Pathophysiology is the study of disordered and altered functions affecting the body's dynamic homeostasis and the concepts of illness development and progression. This course is designed to broaden the nurse's understanding of renal pathophysiology by exploring causes, alterations and physiology adaptations, manifestations, and resolution of disease states. Pathophysiologic symptoms and signs are described in relation to the patient's clinical presentation, allowing the nurse can monitor physical changes and relate them directly to the illness process. Appropriate diagnostic tests and treatments for each problem are included to provide information about disease progression, remission, and resolution.
- INTRODUCTION
- STRUCTURAL AND FUNCTIONAL INTER-RELATIONSHIPS
- PATHOPHYSIOLOGIC INFLUENCES AND EFFECTS
- RELATED SYSTEM INFLUENCES AND EFFECTS
- PSYCHOSOCIAL/LIFESTYLE INFLUENCES AND EFFECTS
- NURSING ASSESSMENT: ESTABLISHING THE DATA BASE
- NURSING DIAGNOSES, PLANNING, AND IMPLEMENTATION
- SPECIFIC DISORDERS OF THE KIDNEYS AND URINARY SYSTEM
- DIALYSIS
- RENAL TRANSPLANTATION
- END-OF-LIFE CARE
- CONCLUSION
- CASE STUDIES
- RESOURCES
- Works Cited
- Evidence-Based Practice Recommendations Citations
This course is designed for nurses working in critical care and general and specialty medical-surgical units in which patients with multiple organ system problems are found.
As health care becomes more complex, it is essential that the theoretical concepts of the basis of illness (pathophysiology) be well understood. The purpose of this course is to reinforce the scientific rationales for the interventions nurses perform and the decisions nurses make as patients move through the ever-changing struggle with their renal illness.
Upon completion of this course, you should be able to:
- Identify the key structures in the renal system.
- Describe the functions of the renal system.
- Evaluate the impact of renal function on blood pressure.
- Discuss the pathophysiologic and environmental influences and effects on the renal system.
- Outline the role of subjective data in completing a full nursing assessment of the renal system.
- Describe objective data compiled during a nursing assessment of the renal system.
- Identify imaging and biopsy studies used in the identification and classification of renal diseases.
- Outline the nursing diagnoses, planning, and management of conditions related to renal dysfunction.
- Evaluate the presentation and management of chronic and acute kidney disease and neurogenic bladder.
- Discuss clinical manifestations of infectious diseases of the renal system.
- Review signs and symptoms of renal neoplasms and related nursing actions.
- Review the clinical presentation and management of nephrolithiasis and urolithiasis.
- Describe the common causes, appearances, and treatment of traumatic disorders of the renal system.
- Describe key concepts related to caring for patients who receive dialysis.
- Analyze the process of renal transplantation and nursing management of transplant recipients.
- Outline key considerations for patients with renal dysfunction at the end of life.
Jane C. Norman, RN, MSN, CNE, PhD, received her undergraduate education at the University of Tennessee, Knoxville campus. There she completed a double major in Sociology and English. She completed an Associate of Science in Nursing at the University of Tennessee, Nashville campus and began her nursing career at Vanderbilt University Medical Center. Jane received her Masters in Medical-Surgical Nursing from Vanderbilt University. In 1978, she took her first faculty position and served as program director for an associate degree program. In 1982, she received her PhD in Higher Education Administration from Peabody College of Vanderbilt University. In 1988, Dr. Norman took a position at Tennessee State University. There she has achieved tenure and full professor status. She is a member of Sigma Theta Tau National Nursing Honors Society. In 2005, she began her current position as Director of the Masters of Science in Nursing Program.
Mary Franks, MSN, APRN, FNP-C, is a board-certified Family Nurse Practitioner and NetCE Nurse Planner. She works as a Nurse Division Planner for NetCE and a per diem nurse practitioner in urgent care in Central Illinois. Mary graduated with her Associate’s degree in nursing from Carl Sandburg College, her BSN from OSF Saint Francis Medical Center College of Nursing in 2013, and her MSN with a focus on nursing education from Chamberlain University in 2017. She received a second master's degree in nursing as a Family Nurse Practitioner from Chamberlain University in 2019. She is an adjunct faculty member for a local university in Central Illinois in the MSN FNP program. Her previous nursing experience includes emergency/trauma nursing, critical care nursing, surgery, pediatrics, and urgent care. As a nurse practitioner, she has practiced as a primary care provider for long-term care facilities and school-based health services. She enjoys caring for minor illnesses and injuries, prevention of disease processes, health, and wellness. In her spare time, she stays busy with her two children and husband, coaching baseball, staying active with her own personal fitness journey, and cooking. She is a member of the American Association of Nurse Practitioners and the Illinois Society of Advanced Practice Nursing, for which she is a member of the bylaws committee.
Contributing faculty, Jane C. Norman, RN, MSN, CNE, PhD, has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Contributing faculty, Mary Franks, MSN, APRN, FNP-C, has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Sharon Cannon, RN, EdD, ANEF
The division planner has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Sarah Campbell
The Director of Development and Academic Affairs has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
The purpose of NetCE is to provide challenging curricula to assist healthcare professionals to raise their levels of expertise while fulfilling their continuing education requirements, thereby improving the quality of healthcare.
Our contributing faculty members have taken care to ensure that the information and recommendations are accurate and compatible with the standards generally accepted at the time of publication. The publisher disclaims any liability, loss or damage incurred as a consequence, directly or indirectly, of the use and application of any of the contents. Participants are cautioned about the potential risk of using limited knowledge when integrating new techniques into practice.
It is the policy of NetCE not to accept commercial support. Furthermore, commercial interests are prohibited from distributing or providing access to this activity to learners.
Supported browsers for Windows include Microsoft Internet Explorer 9.0 and up, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Supported browsers for Macintosh include Safari, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Other operating systems and browsers that include complete implementations of ECMAScript edition 3 and CSS 2.0 may work, but are not supported. Supported browsers must utilize the TLS encryption protocol v1.1 or v1.2 in order to connect to pages that require a secured HTTPS connection. TLS v1.0 is not supported.
The role of implicit biases on healthcare outcomes has become a concern, as there is some evidence that implicit biases contribute to health disparities, professionals' attitudes toward and interactions with patients, quality of care, diagnoses, and treatment decisions. This may produce differences in help-seeking, diagnoses, and ultimately treatments and interventions. Implicit biases may also unwittingly produce professional behaviors, attitudes, and interactions that reduce patients' trust and comfort with their provider, leading to earlier termination of visits and/or reduced adherence and follow-up. Disadvantaged groups are marginalized in the healthcare system and vulnerable on multiple levels; health professionals' implicit biases can further exacerbate these existing disadvantages.
Interventions or strategies designed to reduce implicit bias may be categorized as change-based or control-based. Change-based interventions focus on reducing or changing cognitive associations underlying implicit biases. These interventions might include challenging stereotypes. Conversely, control-based interventions involve reducing the effects of the implicit bias on the individual's behaviors. These strategies include increasing awareness of biased thoughts and responses. The two types of interventions are not mutually exclusive and may be used synergistically.
#38861: Pathophysiology: The Renal System
Each kidney is smaller than a person's fist, but in a single day, the two organs process approximately 22% to 25% of cardiac output, or 1,100 mL/minute [1]. As part of their function, the kidneys filter essential substances, such as sodium and potassium ions, from the blood and selectively reabsorb substances needed to maintain the normal composition of body fluids. Substances that are not needed, or are in excess of normal, pass into the urine. In regulating the volume and composition of body fluids, the kidneys perform excretory and endocrine functions.
This course is designed to broaden the knowledge of nurses, ensuring the understanding of the pathophysiology of renal function and illness by exploring causes, alterations and physiologic adaptations, manifestations, and resolution of disease states. Pathophysiologic symptoms and signs are described in relation to the patient's clinical presentation, so nurses can monitor physical changes and relate them directly to the illness process. Appropriate diagnostic tests and treatments for each problem are included, along with nursing responsibilities for patient teaching.
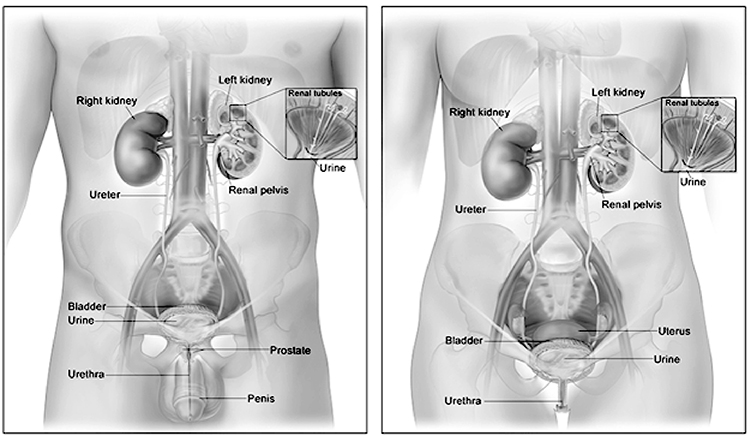
The urinary system consists of two kidneys, which produce urine; two ureters, which carry urine to the urinary bladder, where it is temporarily stored; and the urethra, which transports urine to the outside of the body.
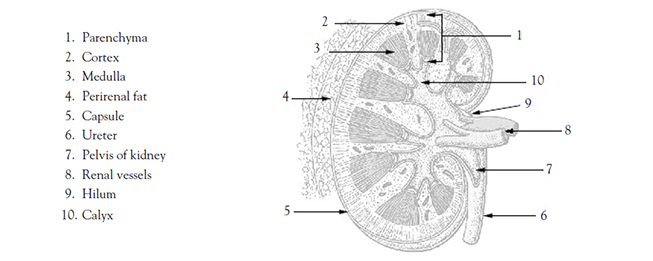
An adult typically has two kidneys, which are reddish-brown and bean-shaped (Image 1). Although their size can vary, the length of the average kidney is about 11 cm, the thickness about 2.5 cm, and the width is about 5 cm. The average weight of a single kidney is 15 g. The kidney's lateral border is convex, whereas the medial border is concave and indented in a depression called the renal hilus. All related structures enter or leave the kidney at the hilus [2,3].
The kidneys lie in the retroperitoneal space of the posterior abdominal cavity (Image 2). Thus, they can be exposed without opening the peritoneal cavity. One kidney lies on either side of the vertebral column. Layers of muscle surround the posterior surfaces of the kidneys, and abdominal organs surround their anterior surfaces. The peritoneal membrane covers most of the anterior surface of each kidney.
The kidneys are protected and supported by renal fascia and layers of perirenal fat. Posteriorly, the psoas, quadratus lumborum, and transverse abdominis muscles provide support. The position of the kidneys is not fixed but varies somewhat with an individual's position. When a patient is in the supine position, the kidneys lie between the 12th thoracic and 3rd lumbar vertebrae. When the patient is standing, the kidneys may descend to the top of the iliac crest. For a patient in Trendelenburg position, the kidneys ascend to the 10th intercostal space [4,5].
The left kidney, which lies near the tail of the pancreas and the splenic flexure of the colon, is normally slightly longer and narrower than the right kidney. The right kidney is lower in the abdomen than the left because of the presence of the liver in the right upper quadrant. The superior pole of the right kidney lies beneath the liver in an area referred to as the renal bed. The right kidney's anterior surface is adjacent to the hepatic flexure of the colon, the duodenum, and the liver. The lower portion of each kidney descends beneath the lower portion of the rib cage [2,6].
Each kidney is surrounded by three layers of tissue. The fibrous renal capsule (the innermost layer) covers the surface of the kidney. The adipose capsule, a mass of perirenal fat, surrounds the renal capsule. The third layer, the renal fascia, surrounds and encloses the kidney and anchors the kidney to the posterior abdominal wall [2,6].
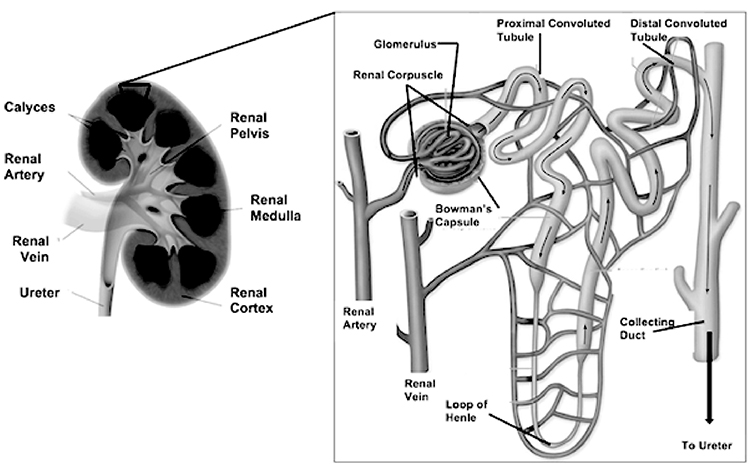
There are essentially three general regions of each kidney: the cortex, the medulla, and the pelvis. These structures are located inside the renal capsule. Two of them—the cortex and the medulla—are often referred to collectively as the renal parenchyma. The cortex is directly beneath the renal capsule. This highly vascularized area of tissue is very sensitive to changes in blood flow. The medulla, located deep in the cortex, consists of 8 to 18 triangular renal pyramids. The renal pyramids are composed of collecting ducts that drain urine into the calyces. The cortex covers the base of the pyramids, and the tips (or papillae) project toward the renal pelvis. Cortical tissue known as renal columns dips into the medulla to separate the pyramids, and blood vessels that supply the cortex and medulla pass through these columns. Urine flows from the papillae into a minor calyx, and several of the funnel-shaped minor calyces emerge to form a major calyx. The major calyces join to form the renal pelvis, which is the expanded upper end of the ureter. At times, a catheter is positioned in the renal pelvis and must be irrigated with 4–6 mL of fluid (as ordered) to maintain catheter patency [7].
The nephron (Image 3) is the functional unit of the kidney and is primarily responsible for most of the mechanisms that provide internal homeostasis. Each kidney contains approximately 1.25 million nephrons, and each nephron in turn is composed of a vascular and tubular system that allows for the formation of urine. The nephrons are located in the renal parenchyma. Most nephrons are in the cortex (referred to as cortical nephrons), but juxtamedullary nephrons begin in the cortex and extend deep into the medulla [7].
The vascular system of the nephron consists of the glomerulus and the Bowman capsule, both located in the cortex of the kidney. The glomerulus is composed of a knot of capillaries. The Bowman (or glomerular) capsule surrounds the glomerulus. The glomerulus derives its blood flow from the renal artery of the abdominal aorta. Each renal artery branches into segmental arteries and increasingly smaller arteriole, arcuate (or arciform), and interlobular (or cortical radial) arteries, which supply progressively smaller areas of renal parenchyma. The smallest branch, the afferent arteriole, feeds blood to the glomerulus. After passing through the capillaries in the glomerulus, the blood exits the glomerulus, not through a venule but via the efferent arteriole. From the efferent arteriole, the blood enters the peritubular capillaries of the cortical nephron or the vasa recta of the juxtamedullary nephron. This plexus of capillaries surrounds the proximal tubule, the Henle loop, and the distal tubule. Finally, blood enters the venous system from either the peritubular capillaries or the vasa recta and returns to the general circulation through a series of renal venules and veins that drain all portions of the kidney. The renal vein of each kidney returns blood into the inferior vena cava [7].
The tubular system of the nephron begins with the Bowman capsule, which is invaginated around each glomerular tuft to form a sac. The Bowman capsule narrows into the proximal convoluted tubule, which changes directions many times until it straightens into the descending limb of the Henle loop and angles downward toward the pelvis of the kidney. The proximal convoluted tubule is largely responsible for the conservation of fluids and electrolytes. The loops of tubular tissue are much longer in juxtamedullary nephrons than in cortical nephrons and are contained within the pyramids of the medulla. The ascending limb of the Henle loop then becomes the distal convoluted tubule. The distal convoluted tubules of several nephrons enter a collecting duct with a pyramid of the medulla; these ducts are responsible for the drainage of formed urine from the nephrons. Pyramids then are drained into the calyceal system of the renal pelvis through the tips of the papillae [1,8].
Urine drips from the collecting tubules into the minor calyces and then the major calyces which, in turn, join the renal pelvis. From the renal pelvis, ureters transport urine to the urinary bladder. Each kidney typically has a single ureter responsible for emptying the urine formed by that kidney. Although the ureter's size varies, an average ureter is around 30 cm (almost 1 foot) long. The ureter's diameter ranges from 2–8 mm at various points in its structure. The ureters descend between the parietal peritoneum and the abdominal wall to the pelvic cavity, where they enter the bladder on its posterior inferior surface. Before opening into the bladder, the ureters travel obliquely through the bladder wall. As a result, pressure in the bladder can compress the ureters and help prevent urine from flowing back into the ureters, especially during bladder emptying [9].
Each ureter is composed of both longitudinal and circular muscular fibers. The interweaving of these fibers is responsible for the peristalsis of urine into the urinary bladder. The narrow points of the ureter are at the junction of the ureter and the renal pelvis (the ureteropelvic junction), the point at which the ureters cross over the iliac vessels, and the point of ureteral enter into the bladder (the ureterovesical junction) [9].
Blood is supplied to the upper portion of the ureters from the renal artery and either the internal spermatic or ovarian artery. The lower portion of the ureters is supplied by branches of the common iliac, hypogastric, and vesical arteries [9].
Multiple sources in a complex distribution provide the nerve supply to the ureters. The ureteric nerves are broadly grouped as superior ureteric, middle ureteric, and inferior ureteric, which supply the upper third, middle third, and lower third of the ureter, respectively. The nerve plexuses that supply the various portions of ureteric nerves have both abdominal (celiac, renal, and mesenteric) and pelvic (gonadal and iliac) origins. Nerve fibers are both sympathetic and parasympathetic [9].
The important relation between the kidneys and the blood vascular system becomes apparent when considering the large size of the renal arteries that supply the kidneys. At rest, these vessels carry about 20% of the total cardiac output to the kidneys, or approximately 600 mL of blood per minute through each kidney. Little of this blood supplies the nutritive needs of the kidneys; the large blood flow is related to the fact that the kidneys can maintain homeostasis of the blood only if a large amount of the blood passes through them [10].
The vascular supply to the kidney consists of several microcirculations:
Glomerular capillaries, where plasma filtration occurs
Peritubular capillaries, which encircle the proximal and distal convoluted tubules, where water, electrolytes, glucose, amino acids, and protein are reabsorbed and some substances are secreted
Medullary circulation (vasa recta), which aids in the concentration of urine
The urinary bladder is a muscular sac capable of significant distention that is used to store formed urine. The bladder rests on the floor of the pelvic cavity and is retroperitoneal. Its anterior surface lies just behind the pubic symphysis. In male individuals, the bladder is in front of the rectum, whereas in female individuals it lies just anterior to the uterus and the superior portion of the vagina [11,12].
The major anatomic areas of the bladder are the fundus, apex, neck, and trigone. The fundus is the upper portion of the bladder, and the apex is the bottom portion of the bladder closest to the pelvic floor. The bladder neck is the most inferior portion of the bladder and contains the internal sphincter. It is composed of a group of thickened fibers of the detrusor muscle that evolves into the smooth muscle of the urethra. The trigone is an area of the posterior wall of the bladder defined by the urethra and the two ureteral orifices or slits, where the ureters enter the bladder. This area of muscle is responsible for separating the upper urinary tract from the lower urinary tract during normal micturition [11,12].
As the bladder fills with urine, its internal pressure increases somewhat initially and then remains fairly constant up to a volume of about 300–400 mL. Beyond this point, the pressure rises rapidly. The bladder can hold 600–800 mL of urine, but it is generally emptied before it reaches this capacity [12].
The nerve supply to the bladder is both sensory and motor. Sympathetic, parasympathetic, and somatic nerves carry sensations to the central nervous system. Sympathetic fibers arise from T9 through L2, and parasympathetic and somatic nerves arise from S2 through S4. The motor nerves of the bladder involve parasympathetic supply to the detrusor muscle and sympathetic supply to the trigone. The pudendal nerves, which are under voluntary control, supply the external sphincter and the muscles of the pelvic floor [12].
The urethra is a muscular tube lined with mucous membranes that exits from the inferior surface of the urinary bladder and carries urine to the exterior of the body. At the junction of the urethra and bladder, the smooth muscle of the bladder surrounds the urethra and acts as a sphincter (the internal urethral sphincter) that keeps the urethra closed. During micturition, a contraction of the bladder opens the sphincter [11,12].
The male urethra is about 21 cm long, approximately the length of the penis, and about 8–9 mm in diameter. The urethra descending from the base of the bladder to the pelvic floor is surrounded by the prostate gland; this is referred to as the prostatic urethra. The portion of the urethra that extends through the pelvic floor is referred to as the membrane urethra, and the cavernous urethra traverses the length of the penis. In female individuals, the urethra is about 4-cm long and about 8 mm in diameter. Because of the close proximity of the anus and vagina to the urethra, micro-organisms found in those regions (most commonly Escherichia coli) may more easily migrate into the bladder in these patients [11,12,124].
The kidneys and urinary system sustain homeostasis of body fluids and their composition. A variety of mechanisms maintain this fluid and electrolyte balance, and the end result is the production of urine. Urine represents the work of the kidneys and results in the removal of nitrogenous waste products as well as the regulations of fluid, electrolyte, and acid-base balances. In addition, the kidneys produce hormones and substances that influence other metabolic and chemical processes [10].
The basic function of the nephron is to cleanse the blood of unwanted substances as it passes through the kidney. This results in the formation of urine and is accomplished through three specific processes that occur in the nephron: glomerular filtration, tubular reabsorption, and tubular secretion. Each process occurs dynamically in the kidneys' continuous efforts to maintain internal equilibrium [10].
Glomerular Filtration
Glomerular filtration is the ultrafiltration of blood whereby fluid, electrolytes, and certain nonelectrolytes are filtered but plasma proteins remain. Glomerular filtration occurs within the glomerulus, across the glomerular capillary membrane. This capillary membrane has anatomic and physical properties that allow the passage of small molecular particles under certain conditions. Small pores within the lining of the capillary loops of the glomerulus (called the basement membrane) allow the passage of fluid and certain particles [10].
In order for glomerular filtration to occur, there must be adequate fluid volume (blood volume or plasma) in the intravascular space, as well as adequate hydrostatic pressure to overcome the forces that oppose glomerular filtration. The pumping of the heart and vascular resistance provide hydrostatic pressure. The vascular tone, or blood flow, within the kidneys is under two types of control: extrinsic factors (e.g., sympathetic nerve fibers from the celiac and renal nerve plexuses) and intrinsic control (i.e., autoregulation of renal blood flow). Each has a definite purpose [10].
The sympathetic nervous system provides extrinsic control of renal blood flow when an emergency situation exists. Normal renal blood flow is 1,200 mL/min, but this flow may be decreased to 200 mL/min when blood is needed to supply the heart, brain, or skeletal muscle. This is a strong vasoconstrictor response to the release of epinephrine and norepinephrine by the sympathetic nervous system [10].
Autoregulation of blood flow maintains a constancy of glomerular filtration through the kidney's unique ability to regulate the resistance of the afferent and efferent arterioles to the flow of blood. Because of this autoregulation, arterial blood pressure can vary widely—80–180 mm Hg—while renal blood flow and glomerular filtration remain basically unchanged [10].
The product of this process is glomerular filtrate. Glomerular filtrate is composed of water, sodium, potassium, calcium, magnesium, chloride, bicarbonate, phosphate, and other anions; glucose; urea; creatinine; and uric and amino acids. In healthy individuals, filtrate does not contain protein, because the glomerular membrane is almost completely impermeable to all plasma proteins.
