Every infant presents uniquely and has certain individual needs. While the vast majority of infants transition without problems, some present with anatomic, physiologic, infectious, and developmental issues that must be addressed. The assessment of the newborn should begin with obtaining a health history and include the initial Apgar assessment, the transitional assessment during the periods of reactivity, the assessment of gestational age, and the systemic physical examination. This systematic approach ensures a thorough exam. Nurses in many different areas of nursing conduct newborn assessments. The information provided includes warning signs, which require immediate attention, as well as basic normal assessment findings in the newborn. Due to the large volume of information, this course will cover only the first 24 hours of life.
- INTRODUCTION
- PRENATAL HISTORY
- PLACENTAL EXAMINATION
- IMMEDIATE POST-BIRTH CARE
- APGAR SCORE
- NEWBORN SCREENING
- PERIODS OF REACTIVITY
- ASSESSMENT OF ATTACHMENT
- GENERAL APPEARANCE ASSESSMENT
- GENERAL MEASUREMENTS
- GESTATIONAL AGE ASSESSMENT
- SKIN ASSESSMENT
- HEAD ASSESSMENT
- EYE ASSESSMENT
- EAR ASSESSMENT
- NOSE ASSESSMENT
- MOUTH ASSESSMENT
- NECK ASSESSMENT
- NEUROLOGIC EXAMINATION
- CHEST ASSESSMENT
- EVALUATING THE RESPIRATORY SYSTEM
- EVALUATING THE CARDIOVASCULAR SYSTEM
- ABDOMEN ASSESSMENT
- GENITOURINARY SYSTEM ASSESSMENT
- EXTREMITIES, BACK, AND SPINE ASSESSMENT
- CONCLUSION
- Works Cited
- Evidence-Based Practice Recommendations Citations
This course is designed for all medical-surgical nurses and ancillary nursing personnel involved in the assessment of newborns.
The purpose of this course is to provide an overview of a newborn assessment for all nurses, especially those who either presently care for newborns or those who come in contact with them occasionally.
Upon completion of this course, you should be able to:
- Outline important points of a prenatal history.
- Describe immediate post-birth care and examination of the placenta.
- Analyze guidelines and strategies for assigning Apgar scores and the implications of maintaining a thermoneutral environment for the newborn.
- Discuss the importance of general measurements and determination of gestational age.
- Identify important aspects of the newborn skin assessment.
- Review key components of the assessment of the newborn's head, face, and neck.
- Evaluate newborns' reflexes and other relevant neurologic findings.
- Outline the steps involved in the assessment of the newborn's chest and respiratory system, including identifying signs of respiratory distress.
- Appropriately evaluate the newborn's cardiovascular system, with attention to potential congenital heart defects.
- Describe key aspects of the newborn abdomen assessment.
- Identify warning signs and normal findings when assessing the newborn's genitourinary system.
- Discuss the inspection of the newborn's extremities, back, and spine.
Nicole F. Keehn, RN, MSN, PsyD, received a Master’s of Science, with emphasis on the pediatric critical care population, from Texas Woman’s University in 1993. She completed her doctorate in clinical and neuropsychology at Argosy University in Dallas, Texas. She was employed as the intensive care educator at Texas Children's Hospital in Houston, Texas, and as a clinical nurse in the intensive care unit at Children's Medical Center of Dallas, Texas. Mrs. Keehn is currently a clinical training director of an APPIC internship program and a pre- and post-doctoral psychology supervisor. She is also the managing partner of Lokahi Life Center, PLLC, in Dallas, Texas.
Katrina Lieben, MSN, CNM, received her Bachelor of Science in Nursing from Humboldt State University, California in 1997 and her Master's of Science in Nursing with a nurse midwifery focus from the Frontier School of Midwifery and Family Nursing in 2008. She has worked with mothers and infants her entire career. Ms. Lieben practiced in Ketchikan, Alaska, for more than 10 years, and currently practices midwifery care in Ukiah, California.
Contributing faculty, Nicole F. Keehn, RN, MSN, PsyD, has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Contributing faculty, Katrina Lieben, MSN, CNM, has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Mary Franks, MSN, APRN, FNP-C
The division planner has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Sarah Campbell
The Director of Development and Academic Affairs has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
The purpose of NetCE is to provide challenging curricula to assist healthcare professionals to raise their levels of expertise while fulfilling their continuing education requirements, thereby improving the quality of healthcare.
Our contributing faculty members have taken care to ensure that the information and recommendations are accurate and compatible with the standards generally accepted at the time of publication. The publisher disclaims any liability, loss or damage incurred as a consequence, directly or indirectly, of the use and application of any of the contents. Participants are cautioned about the potential risk of using limited knowledge when integrating new techniques into practice.
It is the policy of NetCE not to accept commercial support. Furthermore, commercial interests are prohibited from distributing or providing access to this activity to learners.
Supported browsers for Windows include Microsoft Internet Explorer 9.0 and up, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Supported browsers for Macintosh include Safari, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Other operating systems and browsers that include complete implementations of ECMAScript edition 3 and CSS 2.0 may work, but are not supported. Supported browsers must utilize the TLS encryption protocol v1.1 or v1.2 in order to connect to pages that require a secured HTTPS connection. TLS v1.0 is not supported.
The role of implicit biases on healthcare outcomes has become a concern, as there is some evidence that implicit biases contribute to health disparities, professionals' attitudes toward and interactions with patients, quality of care, diagnoses, and treatment decisions. This may produce differences in help-seeking, diagnoses, and ultimately treatments and interventions. Implicit biases may also unwittingly produce professional behaviors, attitudes, and interactions that reduce patients' trust and comfort with their provider, leading to earlier termination of visits and/or reduced adherence and follow-up. Disadvantaged groups are marginalized in the healthcare system and vulnerable on multiple levels; health professionals' implicit biases can further exacerbate these existing disadvantages.
Interventions or strategies designed to reduce implicit bias may be categorized as change-based or control-based. Change-based interventions focus on reducing or changing cognitive associations underlying implicit biases. These interventions might include challenging stereotypes. Conversely, control-based interventions involve reducing the effects of the implicit bias on the individual's behaviors. These strategies include increasing awareness of biased thoughts and responses. The two types of interventions are not mutually exclusive and may be used synergistically.
#32264: Newborn Assessment
Conducting a thorough neonatal assessment is necessary to ensure that the newborn transitions appropriately to extra-uterine life [1]. Skilled observation should begin at the time of birth and continue frequently during the first 24 hours. Nurses should be aware of the normal features of the transition period in order to detect disorders in adaptation soon after birth [2]. The newborn assessment provides much needed information concerning the state of health of the transitioning newborn as well as a basis with which to formulate further care.
Every infant presents uniquely and has certain individual needs. While the vast majority of infants transition without problems, some present with anatomical, physiologic, infectious, and developmental issues that should be addressed. The assessment of the newborn should begin with obtaining a health history and include the initial Apgar assessment, transitional assessment during the periods of reactivity, assessment of gestational age, and a physical examination. This systematic approach ensures a thorough exam.
