrogen therapy when vaginal symptoms are the sole complaint, as it has better efficacy and safety, with minimal systemic absorption [84,107,108]. Potential vaginal estrogen therapy side effects of vaginal bleeding, breast pain, and nausea are dose-related. In women with an intact uterus, endometrial carcinoma risks from unopposed estrogen are unknown beyond one year; no safety data from use longer than 52 weeks has been published [84]. Topical or systemic estrogen may improve vaginal lubrication, dryness, and irritation. Systemic estrogen should be tailored due to safety concerns with long-term use, especially in pre- or perimenopausal women [6].
Patients Receiving Aromatase Inhibitors
Aromatase inhibitors (e.g., anastrozole, letrozole, exemestane) and fulvestrant reduce breast cancer recurrence through complete estrogen blockade. However, the resultant physiologic suppression of estradiol can negatively affect sexual functioning due to unpleasant urogenital and vaginal symptoms, and aromatase inhibitors frequently induce genitourinary syndrome of menopause and dyspareunia [109].
In patients taking aromatase inhibitors, vaginal estrogen therapy can allow resumption of sexual activity, and regular sexual activity or vaginal stimulation also prevents symptom recurrence. However, patients with sexual dysfunction and a history of breast cancer who lack response to conservative therapy should consult their oncologist before starting vaginal estrogen therapy. Treatment of patients with non-hormone-dependent cancers is similar to the treatment of patients without a cancer history. Vaginal estrogen therapy is inappropriate for postmenopausal women with undiagnosed vaginal/uterine bleeding [84,110,111].
Ospemifene is an oral, non-estrogen, tissue-selective estrogen agonist/antagonist approved by the FDA in 2013 for moderate-to-severe dyspareunia associated with genitourinary syndrome of menopause. Daily use at 60 mg is effective and safe, with few side effects [26,112,113]. Vasomotor symptoms (including hot flashes) were the most common adverse event in clinical trials. Data in women with or at risk for breast cancer are lacking, and product information states ospemifene should not be prescribed for these patients [84].
In women, testosterone is a primary precursor for estradiol production and directly acts on androgen receptors. Total and free (bioavailable) testosterone progressively declines during reproductive aging. Declining testosterone, changes in neurochemistry, and indirect changes from loss of estrogen contribute to decreasing levels of desire and increasing difficulties in experiencing arousal from genital stimulation and orgasm that often occur during the period from premenopause to postmenopause [21].
The FDA has not approved testosterone in women due to safety and efficacy concerns, but it is widely prescribed off-label in the United States for postmenopausal women. A 2014 review of studies in menopausal women found consistent positive effect on sexual response, pleasure from masturbation, sexual desire, frequency of sexual activity, sexual satisfaction, and orgasm [103]. Testosterone benefited libido regardless of route of administration, but only transdermal 300 mcg showed efficacy in sexual response. Significant adverse events were not observed; cardiovascular system effects were inconclusive. One of five studies analyzing breast cancer risk from testosterone found significant risk (2.5-fold), but these women received combined estrogen-testosterone [114]. The authors concluded a significant positive effect of testosterone on libido in women with FSIAD, but the maximum 24-week study lengths prohibited conclusions on long-term safety and efficacy [103]. Very little data are available on premenopausal women receiving testosterone for female sexual dysfunction [8].
Four-year safety data of testosterone therapy in postmenopausal women is now available from two studies. Comparison of 2,103 women (mean age: 47 years) prescribed testosterone (72.2% implants, 18.4% tablets, and 7.9% injections) and 6,309 matched controls found, at 4.4-year follow-up, no significant differences between testosterone therapy and controls in rates of breast cancer, acute hepatitis, cardiovascular disease, ischemic heart disease, diabetes mellitus, cerebrovascular disease, or deep venous thrombosis/pulmonary embolism. Mild androgenic events occurred in some women on testosterone [115].
Another study followed 1,094 surgically menopausal women for adverse events during transdermal testosterone patch (300 mcg/day) treatment of HSDD for up to four years. All patients received concurrent estrogen. During follow-up, no increases over time were seen in rates of new onset or severe adverse events, serious adverse events, or study withdrawal from adverse events. The most common adverse events were application site reactions and unwanted hair growth; most were mild and rarely led to study withdrawal. No clinically meaningful changes were observed in serum chemistry, hematology, lipid profile, carbohydrate metabolism, renal and liver function, or coagulation parameters. No important safety or tolerability concerns with transdermal testosterone patch emerged during long-term use up to four years in otherwise healthy oophorectomised women with HSDD receiving concomitant estrogen [116]. This trial used lower-dose testosterone than used in the previous study.
Oral testosterone should not be used because of potential adverse effects on lipids and liver function. Transdermal testosterone therapy delivers testosterone systemically but avoids first-pass liver effects and alterations in lipid metabolism. The most common formulation is a transdermal 1% testosterone cream applied daily to skin on the arms, legs, or abdomen [117,118].
Vaginal Testosterone Therapy
Vaginal testosterone therapy is a testosterone formulation for local application intended to relieve symptoms in a manner comparable to vaginal estrogen. Vaginal testosterone therapy was evaluated in several short trials for efficacy in alleviating unpleasant aromatase inhibitor therapy-induced symptoms. A trial of 13 postmenopausal women with breast cancer experiencing aromatase inhibitor-induced female sexual dysfunction found daily vaginal testosterone therapy 300 mcg cream for four weeks significant improved measures of desire, arousal, lubrication, orgasm, satisfaction, pain, and sexual health quality of life [119]. In 10 women with breast cancer using aromatase inhibitor therapy, vaginal testosterone therapy (300 mcg/day) for four weeks restored vaginal cytology and alleviated dyspareunia. Serum testosterone levels remained within normal range [120].
Twice-weekly vaginal testosterone therapy (0.5 mg 2%) plus conjugated equine estrogen (0.625 mg) was compared with vaginal estrogen therapy for four weeks in 20 patients with genitourinary syndrome of menopause. Vaginal testosterone therapy led to greater improvements in sexual function, and serum testosterone levels did not rise above values within normal premenopausal range [121].