As long as the various mechanisms that regulate renal blood flow maintain adequate hydrostatic pressure, glomerular filtrate will form. However, forces that oppose the formation of glomerular filtrate—plasma oncotic pressure and tubular filtrate pressure—must be overcome. Understanding plasma oncotic pressure is essential to appreciating the shift of body fluids and the formation of edema. Plasma oncotic pressure (also known as colloid osmotic pressure) is the pulling force of the proteins in the plasma that attempts to hold water in the vascular space and prevent its movement into the surrounding tissue. In the kidney, the plasma oncotic pressure attempts to keep water from being pushed onto the Bowman capsule. The typical glomerular filtration rate (GFR) is 130 mL/min/1.73 m2. Thus, roughly 187,000 mL of glomerular filtrate is formed in 24 hours. For all practical purposes, glomerular filtrate is the same as plasma except it has no significant amount of plasma proteins. If all the components found in glomerular filtrate were excreted in the urine, death would occur. Therefore, much of the filtrate is returned to the blood via tubular reabsorption [11,12].
Tubular Reabsorption
In tubular reabsorption (the initial refinement process), water and specific electrolytes and nonelectrolytes from the tubular filtrate, are reabsorbed into the plasma of the peritubular capillaries or vasa recta. Tubular reabsorption occurs throughout the tubular system of the nephron, but much of it occurs within the proximal convoluted tubule [13].
The tubules have a limited capacity for reabsorption of some substances. For example, the threshold for reabsorption of glucose may be limited when the blood level of glucose is exceedingly high or when renal tubular surfaces are altered through injury. Reabsorption is primarily accomplished through passive and active transport mechanisms of diffusion. In passive transport, the development of a pressure gradient causes the movement of molecules or particles. In this case, simple diffusion occurs (i.e., molecules move from an area of greater to lesser concentration or greater to lesser pressure). With some substances, however, expenditure of energy may be necessary to move the molecules. Active transport occurs through this expenditure of energy. Active transport is required when there is not a pressure gradient but a substance must be moved to another area [13].
Normally, reabsorption from the tubular filtrates into the blood is sufficient to maintain normal serum levels of the various electrolytes. However, reabsorption of some nonelectrolytes (i.e., urea, creatinine, and uric acid) is not readily accomplished. The individual benefits from this because these substances are removed or cleared from the body [13].
Reabsorption of water is accomplished by osmosis, the movement of water across a semipermeable membrane from an area of lesser concentration of solute to one of greater concentration. Water is reabsorbed primarily in the proximal convoluted tubule. If greater water reabsorption is needed to maintain balance, however, the permeability of the distal tubule and collecting duct may be increased [13].
The reabsorption of water is reflected in the kidney's ability to concentrate or dilute the urine as necessary. The supraoptic and paraventricular nuclei of the hypothalamic area are able to sense the plasma osmolality—defined by the concentration of particles (electrolytes and nonelectrolytes) in the plasma. When plasma osmolality is out of the normal range (280–295 mOsm/L), the osmoreceptors detect this abnormality and trigger responses to return the osmolality to normal [14].
Only slight alterations in the osmolality of the blood are required to trigger appropriate regulatory mechanisms. A combined response by the neuroendocrine system and the kidneys will ensure that serum osmolality is returned to the normal range by the release of antidiuretic hormone (ADH) from its storage area in the posterior pituitary. ADH alters the permeability of the distal convoluted tubules and collecting ducts so more or less water can be reabsorbed, as needed. Then, ADH secretion and the reabsorption of the water in the tubules increase. When ADH secretion decreases, more water is excreted by the kidneys. Maintenance of intravascular volume is always the primary goal of the homeostatic mechanism. Thus, functioning kidneys concentrate or dilute urine to maintain normal serum osmolality [14].
Tubular Secretion
The third process in urine formation is tubular secretion, the process by which ions in the tubular cells are secreted into the lumen of the tubule to be excreted in the end product (urine). Tubular secretion of potassium and hydrogen regulates the serum potassium level and serves as the kidneys' acid-base balancing mechanism [13,14].
The kidneys regulate acids and bases in conjunction with other regulatory mechanisms (i.e., the blood buffers and the lungs). The kidneys are responsible for the secretion of the fixed acids of normal metabolism, whereas the lungs excrete the volatile acids. The blood buffers provide moment-to-moment regulation. Fixed acids arise primarily from the metabolism of protein. The kidneys regulate their excretion by conserving bicarbonate, excreting sodium in exchange for the secretion of hydrogen, and secreting ammonia [13,14].
Thus, the processes of filtration, reabsorption, and secretion create urine. Urine is composed primarily of water, sodium, potassium, chloride, urea, creatinine, and uric acid. The volume of urine is normally about 1,500 mL per 24 hours, but it depends on the amount of solute that must be excreted [13,14].
The kidneys regulate blood pressure through the maintenance of fluid volume and the release of the hormone renin, which stimulates powerful vasoconstrictive responses. Fluid volume in the extracellular compartment, and specifically the plasma, is controlled by the kidneys' ability to concentrate or dilute urine in response to serum osmolality. Thus, hypertonic plasma stimulates the release of ADH, the reabsorption of water, the expansion of intravascular volume, the decrease of urine output, and the elevation of blood pressure. This primary mechanism of volume expansion is partially responsible for the regulation of blood pressure [15,16].
The renin-angiotensin system is the other kidney-controlled hormonal mechanism that can trigger blood pressure elevation in certain situations. This mechanism is activated when there is a low serum sodium level, decreased cardiac output, or ischemia in the kidneys. Any of these situations can stimulate the release of renin from the juxtaglomerular cells of the afferent arteriole of the glomerulus. It is believed that the macula densa of the distal convoluted tubule is sensitive to both the sodium content and the volume of the tubular filtrate, and that this sensing mechanism stimulates the release of renin [15,16].
When renin is released from the juxtaglomerular cells, it acts on angiotensinogen, a glycoprotein made in the liver and normally found in plasma, converting it to angiotensin I. Another converting enzyme in the pulmonary capillary bed acts on angiotensin I to change it to angiotensin II, a powerful vasoconstrictor that elevates blood pressure through peripheral vasoconstriction. Angiotensin II also triggers the release of aldosterone (a mineralocorticoid that helps control sodium utilization) by the adrenal cortex. With the release of aldosterone, the distal convoluted tubule of the nephron reabsorbs sodium. Water reabsorption follows sodium reabsorption, increasing plasma volume. Thus, angiotensin II has two main effects that help to elevate blood pressure: peripheral vasoconstriction and plasma volume expansion. When ADH production increases, aldosterone production usually does as well [15,16].
When cardiac output is severely decreased because of loss of circulating blood volume, vasoconstriction in the kidneys will severely limit intrarenal blood flow in order to maintain flow to more vital organs (i.e., the heart and brain). This is an excellent example of the kidneys' role in the preservation of the whole body, because if renal vasoconstriction is not abated, death of the renal parenchyma results [16].
Substances have been identified that may be useful in helping overcome severe renal vasoconstriction. These prostaglandins are believed to have vasodilation capabilities. Part of their antihypertensive effect is through the inhibition of norepinephrine and angiotensin II [16].
Several other metabolic and hormonal functions of the kidneys have been identified, including the production of erythropoietin, the production of 1,25-dihydroxycholecalciferol, and the metabolism of insulin. Erythropoietin is a glycoprotein produced in the kidneys that influences red blood cell production. The site of erythropoietin production is in the peritubular cells of the kidney, specifically the renal cortex peritubular cells, while storage in the kidney has not been identified. However, the effects of bilateral nephrectomy and the subsequent decrease in erythropoiesis have been observed. Erythropoietin seems to increase both the rate of production and the rate of release of new red blood cells from the bone marrow and the spleen [16,17,125].
The kidneys also produce 1,25-dihydroxycholecalciferol, the active component of vitamin D. Without this substance, calcium cannot be absorbed properly from the intestines [16,17].
The kidneys play a role in insulin metabolism and excretion. Failure to excrete insulin results in an increased availability of insulin, which has important consequences for the management and control of diabetes. Most patients with diabetes have a decreased need for insulin as renal failure progresses. The reliability of urine testing to calculate insulin needs is limited, as the renal threshold changes constantly with decreasing renal function [16,17].
Micturition, also called urination or voiding, is a complex physical process under a variety of neural controls. For most healthy persons older than 5 years of age, urination is under voluntary control and can be interrupted or initiated upon cerebral command, as long as motor and sensory nerve pathways are intact. Micturition normally is a painless function that occurs five to six times per day and possibly once at night. The average person voids a total of about 1,500 mL of urine per 24 hours. This amount is affected by fluid intake, the ingestion of foods high in water content, diuretics, sweating, temperature, vomiting, and diarrhea [14,18].
Adults usually perceive an initial desire to empty the urinary bladder when about 150 mL of urine has accumulated. However, the bladder can distend to a much larger capacity, and it often does before there is a feeling of bladder fullness. Because urine accumulates gradually in the bladder, the slow distention of the muscular sac accommodates larger and larger quantities of urine. The capacity of the urinary bladder has been calculated at 450 mL, but much larger amounts may accumulate if there is an obstruction to outflow [14,18].
Micturition involves several responses that occur almost simultaneously. Initially, there is the felt need to void and an assessment of the environment. The individual must determine that the environment is appropriate to the release of urine or urination will be prevented [14,18].
After the person has determined that the conditions are satisfactory, a series of nerve impulses are activated to allow the release of urine. First, the muscles of the pelvic floor are relaxed, which opens the urethral opening and allows the descent of the urinary bladder. Next, the trigone contracts, which ensures closure of the ureterovesical junction and prevents the reflux of urine into the ureters. Trigonal contraction also causes contraction of the bladder neck, which makes the bladder more funnel-shaped. Finally, the detrusor muscle of the bladder, which is continuous with the urethral lining, contracts. Detrusor contraction increases the pressure within the bladder and results in bladder emptying [14,18].
The cerebral control mechanism can interrupt the voiding processes at any point. When the bladder is empty, the detrusor muscle relaxes, the bladder neck closes, and the trigone and perineal muscles resume their normal tone [18].
Alterations in urine formation and excretion have profound effects on homeostasis. Failure to maintain the chemical balance of the fluids in the various compartments will result in death unless a balance is restored. Alterations in urine formation and excretion may cause changes in the clearance of rates of substances, changes in the amount and composition of the urine and the pattern of its excretion, elevated blood pressure, decreased maturation of red blood cells, and changes in the excretion of metabolic waste products. Pathophysiologic changes commonly seen in urinary dysfunction include hydronephrosis (enlargement of the kidney), atrophic kidney, alterations in fluid volume, electrolyte imbalances, and accumulation of toxins [4,19,20].
Enlargement of one or both kidneys is most commonly related to the invasion and multiplication of neoplastic cells. If a mass becomes very large, it can put pressure on abdominal nerves or displace other abdominal organs and cause discomfort or pain [1,15].
The kidneys may also become enlarged because of a blockage of the ureters, and occasionally the urethra, by either a stone, tumor, or enlargement of the prostate. This enlargement is due to a build-up of urine and is referred to as hydronephrosis. In these instances, urine backs up into the renal pelvis. The ureters and urethra also may enlarge in diameter as they fill with urine that cannot be voided. If enervation is interrupted, the bladder also can become overdistended with urine [1,15].
In most chronic diseases of the kidneys, the kidneys eventually atrophy because of the destruction of the renal parenchyma. In some instances, the cortex is most affected, whereas in others, the medulla is most affected. The kidneys may decrease to less than one-fifth of their normal size [1,15].
A major goal of renal function is the maintenance of fluid volume necessary to support metabolic and perfusion processes. As such, the inability to control fluid volume may have a variety of sequelae. For example, loss of the ability of the kidneys to concentrate urine may be the earliest sign of renal pathology. Conversely, continuous loss of dilute urine may result in volume depletion and low blood pressure. Hypovolemia (i.e., inadequate circulating blood volume) will eventually alter renal function, as adequate blood volume is required to establish a pressure gradient so glomerular filtration can occur [8,10].
Although the kidneys have the capacity for some autoregulation of blood flow, they will deprive the renal parenchyma of necessary blood volume if the demands are greater elsewhere. This capacity to severely limit renal blood flow is observed in severe shock or stress states. Fluid deficits can also occur with decreased fluid intake or excessive water losses, as with diarrhea, vomiting, or rapid dehydration [8,10].
Failure of the kidneys to excrete water and maintain normal fluid volume has serious consequences for the overall functioning of the body. Hypervolemia (i.e., an increase in circulating and total body water) manifests as an elevation of blood pressure, increased cardiac workload, and the development of fluid in the interstitial spaces and alveoli of the lungs or other body tissues. As such, it puts severe strain on the cardiovascular system. The associated hypertension can cause long-term problems related to ventricular hypertrophy and increased peripheral vascular resistance. More immediate changes due to fluid shifts, particularly into the lungs, can impair adequate gas exchange causing severe hypoxia [1,15].
Ischemia of renal tissue results in the liberation of renin and the stimulation of the renin-angiotensin-aldosterone system (RAAS). The release of renin is triggered to increase renal blood flow in response to hormonal mechanisms, but it is also associated with increased volume retention and volume expansion. Particularly, this can happen when kidney tissue is damaged, which affects the normal formation of urine while aldosterone and ADH are being produced normally. Therefore, overhydration is more common as the patient reaches end-stage renal disease (ESRD) [1,15].
As discussed, the kidneys maintain the normal osmolality of body fluids and, in conjunction with various endocrine mechanisms, control the appropriate balance of electrolytes in body fluids. Excesses and deficits of various electrolytes result in serious problems in the maintenance of normal nerve transmission and muscle conduction. Sodium, potassium, calcium, and magnesium are the major cations under the regulation of the kidneys. Some major anions (e.g., chloride, bicarbonate), as well as other anions such as sulfates, phosphates, and proteinate, are also under direction by the renal system [21,22].
Of the various electrolytes, potassium—the primary intracellular cation—has the most potential for causing harm if disrupted. Hyperkalemia is the most frequently encountered imbalance in chronic renal failure, and failure of the kidneys to excrete potassium will disturb the conduction system of the heart and, if untreated, can terminate myocardial contraction. The kidney tubules also may fail to conserve potassium correctly and thus excrete large amounts. Hypokalemia may result in altered cardiac muscle contractions and alter the medullary interstitium of the kidney, impairing renal function [21,22].
Sodium excesses (hypernatremia) can occur in patients with renal disease when urine volume drops to very low levels. In these cases, sodium and water retention, edema, and pump failure occur, even in patients with restricted salt intake. Because sodium conservation occurs primarily in the renal medulla, deterioration in this area produces excessive sodium loss (hyponatremia), which leads to decreased extracellular fluid. As circulating blood volume decreases, the GFR decreases and the renal function is further compromised [21,22].
Imbalances of calcium and phosphorus are related to the reciprocal relationship between electrolytes and the effect of parathyroid hormone, which regulates serum calcium levels. When the GFR decreases to around 30 mL/min/1.73 m2, the renal excretion of phosphate also decreases. With increases in serum phosphate level (hyperphosphatemia), the body attempts to lower the level by binding phosphate with calcium. The resultant calcium phosphate may then precipitate in various tissues. This response causes the calcium level (non-protein-bound or ionized) to decrease, potentially leading to hypocalcemia. In response to the low calcium level, parathyroid hormone is released and stimulates the release of calcium from stores in bone in an attempt to increase the circulating calcium level. In renal insufficiency, this is compounded by the kidney's inability to produce 1,25-dihydroxycholecalciferol, which is necessary for the intestinal absorption and utilization of calcium in the diet. This further contributes to the development of hypocalcemia [21,22].
The ongoing process of hyperphosphatemia and hypocalcemia with subsequent production of parathyroid hormone results in secondary hyperparathyroidism. Untreated, this leads to serious bone pathology known as renal osteodystrophy [22].
When urine output is low and a normal magnesium intake continues, hypermagnesemia can occur. Hypermagnesemia typically occurs with acute kidney injury (AKI) or chronic kidney disease (CKD). Hypermagnesemia may be aggravated by the administration of magnesium-containing laxatives or antacids, proton pump inhibitors, malnourishment, and/or alcohol use disorder [22,127]. Early findings include diarrhea, nausea, and vomiting. When present, hypermagnesemia can lead to cardiovascular complications, with prolonged PR intervals and abnormal sinoatrial/atrioventricular node block, leading to cardiac arrest. Generalized muscle weakness and loss of deep tendon reflexes will also occur. Respiratory suppression and ultimately respiratory arrest will occur if it continues unaddressed [126].
The kidneys are responsible for excreting the acids produced by the metabolism of amino acids. The excretion of hydrogen ions by the kidneys is accomplished by the conservation of bicarbonate, the secretion of ammonia, and the excretion of hydrogen in exchange for sodium. The kidneys work with blood buffers and the pulmonary system to maintain a normal blood pH of 7.35–7.45. Blood buffers maintain this narrow range of acceptability; they almost instantaneously convert acids for either pulmonary excretion as carbon dioxide or for renal excretion through the conservation of bicarbonate or the exchange of sodium or potassium for hydrogen [23,24].
Without the renal regulation of hydrogen ion excretion, a diminished amount of bicarbonate is available to buffer the fixed acids. In addition, the ability to excrete hydrogen as ammonia or with phosphoric acid is limited. Thus, unless the pulmonary reserve compensates, the pH will fall and metabolic acidosis will result [23,24].
Several factors related to renal impairment cause impaired gas exchange. The problems of fluid overload cause pulmonary edema, ventricular hypertrophy, and hypertension, which can lead to impaired gas exchange in the lungs and at the cellular level. Potassium imbalances can cause cardiac arrhythmias, which also can lead to impaired gas exchange. The lungs of a patient with metabolic acidosis have to work hard to correct the acidosis; if they are not able to do so, gas exchange is impaired. Anemia may result from a decrease in erythropoietin produced by the kidney, and this deficiency makes gas exchange more difficult. Furthermore, patients may encounter bleeding problems as a result of decreased aggregation ability from a defect in platelet factor III [12,13,24].
Patients with impaired renal function are at increased risk for infection as a result of impaired immune responses. These patients may also experience delayed hypersensitivity to antigens. Alterations in immune function with ESRD can be a complex issue. Uremia presents with hypercytokinemia, due to the buildup of pro-inflammatory cytokines resulting from decreased renal elimination following oxidative stress, volume overload, or other comorbidities [128].
The kidneys remove nitrogenous waste products resulting from protein metabolism. The kidneys must continually filter and excrete creatinine and urea nitrogen, whether it is from endogenous protein sources (such as the metabolism of the amine creatinine in skeletal muscle) or from primarily exogenous sources (such as dietary protein) [14,18].
Azotemia is the accumulation of uremic toxins (urea, uric acid, and creatinine) in the blood. Uremia refers to azotemia with clinical symptoms. The accumulation of uremic toxins can result in neurologic complications, gastrointestinal bleeding, and skin changes resulting from urochrome pigments deposited in the skin. This pigmentation, combined with anemia, results in the pale yellow-gray skin color characteristic of patients with renal failure. Pruritus, also common, is thought to be the result of a buildup of the urochrome pigments in the skin as well as the crust of urate crystals that accumulates on the skin (called uremic frost). Increased parathyroid hormone production is also a possible cause of pruritus [14,18].
The accumulation of uremic toxins also causes neurologic changes that range from fatigue, decreased ability to concentrate, irritability, and insomnia to depression, peripheral neuropathy, and retinopathy. Coma, convulsions, and death can occur in the absence of treatment [14,18].
Uremic toxins and gastrointestinal bleeding cause nutrition-related problems. The gastrointestinal tract becomes inflamed and irritated with the accumulation of uremic toxins. This results in loss of appetite, nausea, vomiting, and diarrhea—problems that further complicate fluid and electrolyte imbalances. This inflammation, compounded by altered platelet function, causes gastrointestinal bleeding common in patients with impaired kidney function. The buildup of ammonia in the body results in characteristic uremic fetor—a urine-like odor of the patient's breath accompanied by a bad taste in the mouth (described by some as metallic) that alters the appeal of food. Associated pancreatitis may also impair digestion [25].
Urine output may greatly increase in certain situations, such as in the diuretic phase of acute renal failure. However, decreased urine output is the more common alteration. This may be temporary, as with the oliguria phase of acute renal failure, benign prostatic hyperplasia, or kidney stone obstruction of the urethra, or it may be a long-term sequela of acute tubular necrosis or chronic renal failure. Alterations in the pattern of urination result from the underlying pathologic conditions. Patients who are unable to completely void, at such times as following renal procedures or surgery, should be checked for residual volume of urine. Short-term catheterization may be used to completely empty the bladder. If the amount retained was in the range of 75–100 mL, an indwelling catheter should be placed [1,26].
Because the kidneys and urinary system perform multiple functions inter-related with various body systems, impairment of renal function can affect other body systems to varying extents.
Dry, pale, yellow-grey skin is characteristic of renal failure. Nails and hair are also brittle and dry. Increased parathyroid hormone secretion, uremic frost, and urochrome pigments cause pruritus, which may result in scratches and bleeding. This break in skin integrity increases patients' susceptibility to infection. Edema from sodium and water retention, as well as poor nutritional status, can also make the skin increasingly susceptible to breakdown. Pressure ulcers may form within hours if patients are not frequently repositioned [1,26].
The cardiovascular complications that may occur after a loss of renal function and the resulting uremia include pericarditis, accelerated atherosclerosis, fluid overload, anemia, and the potential for potassium abnormality-related arrhythmias [1,26].
Uremic pericarditis is fairly common for patients with ESRD. Although this condition usually develops within 12 months of the initiation of dialysis, a later onset has also been observed. This inflammatory response is believed to be related to nitrogenous waste products not removed by dialysis. Fluid accumulates within the pericardial sac (pericardial effusion). With increased membrane irritability and platelet aggregation problems, the effusion may be serosanguineous. Massive pericardial effusions (greater than 2,000 mL) may accumulate over a period of days to weeks, seriously altering cardiovascular hemodynamics. Cardiac tamponade (compression of the heart from excessive fluid in the pericardial sac) will result in death unless medical intervention, surgical intervention, or both relieve the fluid accumulation [1,26].
Accelerated atherosclerotic processes have been observed in patients with chronic renal failure. An increased incidence of death from coronary artery disease appears to be associated with the effects of chronic essential hypertension, ventricular hypertrophy, and possible alterations in lipid metabolism associated with urea. In addition, diffuse atherosclerotic processes that include cerebral, aortic, and peripheral vessels are not uncommon [1,26].
Uncontrolled hypertension, either from volume or hormonal response, greatly increases peripheral vascular resistance in small blood vessels throughout the body. The loss of elasticity of the arterioles in the kidney as well as other organs, such as the retina of the eye and the small vessel circulation of the brain, will seriously affect the long-term functioning of these organs [1,26].
As noted, the inter-relation of the renal and respiratory systems is important in maintaining an acid-base balance. Although this balance is partially restored by diet and lifestyle changes, these measures can accomplish only so much. Dialysis can assist, but the lungs must contribute to controlling this narrow range of imbalance minute to minute. Patients with obstructive lung disease have carbon dioxide retention with respiratory acidosis. Thus, when renal and respiratory diseases exist simultaneously, the ability to combat metabolic acidosis is severely impaired. Gas exchange is further hampered by pulmonary edema and anemia states [1,26].
The neurologic manifestations of uremia include uremic encephalopathy and peripheral neuropathy associated with azotemia and metabolic acidosis. Uremic encephalopathy (characterized by altered mentation/intellectual processes, tremors, and myoclonus) is associated with the onset of uremic manifestations. Asterixis (a flapping tremor of the hands) is an early manifestation caused by increased irritability of the central nervous system from elevated serum ammonia levels. This symptom is important, because tonic-clonic seizures may develop if the encephalopathic process is not corrected [1,26].