Nurses in many different areas of nursing conduct newborn assessments. The information provided in this course includes warning signs, which require immediate attention, as well as basic, normal assessment findings in the newborn. Due to the large volume of information, this course primarily covers the first 24 hours of life.
A prenatal history is imperative to the comprehensive care of each newborn and should cover the maternal sequelae, family history, and fetal care history [3]. Maternal history consists of information concerning past pregnancies, including complications, and specifics of labor and maternal illness, including infections and the use of alcohol or drugs [3]. It should also elicit information regarding the current pregnancy, such as the quality and quantity of prenatal care, current laboratory values (including blood type and Rh factor and the results of standard group B streptococcus screening), and the presence of any significant risk factors to the fetus. Obtaining a family history involves inquiring regarding past illnesses, genetic issues, and physiologic problems of parents and siblings [3]. The neonatal history encompasses factors such as vital signs, Apgar scores, required stabilization interventions, and the newborn's general appearance and reaction to the environment [3].
An understanding of the maternal/fetal risk factors is important for the anticipation of possible problems that the infant may experience. Risk factors may be divided into two categories: those that can be modified, such as smoking and drug use, and those that are inherent, such as diabetes and pre-eclampsia.
Smoking during pregnancy continues to be an alarmingly common problem. It has been associated with an increased incidence of ectopic pregnancy, preterm birth, low birth weight infants, placental abruption, premature rupture of membranes, and sudden infant death syndrome (SIDS) [4,5,6]. Studies have shown that low birth weight can be attributed to maternal smoking in 20% to 30% of cases [1]. Smoking causes vasoconstriction, resulting in decreased uterine perfusion and decreased oxygen-carrying capacity for the fetus [4,7]. Smoking has also been implicated in poor fetal nutrition, as it interferes with the ability of the fetus to metabolize key vitamins and minerals [4]. Cigarettes contain nicotine, which is a highly addictive substance. Infants of smokers tend to be fussier than those of nonsmokers, and it is thought that they experience nicotine withdrawal symptoms, especially if they are bottle-fed [6].
Illicit drug use can lead to a multitude of problems for the developing fetus. Cocaine, for example, affects all of the user's body systems, including the cardiovascular system, which can lead to heart attack and stroke. It may cause liver disease and perforation of the nasal septum [4]. It is highly addictive. The effect of cocaine on the fetus is directly related to the effect on the mother, including increased blood pressure, which leads to decreased uterine blood flow and perfusion [1]. These effects may lead to preterm labor, low birth weight and length infants, a decreased head circumference, placental abruption, and stillbirth. Additional effects of prenatal cocaine exposure include cerebral infarcts, renal defects, necrotizing enterocolitis (NEC), cardiac anomalies, and mild facial dysmorphic features [1].
Opioid use during pregnancy can be equally devastating. Maternal signs of opioid use include constricted pupils, slurred speech, euphoria, and respiratory depression [4]. Possible effects on the fetus include meconium aspiration, spontaneous abortion, and low birth weight, which may lead to future developmental problems [8,9]. Infants who are born physically dependent on opioids experience intense acute and subacute withdrawal symptoms [8,9,10].
Studies regarding the effect of cannabis use during pregnancy have shown inconsistent results [2,11]. It is thought that smoking cannabis has similar dangers as smoking cigarettes, including increased carbon monoxide levels, which lead to decreased oxygen levels and fetal hypoxia. Some studies have shown that women who smoke cannabis regularly during pregnancy are more likely to give birth to an infant with fetal alcohol syndrome-like characteristics [2]. Other studies have shown that prenatal exposure to cannabis is directly associated with lower birth weight, reduced birth length, and a smaller head circumference [11,186]. Cannabis readily crosses into breast milk, and women that smoke cannabis should be advised against breastfeeding [2].
Gestational diabetes affects up to 9.2% of all pregnancies [12,13]. Factors that place women at higher risk for developing gestational diabetes include age of 25 years or older, obesity, and a family history of type 2 diabetes [4,14]. The infant of a mother with diabetes, regardless of whether the cause is gestational diabetes or pre-existing disease, is affected in a multitude of ways. The effects are more pronounced in "brittle" cases. Fetuses that are continuously exposed to high blood glucose levels will produce more insulin in response, leading to excessive fetal growth and infants who are large for gestational age (i.e., macrosomia) [1,14]. This in turn can place them at higher risk for birth trauma and shoulder dystocia [4]. In the neonatal period, infants of mothers with diabetes are also more likely to experience hypoglycemia that results from the precipitous drop in available blood sugar while they continue to produce excessive amounts of insulin. This can lead to serious neurologic damage with complications including developmental delay, heart failure, and seizures [13,14,15]. Infants of mothers with diabetes are also at higher risk for neonatal jaundice, and women with diabetes are at higher risk for developing pre-eclampsia [4,14]. These infants are also more likely to be obese and develop type 2 diabetes as adults [13].
Pre-eclampsia is a form of pregnancy-induced hypertension that begins after 20 weeks' gestation. The diagnosis is based on the presence of new-onset hypertension (after 20 weeks' gestation) and at least one of the following: proteinuria; thrombocytopenia; kidney or liver function changes; pulmonary edema; or new-onset, severe, unrelenting headache. Accompanying signs may include headache, visual disturbances, and epigastric pain. Management of pre-eclampsia includes blood pressure control, bed rest, and fluid restriction. The only cure is delivery. The fetus is affected as a result of the hypertension leading to decreased uterine perfusion; chronic hypoxia can result. Infants of women with pre-eclampsia are at higher risk for low birth weight and the catastrophic event of placental abruption [4,16]. Untreated pre-eclampsia may result in eclampsia, which includes the development of seizures. The infant of a woman with eclampsia is at risk for effects of placental abruption, preterm birth, and acute hypoxia [4,16].
Group B streptococcus (GBS) is a leading cause of early-onset neonatal sepsis causing death to newborns [17,18]. Group B streptococcus is found in the mother's genital tract and rectal area [19]. Colonization is common and generally causes no symptoms in the healthy mother. In its 2010 guidelines, the Centers for Disease Control and Prevention (CDC) recommended universal rectovaginal screening of all pregnant women between 35 to 37 weeks' gestation, with subsequent intrapartum antibiotic prophylaxis; however, in 2019, the American Society for Microbiology (ASM) assumed responsibility for maintaining and updating these guidelines [17,18,143]. The ASM currently recommends screening between 36 weeks to 37 weeks/6days [143]. Universal screening has helped decrease the incidence of early-onset neonatal GBS; however, the incidence of late-onset neonatal GBS has not decreased [17,18,143]. Infants generally become infected during labor and birth due to vertical transmission of the bacteria after the membranes have ruptured; therefore, sepsis is a more likely outcome with delayed delivery after rupture of the membranes. Occasionally, fetuses may become infected during pregnancy.