A randomized clinical trial evaluated the safety of vaginal testosterone therapy versus an estradiol-releasing vaginal ring (7.5 ug/d) in patients with early-stage breast cancer, receiving an aromatase inhibitor, and with self-reported vaginal dryness, dyspareunia, or decreased libido [122]. The women were randomized to 12 weeks of testosterone therapy or the estradiol vaginal ring. Estradiol was measured at baseline and at weeks 4 and 12. Gynecologic examinations and sexual quality-of-life assessments were completed at baseline and again at week 12. An intervention was considered unsafe if more than 25% of patients had persistently elevated estradiol (>10 pg/mL). Of the 75 women who began treatment, 92% completed the full 12 weeks of treatment. Persistently elevated estradiol was observed in none of the women with a vaginal ring and in 12% on vaginal testosterone therapy. Transiently elevated estradiol was observed in 11% of women with a vaginal ring. Vaginal atrophy and sexual interest and dysfunction improved for all patients [122].
DHEA is best defined as a prohormone that does not activate androgen receptors until becoming converted to active androgens. DHEA is available in Canada by prescription and in the United States without a prescription as a dietary supplement [84].
Oral DHEA
Comparison of daily oral hormone therapies DHEA (10 mg), estradiol plus dihydrogesterone (1 mg/5 mg), and tibolone (2.5 mg) for 12 months was performed to assess effects on sexual function and relationship quality in 48 postmenopausal women (mean age: 54.5 years) with climacteric symptoms [123]. The DHEA and estradiol/dihydrogesterone groups significantly improved in sexual function relative to baseline; the improvements with DHEA were more robust. However, relationship quality was unchanged. Frequency of sexual intercourse significantly increased with DHEA, estradiol/dihydrogesterone, and tibolone relative to baseline [123].
Response to higher-dose oral DHEA 100 mg/day was studied in 27 postmenopausal women with HSDD. After six weeks, serum DHEA and bioavailable testosterone levels significantly increased in all subjects on the active drug. Significant associations were found between bioavailable testosterone and sexual cognitions, arousal, and orgasm, and between serum DHEA and satisfaction. DHEA significantly improved arousal in women with HSDD, likely mediated by DHEA metabolism to testosterone [124].
Intravaginal DHEA
Evaluation of 12 weeks of topical intravaginal DHEA 1% in 216 women with moderate-to-severe genitourinary syndrome of menopause found improvement with intravaginal DHEA exceeded placebo by 23% and 49% on two measures of desire, 68% on arousal/sensation, 39% on arousal/lubrication, 75% on orgasm, and 57% on dryness during intercourse [125]. All patients on intravaginal DHEA maintained normal postmenopausal serum steroid levels. It appears that intravaginal DHEA exerts local androgenic/estrogenic stimulation in the epithelium, lamina propria, and muscularis vaginal cell layers to improve all four aspects (desire/interest, arousal, orgasm, sexual pain) of female sexual dysfunction [125].
To follow these findings, the efficacy of intravaginal DHEA (0 mg, 3.25 mg, 6.5 mg, or 13 mg daily) was evaluated in postmenopausal women experiencing female sexual dysfunction with or without moderate/severe dyspareunia. Compared with patients receiving placebo, Abbreviated Sexual Function (ASF) arousal/sensation, arousal/lubrication, and summary scores improved by 64.2%, 118%, and 31.1%, respectively, in patients with moderate/severe dyspareunia using intravaginal DHEA and by 58.0%, 67.6%, and 32.1%, respectively, in patients without moderate/severe dyspareunia taking intravaginal DHEA. Compared with patients receiving placebo, those receiving intravaginal DHEA improved from 18.0% to 38.2% on measures of desire, intimacy avoidance, vaginal dryness, and quality of life. Absence of sexual pain did not impede intravaginal DHEA efficacy in female sexual dysfunction [126]. Given the nominal benefit of estrogen in female sexual dysfunction, these results suggest that vulvovaginal atrophy and vulvovaginal sexual dysfunction are distinct manifestations of menopausal sex steroid deficiency with independent treatment response [126].
Microablative CO2 Laser Therapy
A 2015 trial studied the effects of fractional microablative CO2 laser therapy on sexual function and overall sex life satisfaction in 77 postmenopausal women with genitourinary syndrome of menopause. Compared with baseline, evaluation after 12 weeks found significant improvement in total FSFI score and in all FSFI domains as well as significantly improved overall sex life satisfaction. Of 20 women sexually inactive at baseline because of genitourinary syndrome of menopause severity, 17 (85%) regained normal sexual life. Significant improvement was also noted in each genitourinary syndrome of menopause symptom and in quality-of-life scores, with both reflecting scores in physical and emotional domains [127].
For patients with chronic vulvar pain, a multimodal approach is suggested for symptomatic treatment [6,19]. Possible local measures include non-irritating soothing or barrier creams, topical anti-histamines, or anesthetic creams. Down-regulation/denervation/desensitization of sensory nerves may also be useful. This may be accomplished with one or a combination of the following [6,19]:
Pharmacotherapy with imipramine or local or systemic gabapentin
Trigger point injection
Pudendal nerve excision/ablation or injections
Perineal massage
Digital or electric vibration
Use of the Eros-Clitoral Therapy Device (EROS-CTD), a small, battery-powered device used to increase blood flow to the clitoris
Psychologic and somatic therapy should also be included in the treatment plan for chronic vulvar pain. Cognitive-behavioral therapy (CBT) and/or systematic desensitization has been found effective for some patients, as has physiotherapy and graduated vaginal dilators. Non-intercourse sexual intimacy and masturbation should be encouraged.
Management of provoked vestibulodynia includes lidocaine or xylocaine 2% gel or 5% ointment applied to the vestibule 15 to 30 minutes before sexual activity to reduce intercourse-provoked pain. In one trial, lidocaine 5% ointment applied on the vestibule at bedtime for seven weeks resulted in ≥50% pain reduction [128]. Corticosteroid creams are frequently prescribed but are rarely effective [19].
Biofeedback can be very useful in controlling dyspareunia. Exercises of pelvic floor muscles using sequences of contraction and relaxation are helpful in treating vaginismus, which is often associated with provoked vestibulodynia [19].
Surgery is rarely suggested as a first-line approach because of its invasiveness. However, in severe cases, vestibulectomy represents one of the most efficacious treatments, with success rates of roughly 80% [129,130].