Peripheral neuropathies are common in patients with ESRD who require chronic dialysis. These neuropathies are neither clearly understood nor easily treated. Their improvement with dialysis is uncertain, and leg weakness and difficulty with ambulation and maintenance of comfort remain problems. Renal transplantation is potentially the best hope for relief of these symptoms [1,26].
Musculoskeletal manifestations of renal failure are collectively referred to as renal osteodystrophy. A variety of problems, including osteomalacia, osteoporosis, and osteitis fibrosa cystica, may result from the chronic release of parathyroid hormone, which results in elevated phosphorous and decreased calcium levels in the serum. Bone pain, increased fracture risk, and metastatic calcifications result from this imbalance [1,26].
CKD-associated sarcopenia results from the catabolic and anabolic imbalance and can be exacerbated by poor nutritional status [129]. Sarcopenia is associated with increased hospitalizations and mortality in CKD. Loss of skeletal muscle increases the likelihood of sedentary lifestyle, leading to frailty, poor quality of life, fractures, and hospitalizations.
Androgens, specifically testosterone, influence muscle formation and regeneration. Hypogonadism is common in patients with CKD and is associated with a further decrease in muscle mass and strength. Androgen supplementation has been explored in the treatment of CKD, but longer-term studies are needed to determine if the benefits (if any) outweight risks of adverse events [129].
Hematologic effects of uremia include chronic anemia and disorder of platelet aggregation. Each of these alterations can have a variety of effects on patients. Patients with renal failure have chronic normochromic, normocytic anemia. Hematocrit values fall to 20% to 30%, and hemoglobin values drop to 7–8 g/dL. Chronic fatigue is the most common symptom, but patients respond differently and some adjust reasonably well. For patients with coronary artery disease, decreased circulating red blood cells may exacerbate angina or increase the risk of arrhythmias, particularly if hypoxia occurs [1,26].
In general, platelet dysfunction is usually a problem with platelet aggregation—the platelet count is normal, and there is no alteration in the ability to produce platelets. In the uremic environment, however, the platelets do not promote the clotting mechanism as well. The results of other clotting tests (prothrombin time and partial thromboplastin time) are normal, but bleeding time may be prolonged. Impaired platelet function in the uremic environment is believed to be responsible for the frequent and easily induced bleeding into the skin and mucous membranes of the patient with either acute or chronic renal failure. Mucous membrane irritation, particularly of the gastrointestinal tract, along with the frequent presence of occult blood, indicates that a uremic environment persists despite dialysis therapy [1,26].
Uremia profoundly affects the gastrointestinal system. Anorexia, nausea, and vomiting are generally the most bothersome symptoms. Inability to eat or to retain food results in weight loss and breakdown of muscle and fat. Uncorrected, profound debilitation results, because the patient is less able to combat infections and mount appropriate immune responses [1,26].
The effects of uremia may be observed throughout the gastrointestinal system. As described, the patient may develop uremic fetor, a urine-like odor noted on the breath [130]. Hypogeusia may manifest as both loss of acuity and loss of ability to discriminate tastes. Thus, with significant changes in the ability to taste foods, eating is less pleasurable [1,26].
Parotitis, gastritis, pancreatitis, and colitis may occur at varying stages in the development of uremia. The parotid glands may become infected and inflamed. Irritation of the gastric and intestinal mucosa is often accompanied by vague discomfort, nausea, vomiting, belching, and diarrhea. Bleeding from the gastrointestinal lining is common due to platelet abnormalities alter the clotting mechanism. The bleeding generally is occult, but gross bleeding with severe gastritis may also occur [1,26].
The loss of renal function and the development of uremia also affect the reproductive systems of men and women. Both experience a loss of libido, and men frequently become impotent. Fertility is affected as well. Men have lower testosterone levels and a decrease in sperm formation, whereas women ovulate and menstruate less often, if at all. Successfully carrying a pregnancy to term is rare [1,26].
The renal system both influences and is influenced by a variety of psychosocial factors, including sex, lifestyle, pharmacotherapy, and identity issues.
Sex influences the structure of the urinary system in an important way. Because the urethra is shorter in women, they are more prone to cystitis (bladder infection) than men. Stress urinary incontinence is not uncommon in women who have experienced relaxation of the pelvic muscles as a result of pregnancy [27,28].
Sexual dysfunction occurs frequently in CKD and can be the result of a combination of effects of the disease, comorbidities (e.g., diabetes), and sociopersonal factors (e.g., poor self-image). Approximately 71% of those with erectile dysfunction have estimated glomerular filtration rates (eGFR) below 60 mL/min/1.73 m2 [130]. Further, up to 92% of men undergoing dialysis report erectile dysfunction. Patients may be prescribed phosphodiesterase-5 inhibitors (e.g., sildenafil, vardenafil, tadalafil) to improve erectile response [130].
In addition, 56% of women with CKD report decreases and in sexual desire and difficulty achieving orgasm [130]. Estrogen deficiency is believed to be an underlying factor in dysfunctions of sexual drive, as a hypoestrogenic state leads to decreased blood flow to the vulva and vagina, resulting in less vaginal lubrication and dyspareunia. Transdermal estradiol treatments have improved sexual function in these patients [130].
Dietary habits can also affect the function of the kidneys and urinary system. For example, a high-salt diet can contribute to the development of hypertension, which can lead to or exacerbate renal disease. For people with diagnosed renal dysfunction, salt and protein restrictions may limit the pleasure of eating. Individuals can avoid certain foods and beverages in their own homes with some discipline, but visiting friends or dining in restaurants presents difficulties that may not be controlled so easily. People who must strictly limit fluid intake may experience questioning or stigma. Participation and sharing are social expectations, and some people prefer to avoid these situations rather than not do what is expected [27,28].
Among medications that may have a toxic effect on the kidneys, are a number of common antibiotics (e.g., penicillin, neomycin, kanamycin, amphotericin). Probably the most nephrotoxic category of antibiotics is the aminoglycosides (e.g., gentamicin, vancomycin, tobramycin). Other nephrotoxic drugs include sulfonamides, salicylates, thiazides, and furosemide [29,30].
The costs of renal dialysis and transplantation are high. Moreover, a loss of income may result if the patient is a wage earner [31]. Lower rates of transplantation wait list participation and increased risk of mortality is associated with lower socioeconomic status and Medicare/Medicaid insurance (rather than private insurance) [131]. Higher rates of neighborhood poverty are also associated with worsening ESRD and mortality, especially in the Black communities [131].
Certain occupations and hobbies are associated with exposure to nephrotoxic chemicals. Carbon tetrachloride, used in dry-cleaning and various industrial processes, is nephrotoxic, as are methyl alcohol, phenols, and ethylene glycol. Several metals used in the fabrication of jewelry and some electronic components are nephrotoxic, including gold, lead, copper, uranium, arsenic, mercury, and cadmium [31]. Commercially available hair dyes often contain paraphenylenediamine, a chemical linked to acute renal failure [32].
The development of renal disease may severely influence the patient's occupational performance. Jobs requiring travel, flexible hours, or physical energy may be difficult to maintain. The need to change careers or develop new skills, in addition to coping with physical illness, may be an enormous challenge or impossible for patients with renal disease [31]. Patients with higher education levels and/or white-collar jobs with disability benefits were more likely to retain employment than those with lower education and/or blue- or pink-collar jobs. Peritoneal dialysis can provide more flexibility in employment opportunities, with less danger of inadvertent removal of the dialysis catheters. Individuals undergoing dialysis may need to alter work schedules to accommodate appointments [132]. Improving access to care, including early intervention and vocational rehabilitation in patients with ESRD or dialysis, can help to ensure patients remain in the workforce as long as they desire or are medically able to do so [132].
For patients with serious kidney dysfunction, as in ESRD, the psychosocial effects are numerous. Lifestyle, family life, and work commitments usually are interrupted, often in substantial ways. Patients often must make major adjustments in various roles. Stress accompanies the effort to maintain the existing lifestyle in the face of vast uncertainty about the future, and patients and their families (especially spouses and children). Powerlessness, helplessness, and hopelessness are part of the experiences of patients with renal failure [31].
Disorders of the urinary system may profoundly affect the expression of sexuality. Procedures such as urinary diversion may require the placement of an ostomy bag, and patients with such appliances may be reluctant to participate in sexual activity. They and their partners may find it necessary to alter their sexual behaviors to accommodate a catheter or a dialysis fistula. Patients and their sexual partners may have to discuss and experiment with alternate positions for sexual intercourse, and intercourse may be somewhat unpleasant or even uncomfortable or painful [31].
Patients' reproductive ability may also be altered. For example, certain surgical procedures (such as radical cystectomy) may cause impotence. Because hormone levels are not regulated in the presence of chronic renal failure, many female patients have amenorrhea and are unlikely to become pregnant. In men with renal failure, low testosterone levels and oligospermia significantly reduce the ability to fertilize an ovum. Active prevention of pregnancy in women receiving dialysis is indicated for a number of reasons. For one, pregnancy increases the circulating blood volume. In addition, the low hemoglobin values of most patients with chronic renal failure are inadequate to support a healthy fetus. Attempting to conceive while on dialysis can be harmful to both mother and fetus. If the renal problem is genetically transmitted, as in polycystic kidney disease, reproduction may also be discouraged [31].
Because elimination is essentially culturally prescribed, it is intimately linked to our view of ourselves and to our self-concept. A person faced with the problem of disposing of a plastic bag full of urine or who finds that engaging in certain activities means that urine is likely to leak may feel unclean and out of control of basic body functions. The person may also worry about personal odors [31].
To conceal urinary diversion appliances or drainage tubes, patients may find their choice of clothing limited to nonrestrictive and comfortable styles. Not being able to wear a preferred style of clothing may provoke anxiety, depression, and self-isolation in some people. Being unclothed and seen in the nude may also cause great discomfort to the patient concerned about body changes [31].
Patients who have undergone renal transplantation may have concerns about having another person's organ in his or her own body. The knowledge that a donor has sacrificed a healthy kidney for the patient's benefit may become a burden of guilt. This guilt may be increased if the donor kidney is ultimately rejected [31].
The nursing process when caring for patients with problems of the kidneys and urinary tract encompasses a wide range of assessments and interventions.
Assessment of a patient's normal pattern of urination and discussion of changes in the pattern involves questioning the patient about the urinary volume, timing, characteristics, micturition control, and appearance of urine. Patients often experience anxiety discussing this aspect of their lives. Urination is typically private, and language referring to the process frequently involves colloquial expressions or terminology unique to a particular family or group. Thus, clear communication about the exact nature of the problem may be difficult. In addition, the proximity of the urethra to the organs of sexual functioning may cause further embarrassment or anxiety during assessment. Taking a calm, confident approach to the interview and examination may make the patient more comfortable [33,34].
It is important for nurses to question the patient about any changes in micturition. What color is the urine? Is there pain with urination? Does the patient have frequency, urgency, or hesitancy with voiding? Are there problems with incontinence? Does the patient void excessive amounts? Only a little? At night? These questions will elicit information about various alterations in micturition and the characteristics of the underlying problem [33,34].
Changes in the urine's appearance may be the presenting problem. Hematuria can be a serious sign, as it may be indicative of cancer. However, it also may be related to anticoagulant therapy, excessive exercise, infection, or trauma. If the urine is excessively alkaline, patients may describe it as being bright red or coffee-colored. If the urine is excessively acidic, blood can give the urine a cloudy or smoky appearance. In most cases, however, cloudy urine is the result of pus in the urine (pyuria). In severe pyuria, urine is also malodorous. Almost colorless urine usually results from excessive fluid intake, chronic renal disease, diabetes insipidus, or diabetes mellitus. Dark yellow-orange urine suggests dehydration or ingestion of medications or foods that discolor the urine. It is important to know when the change in urine color began and if it is constant, intermittent, or triggered by specific events [27,28]. Certain medications, such as phenazopyridine, can cause discoloration of the urine. It is important for history-taking to include any medications taken prior to urine testing. While dyes in medications can interfere with urine dip testing, they should not interfere with urine microscopy or urine culture [133].
Pain is not always present with chronic disorders of the kidneys and urinary tract; it is more common in acute conditions. If present, the patient's history should illicit descriptions of the character, location, distribution, onset, duration, and frequency of the discomfort [27,28]. Is it related to voiding? What brings it on? What relieves it?
Pain from within the kidney is typically described as a dull ache in the flank area (between the ribs and the ileum) and extending into the lower abdomen or the umbilical area. In most cases, the pain is always present and not interrupted with position changes. Renal and ureteral colic causes severe, excruciating pain of sudden onset. The pain, located in the flank area and radiating to the groin, is accompanied by nausea, vomiting, and paralytic ileus [27,28]. Some patients complain of sudden-onset lower back pain as the only symptom; this is reported primarily in postmenopausal women [134].
Feeling the constant need to void when catheterized is due to the pressure of the catheter in the urethra and spasms of the bladder. Bladder pain in the suprapubic area is usually the result of bladder spasms, which can be contractions of the detrusors muscle responsible for normal micturition. Urgency and burning on urination are also common in patients with cystitis or urethritis. Cystitis may produce burning both during and after urination, whereas urethritis usually causes burning during urination only. Strangury often accompanies severe urinary tract infection [27,28].
Some patients with symptoms of burning on urination may actually have a vaginal infection, so these patients should be assessed for signs of vaginal discharge. Has the patient noted any vaginal or perineal itching or dyspareunia (pain with sexual intercourse)? Pain at the urethral orifice or meatus results from irritation or trauma of the bladder neck or urethra. Renal and perineal fullness and pain suggest prostatitis. Metastasis of prostatic cancer to the pelvis can cause leg and back pain [27].
Questions regarding micturition frequency, dribbling, hesitancy, and incontinence are important. How many times per day does the patient void? Is there trouble initiating the stream or difficulty holding the urine? Does the patient get up at night to void? How many times? When did this begin?
Stress incontinence is a common problem for women. As noted, weakness can develop in the bladder-urethral sphincter mechanism through the stretching of pelvic muscles during childbirth or the pressure of the uterus on the bladder during pregnancy. In the elderly, relaxation of pelvic musculature also contributes to the incontinence. Frequent catheterizations and the use of forceps during delivery increase the chances for development of stress incontinence. When questioned, women may state that they need to wear sanitary pads or incontinence briefs [27].
Nurses should also determine if the patient maintains an adequate fluid intake (1,500–2,000 mL per day). If the patient has an excessive intake of milk and vitamin D, this could lead to hypercalciuria. Proteinuria, hematuria, or both can be a normal finding in people who exercise excessively. Immobility because of disability, fracture, thrombophlebitis, or surgery can predispose patients to the development of renal calculi [25,35].
Obtaining a medication history is essential, because many drugs can damage the kidney. Has the patient taken any prescription drugs recently? What over-the-counter medications does the patient routinely take? It is also important to determine if the patient has had any toxic exposures on their job or in their lives [36].
The patient's health history may be significant [28]. Has the patient ever had problems that could lead to nephropathy, such as frequent streptococcal infections, recurrent urinary tract infections, renal calculi, hyperuremia (as occurs in gout), or hypercalcemia (as occurs in hyperparathyroidism, sarcoidosis, or metastatic bone disease)? Has the patient ever had an indwelling catheter, cystoscopy, or x-rays of the renal system? Is there a history of trauma?
Family history of congenital disorders should also be determined, including polycystic kidney disease and congenital malformations of the urinary tract. A strong family history of diseases such as diabetes or hypertension is also significant because these diseases tend to cause renal problems [37].
Because of common autonomic and sensory nervous system innervation between the gastrointestinal and urinary tracts, as well as the anatomic proximity of the organs of the two systems, gastrointestinal symptoms often accompany renal system conditions. Some of these symptoms are nausea, vomiting, diarrhea, abdominal pain, gastrointestinal hemorrhage, and paralytic ileus. Conversely, urinary symptoms may accompany gastrointestinal conditions [27,28].
Objective data are obtained in the physical assessment of the patient through inspection, auscultation, percussion, and palpation. Objective data are also obtained through a variety of diagnostic studies.
Inspection
The first stage of data collection is inspection of the patient. Examination of the skin is important; patients with uremia have a characteristic ashen, yellow skin coloring, and uremic frost may be visible. The eyes of these patients are often sunken and give the patient a wasted appearance exaggerated by muscle wasting and edema. Edema associated with renal failure is generalized rather than dependent. Bruises are common due to abnormalities with platelets [38].
The skin also provides clues to renal involvement in patients not yet diagnosed as having urinary system problems. In a hypernatremia state, the skin is dry and flushed, and the body temperature is elevated. Examination of the mucous membranes of the nose and mouth is important. With hypernatremia, the mucous membranes are dry and sticky, and the tongue is rough and dry. Skin turgor provides insight regarding the patient's hydration status [38]. Tetany, including carpopedal spasms, occurs with hypocalcemia. Nurses should observe for other signs of various electrolyte imbalances.
Respirations should be observed. Rapid respirations suggest metabolic acidosis, infection, or fluid overload. Shallow respirations and shortness of breath may be signs of hypokalemia. Shortness of breath may also suggest pulmonary edema, congestive heart failure, or both [38].
Inspection of the abdomen may reveal some important findings. Scars may indicate a history of surgical procedures or trauma. There may be urinary or fecal diversions or cutaneous fistulas. The abdominal contour may be altered if the bladder is descended or the kidneys are enlarged, as with polycystic kidney disease. With significant bladder distention, the umbilicus may be displaced [38].
If possible, urine should be inspected for blood, color, cloudiness, and precipitates. If any discharge is present at the urinary meatus, a specimen of that discharge should be obtained before the patient gives a urine sample [38].
Auscultations
Auscultations of the lungs may provide evidence of rales or rhonchi related to fluid overload. Cardiac irregularities and faint heart sounds may be heard with potassium imbalance. Friction rubs, as well as S3 and S4 sounds, also may be heard during auscultation of the heart and lungs. The abdomen should be auscultated for renal bruits. When listening over each costovertebral angle (CVA), a bruit over one or both renal arteries may suggest the possibility of renal artery stenosis.
Percussion
Percussion of the urinary bladder will elicit a dull sound if the suprapubic region of the bladder is distended. Bladder distention may extend to the level of the umbilicus or higher. When bladder tone is diminished because of chronic distention, bladder fullness may be detectable only by percussion [38].
A variation of the normal percussion technique can detect discomfort or pain over the kidney. Tenderness over the CVA suggests infection in the kidney or perinephric space [38].
Palpation
Palpation of normal-sized kidneys is difficult except in individuals who are quite thin or have poorly developed muscles. The right kidney is more readily palpable because it is normally lower in the abdomen and slightly more anterior than the left kidney [38].
As such, deep palpation is necessary to identify the kidney. In this method, the lower pole of the right kidney may be palpated when the patient takes a deep breath. In another method of kidney palpation, called capturing, hand placement is the same, but the right hand exerts greater pressure. The patient is asked to exhale and then hold the breath. If the kidney has been captured, it will be felt as the pressure of the fingers is released. The patient will feel this procedure, but it should not be painful [38].
If abdominal masses are present and are thought to be enlarged kidneys, perform only gentle and light palpation. If the masses are polycystic kidneys, palpation may aggravate bleeding. Examiners in doubt about the nature of the mass should eliminate this aspect of the examination [38].
The laboratory tests most often used to assess kidney function include serum creatinine, blood urea nitrogen (BUN), and creatinine clearance [38].
Serum Creatinine
Serum creatinine measurements primarily reflect the ability of the kidneys to excrete creatinine—the waste product of the breakdown of phosphocreatine during skeletal muscle metabolism. The normal serum creatinine level is 0.6–1.5 mg/dL, but it varies with sex and individual muscle mass characteristics. Serial changes in serum creatinine levels are significant in evaluating and interpreting renal function, because this substance is excreted entirely by the kidneys and is therefore directly proportional to excretory function. Serum creatinine levels are less affected by factors such as dehydration, malnutrition, or hepatic function than BUN levels. Therefore, serum creatinine levels are more accurate than BUN levels in assessing renal function [38].
Blood Urea Nitrogen (BUN)
The BUN level is a general indicator of renal ability to excrete urea nitrogen, and the normal BUN level is 6–20 mg/dL. Urea nitrogen is synthesized by the liver using protein sources for the conversion, so hepatic function is also a factor. Because dietary proteins form the primary source of urea nitrogen, a diet high in protein will increase BUN levels, especially in the presence of renal disease. The metabolism of hemoglobin also results in the production of urea nitrogen [38].
Nearly all primary renal diseases cause BUN levels to rise, as do certain medications (e.g., steroids, tetracycline, tobramycin, gentamicin, certain chemotherapeutic drugs). Hydration changes also alter the BUN level. In dehydration, decreased renal blood flow leads to decreased excretion of urea nitrogen and increased serum BUN levels. The BUN level also may rise when excessive amounts of protein are available for hepatic catabolism or when there is gastrointestinal bleeding. Because urea synthesis relies on a functioning liver, the BUN level may be normal in hepatorenal syndrome or any time there is combined liver and kidney disease. In these cases, the BUN level is normal not because the renal excretory function is good, but because hepatic function is poor and BUN formation is therefore decreased [38].
Uric Acid
Uric acid is a nitrogenous product derived from urine metabolism. Purines are produced by the metabolism of cellular nucleic acids and complex dietary proteins. Elevated serum uric acid levels may indicate renal dysfunction or a defect in purine metabolism. The normal serum uric acid level is 2.1–7.5 mg/dL in men and 2.0–6.6 mg/dL in women [38].
Creatinine Clearance Test
The best test to measure overall renal function is the creatinine clearance test, a mathematical calculation that compares the amount of creatinine filtered in a 24-hour urine collection with the amount of creatinine that remains in the serum. Because almost all creatinine is excreted and other variables do not influence muscle metabolism and renal excretion, creatinine clearance is regarded as the best indicator of renal function. Although a 24-hour collection is preferred, a 12-hour or shorter collection may be acceptable in some situations [38].
The normal creatinine clearance value for men is 107–141 mL/min; for women, it is 87–132 mL/min. For practical purposes, a rate of 100 mL/min may be considered normal to allow comparison for the clearance to a percentage value. For example, a creatinine clearance value of 100 mL/min suggests that 100% is normal. A creatinine clearance value of 50 mL/min suggests that 50% renal function is lost and 50% remains. After the initial calculation, subsequent increases of the serum creatinine level imply that renal function is deteriorating; in other words, the kidneys are clearing less creatinine, and the serum level is increasing. Subsequent creatinine clearance calculations would result in decreasing values, because less creatinine would be measured in the urine as more accumulate in the serum. The trend is what is significant. The serum level will not increase until at least 50% of renal function has been lost [38].
Dialysis or transplantation is generally not indicated until the creatinine clearance value is less than 5 mL/min. Occasionally, however, symptoms may dictate that dialysis be started before the levels get that low [38].
Routine Urinalysis
Routine urinalysis assesses the nature of urine produced, including:
Measurement of color, pH, and specific gravity
Determination of the presence of glucose, protein, blood, and ketones
A microscopic examination of the urine sediment for cells, cysts, bacteria, and crystals
Abnormal findings require further delineation and confirmation.
Specific gravity, a measure of the concentration of particles in the urine, reflects the ability of the kidney tubules to concentrate or dilute urine. The normal specific gravity is 1.016–1.022, but it can range from 1.001–1.040. The specific gravity increases in patients with:
Dehydration, because the kidneys absorb all available free water, making the excreted urine concentrated
Pituitary tumor that causes the release of excessive amounts of ADH, resulting in excessive water absorption
Decrease in renal blood flow, as in hypotension, heart failure, or renal artery stenosis
Glycosuria and proteinuria, because of the increased number of particles in the urine
In contrast, urine specific gravity decreases in patients with:
Overhydration
Diabetes insipidus, which is characterized by inadequate secretion of ADH and resultant decreases in water reabsorption
Renal failure
Pyelonephritis
Acute tubular necrosis
Interstitial nephritis
In patients with chronic renal failure, the specific gravity is usually stable at about 1.010 despite changes in the intake because the kidney can no longer respond to changes.