As noted, there are two different types of GBS infection: early- and late-onset. Early-onset GBS occurs in the first seven days of life and may be detectable in the first six hours [19,20]. It can make a seemingly healthy newborn become ill very quickly. The most common symptoms of early-onset GBS are pneumonia, meningitis, respiratory distress, and sepsis [19,21]. Early-onset GBS has a higher fatality rate than late-onset GBS. Late-onset GBS may not be detected for up to three months and is believed to be transmitted either during birth, through breast milk, or in a nosocomial form [19,21,22]. The most common symptoms are sepsis and meningitis.
GBS sepsis should be considered whenever a newborn demonstrates respiratory distress, temperature instability, or poor feeding. Laboratory values that may be assessed when there is suspicion of GBS include complete blood count with differential, blood culture, chest x-ray, urine culture and analysis, stool culture, and possible lumbar puncture [23]. Treatment of GBS-infected newborns includes IV antibiotics [24].
A thorough assessment of the placenta at the time of delivery may assist with age determination and present significant diagnostic information [3]. The placenta should be assessed for size, color, odor, and the presence and number of membranes [3,25].
The ratio of fresh placental weight to infant weight is normally 1:6 in the last trimester. Very large placentas may be indicative of diabetes; very small placentas may be indicative of chronic hypoxia, perhaps caused by hypertension. The placenta should have a uniform thickness throughout; depressions may be from abruption or infarction [3,25,26].
Color may give an estimate of gestational age, as the placenta will become duller with more calcium deposits postdate. Pallor or plethora should be noted as they may indicate fetal blood volume inadequacy or excess and/or hemoglobin status. Staining by meconium or blood through the membranes or of the cord indicates an insult of longer duration. Vernix nodules (or amnion nodosum) indicate prolonged extreme oligohydramnios and pulmonary hypoplasia. An adherent clot suggests that an abruption has occurred. Any of these abnormal findings should be noted [3,25,26].
The placenta should be essentially odorless except for the slight odor of fresh blood. Foul odor indicates infection. This should be noted, and the physician should be informed [3,25,26].
Membranes should be assessed and noted to be present (i.e., not retained inside the uterus). The number of membranes should be determined in the case of multiple gestations [3,25,26].
The umbilical cord should be assessed for appearance, length, and diameter. The appearance of the insertion site should be documented, noting intactness, the number of vessels, and color. The umbilical cord should be a pearl ivory color, and any deviations from this color should be noted. The umbilical cord length is normally between 55 and 60 cm, with 5% of cords shorter than 35 cm and 5% longer than 80 cm [25,26,27]. Shorter length may be the cause of decreased fetal movement, intrauterine constraint, placental abruption, or cord rupture [27]. A longer cord makes entanglement or prolapse more likely. The cord diameter at term is generally about 1.5 cm and should be relatively uniform throughout, without strictures. The Wharton's jelly should be firm, with compression a likely result if the cord is thin [3,26,27].
Approximately 85% to 90% of infants make the transition from intrauterine to extrauterine life with no assistance necessary [28,29]. However, for the remaining few newborns, some assistance may be required, ranging from simple stimulation to complete resuscitation.
All nurses should be familiar with the ABCs of resuscitation: airway, breathing, and circulation. Because newborns are wet when they are born, they can suffer rapid heat loss if a warm environment is not maintained [28]. Therefore, it is critical to maintain a warm, or thermoneutral, environment for the infant throughout the first hours and days of life. This can be accomplished by placing the infant on the mother's abdomen, with warm blankets placed over them both to maintain body heat. Alternatively, if the need for further intervention is anticipated, or if the caregiver prefers, the infant should be placed on a preheated radiant warmer.
As the infant is being dried with warm blankets, the nurse should also be evaluating the infant's airway, breathing, muscle tone, color, and gestational age. All of these things should be evaluated within the first 30 seconds of life. The airway should be cleared with a bulb syringe or mechanical suction, and the infant should be positioned in such a manner as to facilitate an obstruction-free airway. If, in the initial evaluation, the infant is found to be clear of meconium, is breathing or crying, has good tone, is pink, and appears to be term gestation, then routine care need only be provided [28]. Routine care is comprised of assuring that the infant is warm and dry and keeping the airway clear. According to Neonatal Resuscitation Program standards, further care is warranted if the newborn fails to respond to birth in this positive manner [28].
In the United States, antibiotic eye ointment for the prophylaxis of ophthalmia neonatorum is highly recommended in all newborns [30,31,32]. Ophthalmia neonatorum is the inflammation of the eyes resulting from exposure to gonorrhea or chlamydia as the infant passes through the birth canal and can lead to blindness [30]. Administration of vitamin K intramuscularly is also common in the United States to prevent hemorrhagic disorders [4]. Coagulation alteration is seen predominately in infants in the second or third day of life, specifically because factors VII, VIII, IX, and X are dependent on the synthesis of vitamin K [33,34].
In 1953, an anesthesiologist named Virginia Apgar designed a tool for evaluating newborn infants [35]. The Apgar scores grade the infant's response to extrauterine life in five categories [36]:
Heart rate
Respiratory effort
Muscle tone
Reflex irritability
Color
There are a maximum of 2 points possible in each category, for a total of 10 possible points. The Apgar determination is completed at one and five minutes of life. It is important to note that resuscitative measures should not be delayed while awaiting the one- and five-minute marks for Apgar determination.
Morbidity and mortality findings have been found to correlate with the five-minute Apgar score [37,38]. The one-minute Apgar score correlates with the pH of cord blood. The lower the score, the more acidotic the infant; in addition, infants with lower scores have worsening cardiorespiratory depression [35]. Some studies have suggested that the Apgar score loses clinical significance for infants of 23 to 25 weeks' gestation who survive their first 24 hours of life [39].
Heart rate can be determined either through auscultation of the apical pulsation or by palpating the umbilical cord. A heart rate greater than 100 beats per minute (bpm) is awarded a score of 2 points. A pulse of less than 100 bpm garners 1 point. An absent heartbeat would obtain zero points [35,36].
Assessment of the respiratory effort requires a multifaceted approach. Movement of air in and out of the lungs may be auscultated at the time that a respiratory rate is obtained. An infant with a good cry is awarded 2 points for respiratory effort. An infant that is making some attempt at breathing but may be categorized as slow or irregular will obtain only 1 point. An irregular breathing pattern, also known as periodic breathing, is a normal finding in some newborns. However, if periodic breathing is associated with nasal flaring, grunting, retractions, cyanosis, or decreased rate, further assessment and intervention may be required. A newborn with an absent respiratory drive will receive zero points [35,36].
The nurse considers muscle tone acceptable if the infant's elbows, hips, and knees are flexed and allow active extension of extremities. The infant should return to the gently flexed position after examination. An attitude of flexion is necessary to obtain 2 points. An infant with some flexion should be assigned 1 point. A limp infant would receive zero points [35,36].