In a 2013 study, women (average age: 26 years) with combined hormonal contraception-emergent vestibulodynia received combination therapy with topical estradiol 0.03% and testosterone 0.01%, applied to the vestibule twice daily for 20 weeks. Comparison to pre-therapy values found vestibular pain scores decreased from 7.5 to 2 on a 0–10 scale, SHBG levels decreased from 154 nmol/L to 64 nmol/L, and free testosterone levels increased from 0.193 ng/dL to 0.813 ng/dL. These results support a contraception-induced, hormonally mediated cause of vestibulodynia, with vestibular pain improvement accompanied by cessation of combined hormonal contraception and normalization of free testosterone and SHBG values [80].
Exercises for systematic desensitization to vaginal penetration are the mainstay of vaginismus treatment, with the goal of control over and relaxation of levator ani muscles. Therapy starts with reverse Kegel exercises, progresses to gentle stretching of the vagina using lubricated vaginal dilators of graduated sizes to help restore and maintain vaginal function, followed by introduction a partner's fingers, with intercourse the final step [19].
Many women with vaginismus benefit from pelvic floor physical therapy, which was compared to levator-directed trigger-point injections in one study. Both were found equally effective in pain reduction, with physical therapy showing greater improvement in FSFI sexual pain domain scores [131].
Short-term CBT can be effective for addressing emotional issues that interfere with sexual response or create vaginismus due to inhibition, fear, or trauma. Couples therapy, electromyography, benzodiazepines, neuromodulators, antidepressants (e.g., amitriptyline), hypnotherapy, botulinum toxin A injections, traditional Chinese medicine, and acupuncture may help some patients but have not been evaluated in robust randomized controlled trials [19].
Treatment of deep dyspareunia is aimed at the causative factors [19]. When partner roughness or poor sexual technique is a factor, emphatically stress that no one should be required to tolerate sexual pain and help clarify the relationship goals of the patient [19].
In primary dysmenorrhea, oral nitric oxide donor drugs such as sildenafil can augment the relaxant effects of nitric oxide on myometrial cells, reverse prostaglandin-induced vasoconstriction, and successfully alleviate pain. However, side effect incidence prohibits routine clinical use. Sildenafil inhibits PDE5, preventing degradation of cyclic guanosine monophosphate in muscle, augmenting nitric oxide vasodilatory effects, and allowing tissue relaxation and blood circulation. In randomized controlled trial, vaginal preparations of sildenafil showed enhanced endometrial blood flow with no observed side effects [132].
A single-dose vaginal preparation of sildenafil citrate 100 mg was tested in 25 patients (18 to 35 years of age) with moderate-to-severe primary dysmenorrhea during painful menstruation. Assessment before and throughout the initial four hours post-treatment found significantly greater pain reduction with sildenafil (versus placebo) at every post-treatment point and no observed adverse effects. These results require confirmation in larger studies, but in patients with primary dysmenorrhea and acute menstrual pain, vaginal sildenafil may be a safe, effective option for pain relief when standard therapies lack benefit or tolerability [132].
The complex etiology of desire, arousal, and/or orgasmic dysfunction seen with FSIAD and female orgasmic disorder often dictates a biopsychosocial treatment approach.
As noted, in 2015 flibanserin became the first medication approved for the treatment of female sexual dysfunction (specifically HSDD) and the first medication approved to treat sexual desire disorders in either sex [104]. This medication is a mixed 5-HT1A agonist/5-HT2A antagonist—the first in its class. Its mechanism of action is presumed to be primarily the result of postsynaptic action on 5-HT1A receptors, but the actual action is not clear [133].
In three Phase 3 trials, flibanserin demonstrated improvements over placebo on measurements of satisfying sexual events, FSFI desire domain scores, daily desire, and distress [134]. Possible adverse effects include syncope and hypotension, the risks for which increase with daytime use, concurrent alcohol use, and concomitant use of moderate or strong cytochrome P-450 3A4 inhibitors (e.g., erythromycin, fluconazole, nefazodone, protease inhibitors, grapefruit juice). As such, use of flibanserin is contraindicated in patients taking these agents [135]. Use is also contraindicated in patients with hepatic impairment.
Flibanserin is only approved for premenopausal women whose HSDD is not the result of coexisting medical or psychiatric condition, problems within the relationship, or the effects of a medication or other drug substance. The recommended dosage is 100 mg once daily at bedtime [135]. If no improvements are apparent after eight weeks, the medication should be discontinued.
As noted, in 2019, the FDA approved bremelanotide for the treatment of HSDD in premenopausal women with no known other cause of low sexual desire [106]. The medication is self-injected subcutaneously at least 45 minutes before sexual activity (maximum: one injection per 24 hours). The most common side effects are nausea and vomiting, flushing, injection site reactions, and headache [106].
Bupropion is a norepinephrine and mild dopamine reuptake inhibitor and nicotinic acetylcholine receptor antagonist typically used as an antidepressant and smoking cessation aid. A trial in non-depressed women with sexual dysfunction found a 29% response rate, while a larger randomized, double-blind, placebo-controlled study of bupropion 150 mg/day in 232 non-depressed premenopausal women showed significantly increased desire and decreased distress in the treated group [136,137]. Bupropion is also effective in reversing SSRI-induced sexual dysfunction in premenopausal women.
A study enrolled 400 patients (22 to 62 years of age) with female sexual arousal disorder in blinded trials to evaluate topical alprostadil cream (0, 500, 700, or 900 mcg) efficacy applied to the clitoris and vaginal G-spot before intercourse. Comparisons of alprostadil 900 mcg and placebo showed arousal success rates of 53.9% versus 33.1%, and mean FSFI total score improvement of 22.9% versus 14.7% [138]. Improvements with alprostadil 900 mcg beyond placebo were statistically significant but clinically modest.
Apomorphine activates brain dopamine D2 receptors, known to mediate sexual function. A two-week trial in 62 premenopausal women found improved orgasm and measures of sexual satisfaction during treatment, with better results obtained using daily 3 mg than with 2-mg dosing [139].
Subsequently, subjective and objective changes in female sexual response were studied in 24 patients (average age: 32 years) with female orgasmic disorder [140]. Duplex ultrasound evaluated objective sexual response to vibrator stimulation before and after administration of 3 mg sublingual apomorphine or placebo. Clitoral hemodynamic changes, particularly peak systolic velocity, were significantly higher with apomorphine. Mean increases in post-stimulus peak systolic velocity were 72.50% with placebo versus 139.14% with apomorphine. Subjective rating of changes in arousal and lubrication significantly improved with apomorphine relative to placebo. Apomorphine seemed to produce greater subjective and objective changes than placebo in sexual arousal among women with orgasmic sexual dysfunction. Future research is needed to evaluate the place of this drug in the treatment of female sexual dysfunction [140].