The normal protein content of urine is less than 8 mg/dL. The first voided morning specimen is preferred to detect the presence of protein in the urine, because orthostatic and transient proteinuria generally can be ruled out. However, stress and exposure to cold weather over time also can contribute to transient proteinuria. Higher-than-average protein levels found on routine analysis (albuminuria) should be evaluated further for total protein by the 24-hour collection. Proteins other than albumin may be identified and require investigation by other methods, such as urine electrophoresis [38].
Normally, there are no ketones in the urine. Ketonuria occurs when there is incomplete metabolism of fats. This condition may be observed in diabetic ketoacidosis, dehydration, starvation, or excessive aspirin consumption. Patients who follow a diet high in protein and low in carbohydrates will also form ketones [38].
Normal urine contains only small amounts of glucose—usually less than 15 mg/dL. Glycosuria occurs when the renal threshold for reabsorption of glucose is exceeded. The renal tubule cells are able to reabsorb glucose up to a serum blood glucose level of about 180 mg/dL. When the renal tubules are impaired, glycosuria will occur at lower serum glucose levels. Aging, pregnancy, and diabetes of several years' duration will increase the renal threshold for glucose [38].
Any disruption in the blood-urine barrier, whether at the glomerular or tubular level, will cause hematuria. More than two or three red blood cells found in the sediment of urine is considered abnormal. Hemolysis of red blood cells that results in hemoglobinuria may occur in various hemolytic anemias and following some blood transfusion reactions [38].
Bilirubinuria should be suspected when the color of the urine is dark gold or brown. Normally, there is no detectable bilirubin in the urine, and its presence indicates liver dysfunction or an obstruction of the flow of bile in the biliary tract [38].
As noted, the presence of white blood cells in urine (pyuria) indicates an infection of the urinary tract. However, four or five white blood cells per high power field are not significant [38]. Women may have a small number of epithelial (typically squamous) cells present on urinalysis as epithelial cells line the urethra and vagina. A large number of these cells on a clean-catch urine specimen demonstrates one of two theories: lack of proper cleansing prior to collection or infection. The presence of renal cells suggests a pathologic condition in the kidney [38,135].Casts are abnormal elements formed in and molded to the lumen of the tubules. Mucoproteins and various other cells (e.g., red blood cells, white blood cells, epithelial cells) form the cast. White blood cell casts suggest kidney infection, whereas red blood cell casts suggest damage to glomerular capillaries or ruptured tubular walls. Hyaline casts, composed of various types of protein, are the most common and may be found in people with high fever or who exercise strenuously. This finding indicates the mildest form of tubular damage. As casts degenerate, they may become granular or waxy [38].
Crystals found in the urine sediment indicate that the patient may have or is at risk of forming a renal calculus. Urate crystals occur with gout, while phosphate and calcium oxalate crystals occur in patients with hyperparathyroidism or malabsorptive states. The type of crystal found varies with urine pH. Urate crystals are found in acidic urine, and calcium oxalate crystals occur in alkaline urine [38].
Few if any bacteria are normally present in urine, and large numbers of bacteria suggest infection in the urinary tract. Normal urine contains less than 1,000 colony-forming units/mL; a count greater than 10,000 colony-forming units/mL indicates an infection. However, these cutpoints have been disputed. Some experts use a value of 100,000 colony-forming units/mL as indicating infection, but others assert that infections will be missed using this criterion [38].
Urinary osmolality reflects the ability of the kidneys to concentrate or dilute urine to maintain osmotic balance. Urine osmolality is interpreted in comparison with plasma osmolality. For example, if plasma osmolality is elevated, the kidneys should reabsorb water and excrete more concentrated and smaller volumes of urine. In contrast, if plasma osmolality is low, the kidneys should excrete a less concentrated and larger volume of urine. This maintains the proper osmotic balance among cells, tissue, and plasma. The ratio of urine osmolality to plasma osmolality should be greater than 1:1. Urine osmolality is typically 300–1,090 mOsm/kg, depending upon sex and physical activity. Variations in urinary osmolality also depend on the amount of solute to be excreted [38].
Other Tests
Hemoglobin and hematocrit values of patients with chronic renal failure are low (hemoglobin of 7–8 g/day and hematocrit of 20% to 30%) because of decreased erythropoietin production. White blood cell counts are elevated in the presence of renal infection [38].
Serum potassium and phosphorus levels are elevated with acute renal failure and uremia. Hyperkalemia and metabolic acidosis are especially common in patients with acute renal failure. Elevated phosphorus levels also occur with chronic renal failure. Serum calcium is deceased in renal failure [38].
Ultrasonography of the kidney can locate renal cysts, differentiate renal cysts from solid renal tumors, demonstrate renal or pelvic calculi, and guide a percutaneously inserted needle for cyst aspiration or removal of a biopsy specimen [38]. In addition, ultrasonography may be used to document the size of the kidneys. Chronic kidney disease characteristically results in smaller-than-average kidneys, whereas acute kidney injury is characterized by normal or even enlarged kidneys. Asymmetry may be a result of unilateral renal artery stenosis [27,28].
An abdominal flat-plate x-ray of the abdomen is called a kidney, ureter, and bladder (KUB) or plain film. The KUB will generally determine the presence of two kidneys, as well as provide a general outline of the kidneys, and may also identify tumors, malformations, and calculi. The study is contraindicated during pregnancy [38].
The use of unenhanced computed tomography (CT) imaging is now the standard diagnostic tool to evaluate renal colic. It offers the advantage over intravenous (IV) urography of avoiding contrast and enabling diagnosis of other abdominal abnormalities that can cause pain. A multi-dose CT scan can readily diagnose radiolucent stones, which may not be seen on IV urography, as well as small stones, even in the distal ureter. Apart from some indinavir stones, almost all renal and ureteral stones can be detected on helical CT scan. In the detection of urolithiasis, unenhanced CT has a sensitivity ranging between 96% and 100% and specificity ranging between 92% and 100% [40].
Stones in the distal ureter can be difficult to differentiate from pelvic calcifications. In these cases, the urologist will look for other signs of obstruction indicating the presence of a stone, including ureteral dilation, inflammatory changes in the perinephric fat, hydronephrosis, and a soft tissue rim surrounding the calcification within the ureter. The soft tissue rim around a stone represents irritation and edema in the ureteral wall [40].
A cystoscopy is a procedure in which a cystoscope is inserted into the bladder via the urethra to visualize directly into the internal bladder wall and the contents of the bladder. A cystoscopy is indicated to identify the origin of hematuria as well as to diagnose and remove tumors, stones, or any other foreign material. The application of electrical current to the lesion (fulguration) to remove bladder tumors may be carried out during the cystoscopy examination. A cystoscopy also can be used to implant radium seeds into a tumor, place catheters in the ureters to drain the renal pelvis, or coagulate bleeding areas [38].
A cystoscopy is performed under general or local anesthesia. During the procedure, the patient is supine, with the legs and feet supported in a lithotomy position. Strict aseptic technique is essential during the examination. The cystoscope is available in a variety of sizes from 12 to 26 F. Its wide-angle lens allows viewing of the interior bladder surfaces. A panendoscope will be used to examine the urethra visually, because its lens is more directly in line with the instrument. Retrograde pyelography allows visualization of the bladder, ureters, and renal pelvis, and is used for persons suspected of having an obstruction from a tumor, stone, blood clot, or stricture (narrowing) in the kidney or ureters. It involves the direct injection of dye into each ureter through the cystoscope and may be performed during the procedure. The test evaluates the lower portion of the ureter to which urine flow is obstructed. It is indicated if obstruction is suspected in the ureters or the renal pelvis. Because the dye is not directly injected into the bloodstream, this procedure avoids the risk of reaction to the contrast media [38].
Nursing Implications
Patients should be told that the cystoscope is inserted into the bladder in the same manner as a catheter. Because it is rigid and not flexible, as a catheter would be, the procedure may cause mild-to-moderate discomfort. Patients who are admitted should be given enemas as ordered to clear the bowel. Keep the patient NPO if general anesthesia is planned. A liquid breakfast may be given if local anesthesia is to be used. Administer preprocedure sedatives as ordered to help reduce anxiety as well as bladder spasms [28].
Postprocedure, record careful measurements of urinary output. Vital signs should be measured at least every four hours and any elevation of temperature reported immediately. Urinary instrumentation is a major cause of nosocomial urinary tract infections, but prompt detection and treatment may prevent complications such as sepsis and acute renal failure. Hematuria is a common post-procedure finding, but it will gradually decrease over 24 to 48 hours. Carefully note and monitor the presence of large blood clots, as they may result in obstruction of the urinary drainage system. To avoid these problems, a catheter that permits irrigation and drainage may have been inserted during the cystoscopy [28].
Perforation of the bladder or ureters is possible during cystoscopy. The symptom of severe abdominal pain should alert the nurse to such an occurrence. The patient may also experience pelvic pain, gross hematuria, back pain, bladder spasms, urinary frequency, and burning on urination. Warm sitz baths and mild analgesics may be ordered and given. In some cases, belladonna/opium suppositories are given to relieve bladder spasms. Encourage fluids. Occasionally, antibiotics are ordered one day before and three days after the procedure to reduce the incidence of bacteremia [28]. The American Urological Association recommends that bladder rupture be surgically repaired. Typically, an extraperitoneal bladder rupture can be treated with Foley catheter placement only unless it is complicated [136]. If the rupture is not recognized, intra-abdominal fluid (urine) leakage, sepsis, and abdominal compartment syndrome can develop [137].
Although IV urography was once the standard in urologic imaging, it has essentially been replaced by CT and magnetic resonance imaging (MRI). With the ability of new scanners to perform axial, sagittal, and coronal reconstruction of the upper urinary tract system, essentially all of the data and information obtained by traditional IV urography can be realized with CT imaging. In addition, some parenchymal defects, cysts, and tumors can be better delineated with CT than with IV urography [39].
IV urography may be indicated to assess the renal collecting systems and ureters, including investigation of the level of ureteral obstruction and demonstration of intraoperative opacification of the collecting system during extracorporeal shock wave lithotripsy. It may also be used to demonstrate renal function during emergent evaluation of unstable patients. Finally, it can demonstrate renal and ureteral anatomy after interventions such as transureteroureterostomy and urinary diversion [39].
Percutaneous nephrostomy (PCN) provides a less invasive means to drain the renal collecting system in cases where obstruction of the kidney and ureter has resulted in hydronephrosis. Most often used for patients with kidney stones or bladder or pelvic tumor obstructions, PCN may be used to divert urine from the renal collecting system to allow leaks and fistulas to heal. The procedure is often performed after attempts at placing a ureteral stent through retrograde cystoscopy have proven unsuccessful. Providing drainage for that kidney is an urgent necessity, and PCN provides an exact method of accomplishing this task [39].
The approach is extremely important for PCN, and the procedure is performed under ultrasound or fluoroscopic guidance. In some cases, a small amount of IV iodinated contrast is administered at the start of the procedure to opacify the collecting system. The patient is placed in the prone position with both arms above his or her head or one arm up and the other at the noninvolved side. The entry site is prepped and draped and infiltrated with local anesthetic. A small puncture is made with a scalpel, and a posterior lateral approach is made with a needle and directed toward the lower calyx of the kidney. If the tip of the needle has entered a dilated part of the collecting system, urine will flow back from the needle when the stylet is removed. A specimen should be collected and sent to the laboratory for microscopic and bacterial studies. Obviously, infected urine will be cloudy and turbid [39].
Hemorrhage is the major risk of PCN, but the risk can be reduced substantially with use of a very small needle. Nephrostomies are performed frequently in interventional radiology departments and are a major part of the treatment for patients with malignant obstructions, renal stones, and other kidney problems.
Retrograde pyelograms are performed to visualize the ureters and intrarenal collecting system by the retrograde injection of contrast media. Any contrast media that can be used for excretory urography is also acceptable for retrograde pyelography. It is important that measures are taken to attempt to sterilize the urine before retrograde pyelography, because there is a risk of introducing bacteria into the upper urinary tract or the bloodstream. Although many studies are able to document the presence or absence of dilation of the ureter, retrograde pyelography has the unique ability to document the patency of the ureter distal to the level of obstruction and to help better define the extent of the ureteral abnormality [39].
Retrograde pyelograms are usually performed with the patient in the dorsal lithotomy position. An abdominal plain radiograph (i.e., scout film) is obtained to ensure that the patient is in the appropriate position to evaluate the entire ureter and intrarenal collecting system. Next, the ureteral orifice is identified via cystoscopy, and contrast may be injected through either a non-obstructing or obstructing catheter [39].
Non-obstructing catheters include whistle tip, spiral tip, or open-ended catheters. These catheters allow passage of the device into the ureter and up to the collecting system, over a guidewire if necessary. Contrast can then be introduced directly into the upper collecting system and the ureters visualized as the catheter is withdrawn.
Obstructing ureteral catheters include bulb-tip, cone-tip, and wedge-tip catheters. These catheters are inserted into the ureteral orifice and then pulled back to effectively obstruct the ureter. Contrast is then injected to visualize the ureter and intrarenal collecting system. Depending on the indication for the study, it may be useful to dilute the contrast material with sterile fluid. This prevents subtle filling defects in the collecting system or ureter from being obscured. Care should be taken to evacuate air bubbles from the syringe and catheter before injection, as such artifacts could be mistaken for stones or tumors.
Historically, when a retrograde pyelogram consisted of a series of radiographs taken at intervals, it was important to document various stages of filling and emptying of the ureter and collecting systems. Because of peristalsis, viewing the entire ureter is often not possible with a single static exposure or view. With modern equipment, including tables incorporating fluoroscopy, it is possible to evaluate the ureter during peristalsis in real time, thus reducing the need for static-image documentation. Occasionally, still images may be saved for future comparison. In general, however, urologists interpret retrograde pyelograms in real time as they are performed [39].
Indications for retrograde pyelogram include the evaluation of congenital ureteral obstruction, evaluation of acquired ureteral obstruction, elucidation of filling defects and deformities of the ureters or intrarenal collecting systems, opacification or distention of the collecting system to facilitate percutaneous access (in conjunction with ureteroscopy or stent placement), evaluation of hematuria, surveillance of transitional cell carcinoma, and evaluation of traumatic or iatrogenic injury to the ureter or collecting system.
Retrograde pyelography may be difficult in cases in which there is diffuse inflammation or neoplastic changes of the bladder, especially when bleeding is present. In these cases, identification of the ureteral orifices may be facilitated by the IV injection of indigotindisulfonate sodium (indigo carmine) or methylene blue. Changes associated with bladder outlet obstruction may result in angulation of the intramural ureters, which may make cannulation with an obstructing catheter difficult. Attempts to cannulate may result in trauma to the ureteral orifice and extravasation of contrast material into the bladder wall. The potential for damage to the intramural ureter should be weighed against the potential information obtained by the retrograde pyelogram [39].
Loopography is a diagnostic procedure performed in patients who have undergone urinary diversion. Historically, the term loopogram has been associated with ileal conduit diversion, but it may also be used in reference to any bowel segment serving as a urinary conduit. Because an ileal conduit urinary diversion usually has freely refluxing uretero-intestinal anastomoses, the ureters and upper collecting systems may be visualized. In other forms of diversion, the uretero-intestinal anastomoses may be purposely non-refluxing [40].
The patient is positioned supine and an abdominal plain radiograph is obtained before introduction of contrast material. A commonly employed technique is to insert a small-gauge catheter into the stoma of the loop, advancing it just proximal to the abdominal wall fascia. The balloon on such a catheter can then be inflated to 5–10 mL with sterile water. By gently introducing contrast through the catheter, the loop can be distended, usually producing bilateral reflux into the upper tracts. Oblique films should be obtained in order to evaluate the entire length of the loop. Because of the angle at which many loops are constructed, a traditional anteroposterior view will often show a foreshortened loop and could miss a substantial pathology. A drain film should also be obtained, as this may demonstrate whether there is obstruction of the conduit [40].
Indications for a loopogram include evaluation of infection, hematuria, renal insufficiency, or pain after urinary diversion. It can be used for surveillance of upper urinary tract obstruction or urothelial neoplasia, or it may be used to evaluate the integrity of the intestinal segment or reservoir [40].
A retrograde urethrogram is a study performed to evaluate the anterior and posterior urethra, usually in male patients. It may be particularly beneficial in demonstrating the total length of a urethral stricture that cannot be negotiated by cystoscopy and the anatomy of the urethra distal to a stricture that may not be assessable by voiding cystourethrography. This procedure is performed in the radiology department or in the operating room before performing visual internal urethrotomy or formal urethroplasty [40].
A plain film radiograph is obtained before injection of contrast, and the patient is usually positioned slightly obliquely to allow evaluation of the full length of urethra, with the penis placed on slight tension. A small catheter may be inserted into the fossa navicularis with the balloon inflated to 2 mL with sterile water. Contrast is then introduced via a catheter-tipped syringe. Alternatively, a penile clamp may be used to occlude the urethra around the catheter. Indications for a retrograde urethrogram include evaluation of urethral stricture disease (including location and length of a stricture), assessment for foreign bodies, evaluation of penile or urethral penetrating trauma, and evaluation of traumatic gross hematuria [40].
A voiding cystourethrogram is performed to evaluate the anatomy and physiology of the bladder and urethra. The study provides valuable information regarding the posterior urethra in pediatric patients and has long been used to demonstrate vesicoureteral reflux.
Voiding cystourethrogram may be performed with the patient supine or in a semi-upright position using a table capable of bringing the patient into the full upright position. A preliminary plain pelvic radiograph is obtained. In children, a tube (8 French or smaller) is used to fill the bladder to the appropriate volume, as determined by the radiologist's needs and patient comfort. In the adult population, a standard catheter may be placed and the bladder filled to 200–400 mL. The catheter is then removed and a film is obtained. During voiding, anteroposterior and oblique films are obtained. The bladder neck and urethra may be evaluated by fluoroscopy during voiding. Bilateral oblique views may demonstrate low-grade reflux, which is not able to be appreciated on the anteroposterior film. In addition, oblique films will demonstrate bladder or urethral diverticula, which are not always visible in the straight anteroposterior projection. Post-voiding films should also be performed [40].
Indications for a voiding cystourethrogram include evaluation of the urethra, possible reflux, and structural and functional bladder outlet obstruction. There are certain limitations with a voiding cystourethrogram. Using a catheter may be traumatic in children and difficult in some patients with anatomic abnormalities of the urethra or bladder neck. Filling of the bladder may stimulate bladder spasms at low volumes, and some patients may be unable to hold adequate volumes for investigation. Bladder filling in patients with spinal cord injuries higher than T6 may precipitate autonomic dysreflexia [40].
Renal biopsies are extremely helpful to pinpoint an exact diagnosis in a patient experiencing kidney disease. Lupus-like syndromes and types of tubular interstitial disorders may be diagnosed accurately by examination of kidney tissue. Cellular changes, atrophy, and neutrophil infiltration all may be seen by the pathologist, and these factors aid the nephrologist in treatment planning and prognosis. Renal biopsies are also performed to aid in tracking rejection after a kidney transplant. Some smaller hospitals send their renal specimens out to a laboratory that specializes in analysis of renal tissue because the care and examination of renal specimens are quite involved. The renal biopsy itself, however, may be performed in general interventional radiology departments.
Patients are positioned prone for kidney biopsies. Specimens are collected by means of long sampling needles. The procedure is usually safe, but some caveats apply, as in sampling for lupus [41]. Some referring physicians prefer to admit their patients for overnight observation, to closely monitor for excessive bleeding.
Long needles may also reach tumors of the adrenal gland. Due to the adrenal's location on the superior pole of the kidney, the technique for positioning is almost the same. The physician isolates the solid tumor by CT scan or ultrasound guidance and obtains a sample for analysis. Caution should be practiced with adrenal biopsies because the possibility always exists that the mass is a pheochromocytoma. This tumor consists of cells that secrete adrenaline and other catecholamines and can cause paroxysms of hypertension, tachycardia, headache, nausea, diaphoresis, and a multitude of similar symptoms [42]. Care is always taken not to stimulate a pheochromocytoma, so piercing it with a needle should be avoided.
Assessment of patients with renal dysfunction involves obtaining the history, conducting a physical examination, and reviewing the results of diagnostic studies. The major nursing diagnoses are alterations in comfort, fluid retention, and impairment of gas exchange, nutrition, and urinary elimination. Various nursing interventions may be implemented to address these diagnoses.
Patients with renal or ureteral calculi commonly experience excruciating pain. Renal abscesses and infections of the urinary tract, including cystitis and urethritis, produce moderate pain [33,34].
The pain experienced by patients with renal or ureteral calculi typically requires opioid analgesics for relief. Morphine may be given intravenously to provide immediate pain relief, and subsequent doses may be given subcutaneously until the stone is passed or removed. Non-narcotic analgesics, urinary antiseptics, antibiotics, and increased fluid intake may be prescribed to control the discomfort associated with related infectious processes.
Analgesia may also be required to address the pain associated with the hypocalcemia of renal failure or metastasized renal cancer. Patients with polycystic kidney disease should avoid aspirin and aspirin-containing compounds because of the potential for bleeding with the cysts [33,34,43].
Pain associated with surgical incisions will require opioid analgesia during the initial 24 to 48 hours, and extensive surgical incisions may be quite uncomfortable for a longer period. Combination belladonna/opium suppositories may relieve the pain of bladder spasms following prostate surgery, bladder surgery, or kidney transplant surgery [33,34,43].
Nursing interventions related to comfort are considered successful if the patient is resting, moving, or sleeping comfortably. Facial expressions and body movements should be relaxed and without tension.
Mild or severe generalized itching accompanies ESRD and the development of uremia. The production and elimination of urate crystals by the skin and the increased production of parathyroid hormone are believed to be the causes of intense pruritus. Dry skin or perspiration and other moisture can worsen the pruritus [33,34].
In patients with ESRD and uremia, pruritus is often not relieved with the initiation of dialysis. Interventions that may provide comfort include avoiding agents known to dry the skin (e.g., soaps, lotions that contain alcohol). Bathing without soap will remove the uremic frost that compounds itching; bath water will be yellow from the urochrome pigments. Oil-based lotions and soaps containing lanolin or a high fat content should be encouraged. In addition, patients may be prescribed phosphate-binding agents; control of the phosphorous-calcium balance will moderate the production of parathyroid hormone [27,28]. Keeping the patient's fingernails short can help avoid lacerations and related infections.
Aluminum hydroxide antacids promote phosphate excretion, but they prevent the absorption of iron and should be used with caution, especially in those with anemia. Vitamin D and calcium supplementation should be given to correct the calcium deficit after hyperphosphatemia has been corrected. Possible signs of hypocalcemia are sore feet, muscle weakness, joint pain, generalized bone aching, and spontaneous fractures. Diphenhydramine hydrochloride (Benadryl) and trimeprazine tartrate (Temaril) may provide temporary relief from the itching. Because it is excreted by the kidney, the dose of diphenhydramine hydrochloride may be reduced to avoid toxicity. Ultraviolet light treatments (phototherapy or photochemotherapy) three times per week may control severe pruritus by promoting a more rapid turnover of epithelial skin cells. Parathyroidectomy may be required if excessive parathyroid hormone production and pruritus cannot be controlled with more conservative measures [27,28]. Relief from pruritus is considered successful if the patient does not constantly scratch or ask to be scratched and if the skin is free of excoriation from frequent scratching [33,34].