Reflex irritability is noted as the infant reacts to noxious stimulation. An appropriate response to stimuli, such as suctioning or rubbing the soles of the feet, would be for the infant to cry. This response would be awarded 2 points. If the newborn grimaces in response to such stimuli, 1 point would be awarded for effort. If the infant shows no response, then zero points would be awarded [35,36].
Color can be assessed by noting the color of mucous membranes, the trunk, and the soles of the feet. The infant should be pink and not dusky. An infant who is completely pink, including the hands and feet, would be awarded 2 points in this category. An infant that is pink but is acrocyanotic (i.e., has blue hands and/or feet) would receive 1 point. An infant that is blue, gray, or dusky would receive zero points [35,36].
Newborn screening programs began in the early 1960s with the development of a screening test for phenylketonuria (PKU) [40]. Newborn screening in the United States is a public health program aimed at the early identification of a variety of serious conditions, including genetic disorders (e.g., cystic fibrosis, hearing loss) and endocrine disorders (e.g., congenital adrenal hyperplasia, primary congenital hypothyroidism) [40,41]. In 2003, all but four states were screening for only six of these disorders. By 2022, most states reported screening for at least 31 (of 35 recommended) genetic and endocrine disorders on the standardized uniform panel of core conditions [41,44].
The adoption of a uniform newborn screening panel has led to earlier lifesaving treatment and intervention for newborns with these disorders [42,43]. The number and types of disorders screened for vary by state, but generally are guided by the Recommended Universal Screening Panel, which was developed by the U.S. Department of Health and Human Services [40,44].
All healthy newborns go through predictable periods of alertness and sleep that should be assessed and taken into consideration when performing the comprehensive physical examination. Distressed infants also progress through these stages but at a much slower rate [1]. These stages are called the first and second periods of reactivity.
The first period of reactivity generally lasts six to eight hours. For the first 30 minutes after birth, the newborn is generally very alert and active. The infant will usually have a vigorous suck reflex during this time, and it is generally an excellent time to begin breastfeeding. The infant will have open eyes and will be interested in looking around. Physiologically, the infant's respiratory rate may be increased and the lungs will sound quite wet. The heart rate may be increased, bowel sounds are active, mucus production is increased, and body temperature may be slightly decreased [1,45].
After this initial period of alertness, the newborn will go into a deep sleep that generally lasts from two to four hours, though it may continue much longer. During this period, the infant is very calm. Attempts to stimulate the infant will generally be unsuccessful. Ideally, the physical examination should be completed before this time and the infant can then be left alone to sleep. Physiologically, the infant will experience a decrease in respiratory rate, mucus production, and temperature and will likely not void or stool [1,45].
The second period of reactivity, which usually lasts two to five hours, begins when the newborn wakes from this deep sleep state. The infant is generally very alert once again and showing signs of hunger. This is an excellent opportunity for the infant and family to interact with each other and for the nurse to begin some teaching regarding hunger cues and other ways that the infant may communicate needs. Physiologically, the newborn's heart and respiratory rates increase, the gag reflex is active, and the production of mucus and meconium resumes [1,45].
Throughout the initial postbirth care and physical exam of the newborn, the nurse should be alert for signs of attachment forming between the infant and the parents. It is important for the nurse to look for those behaviors that lead to the successful process of attachment and bonding between parent and infant [1].
Unlike the physical examination, which uses concrete guidelines, the assessment of attachment requires the nurse to observe interactions and speak with the new parents regarding their expectations, dreams, and desires for their infant. This part of the newborn assessment can be challenging in today's hospital environment of shorter stays, but it is imperative in ensuring the well-being of the infant.
The following guidelines may be used when assessing infant-parent attachment [1]:
When the infant is brought to the parents, do they reach out for the infant and call the infant by name?
Do the parents speak about the infant in terms of identification (i.e., who the infant looks like; what appears special about their child over other infants)?
When the parents are holding the infant, what kind of body contact is there? Do they feel at ease in changing the infant's position? Are fingertips or whole hands used? Are there parts of the body they avoid touching or parts of the body they investigate and scrutinize?
When the infant is awake, what kinds of stimulation do the parents provide? Do they talk to the infant, to each other, or to no one? How do they look at the infant (e.g., direct visual contact, avoidance of eye contact, or looking at other people or objects)?
How comfortable do the parents appear in terms of caring for the infant? Do they express any concern regarding their ability or disgust for certain activities, such as changing diapers?
What type of affection do they demonstrate to the newborn, such as smiling, stroking, kissing, or rocking?
If the infant is fussy, what kinds of comforting techniques do the parents use, such as rocking, swaddling, talking, or stroking?
After the prenatal history has been obtained, the placenta has been evaluated, immediate postbirth care has been provided, and Apgar scores assigned, one can proceed with the physical examination. As with other facets of nursing care, the physical assessment always begins with the general appearance.
One important assessment strategy is to discuss the upcoming assessment with the infant's parents. Including the parents during the assessment allows the healthcare provider to point out both normal and abnormal findings. Optimizing this time with the parents assists them in understanding their infant and allows them to ask questions [46].
The following findings are considered warning signs that may be seen during the general assessment [35]:
Axillary temperature less than 36.1°C or greater than 37.2°C
Heart rate less than 100 bpm or greater than 160 bpm
Respiratory rate less than 30 or greater than 60 breaths per minute
Cyanosis
Jaundice
Periods of apnea lasting more than 15 seconds
Lack of movement or responsiveness
Hypotonic or hypertonic position
Lack of interest in environment
Should these findings be noted, they would warrant immediate further investigation and treatment.
As noted, maintaining a thermoneutral environment is an important consideration. The assessment of any newborn should be conducted in a warm, well-lit environment. Cooler environments stress the newborn and may lead to inaccuracies in assessment. For example, incorrect findings may be made if the patient is found to look blue, experience bradycardia, or have cold hands and feet due to a hypothermic environment.
The assessment of the infant can be conducted through inspection, auscultation, and palpation. Inspection should occur before physical contact is made with the infant, though the need may arise to remove blankets, diapers, or clothing in order to complete a thorough inspection. Whenever possible, complete the observation portion of the assessment before touching the newborn. Observe the infant's position, temperament, sleep or wake cycle, color, movement, and respiratory pattern before disturbing the infant. Once the newborn has been disturbed, he or she may become agitated, resulting in a guarded posture, increased respiratory rate, and temperament changes. During the assessment, an inspection of the infant's activity level, color, respiratory effort, ease of movement, and posture should be noted [3]. During inspection, one should also note the appearance of any dysmorphic features. The finding of three or more dysmorphic features may warrant chromosome analysis [29,47].
During inspection, the activity level should be noted for sleep states progressing to irritability during the more intrusive parts of the exam [48]. There are six identified sleep states: deep sleep; active sleep; drowsy, in-between state; awake alert; alert and fussy; and crying [33,48,49]. The infant should be assessed in an awake alert state, which has been identified as the infant being bright, focused, and minimally active [29,33,48,49]. Motor activity, such as tremulousness, irritability, and defensiveness, should be noted both before and during the assessment [48].