Efficacy of the extended-release 5-HT(1A) agonist gepirone in HSDD was evaluated in 875 women (18 to 64 years of age, 80% premenopausal) with major depression [141]. The participants received gepirone-ER (20–80 mg/day), fluoxetine (20–40 mg/day), paroxetine (10–40 mg/day), or placebo. After eight weeks, HSDD was reversed in 63% of women receiving gepirone-ER, compared with roughly 40% of those receiving paroxetine, fluoxetine, or placebo. The onset of gepirone-ER benefit was two weeks. A mildly depressed subgroup also improved at week 2 and week 8, and sexual dysfunction adverse events were significantly less in patients treated with gepirone-ER than with placebo. Gepirone-ER may have efficacy in HSDD among depressed and possibly nondepressed women, perhaps independent of a purely antidepressant effect [141].
A guideline for testosterone therapy in FSIAD was jointly updated in 2014 by the Endocrine Society and other professional organizations [21]. The guideline recommends initiating a three- to six-month trial in postmenopausal women requesting testosterone therapy for verified FSIAD (when not contraindicated). Mid-normal premenopausal testosterone values should be used as the reference standard to avoid testosterone over-administration. Testosterone levels should be measured at baseline, monitored at three- to six-week and six-month follow-up, and then every six months for signs of excessive use and androgen excess. Therapy should be halted in patients lacking response by six months.
If possible, formulations for men and from compounding pharmacies should be avoided because efficacy and safety data are lacking. If available, use non-oral preparations approved for women (e.g., transdermal patch, gel, cream) [21].
Sublingual Testosterone Plus Sildenafil
Low sexual desire in women may result from relative brain insensitivity to sexual cues, and administration of sublingual testosterone 0.5 mg can increase sensitivity to these cues. Because sexual stimulation in the brain is necessary for PDE5 inhibitor-mediated increases in genital sexual response, a single dose of testosterone plus sildenafil may enhance sexual responsiveness in women with low sensitivity for sexual cues [142]. On-demand testosterone/sildenafil 100 mg was evaluated in 56 women with FSIAD. Compared with placebo, testosterone/sildenafil significantly improved physiologic and subjective measures of sexual functioning during ambulatory psychophysiologic laboratory conditions at home and during sexual events [142].
Plant-derived and herbal remedies are a popular alternative to medical treatments for patients with sexual dysfunction. While some preparations seem effective, no safety data are available with long-term use.
Lady Prelox
Lady Prelox is a supplement tablet containing 20 mg pine bark (Pinus pinaster) extract, 200 mg L-arginine, 200 mg L-citrulline, and 50 mg rose hip extract. An eight-week open-label trial of healthy women (37 to 45 years of age) with moderate sexual dysfunction found significantly higher sexual function scores and significantly decreased oxidative stress as measured by plasma free radicals [143]. The relevance of these findings is unclear, as the study lacked placebo control, randomized assignment, and double-blinding.
Ginkgo Biloba Extract
Ginkgo biloba extract efficacy in female sexual dysfunction was evaluated in a study with highly rigorous design, which found gingko biloba extract offered no benefit beyond placebo [144]. A study published in 2014 examined the effect of ginkgo biloba extract on sexual desire in 80 postmenopausal women [145]. Half of the healthy female volunteers received ginkgo biloba extract (120–240 mg) and the other half received placebo for 30 days. Sexual desire was significantly improved in the ginkgo biloba extract group compared with the placebo group.
The following organizations have online tools, information and education, and lists of healthcare providers who specialize in female sexual health issues.
| International Society for the Study of Women's Sexual Health |
| http://www.isswsh.org |
| American Association of Sexuality Educators, Counselors, and Therapists |
| https://www.aasect.org |
| Society for Sex Therapy and Research |
| https://sstarnet.org |
| Sexuality Information and Education Council of the United States |
| https://siecus.org |
| International Society for Sexual Medicine |
| https://www.issm.info |
Sexual dysfunction is distressing for female patients and their partners and can have a profoundly negative impact on patient quality of life and self-image. However, providers often find discussion of patient's sexual concerns difficult, due in part to a lack of knowledge, skills, and confidence in their ability to initiate discussion and assess and treat sexual dysfunction. To compound this, there are few approved options for the treatment of female sexual dysfunction. This course has outlined appropriate approaches to the assessment, diagnosis, and treatment of female sexual dysfunction in order to enhance patient-provider communication and improve patient outcomes and well-being.
1. Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970-978.
2. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281(6):537-544.
3. Laumann EO, Nicolosi A, Glasser DB, et al. Sexual problems among women and men aged 40–80 y: prevalence and correlates identified in the Global Study of Sexual Attitudes and Behaviors. Int J Impot Res. 2005;17(1):39-57.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Text Revision. Arlington, VA: American Psychiatric Association; 2022.
5. Parish SH, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437-447.
6. Giraldi A, Rellini AH, Pfaus J, Laan E. Female sexual arousal disorders. J Sex Med. 2013;10(1):58-73.
7. Woodis CB, McLendon AN, Muzyk AJ. Testosterone supplementation for hypoactive sexual desire disorder in women. Pharmacotherapy. 2012;32(1):38-53.
8. Kingsberg SA, Woodard T. Female sexual dysfunction: focus on low desire. Obstet Gynecol. 2015;125(2):477-486.
9. Angel K. Contested psychiatric ontology and feminist critique: "female sexual dysfunction" and the Diagnostic and Statistical Manual. Hist Human Sci. 2012;25(4):3-24.
10. Althof SE, McMahon CG, Waldinger MD, et al. An update of the International Society of Sexual Medicine's guidelines for the diagnosis and treatment of premature ejaculation (PE). J Sex Med. 2014;11(6):1392-1422.
11. Jannini EA, Isidori AM, Aversa A, Lenzi A. Althof SE. Which is first? The controversial issue of precedence in the treatment of male sexual dysfunctions. J Sex Med. 2013;10(10):2359-2369.
13. Marnash ML, Casey PM. Understanding women's sexual health: a case-based approach. Mayo Clin Proc. 2008;83(12):1382-1387.
14. Nappi RE, Cucinella L. Advances in pharmacotherapy for treating female sexual dysfunction. Exp Opin Pharmacotherapy. 2015;16(6):875-887.