Patients with renal calculi (stones in the kidneys, ureters, or bladder) may develop infections, as the stones are a continuing nidus of bacterial growth. Indwelling urinary catheters, stents, ureteral tubes, and nephrostomy tubes also increase the risk of infection. Urinary stasis associated with a neurogenic bladder increases the risk of ascending urinary tract infections, which can become systemic bacteremia. Any urinary instrumentation required for diagnosis or treatment (e.g., cystoscopy) may introduce pathogens, potentially leading to infection. In patients with uremia from either acute renal failure or ESRD, altered immune processes further contribute to the potential for infection [33,34].
Infections increase the workload of the kidneys, and the prevention of infection is a priority. It is important to maintain sterile technique during dressing changes and at sites where catheters have been inserted into the circulatory or urinary system. Intermittent catheterization is preferable to the placement of a continuous indwelling urinary catheter. If continuous urinary catheter drainage is necessary, however, the continuity of the system should not be disrupted. The tubing and bag should remain below the level of the bladder to ensure that urine does not ascend into the upper urinary tract. Vascular access routes for hemodialysis and catheters for peritoneal dialysis should all be cared for carefully [27,28].
Handwashing is an essential element of aseptic technique and care of patients with catheters. In addition, healthcare staff should be educated and trained in proper techniques of catheter insertion and care.
Loss of fluid volume as a result of diuretics, infection, hemorrhage, or fluid shift to the interstitial space (as with nephritic syndrome) may result in a fluid volume deficit. Patients undergoing dialysis also may have too much fluid removed during the treatments and experience a fluid volume deficit (hypovolemia) [33,34]. For patients with normal renal function, this may contribute to decreased renal perfusion and the development of prerenal azotemia.
Oral or IV fluid replacement will be necessary if the patient becomes fluid-depleted from diuretic therapy, surgical drainage loss, or dialysis. Blood or plasma expanders also may be given to restore intravascular volume. Careful assessment of intake and output, weight, and vital signs is essential to monitor fluid volume status. In addition, the patient's dry weight (i.e., weight after dialysis without evidence of edema and normal blood pressure) may need to be re-evaluated [27,28].
Normalized blood pressure levels and body weight are used to verify successful interventions for stabilization of fluid volume. The patient should demonstrate knowledge of his/her fluid allowance, ability to calculate fluid intake and loss, and correlation of weight changes with physical well-being. For example, the patient who suddenly becomes short of breath should suspect that his/her dry weight has been exceeded and blood pressure is elevated. Other signs that fluid volume has corrected include absence of cerebral manifestations (e.g., syncope, light-headedness) with changes in posture, resolution of nausea or thirst, minimization of peripheral edema, and elimination of rales or lung congestion [33,34].
Excess in circulating fluid volume, or hypervolemia, occurs when a patient is oliguric because of renal failure. This problem may occur acutely with sudden loss of renal function or develop as ESRD progresses. Furthermore, after renal transplant surgery, large volumes of IV fluid are infused at the same rate that urine is produced. This amount often exceeds 4,000 mL/hour the first day [33,34]. Excesses in fluid volume contribute to cardiopulmonary decompensation and must be corrected.
Fluid volume excess may be avoided in patients receiving dialysis if they understand the need to control intake. These patients should restrict fluid intake to the amount of urinary output in 24 hours plus 600 mL. If there is no urine output, fluid should be limited to no more than 1,000 mL and perhaps to no more than 500 mL per day. Intravenous infusions should be administered with micro-drip tubing to avoid excessive fluid administration. Medications administered piggyback are generally dissolved in the smallest possible volume of fluid [27,28].
Providing a glass with lines marking the appropriate amount of fluid will help patients control fluid intake. Fluid restriction may be prescribed as a guideline; however, patients eventually will need to assume responsibility for control of thirst and fluid intake and may impose their own fluid restriction. Intake and output, weight, and blood pressure should be monitored to assess the patient's fluid status. Clinical manifestations of fluid excess include edema, hypertension, and shortness of breath at rest or with exertion [27,28].
Patients with acute or chronic renal failure will have altered nutritional status. Nausea, vomiting, and anorexia are common effects of azotemia. Hypogeusia is also a common problem for patients with ESRD. Dietary modifications (e.g., low-salt, low-potassium diets) prescribed for patients with renal failure complicate efforts to maintain a good nutritional status. The loss of protein via peritoneal dialysis and loss of water-soluble vitamins via dialysis further complicate the issue. Patients with altered renal function who are acutely and critically ill from sepsis or other systemic disease will also experience nutritional deficits [25].
Patients with altered renal function generally require modified or restricted diets. Patients with hypertension should be given a diet of no added salt (generally <4 g sodium per day). If severe hypertension or heart failure is present, dietary sodium intake may be restricted to 2 g/day [25].
Patients who are losing excessive protein (e.g., patients with proteinuria secondary to nephritic syndrome, patients on peritoneal dialysis) require a high-protein diet (1.2–1.3 g/kg body weight/day). In contrast, patients with ESRD who are becoming uremic or are undergoing chronic hemodialysis generally require a protein-restricted diet (0.6–0.8 g/kg/day). Protein in foods should be of high biologic value (i.e., contain a high proportion of essential amino acids). Adequate protein and calories should be provided so the patient's muscle mass is not catabolized [25].
When patients stop excreting urine, potassium intake should be restricted to avoid fatal cardiac arrhythmias. Clinical manifestations of hyperkalemia are chiefly cardiac, although neuromuscular complications can also occur [44,45]. Electrocardiogram (ECG) changes associated with hyperkalemia include peaked T waves (often the first ECG finding), ST-segment depression, widening of the QRS and PR intervals, and loss of the P wave [44]. A late ECG sign is the appearance of a sine-wave pattern, which usually indicates impending ventricular fibrillation and asystole [44,46].
Although cardiac manifestations are obviously the most dangerous sequelae of hyperkalemia, neuromuscular complications, including paresthesias and fasciculations in the extremities, may be seen. Peripheral paralysis can occur, but paralysis of the respiratory muscles is rare [44].
Treatment of acute hyperkalemia with life-threatening symptoms (generally seen with potassium levels ≥7 mEq/L) is accomplished by the administration of IV calcium [44,46,47]. The usual recommended dose is 10 mL of a 10% calcium solution, such as calcium chloride [48]. The ECG should be monitored while calcium is administered, and calcium should be administered only when ECG changes, such as a widening QRS, have occurred [46,47]. Calcium does not correct the underlying hyperkalemia; it only counters the adverse neuromuscular effects [46]. Calcium infusion should always be followed by specific therapy aimed at lowering the plasma potassium level [45].
The administration of IV glucose and insulin is the quickest way to treat acute hyperkalemia that has not yet resulted in life-threatening sequelae [45,46,47]. This results in a shift of extracellular potassium into the cell [46]. Care should be taken in patients with diabetes and hyperkalemia, as glucose infusion that is not accompanied by a matching infusion of insulin can result in increased hyperkalemia due to extracellular hyperosmolarity [44].
When the individual can safely take medication orally and life-threatening sequelae have not developed, treatment with sodium polystyrene sulfate (Kayexalate) in sorbitol solution may be used [48]. This may be the treatment of choice in outpatients who are stable but have potassium levels in the 5.5–6.9 mEq/L range. In patients unable to tolerate oral administration, polystyrene sulfate may be given rectally [47,48]. Studies have raised the possibility of colonic complications from administration of polystyrene sulfate in sorbitol suspension, and there are no controlled studies of safety and efficacy for this use [49]. Due to this concern, concomitant use of sorbitol is no longer recommended [48]. Another strategy to avoid the use of polystyrene sulfate and associated adverse gastrointestinal events (e.g., ischemic colitis, bleeding, perforation, necrosis) would be the use of a loop or thiazide diuretic [50].
The most common cause of chronic hyperkalemia is renal failure; therefore, the most common management of chronic hyperkalemia is dialysis [45]. However, before resorting to dialysis, a combination of medications may be used to increase excretion of potassium, including sodium polystyrene sulfonate, furosemide, and/or IV calcium [51].
Renal dysfunction can lead to impairment of oxygen-carbon dioxide exchange and inadequate oxygenation. If kidneys fail to excrete hydrogen ions and regenerate adequate bicarbonate, the lungs compensate for the increased load of metabolic acids by increasing the rate and depth of breathing (i.e., Kussmaul respirations). Respiratory fatigue from continued compensation may lead to decreased oxygenation, especially if infection or cardiac problems are present.
Flank incisions or thoracoabdominal approaches for renal or urologic surgery also may limit respiratory movements. Hypoventilation following these surgical interventions may precipitate atelectasis or hypostatic pneumonia. Chronic anemia also impairs gas exchange and results in increased cardiac workload (evident as an increased heart rate) [21,22,28].
For patients with metabolic acidosis from acute or chronic renal failure, oxygen-carbon dioxide exchange may be improved with the administration of sodium bicarbonate or with the institution of dialysis. The major risk associated with IV administration of sodium bicarbonate is that it may further expand extracellular fluid volume and worsen problems of hypervolemia, if they are present. Because the correction of acidosis can lead to calcium deficits, it is important to observe for signs of tetany. Dialysate contains acetate, which converts to bicarbonate when absorbed. Thus, dialysis will provide for the replacement of depleted bicarbonate stores [27,28].
Patients with acute or chronic renal failure may experience a variety of changes in the pattern and amount of urine produced.
Retention of urine typically occurs due to obstruction to its outflow. It may result from bladder neck obstruction, as with prostatic hypertrophy, obstruction above the bladder, or neurogenic bladders [27]. The resulting bladder distention may lead to hydroureter or hydronephrosis.
Decreased renal concentrating ability, as occurs with progressive renal insufficiency, results in polyuria. Oliguria or anuria may occur acutely, with sudden loss of renal function. Patients who progress to ESRD often notice that virtually all urine production ceases after the initiation of dialysis [28].
A variety of interventions may be undertaken for patients with incontinence, neurogenic bladder, or urinary diversion. Incontinent men may elect to wear an external catheter (also called a condom catheter), which is preferable to continuous internal catheter drainage. The major drawback of this catheter is that the penis may become excoriated and painful if the area is not kept clean. Even with good hygiene, skin breakdown can occur. A satisfactory external catheter does not exist for women [27,28].
The patient with altered urinary elimination patterns should not have bladder distention or incontinence and should be able to manage the alteration in urine flow without difficulty [27,28].
Severe blood loss, dehydration, or a shift of fluid may cause a decrease in renal tissue perfusion. This alteration in hemodynamics can result in prerenal azotemia. Uremic states also result in a decreased life span of red blood cells and an increased number of immature red blood cells [27,28]. If uncorrected, acute renal failure may result. Patients with chronic renal failure have decreased production of erythropoietin. Because the decrease is gradual, the situation is not life-threatening unless the value suddenly drops further.
Nursing interventions for patients with impaired tissue perfusion include careful monitoring of the patient's vital signs and urinary output. Fluid volume or blood replacement should occur at the prescribed rate, and the nurse should promptly report any inability to administer the fluid as prescribed. If severe hemorrhage is causing a lack of renal tissue perfusion, emergency surgery may be required [27,28].
For patients with renal failure, severe hemorrhage or fluid volume loss may significantly affect cardiac and cerebral tissue perfusion. Patients with ESRD have reduced available hemoglobin. Although patients experience chronic fatigue, vital processes are not typically impaired. If further hemoglobin loss occurs, however, cardiac dysrhythmias, angina, and hypotension may develop [27,28].
Chest pain or arrhythmias indicate alterations in perfusion. Adequate tissue perfusion may be verified through measurements of blood pressure and pulse in the patient's normal range. The patient should demonstrate orientation to person, place, and time [27,28]. Nonacute correction of impaired tissue perfusion may be achieved through the administration of iron supplements, folic acid, anabolic steroids, and judicious administration of blood transfusions [36,52].
Specific disorders of the kidneys and urinary system include life-threatening illnesses with long-term implications. Renal disease can generally be categorized as chronic or acute, although a variety of underlying causes are contributory.
Chronic kidney disease is defined as a reduction in kidney function or kidney damage that has been present for at least three months [53,54,55,56]. However, chronic kidney disease should not be viewed in simple mathematic terms. It is an ongoing process of renal injury that causes compensatory hyperfiltration in less-affected glomeruli, which eventually leads to the destruction of those glomeruli as well [53]. Left untreated, this ongoing destruction results in a steady decline in renal function, which eventually affects not just the renal system but almost every organ system in the body [53,54].
ESRD refers to a disease that requires either dialysis or transplantation services. In the United States, 98% of all patients receiving dialysis or transplantation have a GFR <15 mL/min/1.73 m2 [57]. Therefore, the terms ESRD and kidney failure are often used interchangeably. Although ESRD is generally associated with a GFR of less than 15 mL/min/1.73 m2, it is more importantly an administrative term, as patients receiving dialysis or transplantation services are covered by the Medicare ESRD program [58]. In some cases, ESRD is also used rather loosely to refer to patients who are experiencing progressive chronic kidney disease and are expected to begin dialysis in a matter of days or weeks.
The two leading causes of ESRD are diabetes (44% of new patients) and hypertension (29% of new cases) [59]. New cases of ESRD with diabetes or hypertension listed as the primary cause had been rising rapidly since 1980, but each has declined from 2010 to 2013 [60,61]. However, in 2019, 60.6% of those with ESRD had comorbid diabetes [138]. Approximately 6% of those with CKD and diabetes were hospitalized in 2020 [139]. Other less common causes of ESRD include glomerulonephritis, interstitial nephritis, autosomal dominant polycystic kidney disease (the leading genetic cause), and collagen vascular disease. Due to the prevalence of kidney transplantation, post-transplantation kidney disease has become the fourth largest cause of ESRD in the United States; however, these patients are reported within their original disease category for epidemiologic purposes [62]. New cases of diabetic ESRD are expectedly higher with increasing age in all racial groups, but generally stable or only slightly higher among younger individuals [63]. Statistically, non-White individuals are four times more likely to require dialysis. Compared with White patients, the prevalence of ESRD per million is 9.5 times greater in Native Hawaiians/Pacific Islanders, 3.7 times greater in Black/African Americans, 1.5 times greater in American Indians/Alaska Natives, and 1.3 times greater in Asian Americans [63]. The cost of treating ESRD was $35.4 billion in 2016 [63].
The pathophysiology of chronic kidney disease is dependent on the underlying cause, the most common of which are the disease processes of diabetes and hypertension. Diabetic nephropathy has various proposed hypotheses for mechanisms of kidney damage, though it is most often and broadly attributed to hyperglycemic end-organ damage to the glomerulus, eventually to the point of proteinuria. Experts have suggested that there may be disadvantaged nephron development in those born to mothers with diabetes, predisposing the offspring to chronic kidney disease during their lives. Some also posit that hyperglycemia sensitizes end-organs to hypertensive damage, and because diabetes and hypertension often occur together, this has an additive deleterious effect on the kidney [64]. The initial manifestation is often albuminuria and hyperfiltration (i.e., an elevation in GFR) [65]. Over time, albuminuria increases to the point of overt nephropathy, accompanied by a decline in GFR. Hyperglycemia-mediated overactivation of protein kinase C is also thought to be involved in progressive renal parenchymal damage, resulting in the loss of selective permeability in the glomerulus and an increase in local inflammation [66]. In addition to glomerular damage, thickening of the basement membrane and afferent and efferent arterioles may be noted [66].
Hypertensive nephropathy is another common cause of chronic kidney disease and induces renal damage through a variety of mechanisms. One mechanism is sympathetic nervous overactivity resulting in constriction of efferent arterioles and decreased outflow from the glomerulus, allowing for increased oncotic pressure in the nephron. Activation of the RAAS may also occur as a response to sympathetic nerve activity. Arterial stiffness, a central component of hypertension, is a contributing mechanism as well. Impaired salt and water excretion from sympathetic nervous overactivity or from RAAS activation serves to increase hypertension and thereby increase renal damage.
Other underlying causes of the renal damage leading to chronic kidney disease are generally associated with unique mechanisms, including immune complex deposition and interstitial damage from prolonged use of nephrotoxic drugs. Acute kidney injury may also lead to chronic kidney disease if the initial insult has not been removed or if the initial injury has not been completely reversed. Even in cases of full recovery after acute kidney injury, the risk of developing chronic kidney disease is increased [67]. Renal ischemia-reperfusion injury may also cause lasting damage [68].
Many of the complications and comorbidities associated with chronic kidney disease stem from this initial damage and begin to manifest as renal damage progresses. Hypertension, a common comorbidity of chronic kidney disease, can cause renal damage as well as be exacerbated by it, as discussed. Anemia is a common complication of chronic kidney disease and is likely due to reduced renal production of erythropoietin, though other factors, such as uremia-induced inhibition of erythropoiesis, shortened erythrocyte survival, and disordered iron homeostasis, also play a role [69]. A study of patients with stage 3 chronic kidney disease determined that renal anemia is associated with rapid progression to stage 4 and a higher risk of cardiovascular disease and hospitalization [70].
Alterations in bone and mineral metabolism are commonly seen in patients with chronic kidney disease, starting in early-stage disease. Hypocalcemia is common in these patients, often leading to parathyroid hyperplasia and secondary hyperparathyroidism [71]. The hypocalcemia is likely attributable to phosphate retention, skeletal resistance to parathyroid hormone, altered vitamin D metabolism, or a combination of these factors [72]. In line with altered vitamin D metabolism, patients with chronic kidney disease also have defective intestinal calcium absorption. Hyperphosphatemia is also common, and the resultant elevation in the plasma calcium-phosphate product often leads to precipitation of calcium phosphate in soft tissues and to calcific changes in the walls of arterioles and small arteries [72]. These electrolyte derangements are largely attributable to intrarenal damage and hormonal abnormalities, both of which affect electrolyte excretion and reabsorption. Activation of fibroblast growth factor 23 (FGF-23) and parathyroid hormone is implicated in the regulation of phosphate reabsorption in the tubules. FGF-23 also inhibits vitamin D production and promotes catabolism of vitamin D stores [73].
The clinical presentation of chronic kidney disease is often subtle, and symptoms are uncommon with a GFR greater than about 35 mL/min/1.73 m2. Therefore, suspicion for mild renal disease should be based on recognition of the primary pathologic mechanism responsible for renal injury, particularly in patients with diabetes and/or hypertension. It is equally important to begin early screening for the complications of renal disease to prevent morbidity and to establish a credible baseline for the individual patient.
After the GFR falls to less than 35 mL/min/1.73 m2, a variety of metabolic, psychiatric, hematologic, cardiovascular, and acid-base regulatory problems occur. Clinical presentation at this point depends on the particular complication and the underlying cause of renal failure [44,53,54,55,75].
The management of chronic kidney disease is multifaceted, involving a series of tactical measures (including the effective management of comorbidities) designed to reduce the risk of further damage and slow the progression of kidney disease. The clinician should first seek to identify and treat reversible causes, such as lower urinary tract obstruction, which should be considered in any patient with unexplained deterioration in renal function. Optimal glucose control in the patient with diabetes and blood pressure control in those with hypertension, as well as initiation of angiotensin-converting enzyme (ACE) inhibitor or angiotensin II receptor blocker (ARB) therapy, are important to limit progression [76,77]. Certain nephrotoxic agents should be avoided if at all possible, especially in the patient with diabetes or receiving loop diuretics. These include nonsteroidal anti-inflammatory drugs, aminoglycoside antibiotics, and radiographic contrast material. Additional measures to protect the kidney and slow progression include smoking cessation, statin therapy to control hyperlipidemia, dietary protein restriction, and satisfactory treatment of metabolic acidosis.
For cases of chronic kidney disease that do progress to late-stage kidney disease, it is also important to anticipate and prepare patients for renal replacement therapy [76]. Patients with a GFR less than 30 mL/min/1.73 m2 should be referred to a nephrologist to make preparations for impending end-stage disease and renal replacement therapy [76]. It is well to keep in mind, however, that acute, intercurrent declines in GFR are often due to reversible factors such as volume depletion, radiographic contrast or nephrotoxic drug use, and urinary tract obstruction; efforts should be made to correct these in order to appropriately address declines in GFR and determine whether true progression of the disease has occurred [76].
An intensive and multifactorial management approach is required for patients with renal disease in order to address all risk determinants. The mainstays of treatment are management of complications and/or comorbidities, lifestyle modification, and dialysis for patients with severe or late-stage disease. Some patients may be candidates for kidney transplant, although the wait for a non-related donor can be long. Psychosocial issues and patient education (primarily to ensure compliance with the established treatment plan) are important as well.
An essential component of renal disease management is diet modification and dietary referral is beneficial for optimum care. This is especially true in patients with underlying diabetes and in patients who are under- or overweight, as the dietary recommendations must be modified in these cases. The American Dietetic Association has established guidelines for renal failure diets, which are published in the Manual of Clinical Dietetics [78].
Although the medical management of chronic kidney disease may seem overwhelming to even veteran healthcare providers, it is often devastating to patients. Therefore, it is essential to provide adequate social and psychiatric support. Patients with ESRD (especially patients on hemodialysis) are known to suffer from high rates of depression, insomnia, and anxiety [79]. Often ignored, sexual dysfunction occurs at high rates in both male and female patients with ESRD [79]. The treatment of these and other psychiatric complications should begin before the onset of ESRD, when possible, and continue as long as necessary.
Acute kidney injury is primarily a disease of hospitalized patients and often is the result of pre-existing chronic kidney disease [63]. Acute kidney injury in the Medicare population has reached an annual average of approximately 4.7%, compared with 1.5% in 2005 [63]. In 2020, the hospitalization rate for AKI was 6.2%, a 7% decrease compared with 2019; however, the hospitalization rate among those on Medicare was 34% [139]. Acute kidney injury rates are significantly associated with aging and with Black/African American race [63]. The most common causes of acute kidney injury vary according to pathophysiology, but possible etiologies include trauma, dehydration/volume depletion, tubulointerstitial disease, glomerulonephritis, and obstruction [53,80].
The usual clinical presentation of acute kidney injury includes signs and symptoms of accumulation of nitrogen in the blood, such as fatigue, headache, anorexia, nausea, and vomiting. Potassium imbalances can result in tachycardia. Patients at highest risk for prerenal acute kidney injury have pre-existing chronic kidney disease, have recently undergone surgery, been exposed to radiocontrast dye, have received aminoglycoside antibiotics, or have developed sepsis [80]. Postrenal acute kidney injury is associated with flank or abdominal pain and possibly neurologic symptoms. Most patients with acute kidney injury have identifiable risk factors, such as chronic kidney disease, advanced age, liver disease, diabetes, or vascular disease [80]. Therefore, it is possible and essential to identify those at high risk before acute kidney injury develops in order to minimize damage.
A neurogenic bladder occurs in patients whose normal neural innervation of bladder contraction is interrupted. The result may be sensory disruption, motor disruption, or both. A neurogenic bladder may have a variety of causes. Diabetes-related autonomic neuropathy may result in a sensory deficit. Neurologic disease (e.g., multiple sclerosis, amyotrophic lateral sclerosis), spinal cord injury, or central nervous system malignancy may also result in a neurogenic bladder [81,82].
Patients with lower motor neuron neurogenic bladder have lost the perception of bladder fullness and do not experience a desire to urinate. As a result, overflow incontinence occurs when bladder capacity is exceeded. The distended bladder may be palpated and percussed. Patients with upper motor neuron neurogenic bladder experience spontaneous voiding when the bladder is stimulated. However, the bladder is hyperirritable, and voiding is not complete [84].