Proper lighting is important as the accurate assessment of a newborn's skin can be useful in determining problems. Even if a newborn is slightly icteric at birth, making this determination can be useful in following the infant for potential problems related to jaundice. If the lighting of the examination room is inadequate, the slight appearance of jaundice may be overlooked. Color is important in determining pulmonary and cardiac involvement and thermoregulation, as well as other organ function in the newborn. Changes in the infant's color may occur throughout the exam when the infant cries, becomes cool, or is irritable. Assessment should be made for jaundice, cyanosis, pallor, and plethora [3]. It is important to note that acrocyanosis, the blue or dusky hands and feet with a pink trunk and mucous membranes, is frequently found and does not indicate a major problem.
Respiratory effort should be assessed by noting the rate and quality of breaths. Depth of breathing, retracting, grunting, nasal flaring, head bobbing, and posture changes associated with inadequate effort should be noted. Periodic breathing by the newborn (i.e., taking several breaths in a row then pausing for up to 15 seconds) is a normal finding. However, grunting, flaring, retractions, and head bobbing warrant further investigation [29]. A full discussion of respiratory assessment follows later in this course.
During inspection, resting posture offers many clues to the health of a newborn. In a healthy, full-term newborn, the posture should be that of flexion [1,29]. Muscle tone, including the amount of flexion or extension, should be assessed. Asymmetry of extremities, comparison of upper and lower extremities, and flaccid posture or contraction should be noted and further evaluated during the exam. The newborn's ease of movement should be noted throughout the assessment. The levels of fluidity and spasticity should be observed.
General measurements should be performed on each newborn. Infants who are found to have values outside the accepted range may require further evaluation and treatment. Weight, length, head circumference, and chest circumference measurements allow the practitioner to find abnormalities. Plotting these abnormalities provides a quick reference for comparisons with acceptable ranges.
Birth weight is an important indicator of perinatal morbidity and mortality[2]. When weighing a newborn, it is important to do so when the infant is not wearing a diaper. If a diaper is in place, subtract the weight of the diaper from the total weight. A strategy to prevent weight inaccuracies is to use the same scale each time the patient is weighed. This will control for differences in zero balancing between two scales. If an infant is being weighed on a bed that has a built-in scale, it is important to remove any extra sheets, toys, or diapers. A further consideration in weighing the critically ill newborn is lifting the intravenous infusion lines, as well as other pieces of equipment, such as ventilator tubing, so they do not cause an inaccurately high measurement. A list of the items that the bed was initially zeroed with should be recorded for easy access. These items should remain on the bed during each weighing, so the zero balance of the scale remains at a constant and only the infant's weight is measured. For example, removing a critically ill newborn from the bed each time the weight must be assessed may not be feasible. Instead, the foam mattress and gel devices need not be removed each time the infant is weighed. One of the most important factors in monitoring an infant's fluid balance is weight[50]. Birth weight should be measured soon after birth because the fluid loss that occurs after birth begins fairly rapidly[1].
Classification of weight may be used independent of gestational age. Extremely low birth weight infants weigh less than 1,000 grams, very low birth weight newborns weigh less than 1,500 grams, and low birth weight newborns weigh less than 2,500 grams[51]. Normal weight in a term newborn ranges from 2,500 to 4,000 grams[52,53].
Another common classification system for identifying birth weight-related risk factors uses the terms large for gestational age (LGA), appropriate for gestational age (AGA), and small for gestational age (SGA) (Table 1). An LGA infant weighs more than the 90th percentile at any given gestational age[54]. At term, an LGA infant would be considered one that weighs more than 4,000 grams. An AGA infant is one that falls anywhere between the 10th and the 90th percentile for his or her given age[53]. At term, this would be any infant weighing between 2,500 and 4,000 grams. An SGA infant falls below the 10th percentile for his or her gestational age[53]. At term, an SGA infant weighs less than 2,500 grams. Infants are categorized as term when they are born between the first day of week 37 to 42 weeks of gestation[55]. Before 37 weeks, the newborn may be considered premature, and after 42 weeks, the newborn should be classified as post-term[55,145](Table 2). Correctly categorizing the newborn can aid in determining future risk factors.
The most accurate way to measure length is to fully extend the newborn's leg and record the length from the crown of the head to the heel. To establish an accurate measurement, one person should hold the infant in place while another person completes the measurements. To ensure accurate measurements, mark the sheet or the paper on which the infant is lying, at the infant's crown and heel. Acceptable newborn length ranges from 48–53 cm or 19–21 inches [37]. An adjunct to crown-heel measurement is the crown-rump measurement. This particular assessment is useful in determining anatomical abnormalities such as dwarfism [3].
Head circumference, often referred to as occipital-frontal circumference, may be determined by measuring the circumference of the skull from the frontal to occipital area by placing the tape measure above the ears. It is important to measure the largest part of the head when measuring occipital-frontal circumference. Because of edema and molding due to the birth process, the subsequent occipital-frontal circumference measurements may increase or decrease as much as 2 cm during the first week of life [3]. The head circumference is larger than the abdominal circumference until 32 through 36 weeks' gestation, when it is equal. Acceptable head circumference is 33–37 cm or 13–15 inches [3,37,57,184]
Chest circumference is obtained by measuring around the infant's chest at nipple line midway between inspiration and expiration. Acceptable chest circumference is 30.5–33 cm or 12–13 inches [37,57]. As noted, the head circumference should generally be larger than the chest circumference.
General measurements should be plotted on a standard growth chart and followed over time. Growth charts have been developed to detect nutritional and growth disturbances and are available through the National Center for Health Statistics [58]. The CDC recommends that healthcare providers use the World Health Organization (WHO) growth standards to monitor growth for infants and children ages 0 to 2 years in the United States. For children 2 years of age and older, the CDC recommends that providers use the CDC growth charts [59,60]. For newborns, these charts record [58]:
Length for age
Weight for age
Head circumference for age
Weight for length
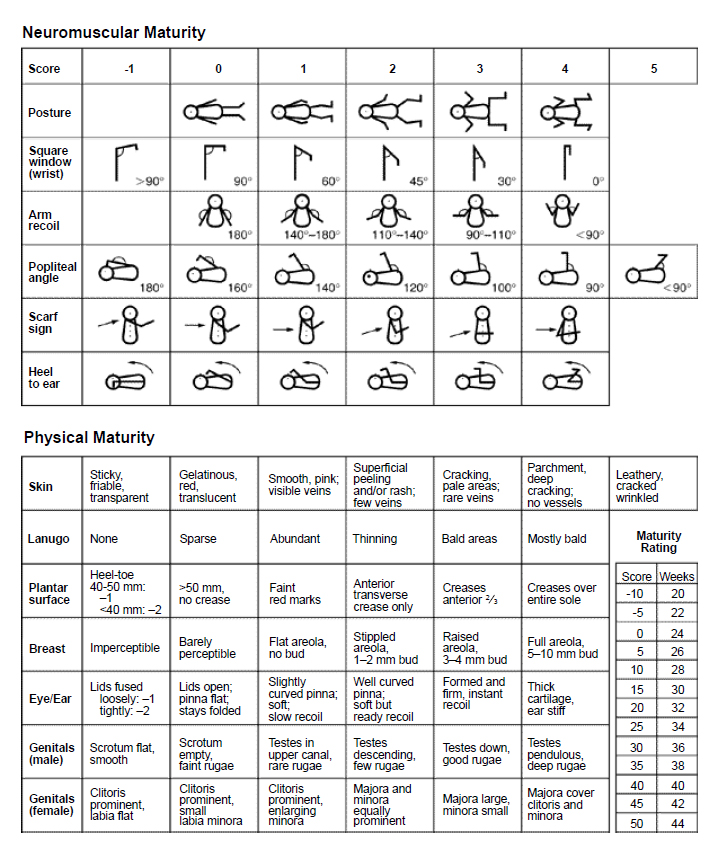
Protocols vary regarding which infants require gestational age assessment. Some clinicians feel that this assessment should be standard for all newborns, while others use this tool on a case-by-case basis. A gestational age assessment should be performed on any infant that is thought to be premature or when there is a question of gestation. When assessing the gestational age of newborns, the New Ballard Score is often used [3,61]. The New Ballard Score takes into account both neuromuscular and physical maturity [45]. The score sheet includes scales that have grades of -1 through 5. Once the assessment is complete and the scores assigned, the scores are simply added. The total score is then correlated with the corresponding number of weeks to give the clinical determinant of the newborn's age.