17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Text Revision. Arlington, VA: American Psychiatric Association; 2000.
18. Poels S, Bloemers J, van Rooij K, Kopperschaar H, Olivier B, Tuiten A. Two novel combined drug treatments for women with hypoactive sexual desire disorder. Pharmacol Biochem Behav. 2014;121:71-79.
19. Lamont J, Bajzak K, Bouchard C, et al. No. 279-female sexual health consensus clinical guidelines. J Obstet Gynaecol Can. 2018;40(6):e451-e503.
20. Meston CM, Hull E, Levin RJ, Sipski M. Disorders of orgasm in women. J Sex Med. 2004;1(1):66-68.
21. Wierman ME, Arlt W, Basson R, et al. Androgen therapy in women: a reappraisal. An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(10):3489-3510.
24. Portman D, Palacios S, Nappi RE, Mueck AO. Ospemifene, a non-oestrogen selective oestrogen receptor modulator for the treatment of vaginal dryness associated with postmenopausal vulvar and vaginal atrophy: a randomised, placebo-controlled, phase III trial. Maturitas. 2014;78(2):91-98.
25. Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (Real Women's Views of Treatment Options for Menopausal Vaginal Changes) survey. J Sex Med. 2013;10(7):1790-1799.
26. Constantine G, Graham S, Portman DJ, Rosen RC, Kingsberg SA. Female sexual function improved with ospemifene in postmenopausal women with vulvar and vaginal atrophy: results of a randomized, placebo-controlled trial. Climacteric. 2015;18(2):226-232.
27. Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(2):191-208.
28. Derogatis LR, Rosen R, Leiblum S, Burnett A, Heiman J. The Female Sexual Distress Scale (FSDS): initial validation of a standardized scale for assessment of sexually related personal distress for women. J Sex Marital Ther. 2002;28(4):317-330.
29. Lewis RW, Fugl-Meyer KS, Corona G, et al. Definitions/epidemiology/risk factors for sexual dysfunction. J Sex Med. 2010;7 (4 Pt 2):1598-1607.
30. West SL, D'Aloisio AA, Agans RP, Kalsbeek WD, Borisov NN, Thorp JM. Prevalence of low sexual desire and hypoactive sexual desire disorder in a nationally representative sample of U.S. women. Arch Intern Med. 2008;168(13):1441-1449.
31. Harlow BL, Wise LA, Stewart EG. Prevalence and predictors of chronic lower genital discomfort. Am J Obstet Gynecol. 2001;185(3):545-550.
32. Goetsch M. Vulvar vestibulitis: prevalence and historic features in a general gynecologic practice population. Am J Obstet Gynecol. 1991;164(6 Pt 1):1609-1614.
33. Arnold LD, Bachmann GA, Rosen R, Rhoads GG. Assessment of vulvodynia symptoms in a sample of U.S. women: a prevalence survey with a nested case control study. Am J Obstet Gynecol. 2007;196(2):128.e1-6.
34. Rosen RC, Connor MK, Maserejian NN. The HSDD registry for women: a novel patient registry for women with generalized acquired hypoactive sexual desire disorder. J Sex Med. 2010;7(5):1747-1756.
35. Bhasin S, Enzlin P, Coviello A, Basson R. Sexual dysfunction in men and women with endocrine disorders. Lancet. 2007;369(9561):597-611.
36. Atis G, Dalkilinc A, Altuntas Y, et al. Hyperthyroidism: a risk factor for female sexual dysfunction. J Sex Med. 2011;8(8):2327-2333.
37. Gulmez H, Akin Y, Savas M, et al. Impact of iron supplementation on sexual dysfunction of women with iron deficiency anemia in short term: a preliminary study. J Sex Med. 2014;11(4):1042-1046.
38. Hallikas J, Weller R, Morse C. Effects of regular marijuana on sexual performance. J Psychoactive Drugs. 1982;14(1-2):59-70.
39. Kasman AM, Bhambhvani HP, Wilson-King G, Eisenberg ML. Assessment of the association of cannabis on female sexual function with the Female Sexual Function Index. Sex Med. 2020;8(4):699-708.
40. Palha A, Esteves M. A study of the sexuality of opiate addicts. J Sex Marital Ther. 2002;28(5):427-437.
41. Farnia V, Shakeri J, Tatari F, AhmadiJuibari T, Yazdchi K, Abdoli N. The evaluation of sexual dysfunction among male patients with methamphetamine abuse and withdrawal. J Subst Use. 2015;20(5):363-366.
42. Henderson D, Boyd CJ, Whitmarsh J. Women and illicit drugs: sexuality and crack cocaine. Health Care Women Int. 1995;16(2):113-124.
43. Lorvick J, Bourgois P, Wenger LD, et al. Sexual pleasure and sexual risk among women who use methamphetamine: a mixed methods study. Int J Drug Policy. 2013;23(5):385-392.
44. Derogatis LR, Psychiatric Times. Female Sexual Dysfunction. What We Know, What We Suspect, and Enduring Enigmas. Available at https://www.psychiatrictimes.com/articles/female-sexual-dysfunctionwhat-we-know-what-we-suspect-and-enduring-enigmas. Last accessed November 15, 2022.
45. Harte CB, Meston CM. The inhibitory effects of nicotine on physiological sexual arousal in nonsmoking women: results from a randomized, double-blind, placebo-controlled, cross-over trial. J Sex Med. 2008;5(5):1184-1197.
46. Wouters H, Van Dijk L, Van Geffen EC, Gardarsdottir H, Stiggelbout AM, Bouvy ML. Primary-care patients' trade-off preferences with regard to antidepressants. Psychol Med. 2014;44(11):2301-2308.
47. Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol. 2009;29(3):259-266.
48. Ali SK, Reveles KR, Davis R, Mortensen EM, Frei CR, Mansi I. The association of statin use and gonado-sexual function in women: a retrospective cohort analysis. J Sex Med. 2015;12(1):83-92.
49. Levine KB, Williams RE, Hartmann KE. Vulvovaginal atrophy is strongly associated with female sexual dysfunction among sexually active postmenopausal women. Menopause. 2008;15(4 Pt 1):661-666.
50. Dennerstein L, Lehert P, Burger H, Dudley E. Factors affecting sexual functioning of women in the mid-life years. Climacteric. 1999;2(4):254-262.