Patients with neurogenic bladder are more susceptible to the development of urinary tract infection as a result of ineffective bladder emptying and catheterization. In turn, repeated infections increase the risk for chronic renal failure. Urinary tract obstruction from struvite stones (a type of renal calculi) is more likely in patients with neurogenic bladder [84].
Parasympathomimetic agents (e.g., bethanechol chloride, neostigmine) may be prescribed for patients with a lower motor neuron neurogenic bladder to improve contraction of the detrusor muscle. For patients with upper motor neuron neurogenic bladder, parasympatholytics (e.g., propantheline bromide, methantheline bromide) may be indicated. Muscle relaxants (e.g., diazepam) may reduce skeletal muscle spasms. In addition to pharmacologic agents, voiding or catheterization programs may be prescribed [84].
Emotional support and educational interventions are important nursing measures for patients with neurogenic bladder. Patients may be instructed to perform the Credé maneuver to promote bladder emptying. This consists of placing the palm of the hand on the lower part of the abdomen, over the bladder. The palm of the hand is flattened, gradually increasing pressure as the palm is rotated [28].
Intermittent self-catheterization may also be necessary. A clean (rather than aseptic) technique is adequate and should not result in an increased risk of urinary tract infection. Catheterization is generally required every four hours. Limiting fluid intake after 6 p.m. will help minimize problems with nocturnal incontinence or the need for awakening at night for self-catheterization [28].
Renal and urinary infections are a common clinical problem and may occur anywhere in the urinary tract.
Cystitis, or inflammation of the urinary bladder, is usually the result of bacterial contamination; however, it may also be the result of fungal infection or fibrosis of the bladder wall. Bladder calculi, urinary diverticulum, or an indwelling urethral or suprapubic catheter will increase the likelihood of cystitis. Because of the relatively short female urethra and its proximity to the rectum and vagina, women are much more susceptible to cystitis than men. In healthy subjects, bacteriuria increases following sexual intercourse; however, this increase is transient [24,81,82].
Interstitial cystitis (also known as painful bladder syndrome) is a condition also characterized by painful bladder and urinary frequency and urgency. This is a chronic condition, and the diagnosis is one of exclusion; there are no effective diagnostic tests. The cause of the condition is not clear, but it is not believed to be related to infection. Instead, the pathogenesis is believed to be multifactorial, with immune system and neurogenic involvement [140].
Clinical Manifestations
Cystitis is characterized by a burning discomfort upon urination. Frequency, urgency, nocturia, bladder spasms, or incontinence may also be present. The urine may be cloudy or cola-colored from the presence of white and/or red blood cells. Other symptoms may include fever, fatigue, and pelvic and abdominal discomfort. When cystitis is associated with urinary tract obstruction at the bladder neck, the patient may have symptoms of urinary obstruction and/or uremia [24,81,82].
Therapeutic Measures
If cystitis has resulted in obstruction or acute renal failure, the obstruction should be removed immediately. Failure to remove the obstruction and treat the infection can result in permanent renal damage [24,81,82]. Antibiotics effective in eradicating the bacteria causing cystitis will be prescribed.
Patients with suspected acute cystitis caused by bacterial infection should be assessed with a urine dip or urine microscopic test. If the urine dip indicates possible bacterial contamination, the urine culture should be sent to obtain the sensitivity report. The antibiogram will detect the most susceptible and resistant medications for treatment. Prescribing prior to receiving the antibiogram can result in misdiagnosis or under- or overtreatment. The antibiogram will also allow prescribers to treat based on resistance patterns and mitigate the chances of antibiotic resistance [141].
Patients with cystitis should have a liberal fluid intake of at least 3 L/day. Patients should be encouraged to void frequently, even if it is uncomfortable, to help flush contaminating micro-organisms. Surgery to remove obstructions is rare but may be indicated [24,81,82].
Specific Nursing Measures
Nursing interventions for the care of patients with cystitis are supportive and educational. Phenazopyridine may be taken for the first two to three days to lessen pain and bladder spasms. This drug colors the urine red or orange and may stain fabrics. Warm compresses or sitz baths may help patients achieve adequate relaxation for natural micturition. Urethral catheterization should be avoided whenever possible, because additional contamination may result [27,28,83].
Patients should receive education on the prevention of future infections. Moisture around the urethral meatus provides a medium for enhanced bacterial growth and increases the risk for an ascending urinary tract infection. Those who utilize panty-liners for stress incontinence protection should be advised to change the liner frequently throughout the day, not just when they feel they have been incontinent. Cotton underwear is less likely to trap moisture than synthetic fabrics. Acidification of the urine can inhibit bacterial growth and is achieved through drinking cranberry or prune juice. Conversely, urine may become alkaline through the ingestion of tomato, orange, grapefruit, or apple juices [27,28,83,85].
Pyelonephritis is inflammation of the renal pelvis. Acute pyelonephritis is the result of bacterial invasion of the renal pelvis and medulla—usually an infection that has ascended from the lower urinary tract. A major cause of chronic pyelonephritis is believed to be ureterovesical reflux, in which infected urine ascends into the ureter and, consequently, the renal pelvis due to inadequate closure of the ureterovesical valve during voiding [24,81,82,86].
Acute Pyelonephritis
Patients with acute pyelonephritis generally present with fever, chills, nausea, and vomiting. Unilateral or bilateral severe pain or constant dull aching over the kidney in the flank area may be present. Urine of these patients will contain white blood cells [24,86].
Appropriate antibiotics should be administered, with follow-up cultures to ensure that the urine is sterile. Antispasmodics, rest, nutrition, and adequate fluid intake are necessary for the patient's condition to improve [84,86,87].
Chronic Pyelonephritis
Unless there is an acute episode, patients are generally unaware of chronic pyelonephritis. If symptoms are present, they are typically bladder irritability, chronic fatigue, or a slight aching over one or both kidneys. Eventually, patients will develop hypertension and the kidneys will atrophy [24,86].
In addition to antibiotics to treat the suspected bacterial infection, agents that suppress new infections are generally prescribed. The major goal of medical management of chronic pyelonephritis is to prevent farther damage to the renal parenchyma. If ESRD develops, dialysis or transplantation will be necessary [84,86,87].
A renal abscess is an infection that develops within the kidney. Single or multiple sites of bacterial abscess may be present. Renal abscesses are most common in patients with a history of pyelonephritis, chronic obstruction, or calculous disease [84,86,87].
Clinical Manifestations
The patient usually has pain in the costovertebral angle, fever, and chills. Physical examination may reveal edema and a palpable mass.
Therapeutic Measures
Antibiotics should be given immediately. If the infection is associated with chronic pyelonephritis, a broad-spectrum antibiotic should be selected and administered, typically intravenously with stepdown to oral as appropriate. If severe bacteremia develops, sepsis and shock may follow. Surgical drainage of the abscess may be necessary, and partial or complete nephrectomy may be required if significant renal deterioration has occurred [23,24].
Specific Nursing Measures
Patients with renal abscess are acutely and potentially critically ill. Administration of the prescribed antibiotics is a priority. Nursing interventions also include careful monitoring of vital signs, including temperature. Changes in level of consciousness and/or blood pressure may signify systemic infection. Measurement of intake, output, and daily weight should be part of the nursing care plan [27,28,83].
In general, nursing interventions for patients with kidney or urinary tract cancers include providing comfort; providing emotional support during the diagnostic, therapeutic, and convalescent periods; and educating the patient in self-care and decision making. Patients should also be regularly assessed for potential complications related to cancer or therapeutic interventions (e.g., infection, urinary tract obstruction, acute renal failure) [18,86,88].
In 2020, 73,750 new cases of kidney cancer (45,520 in men and 28,230 in women) will be diagnosed [89]. The average age at diagnosis is 64 years; appearance in adults younger than 45 years of age is rare. Diagnosis at younger ages is often incidental to other testing. Risk factors include smoking, hypertension, obesity, and hepatitis C. Because the malignancy frequently grows for months before detection, the tumor may be quite large at initial presentation. Metastasis is relatively common, and the most common sites are the liver, lungs, long bones, and other kidney [84,87,90].
Clinical Manifestations
When symptoms are present, gross hematuria is the most common manifestation of renal cell carcinoma. Dull flank pain may be present, but this is generally a late symptom that develops as the tumor enlarges and presses upon adjacent structures. Occasionally, the patient may identify a mass in the flank region, but this is rare. Other manifestations may include nausea or vomiting, which result from the displacement of abdominal contents. Metastatic symptoms include weakness, weight loss, and bone pain [84,87,90].
Therapeutic Measures
Surgery is the primary treatment of renal cell carcinoma, and the surgical procedure of choice is a nephrectomy. In some cases, especially if the cancer has metastasized, immunotherapy and/or chemotherapy may be necessary.
Bladder malignancies are the most common tumors of the genitourinary tract, with the exception of prostatic tumors. An estimated 81,400 new cases of bladder cancer (62,100 in men and 19,300 in women) will be diagnosed in 2020 [91]. Bladder tumors commonly involve the ureteral orifices or bladder neck. Metastases are common [84,87,90].
Considerable evidence shows that exposure to certain compounds is associated with an increased incidence of bladder cancer. For example, there is a well-established link between prolonged exposure to industrial compounds (e.g., aniline dyes) and the development of bladder cancer. Tryptophan and the tars of tobacco smoking have been strongly linked with bladder cancer [84,87,90]. An estimated 50% of all cases of bladder cancer are found in smokers.
Clinical Manifestations
Painless hematuria is the primary clinical manifestation of bladder cancer. The hematuria is typically intermittent, so it may be ignored initially. Obstruction of the urinary tract may alter the outflow of urine, causing intermittent anuria/polyuria, a decrease in the force or volume of the urinary stream, and potentially bladder distention. Infection may cause symptoms of dysuria, such as burning, frequency, or urgency [84,87,90].
Therapeutic Measures
Treatment of nonmuscle-invasive bladder cancers is based on risk stratification. Essentially all patients are initially treated with a transurethral resection of the bladder tumor followed by a single immediate instillation of intravesical chemotherapy (usually mitomycin C) [92].
Standard treatment for patients with muscle-invasive bladder cancers whose goal is cure is either neoadjuvant multiagent cisplatin-based chemotherapy followed by radical cystectomy and urinary diversion or radiation therapy with concomitant chemotherapy [92].
Obstructive disorders of the kidneys and urinary tract are fairly common in adults. The most common nonmalignant obstructive disorders result from the formation of calculi or the effects of their obstruction or from hyperplasia of prostatic tissue (benign prostatic hyperplasia) [18,86,88].
Nephrolithiasis and urolithiasis refer to the presence of calculi (stones) in the urinary tract. Calculi in the urinary tract are a relatively common problem, especially for men, and they often occur within the same family, indicating a possible heritable factor. There is also a high incidence of recurrence, with as many as 40% of patients reporting a recurrence within two years. Renal calculi are extremely painful, and individuals are incapacitated during an acute episode. When associated with infection or obstruction, renal calculi may be life-threatening [18,86].
Most calculi originate in the renal parenchyma (nephrolithiasis) and are passed out into the ureters or bladder (urolithiasis). Calculi are usually composed of calcium salts (calcium oxalate or phosphate, uric acid, or struvite [magnesium ammonium phosphate]). A number of factors contribute to the formation of calculi, primarily the degree to which the urine is supersaturated with a normally excreted element, the pH of the urine, the presence of substances that inhibit the formation of crystals, the stasis of urine, and the pre-existing environment [18; 86].
Immobilization, primary hyperparathyroidism, hypervitaminosis D, renal tubular acidosis, and dietary excess of foods containing calcium or purine may result in hypercalciuria. Medications or supplements taken in excess, such as antacids or vitamin C, may also result in hypercalciuria or excess calcium oxalate. However, most cases of calculus formation are idiopathic [93,94,95].
With infection and the consumption of some foods/beverages, the urine may become slightly alkaline or acidic. Uric acid and cystine calculi will form in acidic urine; calcium phosphate calculi will dissolve in acidic urine; and calcium oxalate calculi are not influenced by the pH of the urine. Urea-splitting bacteria (e.g., Proteus, Xanthomonas, Pseudomonas, Klebsiella, Staphylococcus, Mycoplasma) convert urea to ammonia, and the alkaline urine contributes to the formation of struvite calculi. Calculus formation is enhanced when there is urinary tract scarring (either from infection or surgical procedures in the urinary tract) or when urinary stasis or urinary crystallization is present [93,94,95].
Clinical Manifestations
The primary clinical manifestation of nephrolithiasis/urolithiasis is pain, and its location may suggest the location of the calculus. In some situations, there may be no discomfort at all. However, if the calculus descends into the ureters, the patient may describe the pain as excruciatingly severe (i.e., 10+ on a 10-point scale). The ureteropelvic junction, the point at which the ureter crosses anterior to the iliac vessels, and the ureterovesical junction—the narrowest points in the ureters—are the sites at which calculi are most likely to become lodged. Severe intermittent pain results as the musculature of the ureters goes into spasm (colic) in an attempt to move the calculus out of the ureters by peristalsis. The pain of urethral colic may traverse along the route of the ureter and extend into the lower abdomen, the vulva, or the testes. Patients commonly experience nausea and vomiting. They may also report blood in the urine and, if infection is present, chills and fever [93,94,95].
Therapeutic Measures
Opioids and NSAIDs are generally necessary to relieve pain associated with renal or urinary calculi; NSAIDs (e.g., ketorolac) are associated with fewer adverse effects. If analgesia is insufficient for pain management, referral to the emergency department with urology consultation is warranted [142]. Antispasmodics may also be prescribed to relax the urethral musculature. If infection is present or if instrumentation is necessary to remove the calculus, antibiotics will be prescribed. Other agents may be prescribed to alter the urinary pH to create the desired environment (e.g., sodium acid phosphate, potassium acid phosphates, or ascorbic acid to promote the formation of acidic urine). Thiazide diuretics increase calcium reabsorption and thus may be prescribed for patients who form calcium calculi. However, these diuretics may cause hyperuricemia, so agents to manage excess uric acid (e.g., allopurinol) may also be necessary. For patients who require more alkaline urine, 50% sodium citrate may be prescribed [93,94,95]. Other considerations for referral to the emergency department include suspected sepsis, anuria, or obstruction (particularly in those with a history of nephrectomy). When immediate referral is not indicated from exam, the provider should evaluate the urinalysis and culture, if necessary, to rule out infection. Non–contrast-enhanced computed tomography (CT) of the abdomen and pelvis has superior sensitivity and specificity and is commonly performed in the emergency department for concerns of renal calculi; it can also be ordered outpatient, if needed. In the outpatient setting, ultrasonography has acceptable performance and is more cost-effective [142]. Patients with a history of calculi should focus on producing high-volume, dilute urine. This may be achieved by consuming 4 L fluid (preferably water) per day. It is also important to void before bedtime and to awaken in the night to empty the bladder. When awakened, the patient should drink more water to ensure a constant dilution of urine [93,94,95].
Dietary modifications will depend on the content of the calculi. A low-calcium diet is only recommended for patients with active hypercalciuria. For patients who should limit calcium oxalate, foods to avoid include tea, cola, spinach, and citrus fruits.
Patients who are hyperuricosuric and form uric acid calculi may benefit from a diet low in purines. This means limiting intake of meat, fish, poultry, beer, and wine. Struvite calculi contain phosphates; therefore, a diet that limits phosphorus (from seafood, chicken, pork, nuts, dairy, and grains) may be indicated. Medications that alter urine composition are often more effective than dietary modification [25].
Patients with calculi too large to pass spontaneously may be candidates for extracorporeal shock wave lithotripsy. In this treatment, the patient is supported and suspended in lukewarm water or on a cushion or membrane. An extracorporeal shock wave lithotripter sends shock waves to the calculus, which shatters and is excreted within several days [93,94,95]. This procedure generally takes one hour and requires thousands of waves to be sent to the site of the calculi.
In percutaneous nephrolithotomy, a small incision is made and a scope is introduced to visualize the calculus. The stone(s) can then be pulverized with ultrasound vibration and flushed.
In some cases, the calculus may be retained and additional treatment necessary. A variety of surgical procedures may be used to remove the calculus; the method selected depends somewhat on its location [93,94,95].
Specific Nursing Measures
Care of the patient with renal or urinary calculi is primarily focused on comfort and the prevention of infection. In addition to measuring intake and output carefully, urine should be strained through fine mesh in order to obtain a calculus or calculus fragment that may be analyzed to determine its chemical characteristics [27,28,83].
Initially, patients may require IV administration of morphine to obtain pain relief. Antibiotics should be administered as prescribed. A high fluid intake (3–4 L/day) is usually recommended for the remainder of the patient's lifetime [25,36,52].
Direct or indirect injury to the organs of the urinary system can interrupt the structural integrity of the kidney(s), the ureter(s), and/or the bladder. A major interruption of vascular supply may occur concomitantly. Traumatic injuries are commonly classified as either penetrating or nonpenetrating (blunt) [93,94,95,96]. Abdominal gunshot or stab wounds are the most common sources of penetrating trauma. Automobile accidents, automobile-pedestrian accidents, motorcycle accidents, assault, and injuries from sporting events may result in blunt trauma [93,94,95,96].
Injuries to the renal system may significantly affect patients' overall health. Preservation of renal function is the primary goal. Loss of a single kidney from trauma may not be life-threatening, assuming the other kidney is functioning adequately, but it can have serious consequences. Patients who experience renal trauma are often victims of other abdominal trauma as well [93,94,95,96].
Obtaining a careful patient history is often the most important part of diagnosis. Gunshot or stab wounds are obvious, but the history may disclose less obvious traumatic events. Physical sports involving heavy direct contact, such as football or ice hockey, may result in injuries that did not seem significant at the time. A history of rib fractures, chest injuries, or abdominal injuries suggests the potential for renal trauma.
Physical assessment includes the collection of a urine specimen to determine the presence of blood. Flank pain or suprapubic tenderness may or may not be present. Alterations in urinary output and patterns of voiding are important [93,94,95,96].
Renal trauma is generally categorized as minor, major, or critical. Minor injuries include contusions, hematomas, and simple lacerations. Major injuries involve significant laceration of the renal parenchyma, loss of the renal parenchyma, and injury to a major branch of the areal artery. Critical injuries involve lacerations of the renal artery, renal vein, or renal pelvis. A transplanted kidney is not as well protected from injury, so abdominal injuries to transplant recipients may result in significant traumas. Other abdominal organ trauma should be explored in patients with known or suspected renal trauma [23,93,94,95,96].
Clinical Manifestations
Gross or microscopic hematuria, flank pain, and abdominal pain are the most common manifestations of renal trauma. A significant hematoma may develop in the retroperitoneal space. Blood clots that form and descend into the ureter may result in pain that mimics renal colic and obstruction from nephrolithiasis/urolithiasis [88].
Therapeutic Measures
Patients with renal trauma should undergo appropriate radiologic testing to evaluate the extent of traumatic injury; abdominal ultrasonography, CT scan, and renal arteriography are among the most useful tests. Significant hemorrhage occurs with renal pedicle injuries; therefore, blood, volume expanders, and fluid are administered parenterally to support blood pressure and perfusion of vital organs. Surgical intervention is required to control hemorrhage, preserve renal function, and prevent death [88].
For nonpenetrating and minor renal injuries, a conservative approach is generally indicated, including bed rest, monitoring serial laboratory data (e.g., hemoglobin, hematocrit, serum creatinine, BUN), and evaluation of fluid balance. Minor bleeding ceases from the tamponade effect within the kidney capsule [88].
Penetrating injuries, in contrast, necessitate surgical exploration to ensure that bleeding is controlled and other tissue tears, especially intraperitoneal lacerations, are repaired. Nephrectomy, partial nephrectomy, and renal bench surgery with auto-transplantation may be required. Antibiotics are generally prescribed for any penetrating traumas. Perforating wounds often perforate the peritoneum and abdominal organs and cause a significant amount of pain [88]. Dosages of any medications prescribed should be adjusted for renal dysfunction, if necessary.
Specific Nursing Measures
Urine output and vital signs should be monitored. Patients should be assessed for hypovolemic shock that may occur secondary to hemorrhage or peritonitis. The administration of food, fluids, volume expanders, antibiotics, and education for pain relief is a primary nursing responsibility. If surgery is done to control hemorrhage or repair the kidney, preoperative and postoperative nursing care will be necessary [83].
As with renal trauma, penetrating (e.g., gunshot, stab wounds) or nonpenetrating (e.g., automobile injuries, automobile-pedestrian accidents) injuries may result in bladder trauma. A full bladder is at increased risk of injury from perforation during a traumatic or compression injury. Keeping the bladder non-distended reduces the risk of injury from seat belt use—it should not be used as justification to avoid seat belts [88,96].
Clinical Manifestations
Manifestations of bladder injury include hematuria, difficulty with voiding, or absence of urine output. Suprapubic pain or tenderness is common, as is scrotal or perineal swelling from the extravasation of urine into these tissues [88,94,95,96].
Therapeutic Measures
The immediate recognition and treatment/repair of bladder rupture are vital to avoiding death. For bladder contusions, however, conservative treatment (e.g., rest, drainage via a Foley catheter) is indicated. Suspected bladder tears or lacerations require surgical exploration and potentially repair. Antibiotics are usually required with leakage of urine into the peritoneum or other tissues [88,94,95,96].
Specific Nursing Measures
Nurses should be vigilant of symptoms that may identify previously undetected bladder rupture; for example, persistent, unidentified fever may result from leakage of urine into the abdominal cavity.
Intake and output should be monitored to ensure that fluid balance is achieved. Alterations in urinary output should be detected as soon as possible, so corrective measures may be initiated. Complications associated with stasis of pulmonary secretions or venous congestion in the extremities are possible but may be avoided with appropriate nursing measures. In addition, appropriate health teaching and emotional support should be provided [83].
There are two major types of dialysis currently in use in the United States: hemodialysis and peritoneal dialysis. Though generally used for patients with ESRD, it may also be a short-term option in the treatment of patients with acute kidney injury or in post-transplant patients with delayed graft function. It is estimated that nearly 460,000 patients are receiving hemodialysis in the United States, an increase of 80.2% since 2000 [63]. It is estimated that 786,000 individuals are living with ESRD, with 71% of those on dialysis [143]. Despite this large number, dialysis is a relatively new therapy and has only been routinely provided for approximately 40 years [55].
Planning for access should occur well before the need for dialysis, as proper access may take months to properly heal. Up to 80% of patients presenting for initial dialysis are dialyzed via temporary venous catheters due to a lack of established access [63].
Hemodialysis can be provided via three major different types of access: an AV fistula, an AV graft, or a temporary venous catheter. The National Kidney Foundation endorsed a goal for at least 65% of all patients on hemodialysis to have a working AV fistula by 2009, but this goal was not met [97]. As of 2017, reporting institutions dialyzed 62.8% of all patients on hemodialysis via AV fistulas [63].
AV fistulas are created surgically by attaching an artery directly to a vein. Generally placed in the forearm, AV fistulas may be categorized as radial-cephalic, brachial-cephalic, or brachial-basilic based on the vein and technique used [55,98]. Generally, the radial-cephalic, having a lower anatomical position in the forearm, is preferred for first access as it preserves the higher veins for later use. After anastomosis, the resultant increase in blood flow to the vein results in thickening of the venous wall, allowing it to withstand the numerous punctures required for hemodialysis [98]. AV fistulas generally require at least three months to fully mature. AV fistulas are recommended as the first form of access, and they should be promoted in all eligible patients who choose hemodialysis, as they improve outcomes and reduce costs compared with central venous catheters [99]. They also offer the best access for longevity and have the lowest association with morbidity and mortality [100]. However, multiple studies suggest that certain subgroups of patients (i.e., the elderly and those with limited life-expectancy) may benefit from alternative forms of access. A patient-centered, individualized approach to the choice of access may indicate the use of a method other than AV fistula [101,102].