Gestational age should be assessed within the first four hours of birth for maximum reliability [33]. The nurse should initially conduct the parts of the examination that can be done without disturbing the infant. This includes the physical maturity section of the New Ballard Score as well as the resting posture component of the neuromuscular maturity section.
The following discussion appears in the order in which the examination should be conducted. Please follow along with the New Ballard Score (Figure 1).
Resting posture should be evaluated first before the infant is disturbed. The infant should be supine on a flat surface [33]. There are five choices, ranging from 0 points, for a flaccid infant, to 4 points, for a completely flexed infant. Infant A is lying quietly on her back with her arms out to her sides at a greater than 90-degree angle. Her legs are bent at the knee with only a small amount of flexion. She should be awarded 2 points for posture.
The skin should be evaluated next. There are seven subclassifications in the skin category. The extremely premature infant has transparent, friable skin and woulsd be awarded -1 points. The postmature infant presents with leathery, tough, cracked skin and would receive 5 points. Infant A has a fine rash. There appears to be some peeling, with few veins visible on the abdomen. She is awarded 2 points for her skin maturity.
The presence or absence of lanugo (i.e., fine body hair) is evaluated next, with six categories from which to choose. The extremely premature infant is bald, not having had the opportunity to develop lanugo. The postmature infant is also mostly bald because the majority of lanugo has fallen out prior to birth. Infant A has large bald patches but still has a significant amount of hair on her back and ears. She receives 3 points.
Next, the soles of Infant A's feet are examined. Creases begin forming at the top of the newborn's foot and progress to the sole with maturity [33]. There are seven categories from which to choose in this area of assessment, ranging from -2 to 4 points. The extremely premature infant has a smooth sole and should be given points based on the size of the foot. The postmature infant has creases over the entire foot, which often appears cracked and leathery. Infant A has creases over the majority of her foot but not the entire sole. She receives 3 points for her feet.
Observing the presence and size of the breast bud is the next indicator of physical maturity. There are six categories from which to choose in this area. The extremely premature infant will have imperceptible breast buds, while the postmature infant will have well-developed 5–10 mm breast buds and a full areola. Infant A has a noticeable areola and a small bud measuring 2 mm. She is given 2 points.
The development of the newborn's eyes and ears are important markers of gestational age. There are seven categories from which to choose in this area. The extremely premature infant will have fused eyelids; the scoring on these infants is dependent on how tightly or loosely the eyes are closed. The postmature infant will have thick cartilage in the ears and they will be stiff. Infant A has open eyes, and her ears are soft but they do recoil easily. She is awarded 2 points for her eyes and ears.
The last area in the physical maturity assessment is genitalia. There are six categories from which to choose in this area. The extremely premature male infant will have a flat and smooth scrotum; the testes will not have descended into the scrotum. The extremely premature female infant will have an extremely prominent clitoris and flat labia. The postmature male infant will have descended testes and pendulous scrotum with deep rugae. The postmature female will show a labia majora that completely covers her clitoris and labia minora. Infant A has a large labia majora and a small labia minora. Her clitoris is not visible. She is awarded 3 points.
Returning to the neuromuscular maturity section of the New Ballard Score, the square window is measured by bending the wrist and visualizing how far forward the infant's hand can go. The extremely premature infant will have little flexibility in the wrist and show a greater than 90-degree angle. The postmature infant will have great flexibility and the hand will be completely in contact with the forearm. Infant A demonstrates a 30-degree angle when assessing the square window sign. She receives 3 points.
Arm recoil has much to do with the infant's flexion. One performs this test by actively extending the infant's arm to a straight position and letting go in order to evaluate how far back to full flexion the arm returns. In the extremely premature infant, there will be no recoil, and in the postmature infant, there will be full recoil. Again, an observation of the angle determines the point assignment. Infant A recoils to a 140-degree angle. She is given 2 points.
Actively extending the infant's leg and placing the foot near the head may measure popliteal angle. The extremely premature infant will have great flexibility and will be able to demonstrate a completely straight leg in this posture. The postmature infant will show little flexibility and will be unable to extend the knee to greater than 90 degrees. Infant A extends her leg to a 100-degree angle and is given 3 points.
Scarf sign may be measured by extending the infant's arm across the body and measuring how far across the elbow falls. The extremely premature infant will demonstrate great flexibility, and the elbow will be able to stretch all the way across the body. The postmature infant will show little flexibility and will barely get the elbow to meet the inner chest wall. Infant A is able to get her elbow to midline and is awarded 2 points.
The last marker of neuromuscular maturity is the heel to ear sign. This sign is measured by actively extending the infant's foot and attempting to reach the ear. The extremely premature infant will be able to touch his or her foot to the ear, while the postmature infant will be unable to extend a foot anywhere near the ear. Infant A can only extend her foot to a right angle above her body and is given 3 points.
Finally, the points for each category are added to give an estimated gestational age. In our example, Infant A received 15 points in the neuromuscular maturity area and 15 points in the physical maturity section. This is a grand total of 30 points, making her approximately 36 weeks' gestation.
A thorough skin assessment can be an important tool that provides invaluable information. Skin can deliver insight into the newborn's thermoregulation system, tell the experienced practitioner about the newborn's cardiac and respiratory functioning, be an invaluable indicator of gestational age, and be the first indicator of an infectious process.