51. von Hippel C, Adhia A, Rosenberg S, Austin SB, Partridge A, Tamimi R. Sexual function among women in midlife: findings from the Nurses' Health Study II. Womens Health Issues. 2019;29(4):291-298.
52. Fisher WA, Byrne D, White LA, Kelley K. Erotophobia-erotophilia as a dimension of personality. J Sex Res. 1988;25(1):123-151.
53. Weaver AD, Byers ES. The relationships among body image, body mass index, exercise and sexual functioning in heterosexual women. Psychol Women Q. 2006;30(4):333-339.
54. de Lucena BB, Abdo CHN. Personal factors that contribute to or impair women's ability to achieve orgasm. Int J Impot Res. 2014;26(5):177-181.
55. Afari N, Ahumada SM, Wright LJ, et al. Psychological trauma and functional somatic syndromes: a systematic review and meta-analysis. Psychosom Med. 2014;76(1):2-11.
56. Cichowski SB, Dunivan GC, Komesu YM, et al. Sexual abuse history and pelvic floor disorders in women. South Med J. 2013;106(12):675-678.
57. Stephenson KR, Hughan CP, Meston CM. Childhood sexual abuse moderates the association between sexual functioning and sexual distress in women. Child Abuse Negl. 2012;36(2):180-189.
58. Schloredt K, Heiman J. Perceptions of sexuality as related to sexual functioning and sexual risk in women with different types of childhood abuse histories. J Trauma Stress. 2003;16(3):275-284.
59. Baldwin DS, Foong T. Antidepressant drugs and sexual dysfunction. Br J Psychiatry. 2013;202(6):396-397.
60. Williams K, Reynolds MF. Sexual dysfunction in major depression. CNS Spectr. 2006;11(Suppl 9):19-23.
61. Leonard LM, Follette VM. Sexual functioning in women reporting a history of child sexual abuse: clinical and empirical considerations. Annu Rev Sex Res. 2002;13:346-388.
62. Najman JM, Dunne MP, Purdie DM, Boyle FM, Coexter PD. Sexual abuse in childhood and sexual dysfunction in adulthood: an Australian population-based study. Arch Sex Behav. 2005;34(5):517-526.
63. Barlow DH. Causes of sexual dysfunction: the role of cognitive interference. J Consult Clin Psychol. 1986;54(2):140-148.
64. Elliott AN, O'Donahue WT. The effects of anxiety and distraction on sexual arousal in a nonclinical sample of heterosexual women. Arch Sex Behav. 1997;26(6):607-624.
65. Purdon C, Holdaway L. Non-erotic thoughts: content and relation to sexual functioning and sexual satisfaction. J Sex Res. 2006;43(2):154-162.
66. Pujols Y, Seal BN, Meston CM. The association between sexual satisfaction and body image in women. J Sex Med. 2010;7 (2 Pt 2):905-916.
67. Brotto LA, Bitzer J, Laan E, Leiblum S, Luria M. Women's sexual desire and arousal disorders. J Sex Med. 2010;7(1 Pt 2):586-614.
68. Lawrence K, Byers ES. Sexual satisfaction in long-term heterosexual relationship: the interpersonal exchange model of sexual satisfaction. Pers Relatsh. 1995;2(4):267-285.
69. Basson R. Female sexual response: the role of drugs in the management of sexual dysfunction. Obstet Gynecol. 2001;98(2):350-353.
70. MacNeil S, Byers ES. Dyadic assessment of sexual self-disclosure and sexual satisfaction in heterosexual dating couples. J Soc Pers Relat. 2005;22(2):169-181.
71. MacNeil S, Byers ES. Role of sexual self-disclosure in the sexual satisfaction of long-term heterosexual couples. J Sex Res. 2009;46(1):1-12.
72. Nappi RE, Kokot-Kierepa M. Vaginal health: insights, views & attitudes (VIVA)—results from an international survey. Climacteric. 2012;15(1):36-44.
73. Goldstein I, Dicks B, Kim NN, et al. Multidisciplinary overview of vaginal atrophy and associated genitourinary symptoms in post-menopausal women. Sex Med. 2013;1(2):44-53.
74. Gordon D, Gardella C, Eschenbach D, Mitchell CM. High prevalence of sexual dysfunction in a vulvovaginal specialty clinic.J Low Genit Tract Dis. 2016;20(1):80-84.
75. Burrows LJ, Klingman D, Pukall CF, Goldstein AT. Umbilical hypersensitivity in women with primary vestibulodynia. J Reprod Med. 2008;53(6):413-416.
76. Farmer MA, Meston CM. Predictors of genital pain in young women. Arch Sex Behav. 2007;36(6):831-843.
77. Bouchard C, Brisson J, Fortier M, Morin C, Blanchette C. Use of oral contraceptive pills and vulvar vestibulitis: a case-control study. Am J Epidemiol. 2002;156(3):254-261.
78. Greenstein A, Ben-Aroya Z, Fass O, et al. Vulvar vestibulitis syndrome and estrogen dose of oral contraceptive pills. J Sex Med. 2007;4(6):1679-1683.
79. Bohm-Starke N, Johannesson U, Hilliges M, Rylander E, Torebjörk E. Decreased mechanical pain threshold in the vestibular mucosa of women using oral contraceptives: a contributing factor in vulvar vestibulitis? J Reprod Med. 2004;49(11):888-892.
80. Burrows LJ, Goldstein AT. The treatment of vestibulodynia with topical estradiol and testosterone. Sex Med. 2013;1(1):30-33.
81. Edgardh K, Abdelnoor M. Vulvar vestibulitis and risk factors: a population-based case-control study in Oslo. Acta Derm Venereol. 2007;87(4):350-354.
82. Lee M, Morgan M, Rapkin A. Clitoral and vulvar vestibular sensation in women taking 20 mcg ethinyl estradiol combined oral contraceptives: a preliminary study. J Sex Med. 2011;8(1):213-218.
83. Watts G, Nettle D. The role of anxiety in vaginismus: a case-control study. J Sex Med. 2010;7(1 Pt 1):143-148.
84. North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888-902.
85. Althof SE, Rosen RC, Perelman MA, Rubio-Aurioles E. Standard operating procedures for taking a sexual history. J Sex Med. 2013;10(1):26-35.