AV fistulas are not without complications, and the overall patency rate is only 50% after five years [98]. Fistula failure can be classified as early (in the first three months) or late (after three months) [98]. Early failure is generally due to infection, stenosis, or obstruction (either of inflow or outflow). Late failure is generally due to either venous stenosis or arterial lesions, with venous stenosis being the most common cause of late AV fistula failure [55,98]. Steal syndrome is another possible complication of AV fistulas and results in too high of a diversion of blood flow from the extremity distal to the fistula. Patients with steal syndrome may present with signs and symptoms of decreased blood flow to the affected extremity, particularly in the digits of the hand. Treatment of this complication includes the takedown of the fistula or placement of coils in the fistula to decrease diversion. Patients deemed at risk for imminent limb or digit loss should be referred immediately to the emergency department for evaluation by a vascular surgeon.
Fistula patency can be assessed by palpation of "thrill" and auscultation of bruit. A palpable motion in the surface of the fistula and a bruit when a stethoscope is placed over the fistula should both be present. The bruit should be loudest at the arterial side of the fistula. Patients found to have nonpatent grafts should be referred immediately to interventional radiology, as patency can often be restored by the use of antithrombotics (e.g., tissue plasminogen activators) and/or balloon angioplasty. Unfortunately, the use of contrast medium is usually necessary in order to restore patency. In these cases, the patient should be dialyzed within 24 hours of receiving the contrast. If the patient is to receive dialysis immediately after angioplasty, catheters may be left in place so it is not necessary to recannulate immediately after the procedure. If the patient is to return the following day for dialysis, temporary catheters should be removed to prevent potentially deadly hemorrhage. At no point should any patient with ESRD receive gadolinium-based contrast dye due to the risk of nephrogenic systemic fibrosis. Postexposure dialysis does not decrease the risk of developing nephrogenic systemic fibrosis in at-risk patients.
AV grafts are generally considered to be inferior to AV fistulas and superior to temporary catheters, but fewer than half are patent after five years [103]. These grafts are often used for patients who lack veins large enough to create fistulas. The determination of need for graft placement versus fistula is accomplished via vein mapping and clinical evaluation.
AV graft formation is accomplished by implantation of a synthetic tube that connects a vein and an artery. Dialysis is then performed by cannulating the synthetic graft. Synthetic grafts have higher rates of infection and clotting and, as noted, generally fail sooner than fistulas [104].
Both AV fistulas and grafts should be cannulated only by trained practitioners. Repeated same-site cannulation can result in destruction of grafts, aneurisms, and/or infiltrates. Although not all these complications are entirely preventable, trained inserters can minimize their occurrence.
Temporary catheters are the least preferred method of access for hemodialysis, but one of the most frequently used. However, according to data from the Centers for Medicare and Medicaid Services, catheter use in the United States declined slightly from approximately 28% in 2006 to 24% in 2007 [105]. Central venous catheters for dialysis (CVCDs) are generally tunneled catheters with two external access ports ("pigtails") and a tunneled lumen under the skin that extends into the vena cava; they may last up to one year. The introduction of advanced CVCDs has helped make temporary catheters a viable solution for patients with very poor vascular status. Patients often prefer the temporary catheters to other forms of access, as they do not involve the pain of subcutaneous cannulation and are often cosmetically more acceptable. Using a tunneled catheter helps reduce the risk of infection but does not eliminate it.
The peritoneal catheter is the only option for peritoneal dialysis. The invention of the Tenckhoff catheter in 1968 helped reduce high rates of peritonitis associated with peritoneal dialysis. It is made of silicone rubber and Dacron cuffs and can be coiled or straight. Compared with coiled cuffs, straight cuffs have higher catheter survival and fewer complications [106]. Coiled catheter use has been associated with lower rates of failure due to outward migration of the catheter [107,108]. Most catheters in use today have two cuffs: one in the musculature of the abdominal wall and one in the subcutaneous tissue nearer the exit. Placement is generally performed operatively and can now be accomplished laparoscopically. Other methods, such as blind insertion via Tenckhoff trocar or guidewire, are available.
After insertion, approximately 7 inches of catheter extend beyond the surface. The catheter will usually have an external suture in place for the first two weeks after implantation. An external flow switch that is capped when not in use is present at the end of the catheter.
Hemodialysis is a risk factor for a variety of complications, and patients must be closely monitored. Mortality rates for patients with ESRD are high in part due to uncontrolled complications associated with dialysis.
Patients on hemodialysis often experience hypotension during and after dialysis sessions. A fluid gain of 4 or more liters between sessions is not uncommon, and this same amount of fluid must then be removed over a four-hour dialysis session. Most of this fluid is situated in the extravascular space, and dialysis removes fluid only from the intravascular space. So, changes in osmotic pressure between the intravascular and extravascular must allow for fluid movement during dialysis. If the patient becomes acutely hypotensive, the dialysis may need to be interrupted and the patient placed in Trendelenburg (supine) position with elevated feet. Rarely, fluid replacement may be required.
Cramping is also common, especially at the end of the dialysis session after large amounts of fluid and electrolytes have been removed. Severe cramping may require administration of normal saline. For patients who are significantly bothered by cramping, hydromorphone (Dilaudid) 1–2 mg orally may be given one hour before the usual onset of cramping. Hydromorphone has the advantage of not being dialyzed out of the patient's system.
Renal transplantation involves the surgical implantation of a kidney from either a living or deceased donor into the body of a patient with ESRD. Of the 786,000 persons in the United States with ESRD, 29% have had a kidney transplant [142]. Generally, candidates must have GFRs less than 20 mL/min/1.73 m2. Transplants are classified as living donor related, living donor unrelated, or deceased (cadaveric) donor. The first truly successful human kidney transplant was in 1954 by Dr. Joseph Murray at Brigham Hospital in Boston. This first transplant involved identical twins, as anti-rejection medications had yet to be discovered. In the 1960s, tissue typing and anti-rejection drugs made the use of deceased donor kidneys and non-twin living donors possible.
In 1980, more than 3,000 kidney transplants were performed annually. By 2013, this figure had risen to more than 17,600 [109]. Fewer than one-third of transplanted kidneys were from living donors in 2013. From 2012 to 2013, there was a 3.1% increase in the cumulative number of recipients with a functioning kidney transplant [109]. According to the United Network for Organ Sharing, total kidney transplants exceeded 25,000 in 2022 [144].
The benefits of renal transplantation should not be underestimated. The survival of transplant patients is far higher than patients receiving dialysis. Some of this may be attributable to patient selection; healthier patients are selected for transplantation and terminally ill patients are rarely selected. Still, the statistics are impressive. At 80.5%, the five-year survival rate for transplant patients is significantly greater than the rate for patients receiving dialysis (47.1%) [63]. However, patient survival is not the same as donor graft survival. For the most recent years of data available, the probability of graft survival for living-donor transplants was 99%, 92%, and 79%, for 1-, 5-, and 10-year periods post-transplant, respectively [109].
The indication for transplantation is ESRD, or a GFR of <15 mL/min/1.73 m2 [57]. In recent years, the process for selection of a kidney transplant has liberalized, with larger numbers of elderly patients being accepted for transplantation. In 1991, 30% of kidney transplant recipients were older than 50 years of age; by 2016, this number had increased to more than 70% [63]. Transplant counts for recipients 65 years of age and older have been steadily increasing [63]. Only 3% of individuals older than 65 years of age on the waiting list received a kidney in 2017 [145].
One study assessed transplantation rates among 4,445 patients with a mean age of 52.2 years [146]. The survival time with transplant for all ages within a 10-year follow-up period was increased with transplantation versus dialysis only [146].
Guidelines for selection of patients for transplantation vary from program to program. Generally, metastatic disease or severe pulmonary or heart disease are exclusionary factors. Human immunodeficiency virus (HIV) is no longer considered an absolute contraindication, and studies have shown that patients with ESRD and HIV nephropathy have an increased life expectancy when treated with transplantation versus dialysis [110].
In January 2023, the Board of Directors of the Organ Procurement and Transplantation Network (OPTN) updated its policy on the use of eGFR to evaluate kidney function in candidates, increasing the cutpoint by approximately 16%. This update is meant to better reflect kidney function in Black/African American kidney candidates who were previously excluded [146].
Allocation of all transplants in the United States is managed by the UNOS. Kidney transplant guidelines place the highest considerations on histocompatibility and time spent on the transplant list [111]. Children suffering from ESRD will lose growth (and possibly other milestones) while on dialysis, so they are given priority. The median adult wait time for a cadaver kidney is four years [63].
One result of this method of allocation is a potential mismatch between grafts and patients. If a patient with a life expectancy of 10 to 20 years receives a kidney from a donor who is/was 20 years of age (with a potential for 50 to 60 years of function), the transplanted kidney will potentially be underutilized. Worse, if a patient with a 40- to 50-year life expectancy receives a kidney from an older donor, he or she will likely outlive the donated kidney. In fact, approximately 15% of all patients awaiting transplantation have already received at least one transplant [63].
To address these problems and others, in 2011, the UNOS released a concept document that proposes changes to the allocation system in order to more accurately match donors and recipients [112]. While this proposal has been met with some controversy, UNOS believes that it could actually increase the number of functioning years obtained from the current pool of donated kidneys. This gain would occur both from increased age matching as well as from eliminating the current designation of "expanded donor criteria" kidneys. These kidneys do not meet all donation criteria, either due to existing disease in the graft or risk factors of the donor. An estimated 12% of all donated kidneys are not used because they are classified as expanded donor criteria kidneys, meaning they have an increased potential for rejection [112].
A 2020 study reviewed data on kidneys discarded in the United States between 2015 and 2016. These kidneys underwent a "procurement biopsy" and were rejected based on abnormalities noted in the tissue. During this time, 1,103 kidneys were rejected; of these, 493 would have been matchable based on French criteria, which are less restrictive. The 493 kidneys that passed the French criteria had a one-year survival rate of 93%, a five-year survival rate of 81%, and a 10-year rate of 69%. This suggests that many donor kidneys in the United States are being discarded unnecessarily and may be worsening the already-severe deficiency of transplant-eligible kidneys [147]. An elderly recipient may be better off receiving a kidney with only 10 to 15 years of expected graft survival, as they are unlikely to live long without a transplant.
Transplanted kidneys are generally placed in the extraperitoneal space in the right iliac fossa. Pediatric patients can have their grafts placed in the intraperitoneal space. The major consideration for placement of the graft is surgical access to the renal arteries, renal veins, and the ureter. Failed kidneys are rarely removed unless infection or carcinoma is present. The donor's renal artery is usually anastomosed to the recipient internal iliac artery, and the donor renal vein is connected to the recipient external iliac vein. Lastly, the donor ureter is attached to the recipient bladder.
Immediate postoperative care of the patient post-transplant involves all the usual issues involved in major surgery (e.g., bleeding, pain management, bowel function, infection, postoperative cardiac or pulmonary complications) as well as issues specific to kidney transplantation. These issues include graft function, acute rejection, urine leakage from the ureter anastomoses, and complications from immunosuppression. Acute rejection is not nearly the problem it was prior to the development of tacrolimus and cyclosporine, although chronic rejection remains a problem in long-term graft survival.
After transplantation, the graft may function immediately, have delayed graft function, or have complete non-function. Delayed graft function is a common problem in transplants, affecting up to 23% of cadaver transplants and 6% of living transplants [113]. The delay may last from days to weeks and may require temporary dialysis. Complete graft non-function, by definition, does not resolve and requires a return to dialysis.
An end-of-life discussion is recommended in the presence of stage 4 or 5 chronic kidney disease or ESRD [114,115,116]. Hospice is generally approved when patients with ESRD are not candidates for dialysis, have a creatinine clearance less than 15 mL/minute, and/or have a serum creatinine level greater than 8 mg/dL (or 6 mg/dL in patients with diabetes) [117]. Guidelines from the Renal Physicians Association note that prognosis should be fully discussed with all patients who have stage 4 or 5 disease or ESRD [115]. Clinicians should carefully prepare for the discussion of prognosis by reviewing the patient's medical record and talking to other healthcare professionals involved in the care of the patient [118]. Because there is variation among patients regarding their desire for information, clinicians should follow the "ask-tell-ask" approach: ask the patient if he or she is willing to discuss prognosis; if yes, discuss the prognosis and then ask the patient to confirm his or her understanding [114,119]. When discussing prognosis, quantitative estimates are more understandable for patients and families than qualitative ones (e.g., "poor"), and general timeframes for survival should be given [114,119,120,121]. In addition, clinicians should emphasize that prognosis is determined by looking at large groups of patients, and that it is harder to predict survival for an individual [114,115]. The discussion of prognosis is often not documented in the patient's record, but it should be [118].
Clinical guidelines have begun to address the use of aggressive treatment at the end of life. The Renal Physicians Association recommends forgoing dialysis for patients with chronic kidney disease or ESRD who have a "very poor prognosis" [115]. Early discussion of preferences for life-sustaining measures is especially important. Nearly three-quarters of people will be unable to participate in some or all of the decisions about their care at the end of life [122]. Documentation of preferences helps inform decision-making by the physician and the patient's healthcare proxy (surrogate decision maker). Clinicians should encourage their patients to designate a healthcare proxy early in the course of a life-limiting disease [74,114,123]. Patients should be urged to clarify their wishes with their chosen proxy, as a proxy often inaccurately predicts a patient's wishes or may have values that conflict with those of the patient [122].
With knowledge of renal structure and function and the dynamic pathology that intrudes and impedes normal function, nurses can readily provide quality and often life-saving care. The awareness of the pathophysiology underlying symptoms leads to quicker reporting and optimal patient care. Nurses can also perform immediate interventions based on standing orders and the recognition of what needs to be done in order to provide safe, quality care. This knowledge changes what could be only technical care to professional care through use of decision-making skills built upon the knowledge of pathophysiology.
Patient A, a man 27 years of age, presents to an outpatient clinic complaining of two days of facial and hand swelling. He first noticed swelling around his eyes, along with difficulty putting on his wedding ring because of swollen fingers. Around the same time, he began to notice that his urine appeared reddish-brown. He reports having less urine output over the past several days. He has no significant medical history. His only medication is ibuprofen, which he took more than one week ago for a fever and sore throat that have since resolved. On examination, he is afebrile, with a heart rate of 85 beats per minute and a blood pressure of 172/110 mm Hg. Patient A has periorbital edema; his funduscopic examination is normal, without arteriovenous nicking or papilledema. His chest is clear to auscultation. His heart rhythm is regular, with a nondisplaced point of maximal impulse, and he has no abdominal masses or bruits. The patient also has edema of his feet, hands, and face. A dipstick urinalysis in the clinic shows specific gravity of 1.025 with 3+ blood and 2+ protein but is otherwise negative. A fresh-spun urine specimen is positive for red blood cell casts.
The physician diagnoses acute (likely poststreptococcal) glomerulonephritis. This condition is most likely to affect children with a history of upper respiratory infection or a history of strep throat one to three weeks earlier, although adults may also be affected. Patients present with edema, hypervolemia, hypertension, hematuria, and oliguria. Red blood cell casts on urinalysis verify the diagnosis. Systemic lupus erythematosus is another possible cause, but this patient has no signs or symptoms indicating this etiology.
Treatment of poststreptococcal glomerulonephritis focuses on managing hypertension and edema. Patient A is placed on a low-salt diet and liquid intake is restricted. If the edema was more severe, loop diuretics would be prescribed. However, the edema begins to resolve, and his blood pressure begins to lower with conservative management. Additionally, Patient A is prescribed oral penicillin G at a dosage of 250 mg four times per day for 7 to 10 days to eradicate the nephritogenic strain. The patient is instructed to rest and avoid vigorous physical activity for a day or two.
Patient B, a man 40 years of age with no past medical history, presents to the clinic to establish care. He reports that he had a prior urinalysis that revealed blood as an incidental finding. The urinalysis was done as a standard screening test by his former employer. He denies ever seeing any blood in his urine and denies any voiding difficulties, dysuria, sexual dysfunction, or any history or risk factors for sexually transmitted infections. His review of systems is otherwise negative. He reports having smoked a half-pack of cigarettes per day for the past 10 years and jogging 15 minutes and lightweight training daily. On examination, his vital signs are normal, and the entire physical examination is unremarkable. A complete blood count and a chemistry panel (electrolytes, blood urea nitrogen, and creatinine) are normal. The results of the urinalysis are:
Specific gravity: 1.015
pH: 5.5
Leukocyte esterase: Negative
Nitrites: Negative
White blood cell count: 0
Red blood cell count: 4–5 per high-power field
The likely diagnosis is determined to be asymptomatic microscopic hematuria. Repeat urinalysis is ordered to assess for risk factors. A urine culture is necessary to rule out infection. Imaging studies are also ordered. The primary concern is to rule out malignancy, including renal cell carcinoma and transitional cell carcinoma. The nurse counsels Patient B on the importance of an appropriate work-up but reassures him that the prevalence of renal cancers is low. Because the patient is a smoker, bladder cancer should be ruled out (or identified) as soon as possible.
Patient C, a Hispanic woman 48 years of age, presents to a medical office complaining of persistent swelling of her feet and ankles, to the degree that she cannot put on her shoes. She first noted mild ankle swelling approximately two to three months previously. She borrowed some diuretic pills from a friend, and the pills seemed to help, but now she has run out. She also reports that she has gained 20 pounds over the last few months, despite regular exercise and trying to adhere to a healthy diet. Her medical history is significant for type 2 diabetes, for which she takes a sulfonylurea agent. She neither sees a physician regularly nor monitors her blood glucose at home. She denies dysuria, urinary frequency, or urgency, but she does report that her urine has appeared foamy. She has had no fever, joint pain, skin rashes, or gastrointestinal symptoms.
Her physical examination is significant for mild periorbital edema, multiple hard exudates, and dot hemorrhages on funduscopic examination. She also has pitting edema of her hands, feet, and legs. Her heart rhythm is regular without murmurs, and her abdominal examination is benign. She has diminished sensation to light touch in her feet and legs to mid-calf. A urine dipstick performed in the office shows 2+ glucose, 3+ protein, and negative leukocyte esterase, nitrates, and blood.
The presumed diagnosis is nephrotic syndrome secondary to diabetic nephropathy. A 24-hour urine collection is taken to measure proteinuria and to verify the diagnosis. Usual causes in adults include diabetes, hepatitis B, amyloidosis, lupus erythematosus, and drugs such as penicillamine and captopril.
Maintenance of glycosylated hemoglobin (HbA1c) to 7.0% or lower is recommended, and Patient C is started on diabetes care (with regular follow-up and screening). To address the nephrotic syndrome, an ACE inhibitor is prescribed to control her blood pressure. In order to meet her blood pressure targets more quickly, a diuretic is also prescribed. Patient C is instructed to limit her intake of fluids and protein (to less than 2 g/day). She is asked to return in several weeks for additional screening and to call if the edema does not resolve.
The emergency resident calls up a new admission, describing a female patient, 84 years of age. Patient D was brought to the emergency department by ambulance from her long-term care facility for increased confusion, combativeness, and fever. Her medical history is significant for Alzheimer disease and well-controlled hypertension; otherwise, she has been healthy. The resident states that the patient is "confused" and combative with staff, which, per her family, is not her baseline mental status. Her temperature is 100° F, heart rate is 130 beats per minute, blood pressure 76/32 mm Hg, respiratory rate 24 breaths per minute, and oxygen saturation is 95% on room air. On examination, Patient D is lethargic but agitated when disturbed. Her neck veins are flat, her lung fields are clear, and her heart rhythm is tachycardia but regular, with no murmur or gallops. Her abdominal examination is unremarkable, and her extremities are warm and pink.
After administration of 2 L of normal saline over 30 minutes, her blood pressure is 95/58 mm Hg. The initial laboratory work indicates a white blood cell count of 14,000/mm3, with 67% neutrophils, 3% bands, and 24% lymphocytes. No other abnormalities are noted. The chest x-ray obtained in the emergency department is normal. Urinalysis of a catheterized sample shows 2+ leukocyte esterase, negatives nitrite, and trace blood. Microscopy shows 20–50 white blood cells per high-power field, 0 to 3 red blood cells, and many bacteria.
Patient D is diagnosed with urinary tract infection that has progressed to sepsis and possibly shock. She has several risk factors that predispose her to urinary tract infection, including older age, female sex, cognitive dysfunction, and fecal incontinence. Blood pressure support is continued, with IV fluids or vasopressors as necessary. Broad-spectrum antibiotics are started immediately. As the infection clears, the patient's combativeness resolves as well.
Patient E is a man, 42 years of age, who has driven to the mountains to hunt. While tramping through thick woods, a stray bullet (perhaps from another hunter) hits Patient E's thigh, tearing into muscles and blood vessels. He is thrown to the ground and temporarily loses consciousness. When he awakens a few minutes later, his leg is bleeding profusely. He yells for help, but there is no reply.
The patient slows the bleeding somewhat by tying his shirt around his leg and manages, with great effort, to get to a rural roadside where he again loses consciousness. A driver finds him a few hours later, pulls him into his car, and takes him to the hospital.
When he arrives, Patient E is immediately treated for hypovolemic shock that resulted from extensive blood loss. He is taken to surgery, and his leg is repaired. Although the patient's blood pressure increases and his pulse rate decreases with appropriate fluid replacement and cessation of blood loss, his urine output remains low.
Two days after admission, Patient E's total urine output has been 320 mL, with an average specific gravity of 1.013. His blood pressure is 150/95 mm Hg (an increase from his previous average of 125/80 mm Hg). His pulse is 90 beats per minute, and his respirations are 18 breaths per minute. He is complaining of nausea, which seems unrelated to the administration of his pain medication. Significant blood tests reveal a BUN of 48 mg/dL and elevations in serum creatinine and potassium. Urinalysis results are consistent with renal failure findings. A diagnosis of acute renal failure secondary to prolonged hypovolemic shock is made. Patient E's physicians administer trial doses of mannitol and furosemide (Lasix) in an effort to produce diuresis. Because no increase in urine results from this therapy, further diuretics are not employed. However, his physicians hope to manage him conservatively and avoid dialysis, if possible.
Patient F is a White woman, 35 years of age, with a history of frequent urinary tract infections who now presents with gross hematuria. On physical exam, she is thin with a palpably enlarged right kidney. On questioning, she states that her mother had some sort of "cyst disease" of the kidney. She further states that her mother died of a heart attack several years ago, at 62 years of age. A renal ultrasound reveals numerous large, fluid-filled cysts on the right kidney and several cysts on the left kidney. Further imaging also reveals a fluid-filled cyst visible on the liver. A consultation with nephrology and a geneticist results in a diagnosis of autosomal dominant polycystic kidney disease (ADPKD).
ADPKD is the fourth leading cause and the leading genetic cause of ESRD, and the most common life-threatening hereditary disease in the United States. ADPKD occurs in approximately one of every 1,000 live births. Children of affected individuals have a 50% chance of inheriting the disorder.
Patients with ADPKD can present with flank pain, hematuria, and/or palpable kidneys. Individuals with a family history are considered to have ADPKD if ultrasound reveals two unilateral or bilateral cysts in patients 15 to 30 years of age, two or more cysts in each kidney for patients 30 to 59 years of age, or four or more cysts in each kidney in patients older than 60 years of age. For patients with no known genetic risks (either from family history or genetic testing), the diagnostic criteria are three or more unilateral or bilateral cysts in patients 15 to 39 years of age or two or more cysts in each kidney for patients 30 to 59 years of age.