Vernix caseosa is a lubricant found on the skin or in the skin folds. While usually white, it may be yellow from bilirubin stains or green from meconium staining [34]. It disappears as the fetus ages and, by term, is generally found only in the folds such as the armpit or the groin. Vernix caseosa is almost entirely absent in postmature fetuses and may be an important indicator of gestational age.
Lanugo is the name for the fine hair that covers the body, ears, and forehead of many newborns [3]. Lanugo first develops at 19 weeks' gestation and becomes most obvious at 27 to 28 weeks' gestation. As such, lanugo is an important indicator of gestational age. It may be important for parents to understand that the hair will fall off within the first few weeks of life.
Warning signs of the skin assessment that would warrant further investigation and/or immediate intervention include:
Long nails and desquamation, indicating postmaturity
Thin translucent skin with abundant vernix and lanugo, indicating prematurity
Pallor, possibly caused by hypothermia, anemia, sepsis, or shock
Cyanosis, possibly caused by cardiorespiratory disease, hypoglycemia, polycythemia, sepsis, or hypothermia
Petechiae, possibly caused by thrombocytopenia, sepsis, congenital infection, or pressure sustained during delivery
Plethora, possibly caused by polycythemia
Meconium staining, possibly caused by intrauterine asphyxia
Abnormal hair distribution or extra skin folds, possibly associated with genetic abnormalities
Poor skin turgor associated with intrauterine growth retardation and hypoglycemia
Large hemangiomas, which may trap platelets within their borders and cause thrombocytopenia
Bullae or pustules, possibly caused by staphylococcal infection
During the first few moments of life, when the infant is first exposed to the sensation of cold, skin receptors become stimulated. These receptors aid in stimulating the respiratory center to begin the first sequences of breaths and pulmonary gas exchange [37]. A skin temperature range of 36°C to 36.5°C (96.8°F to 97.7°F) is acceptable for the term newborn [37].
In a preterm infant, one will find the skin to be more translucent as opposed to the thick, cracked appearance of the term infant's skin. Being able to visualize vessels through the skin on the abdominal wall is also an age marker [3]. It is easy to see abdominal vessels in preterm infants because of the transparency of the skin. Creases in the sole develop from toe to heel. A decrease in the amount of creasing of the soles of the feet may be a sign of motor deficits [3]. This is based on the belief that foot creasing is caused by movement of the lower extremities, movement of the fetus, and uterine compression. Increased creasing of the sole, generally seen in postmaturity, is also a sign that warrants further investigation.
The assessment of skin turgor may be easily completed along with the assessment of the infant's hydration status, fontanelles, and mucous membranes. The skin turgor test is simple and corroborates the other findings of dehydration. To test the infant's skin turgor, simply pinch the skin. The skin should automatically recoil. If the skin remains "pinched," the infant has poor skin turgor [62].
Color is a valuable finding in the assessment of a newborn. Due to variations in newborns' skin tones, an assessment may vary between healthcare personnel. In this case, an agreement of what to call the color should be noted between personnel so that fluctuations from the baseline color may be quickly identified. If an infant has a normal cardiorespiratory function, the mucous membranes, nail beds, palms of hands, and soles of feet will be pink in lighter-pigmented infants [63]. In darker-pigmented infants, color may be light pink with a yellow or red tinge. It is important to keep in mind that acrocyanosis is a normal finding in the first 24 to 48 hours of life, and an infant can still be considered pink if his/her mucous membranes and trunk remain so, even if the extremities remain blue or pale [64]. Alterations in cardiovascular or respiratory function may present in the form of mottled or dusky skin color. Pallor can be a sign of poor perfusion or anemia. If the infant's skin appears to be pale, then it should be noted and reasons should be identified. Other symptoms of poor skin perfusion are mottling and delayed capillary refilling [63]. Central cyanosis, or a blue color of the face, trunk, or mucous membranes, is not a normal finding and should be acted upon immediately.
Neonatal hyperbilirubinemia, or jaundice, is the yellowish discoloration of an infant's skin and sclera caused by a buildup of the bile pigment bilirubin. Hyperbilirubinemia that is noted in the first 24 hours of life is considered pathologic and should be treated appropriately [65]. Hyperbilirubinemia is a normal variation to a certain degree, and most infants will show some signs during the first week of life [65]. The severity and diagnosis of hyperbilirubinemia is dependent on the bilirubin level at a certain newborn age as well as the presence of risk factors [65,66].
Newborns become jaundiced for two main reasons: immaturity of the liver and/or the excessive amount of fetal hemoglobin that is required in utero. The liver is thought to be one of the last organs to fully mature; therefore, even the full-term infant may be considered to have an immature liver. The liver is where bilirubin, a byproduct of hemoglobin metabolism, is processed, and the immature organ may not be able to keep up with the increased demand that occurs shortly after birth. The increased demand is caused by the breakdown of fetal hemoglobin.
In utero, fetuses are exposed to a much lower partial oxygen pressure (PO2) than that of the air they begin to breathe as soon as they are born. For this reason, they are in need of a much higher amount of fetal hemoglobin than is necessary after they are born. Fetal hemoglobin has unique characteristics. It has less oxygen carrying capacity, though it does have a greater affinity for the oxygen molecule. Because of the strong oxygen affinity, the oxygen does not unload until the tissue oxygen levels in an infant are lower than they would be in an adult. After they are born, infants begin to rapidly break down the excess hemoglobin to adjust to the much higher PO2 of the air. When fetal hemoglobin is broken down, the heme is converted to biliverdin and then to unconjugated bilirubin. When the unconjugated bilirubin level exceeds normal, there becomes an increased chance of it depositing in the basal ganglia. Deposition of unconjugated bilirubin in the brain is known as kernicterus, a chronic and permanent form of hyperbilirubinemia that can be life-threatening [66].
For newborns that present with jaundice, the American Academy of Pediatrics (AAP) has several recommendations. Total serum bilirubin or transcutaneous bilirubin levels should be measured within the first 24 hours of life, as observational assessment of the degree of jaundice severity is inaccurate and does not take into account the appearance of infants with darker skin pigmentation [66]. Bilirubin levels should be considered in relation to the age of the newborn (i.e., measured by hours) [66]. Infants younger than 38 weeks' gestation who are breastfed are at greater risk of developing hyperbilirubinemia; these infants require additional observation [66]. All infants should be screened for the risk of developing severe hyperbilirubinemia, and parents should be provided with written and verbal information about neonatal jaundice prior to hospital discharge [66].
Obtaining a thorough history for risk factors of hyperbilirubinemia is essential. The causes of jaundice may be related to Rh incompatibility, abnormal blood cell structures, birth injury, polycythemia, glucose-6-phosphate dehydrogenase or pyruvate kinase deficiency, infection, certain medications, and prematurity [65]. Depending on the cause of the hyperbilirubinemia, treatment includes more frequent feedings, phototherapy, maintaining active bowel routines, and, occasionally, exchange transfusions [65,66].
When assessing for dysmorphic features of the skin, findings of birthmarks, missing skin, skin tags, vesicles, and lesions should be documented [47]. The use of drawings to better describe abnormal skin findings are helpful [47].