86. Bitzer J, Giraldi A, Pfaus J. A standardized diagnostic interview for hypoactive sexual desire disorder in women: standard operating procedure (SOP Pt 2). J Sex Med. 2013;10(1):50-57.
87. Arnold LD, Bachmann GA, Rosen R, Kelly S, Rhoads GG. Vulvodynia: characteristics and associations with comorbidities and quality of life. Obstet Gynecol. 2006;107(3):617-624.
90. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009;113(5):1124-1136.
91. Wylie K. Assessment and management of sexual problems in women. J R Soc Med. 2007;100(12):547-550.
92. Nappi R, Salonia A, Traish AM, et al. Clinical biologic pathophysiologies of women's sexual dysfunction. J Sex Med. 2005;2(1): 4-25.
93. Berman JR. Physiology of female sexual function and dysfunction. Int J Impot Res. 2005;17(Suppl 1):S44-S51.
94. Kennedy SH, Dickens SE, Eisfeld BS, Bagby RM. Sexual dysfunction before antidepressant therapy in major depression. J Affect Disord. 1999;56(2-3):201-208.
95. Stimmel GL, Gutierrez MA. Sexual dysfunction and psychotropic medications. CNS Spectr. 2006;11(8 Suppl 9):24-30.
96. Doumas M, Tsiodras S, Tsakiris A, et al. Female sexual dysfunction in essential hypertension: a common problem being uncovered. J Hypertens. 2006;24(12):2387-2392.
97. Binik YM, Hall KSK (eds). Principles and Practice of Sexual Therapy. 5th ed. New York, NY: Guilford Press; 2014.
99. Althof S. When an erection alone is not enough: biopsychosocial obstacles to lovemaking. Int J Impot Res. 2002;14(Suppl 1): S99-S104.
100. Bradford A. Inhibited sexual desire in women. In: Grossman LR, Walfish S, (eds). Translating Psychological Research into Practice. New York, NY: Springer; 2014: 515-518.
101. Frühauf S, Gerger H, Schmidt HM, Munder T, Barth J. Efficacy of psychological interventions for sexual dysfunction: a systematic review and meta-analysis. Arch Sex Behav. 2013;42(6):915-933.
102. Günzler C, Berner MM. Efficacy of psychosocial interventions in men and women with sexual dysfunctions—a systematic review of controlled clinical trials: part 2—the efficacy of psychosocial interventions for female sexual dysfunction. J Sex Med. 2012;9(12):3108-3125.
103. Reis SLB, Abdo CHN. Benefits and risks of testosterone treatment for hypoactive sexual desire disorder in women: a critical review of studies published in the decades preceding and succeeding the advent of phosphodiesterase type 5 inhibitors. Clinics. 2014;69(4):294-303.
104. U.S. Food and Drug Administration. Archive: Press Release. FDA Approves First Treatment for Sexual Desire Disorder. Available at https://wayback.archive-it.org/7993/20170112023753/http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm458734.htm. Last accessed November 15, 2022.
105. BioSpace. Sprout Pharmaceuticals Resubmits Flibanserin New Drug Application For The Treatment Of Hypoactive Sexual Desire Disorder In Premenopausal Women. Available at https://www.biospace.com/article/releases/sprout-pharmaceuticals-resubmits-flibanserin-new-drug-application-for-the-treatment-of-hypoactive-sexual-desire-disorder-in-premenopausal-women-/. Last accessed November 15, 2022.
106. U.S. Food and Drug Administration. FDA Approves New Treatment for Hypoactive Sexual Desire Disorder in Premenopausal Women. Available at https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-hypoactive-sexual-desire-disorder-premenopausal-women. Last accessed November 15, 2022.
107. Long CY, Liu CM, Hsu SC, Wu CH, Wang CL, Tsai EM. A randomized comparative study of the effects of oral and topical estrogen therapy on the vaginal vascularization and sexual function in hysterectomized postmenopausal women. Menopause. 2006;13(5):737-743.
108. Cardozo L, Bachmann G, McClish D, Fonda D, Birgerson L. Meta-analysis of estrogen therapy in the management of urogenital atrophy in postmenopausal women: second report of the Hormones and Urogenital Therapy Committee. Obstet Gynecol. 1998;92(4 Pt 2):722-727.
109. Baumgart J, Nilsson K, Evers AS, Kallak TK, Poromaa IS. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20(2):162-168.
110. Whelan TJ, Goss PE, Ingle JN, et al. Assessment of quality of life in MA.17: a randomized placebo-controlled trial of letrozole after 5 years of tamoxifen in postmenopausal women. J Clin Oncol. 2005;23(28):6931-6940.
111. Whelan TJ, Pritchard KI. Managing patients on endocrine therapy: focus on quality-of-life issues. Clin Cancer Res. 2006;12(3 Pt 2):1056s-1060s.
112. Cui Y, Zong H, Yan H, Li N, Zhang Y. The efficacy and safety of ospemifene in treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy: a systematic review and meta-analysis. J Sex Med. 2014;11(2):487-497.
113. Goldstein SR, Bachmann GA, Koninckx PR, Lin VH, Portman D, Ylikorkala O. Ospemifene 12-month safety and efficacy in postmenopausal women with vulvar and vaginal atrophy. Climacteric. 2014;17(2):173-182.
114. Tamimi RM, Hankinson SE, Chen WY, Rosner B, Colditz GA. Combined estrogen and testosterone use and risk of breast cancer in postmenopausal women. Arch Intern Med. 2006;166(14):1483-1489.
115. van Staa T, Sprafka, J. Study of adverse outcomes in women using testosterone therapy. Maturitas. 2009;62(1):76-80.
116. Nachtigall L, Casson P, Lucas J, Schofield V, Melson C, Simon JA. Safety and tolerability of testosterone patch therapy for up to 4 years in surgically menopausal women receiving oral or transdermal oestrogen. Gynecol Endocrinol. 2011;27(1):39-48.
117. Davis SR, Braunstein GD. Efficacy and safety of testosterone in the management of hypoactive sexual desire disorder in post-menopausal women. J Sex Med. 2012;9(4):1134-1148.
118. Davis SR. Cardiovascular and cancer safety of testosterone in women. Curr Opin Endocrinol Diabetes Obes. 2011;18(3):198-203.
119. Dahir M, Travers-Gustafson D. Breast cancer, aromatase inhibitor therapy, and sexual functioning: a pilot study of the effects of vaginal testosterone therapy. Sex Med. 2014;2(1):8-15.