While the predominant clinical feature of ADPKD is renal disease (50% of affected patients have ESRD by 60 years of age), extrarenal manifestations are also common, which suggests that the disease may involve a generalized collagen disorder. As well as liver and pancreatic cysts, patients have an increased risk of cerebral hemorrhage due to intracranial aneurysms, cardiac valve abnormalities, aortic root dilation, and abdominal hernias.
| American Association of Kidney Patients |
| https://aakp.org |
| American Kidney Fund |
| https://www.kidneyfund.org |
| American Society of Nephrology |
| https://www.asn-online.org |
| Dialysis Patient Citizens |
| https://www.dialysispatients.org |
| International Society for Hemodialysis |
| http://www.ishd.org |
| Kidney and Urology Foundation of America, Inc. |
| http://www.kidneyurology.org |
| National Kidney Foundation |
| https://www.kidney.org |
| National Kidney Foundation Disease Outcomes Quality Initiative |
| https://www.kidney.org/professionals/guidelines |
| Renal Support Network |
| https://www.rsnhope.org |
| United Network for Organ Sharing |
| https://unos.org |
4. Copstead-Kirkhorn LE, Banasik JL. Pathophysiology. 6th ed. St. Louis, MO: Elsevier Health Sciences; 2019.
5. Mitchell R. Pocket Companion to Robbins and Cotran Pathologic Basis of Disease. 9th ed. Philadelphia, PA: Elsevier; 2017.
6. Avram M, Kiahr S. Renal Disease Progression and Management: Role of Vasoactive Agents, Cytokines, Lipids, and Nutrition. Philadelphia, PA: Elsevier Health Sciences; 1996.
8. Xin JZ, Laszidk ZG, Nadasdy T, D'Agati VD. Silva's Diagnostic Renal Pathology. 2nd ed. Cambridge: Cambridge University Press; 2018.
10. Nowak T. Pathophysiology: Concepts and Applications for Health Care Professionals. 2nd ed. Boston, MA: McGraw-Hill; 1999.
11. Rennke H, Denton M, Magee C. Renal Disease: Prevention and Treatment. New York, NY: CRC Press; 1998.
12. Johnson G. On the Diseases of the Kidney, Their Pathology, Diagnosis, and Treatment. London: J.W. Parker and Son; 1852.
13. Clark WB. The Diagnosis and Treatment of Diseases of the Kidney Amenable to Direct Surgical Interference. London: Lewis Publishing; 1886.
14. Wilcox CS. Therapy in Nephrology and Hypertension: A Companion to Brenner and Rector's The Kidney. 3rd ed. Philadelphia, PA: Saunders/Elsevier; 2008.
15. Moorthy V. Pathophysiology of Kidney Disease and Hypertension. Philadephia, PA: Elsevier Health Sciences; 2009.
16. Brenner BM, Coe FL, Rector FC. Renal Physiology in Health and Disease. Philadelphia, PA: Elsevier Health Sciences; 1987.
17. Gonick HC, Buckalew VM. Renal Tubular Disorders: Pathophysiology, Diagnosis and Management. New York, NY: Marcel Dekker; 1985.
20. McCance KL, Felver L. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 8th ed. St. Louis, MO: Elsevier Health Sciences; 2018.
22. Humes HD. Pathophysiology of Electrolyte and Renal Disorders. New York, NY: Churchill Livingstone; 1986.
23. Tisher CC, Brenner BM. Renal Pathology with Clinical and Functional Correlations. 2nd ed. Philadelphia, PA: Lippincott, Williams, and Wilkins; 1994.
24. Price SA. Pathophysiology: Clinical Concepts of Disease Processes. 7th ed. St. Louis, MO: Mosby; 2014.
26. Brashers VL. Clinical Applications of Pathophysiology: Assessment, Diagnostic Reasoning and Management. 2nd ed. St. Louis, MO: Mosby; 2002.
27. Black J, Hawks JH, Keene AM. Medical Surgical Nursing: Clinical Management for Continuity of Care. 8th ed. Philadelphia, PA: W.B Saunders, 2011.
28. LeMone P, Burke KM, Bauldoff G. Medical-Surgical Nursing: Critical Thinking in Patient Care. 5th ed. Upper Saddle River, NJ: Prentice Hall; 2014.
29. Ginès P. Ascites and Renal Dysfunction in Liver Disease: Pathogenesis, Diagnosis, and Treatment. Walden, MA: Blackwell; 2005.
30. Suki WN, Eknoyan G. The Kidney in Systemic Disease. New York, NY: Wiley, John and Sons, Inc.; 1981.
32. Al-Shaikh TM, Alkaseb KS, Mudawi MME, Yassin AYA, Habeballa RS, Alzain SD. Toxicological studies on commercial hair dye brand commonly used in Saudi Arabia: histological study of the liver, kidney, heart, and testis. J Basic Appl Zoology. 2018;79:22.
33. Ackley BJ, Ladwig GB, Flynn Makic MB, Martinez-Kratz MR, Zanotti M. Nursing Diagnosis Handbook: An Evidence-Based Guide to Planning Care. 12th ed. St. Louis, MO: Elsevier; 2020.
34. Ladwig GE, Ackley BJ, Flynn Makic MB, Martinez-Kratz MR, Zanotti M. Mosby's Guide to Nursing Diagnosis. 6th ed. St. Louis, MO: Elsevier; 2020.
36. Zerwekh, JG, Claborn JC, Gaglione T, Gameau AZ, Souter S. Mosby's Pharmacology Memory NoteCards: Visual, Mnemonic, and Memory Aids for Nurses. 3rd ed. Philadelphia, PA: Elsevier Mosby; 2011.
37. Mount DB, Pollak MR. Molecular and Genetic Basis of Renal Disease. Philadelphia, PA: Elsevier Health Sciences; 2008.
40. McDougal WS, Wein AJ, Kavoussi LR, Partin AW, Peters C. Campbell-Walsh Urology 11th Edition Review. 2nd ed. Philadelphia, PA: Elsevier; 2015.
41. Bihl GR, Petri M, Fine DM. Kidney biopsy in lupus nephritis: look before you leap. Nephrol Dial Transplant. 2006;21(7):1749-1752.
42. Blake MA, Sweeney AT. Pheochromocytoma. Available at https://emedicine.medscape.com/article/124059-overview. Last accessed June 28, 2023.
43. Ferrell BR, Paice JA. Oxford Textbook of Palliative Nursing. 5th ed. New York, NY: Oxford University Press; 2019.
45. Lederer E, Alsauskas ZC, Mackelaite L, Nayak V. Hyperkalemia. Available at https://emedicine.medscape.com/article/240903-overview. Last accessed June 28, 2023.
46. Kasper DL, Braunwald E, Hauser S, Longo D, Jameson JL, Fauci AS. Harrison's Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill; 2015.
48. Wu Y, Fu YY, Zhu HD, Xu J, Walline JH. Treatment of hyperkalemic emergencies. World J Emerg Med. 2022;13(3):232-236.
49. Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective?J Am Soc Nephrol. 2010;21(5):733-735.
50. Tamargo J. New Drugs for the Treatment of Hyperkalemia in Patients Treated with Renin-Angiotensin-Aldosterone System Inhibitors: Hype or Hope? Available at https://www.discoverymedicine.com/Juan-Tamargo/2014/11/new-drugs-for-the-treatment-of-hyperkalemia-in-patients-treated-with-renin-angiotensin-aldosterone-system-inhibitors-hype-or-hope. Last accessed June 28, 2023.
52. DiPiro JT. Pharmacotherapy A Pathophysiologic Approach. 11th ed. New York, NY: McGraw Hill Medical; 2020.
53. Longo DL, Kasper DL, Jameson JL, et al. Harrison's Principles of Internal Medicine. 19th ed. New York, New York: McGraw-Hill; 2015.
54. Malhotra D, Tzamaloukas AH. Non-dialysis management of chronic renal failure. Med Clin North Am. 1997;81(3):749-766.
55. Skorecki K, Chertow GM, Marsden PA, Taal MW, Yu ASL. Brenner and Rector's The Kidney. 10th ed. Philadelphia, PA: Saunders; 2015.
56. International Society of Nephrology. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Available at https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf. Last accessed June 28, 2023.
57. NKF KDOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. Available at https://www.kidney.org/sites/default/files/docs/ckd_evaluation_classification_stratification.pdf. Last accessed June 28, 2023.
58. Centers for Medicare and Medicaid Services. Medicare Coverage of Kidney Dialysis and Kidney Transplant Services. Available at https://www.medicare.gov/Pubs/pdf/10128-Medicare-Coverage-ESRD.pdf. Last accessed June 28, 2023.
59. Centers for Disease Control and Prevention. National Chronic Kidney Disease Fact Sheet, 2017. Atlanta, GA: US Department of Health and Human Services; 2017.
60. Bullock A, Burrows NR, Narva AS, et al. Vital signs: decrease in incidence of diabetes-related end-stage renal disease among American Indians/Alaska Natives—United States, 1996–2013. MMWR. 2017;66(01):26-32.
61. Burrows NR, Li Y, Geiss LS. Incidence of treatment for end-stage renal disease among individuals with diabetes in the U.S. continues to decline. Diabetes Care. 2010;33(1):73-77.
62. Whaley-Connell AT, Vassalotti JA, Collins AJ, Chen SC, McCullough PA. National Kidney Foundation's Kidney Early Evaluation Program (KEEP) annual data report 2011: executive summary. Am J Kidney Dis. 2012;59(3 Suppl 2):S1-S4.
63. United States Renal Data System Annual Data Report 2018: Epidemiology of Kidney Disease in the United States. Available at https://www.usrds.org/adr.aspx. Last accessed June 28, 2023.
64. Gross ML, Dikow R, Ritz E. Diabetic nephropathy: recent insights into the pathophysiology and the progression of diabetic nephropathy. Kidney Int. 2005;(94):S50-S53.
65. Shumway JT, Gambert SR. Diabetic nephropathy: pathophysiology and management. Int Urol Nephrol. 2002;34(2):257-264.
66. UK Prospective Diabetes Study Group. Effect of intensive blood glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854-865.
67. Jones J, Holmen J, De Graauw J, Jovanovich A, Thornton S, Chonchol M. Association of complete recovery from acute kidney injury with incident CKD stage 3 and all-cause mortality. Am J Kidney Dis. 2012;60(3):402-408.
68. Hart PD, Bakris GL. Hypertensive nephropathy: prevention and treatment recommendations. Expert Opin Pharmacother. 2010;11(16):2675-2686.
70. Portolés J, Gorriz JL, Rubio E, et al. The development of anemia is associated to poor prognosis in NKF/KDOQI stage 3 chronic kidney disease. BMC Nephrol. 2013;14:2.
71. National Kidney Foundation. KDOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(Suppl 3):S1-S201.
72. Sarafidis PA, Blacklock R, Wood E, et al. Prevalence and factors associated with hyperkalemia in predialysis patients followed in a low-clearance clinic. Clin Am J Soc Nephrol. 2012;7(8):1234-1241.
73. Block GA, Ix JH, Ketteler M, et al. Phosphate homeostasis in CKD: report of a scientific symposium sponsored by the National Kidney Foundation. Am J Kidney Dis. 2013;62(3):457-473.
74. Lanken PN, Terry PB, Delisser HM, et al. An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illnesses. Am J Respir Crit Care Med. 2008;177(8):912-927.
75. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851-860.
76. National Kidney Foundation. KDOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis. 2002;39(Suppl 1):S1-S266.
77. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3:1-150.
78. American Dietetic Association. Manual of Clinical Dietetics. 6th ed. Chicago, IL: Academy of Nutrition and Dietetics; 2000.
79. London GM. Left ventricular alterations and end-stage renal disease. Nephrol Dial Transplant. 2002;17(1):S29-S36.
80. Workeneh BT, Agraharkar M, Gupta R. Acute Kidney Injury. Available at https://emedicine.medscape.com/article/243492-overview. Last accessed June 28, 2023.
81. Huether SE, McCance KL, Brashers VL. Understanding Pathophysiology. 7th ed. St. Louis, MO: Elsevier; 2020.
83. Baird MS. Manual of Critical Care Nursing: Nursing Interventions and Collaborative Management. 7th ed. St. Louis, MO: Mosby; 2016.
84. Norris TL. Porth's Pathophysiology: Concepts of Altered Health States. 10th ed. Philadelphia, PA: Wolters Kluwer; 2019.
85. National Institute of Diabetes and Digestive and Kidney Diseases. National Kidney Disease Education Program. Available at https://www.niddk.nih.gov/health-information/community-health-outreach/information-clearinghouses/nkdep. Last accessed June 28, 2023.
87. Bruyere HJ Jr. 100 Case Studies in Pathophysiology. Philadelphia, PA: Lippincott, Williams and Wilkins: 2009.
88. Berg SM, Bittner EA, Lee J. Massachusetts General Hospital Review of Critical Care Medicine. Philadelphia, PA: Wolters, Kluwer, Lippincott, Williams & Wilkins; 2014.
89. American Cancer Society. Key Statistics About Kidney Cancer. Available at https://www.cancer.org/cancer/kidney-cancer/about/key-statistics.html. Last accessed June 28, 2023.
90. Smith S. Kidney Cancer: Causes, Symptoms, Signs, Diagnosis, Treatments, Stages of Kidney Cancer. Charleston, SC: CreateSpace; 2012.
91. American Cancer Society. Key Statistics About Bladder Cancer. Available at https://www.cancer.org/cancer/bladder-cancer/about/key-statistics.html. Last accessed June 28, 2023.
92. National Cancer Institute. Bladder Cancer Treatment (PDQ)–Health Professional Version. Available at https://www.cancer.gov/types/bladder/hp/bladder-treatment-pdq. Last accessed June 28, 2023.
93. Galvagno SM. Emergency Pathophysiology: Clinical Applications for Prehospital Care. Jackson, WY: Teton NewMedia; 2013.
95. Markovchick VJ, Pons PT, Bakes KM, Buchanan JA. Emergency Medicine Secrets. 6th ed. Philadelphia, PA: Elsevier; 2016.
96. Goldstein RS. Mechanisms of Injury in Renal Disease and Toxicity. Boca Raton, FL: CRC Press; 1994.
97. KDOQI Clinical Practice Guidelines for vascular access. Am J Kidney Dis. 2006;48(Suppl 1):S176-S247.
98. Beathard G. A practitioner's resource guide to hemodialysis arteriovenous fistulas. ESRD Network of Texas. 2003;(Suppl).
99. Lomonte C, Basile C. Preoperative assessment and planning of hemodialysis vascular access. Clin Kidney J. 2015;8(3):278-281.
100. Santoro D, Benedetto F, Mondello P, et al. Vascular access for hemodialysis: current perspectives. Int J Nephrol Renovasc Dis. 2014;7:284-294.
101. Drew DA, Lok CE. Strategies for planning the optimal dialysis access for an individual patient. Curr Opin Nephrol Hypertens. 2014;23(3):314-320.
102. Tordoir JH, Bode AS, van Loon MM. Preferred strategy for hemodialysis in access creation in elderly patients. Eur J Vasc Endovasc Surg. 2015;49(6):738-743.
103. Mansilla AV, Toombs BD, Vaughn WK, Zeledon JI. Patency and life-spans of failing hemodialysis grafts in patients undergoing repeated percutaneous de-clotting. Tex Heart Inst J. 2001;28(4):249-253.
104. Zaleski G. Declotting, maintenance, and avoiding procedural complications of native arteriovenous fistulae. Semin Intervent Radiol. 2004;21(2):83-93.
105. Centers for Medicare and Medicaid Services. Center for Clinical Standards and Quality. CMS ESRD Measured Manual for the 2018 Performance Period/2020 Payment Year v3.0. Available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/ESRDQIP/Downloads/ESRD-Manual-v30.pdf. Last accessed June 28, 2023.
106. Hagen SM, Lafranca JA, Ijzemans JN, Dor FJ. A systematic review and meta-analysis of the influence of peritoneal dialysis catheter type on complication rate and catheter survival. Kidney Int. 2014;85(4):920-932.
107. Lye WC, Kour NW, van der Straaten JC, Leong SO, Lee EJ. A prospective randomized comparison of the Swan neck, coiled, and straight Tenckhoff catheters in patients on CAPD. Perit Dial Int. 1996;16(Suppl 1):S333-S335.
108. Xie J, Kiryluk K, Ren H, et al. Coiled versus straight peritoneal dialysis catheters: a randomized controlled trial and meta-analysis.Am J Kidney Dis. 2011;58(6):946-955.
109. National Institute of Diabetes and Digestive and Kidney Diseases. Kidney Disease Statistics for the United States. Available at https://www.niddk.nih.gov/health-information/health-statistics/kidney-disease. Last accessed June 28, 2023.
110. Stock PG, Barin B, Murphy B, et al. Outcomes of kidney transplantation in HIV-infected recipients. N Engl J Med. 2010;363(21):2004-2014.
111. Organ Procurement and Transplantation Network. Policies: Allocation of Kidneys. Available at https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf. Last accessed June 28, 2023.
112. Organ Procurement and Transplantation Network. Kidney Allocation System. Available at https://optn.transplant.hrsa.gov/learn/professional-education/kidney-allocation-system. Last accessed June 28, 2023.
113. Tapiawala SN, Tinckam KJ, Cardella CJ, et al. Delayed graft function and the risk for death with a functioning graft. J Am Soc Nephrol. 2010;21(1):153-161.
114. Curtis JR. Palliative and end-of-life care for patients with severe COPD. Eur Respir J. 2008;32(3):796-803.
115. Renal Physicians Association. Shared Decision-Making in the Appropriate Initiation and Withdrawal from Dialysis. Clinical Practice Guideline. 2nd ed. Rockville, MD: Renal Physicians Association; 2010.
116. Larson AM, Curtis JR. Integrating palliative care for liver transplant candidates: "too well for transplant, too sick for life." JAMA. 2006;295(108):2168-2176.
117. U.S. Centers for Medicare and Medicaid Services. Hospice Determining Terminal Status. Available at https://www.cms.gov/medicare-coverage-database/view/lcd.aspx?LCDId=34538. Last accessed June 28, 2023.
118. Clayton JM, Hancock KM, Butow PN, et al. Clinical practice guidelines for communicating prognosis and end-of-life issues with adults in the advanced stages of a life-limiting illness, and their caregivers. Med J Aust. 2007;188(12 Suppl):S77, S79, S83-S108.
119. Buckman R. Communication skills in palliative care: a practical guide. Neurol Clin. 2001;19(4):989-1004.
120. Finlay E, Casarett D. Making difficult decisions easier: using prognosis to facilitate transitions to hospice. Ca Cancer J Clin. 2009;59:250-263.
121. Stapleton R, Curtis JR. End-of-life considerations in older patients who have lung disease. Clin Chest Med. 2007;28(4):801-811.
122. Silveira MJ, Kim SY, Langa KM. Advance directives and outcomes of surrogate decision making before death. N Engl J Med. 2010;362(13):1211-1218.
123. Goodlin SJ. Palliative care in congestive heart failure. J Am Coll Cardiol. 2009;54(5):386-396.
124. Lee D, Lee S, Choe H. Community-acquired urinary tract infection by Escherichia coli in the era of antibiotic resistance. Biomed Res Int. 2018;2018:7656752.
125. Schoener B, Borger J. Erythropoietin-stimulating agents. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2023.
126. Ajib F, Childress J. Magnesium toxicity. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2023.
127. Cascella M, Vaqar S. Hypermagnesemia. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2023.
128. Kato S, Chmielewski M, Honda H, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3(5):1526-1533.
129. Avin KG, Moorthi RN. Bone is not alone: the effects of skeletal muscle dysfunction in chronic kidney disease. Curr Osteoporos Rep. 2015;13(3):173-179.
130. Pagonas N, Vautz W, Seifert L, et al. Volatile organic compounds in uremia. PLoS ONE. 2012;7(9):e46258.
131. Nicholas SB, Kalantar-Zadeh K, Norris KC. Socioeconomic disparities in chronic kidney disease. Adv Chronic Kidney Dis. 2015;22(1):6-15.
132. Hallab A, Wish JB. Employment among patients on dialysis: an unfulfilled promise. Clin J Am Soc Nephrol. 2018;13(2):203-204.
133. Eastham J, Patel P. Phenazopyridine. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2023.
134. Arinzon Z, Shabat S, Peisakh A, Berner Y. Clinical presentation of urinary tract infection (UTI) differs with aging in women. Archives of Gerontology and Geriatrics. 2012;55(1):145-147.
135. Urinalysis: Reference Range, Interpretation, Collection and Panels. Available at https://emedicine.medscape.com/article/2074001-overview. Last accessed June 28, 2023.
136. Simon L, Sajjad H, Lopez R, et al. Bladder rupture. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2023.
137. Licina A. Acute abdominal compartment syndrome following extraperitoneal bladder perforation. Case Reports in Anesthesiology. 2017;2017:1-5.
138. U.S. Renal Data System. 2021 Annual Data Report. Available at https://www.supremecourt.gov/opinions/URLs_Cited/OT2021/20-1641/20-1641-1.pdf. Last accessed June 28, 2023.
139. U.S. Renal Data Reporting System. Chronic Kidney Disease: Chapter 4. Available at https://usrds-adr.niddk.nih.gov/2022/chronic-kidney-disease/4-acute-kidney-injury/#figure-4-1-section-wrapper. Last accessed June 28, 2023.
140. Lim Y, Leslie S, O'Rourke S. Interstitial cystitis. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2023.
141. Velez R, Richmond E, Dudley-Brown S. Antibiogram, clinical practice guidelines, and treatment of urinary tract infection. Journal for Nurse Practitioners. 2017;13(9):617-622.
142. Fontenelle LF, Sarti TD. Kidney stones: treatment and prevention. Am Fam Physician. 2019;99(8):490-496.
143. National Institute of Diabetes and Digestive and Kidney Diseases. Kidney Disease Statistics for the United States. Available at https://www.niddk.nih.gov/health-information/health-statistics/kidney-disease. Last accessed June 28, 2023.
144. United Network for Organ Sharing. 2022 Organ Transplants Again Set Annual Records. Available from https://optn.transplant.hrsa.gov/media/njsllhg4/policy-notice_egfrwtmods_mac_ki.pdf. Last accessed June 28, 2023.
145. Dreyer GJ, de Fijter JW. Transplanting the elderly: mandatory age- and minimal histocompatibility matching. Frontiers in Immunology. 2020;11.
146. Strohmaier S, Wallisch C, Kammer M, et al. Survival benefit of first single-organ deceased donor kidney transplantation compared with long-term dialysis across ages in transplant-eligible patients with kidney failure. JAMA Network Open. 2022;5(10):e2234971.
147. Penn Medicine. Too Many Donor Kidneys Are Discarded in U.S. Before Transplantation. Available at https://www.pennmedicine.org/news/news-releases/2020/december/too-many-donor-kidneys-are-discarded-in-us-before-transplantation. Last accessed June 28, 2023.
1. Management of Chronic Kidney Disease Working Group. VA/DoD Clinical Practice Guideline for the Management of Chronic Kidney Disease in Primary Care. Washington, DC: Department of Veterans Affairs, Department of Defense; 2019. Available at https://www.healthquality.va.gov/guidelines/CD/ckd/VADoDCKDCPGFinal5082142020.pdf. Last accessed July 27, 2023.
2. Ikizler TA, Burrowes JD, Byham-Gray LD, et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis. 2020;76(3 Suppl 1):S1-S107. Available at https://www.ajkd.org/article/s0272-6386(20)30726-5/pdf. Last accessed July 27, 2023.
3. Kasiske BL, Zeier MG, Chapman JR, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int. 2010;77(4):299-311. Available at https://kdigo.org/wp-content/uploads/2022/09/KDIGO-2009-Transplant-Recipient-Guideline-English.pdf. Last accessed July 27, 2023.
Mention of commercial products does not indicate endorsement.