120. Witherby S, Johnson J, Demers L, et al. Topical testosterone for breast cancer patients with vaginal atrophy related to aromatase inhibitors: a phase I/II study. Oncologist. 2011;16(4):424-431.
121. Raghunandan C, Agrawal S, Dubey P, Choudhury M, Jain A. A comparative study of the effects of local estrogen with or without local testosterone on vulvovaginal and sexual dysfunction in post-menopausal women. J Sex Med. 2010;7(3):1284-1290.
122. Melisko ME, Goldman ME, Hwang J, et al. Vaginal testosterone cream vs estradiol vaginal ring for vaginal dryness or decreased libido in women receiving aromatase inhibitors for early-stage breast cancer: a randomized clinical trial. JAMA Oncol. 2017;3(3):313-319.
123. Genazzani AR, Stomati M, Valentino V, et al. Effect of 1-year, low-dose DHEA therapy on climacteric symptoms and female sexuality. Climacteric. 2011;14(6):661-668.
124. Bloch M, Meiboom H, Zaig I, Schreiber S, Abramov L. The use of dehydroepiandrosterone in the treatment of hypoactive sexual desire disorder: a report of gender differences. Eur Neuropsychopharmacol. 2013;23(8):910-918.
125. Labrie F, Archer D, Bouchard C, et al. Effect of intravaginal dehydroepiandrosterone (Prasterone) on libido and sexual dysfunction in postmenopausal women. Menopause. 2009;16(5):923-931.
126. Labrie F, Archer D, Bouchard C, et al. Lack of influence of dyspareunia on the beneficial effect of intravaginal prasterone (dehydroepiandrosterone, DHEA) on sexual dysfunction in postmenopausal women. J Sex Med. 2014;11(7):1766-1785.
127. Salvatore S, Nappi RE, Parma M, et al. Sexual function after fractional microablative CO2 laser in women with vulvovaginal atrophy. Climacteric. 2015;18(2):219-225.
128. Zolnoun D, Hartmann KE, Steege JF. Overnight 5% lidocaine ointment for treatment of vulvar vestibulitis. Obstet Gynecol. 2003;102(1):84-87.
129. Goldstein A. Surgical techniques: surgery for vulvar vestibulitis syndrome. J Sex Med. 2006;3(3):559-562.
130. Goldstein AT, Klingman D, Christopher K, Johnson C, Marainoff SC. Surgical treatment of vulvar vestibulitis syndrome: outcome assessment derived from a postoperative questionnaire. J Sex Med. 2006;3(5):923-931.
131. Zoorob D, South M, Karram M, et al. A pilot randomized trial of levator injections versus physical therapy for treatment of pelvic floor myalgia and sexual pain. Int Urogynecol. 2015;26(6):845-852.
132. Dmitrovic R, Kunselman AR, Legro RS. Sildenafil citrate in the treatment of pain in primary dysmenorrhea: a randomized controlled trial. Hum Reprod. 2013;28(11):2958-2965.
133. Stahl SM, Sommer B, Allers KA. Multifunctional pharmacology of flibanserin: possible mechanism of therapeutic action in hypoactive sexual desire disorder. J Sex Med. 2011;8(1):15-27.
134. Joffe HV, Chang C, Sewell C, et al. FDA approval of flibanserin: treating hypoactive sexual desire disorder. N Engl J Med. 2016;374(2):101-104.
135. LexiComp Online. Available at https://online.lexi.com. Last accessed November 15, 2022.
136. Segraves RT, Croft H, Kavoussi R, et al. Bupropion sustained release (SR) for the treatment of hypoactive sexual desire disorder (HSDD) in nondepressed women. J Sex Marital Ther. 2001;27(3):303-316.
137. Safarinejad MR, Hosseini SY, Asgari MA, Dadkhah F, Taghva A. A randomized, double-blind, placebo-controlled study of the efficacy and safety of bupropion for treating hypoactive sexual desire disorder in ovulating women. BJU Int. 2010;106(6):832-839.
138. Liao Q, Zhang M, Geng L, et al. Efficacy and safety of alprostadil cream for the treatment of female sexual arousal disorder: a double-blind, placebo-controlled study in Chinese population. J Sex Med. 2008;5(8):1923-1931.
139. Caruso S, Agnello C, Intelisano G, Farina M, Di Mari L, Cianci A. Placebo-controlled study on efficacy and safety of daily apomorphine SL intake in premenopausal women affected by hypoactive sexual desire disorder and sexual arousal disorder. Urology. 2004;63(5):955-959.
140. Bechara A, Bertolino MV, Casabé A, et al. A double-blind randomized placebo control study comparing the objective and subjective changes in female sexual response using sublingual apomorphine. J Sex Med. 2004;1(2):209-214.
141. Fabre LF, Brown CS, Smith LC, Derogatis LR. Gepirone-ER treatment of hypoactive sexual desire disorder (HSDD) associated with depression in women. J Sex Med. 2011;8(5):1411-1419.
142. Poels S, Bloemers J, van Rooij K, et al. Toward personalized sexual medicine (part 2): testosterone combined with a PDE5 inhibitor increases sexual satisfaction in women with HSDD and FSAD, and a low sensitive system for sexual cues. J Sex Med. 2013;10(3):810-823.
143. Bottari A, Belcaro G, Ledda A, Luzzi R, Cesarone MR, Dugall M. Lady Prelox® improves sexual function in generally healthy women of reproductive age.Minerva Ginecol. 2013;65(4):435-444.
144. Meston CM, Rellini AH, Telch MJ. Short- and long-term effects of Ginkgo biloba extract on sexual dysfunction in women. Arch Sex Behav. 2008;37(4):530-547.
145. Pebdani MA, Taavoni S, Seyedfatemi N, Haghani H. Triple-blind placebo-controlled trial of ginkgo biloba extract on sexual desire in postmenopausal women in Tehran. Iran J Nurs Midwifery Res. 2014;19(3):262-265.
1. American College of Obstetricians and Gynecologists. Female sexual dysfunction: ACOG Practice Bulletin summary, number 213.Obstet Gynecol. 2019;134(1):203-205. Available at https://journals.lww.com/greenjournal/Abstract/2019/07000/Female_Sexual_Dysfunction__ACOG_Practice_Bulletin.42.aspx. Last accessed November 21, 2022.
Mention of commercial products does not indicate endorsement.