Birthmarks are common in the newborn but may cause considerable anxiety in parents. Some birthmarks involute voluntarily, while others may persist into adulthood [67,68]. The majority of birthmarks are benign; however, some birthmarks may necessitate further investigation to assess their potential for future malignancy or possible underlying conditions. Birthmarks may be divided into three etiologic categories [67]:
Vascular
Pigmented
Abnormal development
Vascular nevi include hemangiomas, nevus flammeus (i.e., port wine stain), and nevus simplex (i.e., stork bite, salmon patch). Pigmented nevi include congenital melanocytic nevi and dermal melanosis (i.e., Mongolian spots). Nevi caused by abnormal development, such as supernumerary nipples and lesions along the spine associated with spinal dysraphism, will be discussed in the chest and back assessment sections of this course, respectively.
Strawberry Hemangiomas
Also referred to as strawberry mark, nevus vascularis, capillary hemangioma, or hemangioma simplex, strawberry hemangiomas consist of newly formed capillaries occupying both the dermal and subdermal layers. Strawberry marks are typically raised, sharply demarcated, and bright red. However, these lesions may also present as a patch of pale skin or may not be visible at all [67]. Hemangiomas may be present at birth but most often appear in the first several months of life. They occur most often on the neck and face [68]. Generally, no intervention is required, though many parents will need reassurance that the lesion will involute spontaneously in most cases [67,68]. It is possible for these lesions to compress the eyes, airway, or vital organs, in which case the infant should be referred immediately for treatment, usually with steroid injections or laser therapy [67].
Cavernous Hemangiomas
Cavernous hemangiomas are located in the subcutaneous tissue and generally do not involve the overlying skin, though they may be topped by a strawberry hemangioma or nevus flammeus. Cavernous hemangiomas are composed of a communicating network of interconnected venules. They are often present at birth and undergo a period of rapid growth before they begin to recede on their own. They appear as a reddish-blue, spongy swelling filled with blood. Steroids and/or laser therapy may be used to reduce the size of hemangiomas, especially if their location is obstructive or makes them prone to bleeding [68].
Nevus Flammeus
Nevus flammeus, or port wine stain, is usually observed at birth and is composed of dilated or distended dermal capillaries and postcapillary venules [69]. Most frequently found on the face, nevus flammeus are generally red-to-purple in color, can be of varying size, and are generally unilateral [67]. They are not raised and do not blanch with pressure. Treatment is usually unnecessary unless the lesion is very large or associated with an underlying condition [67]. When it appears along with glaucoma and seizures, nevus flammeus is associated with Sturge-Weber syndrome [69]. An infant with nevus flammeus near the eye should be referred to an ophthalmologist [67].
Nevus Simplex
Nevus simplex, also referred to as stork bites, angel kisses, or salmon patches, appear in 30% to 50% of all newborns [67]. Nevus simplex is generally found at the nape of the neck, but may be also found on the face and scalp. It appears pink in color, blanches with pressure, and is commonly bilateral [67,68]. It has no clinical significance and fades quickly, often having disappeared entirely by 18 months of age [70].
Congenital Melanocytic Nevi
Most nevi, or moles, do not appear until after birth. However, 0.2% to 2.1% of infants are born with congenital melanocytic nevi [67]. These lesions are usually flat, but some may be raised. They appear brown or black in color and may be hairy. Due to their potential for malignancy, careful consideration regarding management is required. A hairy nevus discovered along the base of the spine is often associated with spina bifida. Infants with large or giant melanocytic nevi should be referred to a surgeon [67].
Dermal Melanosis
Dermal melanosis, or Mongolian spot, is a common finding in infants of Asian, East Indian, or African descent. Caused by melanocytes trapped deep in the skin, these lesions appear flat and bluish-gray or brown and are most commonly found on the back or buttocks. Due to their resemblance to bruises, which may lead to unsubstantiated allegations of child abuse, it is important to document all dermal melanoses in the infant's medical record and explain their presence to the infant's parents. No intervention is required, and most cases resolve by 2 years of age [71].
Erythema Toxicum Neonatorum
It is estimated that 50% of infants are born with erythema toxicum neonatorum, commonly referred to as newborn rash [72]. The lesions, which may appear as erythematous macules, papules, or vesicles, can appear suddenly in the first three weeks on any part of the body, with the exception of the palms and soles. Although it is not cause for concern in healthy infants, infants who appear ill and have an atypical rash should be tested for fungal, viral, and bacterial infections. The rash has an unpredictable occurrence and presentation. Though it may appear significant to the parents, it requires no treatment and typically resolves within seven days [72,73].
Acne Neonatorum
Acne neonatorum, consisting of closed comedones, is normally found on the forehead, nose, and cheeks. An estimated 20% of infants have acne neonatorum, which is believed to be caused by infant or maternal androgen levels [73]. Parents should be reassured that the acne will resolve with no residual scarring within approximately four months [73].
Milia
Milia are sebaceous glands that are occluded with keratin. They appear as tiny white or yellow papules, approximately 1–2 mm in size, and are generally found on the nose, chin, forehead, and cheeks. They require no special care and usually resolve by 4 weeks of age [34,73].
When assessing the newborn's head, one begins with the general appearance of the head, including the shape, circumference, suture lines, and fontanelle size [74]. Symmetry or asymmetry should be noted, though asymmetry can be a normal variation resulting from the fetal lie in utero. Facial bruising is commonly caused by birth trauma and should be noted. The following section addresses assessment of the newborn's head shape, size, and fontanelles.
During physical examination, the head should be supported appropriately. The head should move easily from side to side and up and down. Infants may or may not be able to support their heads initially. The shape of each infant's head is unique. After a vertex vaginal delivery, a newborn's head is generally flattened over the forehead and rises to a point at the posterior of the skull over the occiput [1]. This shape reflects the process of molding, where the presenting part engages the cervix and becomes molded to the shape of the cervix. Molding is generally symmetrical in nature and is caused by the skull bones coming together to facilitate birth. The infant is born with a classic "cone head" appearance. This occurrence resolves spontaneously within three to five days and requires no intervention, beyond reassuring parents that their infant's head shape is not permanent [74].
Caput succedaneum and cephalohematoma may occur as a result of birth trauma. Caput succedaneum is the formation of edema of the scalp at the presenting part of the head [34,75]. It has a generally symmetrical appearance and crosses the suture lines. Cephalohematoma, a collection of blood beneath the periosteum, may also occur as a result of increased force to the newborn's head during vaginal birth. It has a generally asymmetrical appearance and does not cross suture lines [75]. It may look like a large "goose egg." It can be very alarming to parents, and they should be reassured that it is normal and will go away without treatment [76].
The occipital-frontal circumference in an AGA infant should measure 33–38 cm [77]. The inexperienced practitioner may require assistance if the infant is moving at the time of examination. As noted, the head circumference is approximately 2–3 cm larger than the chest circumference in newborns. The circumference should be plotted on a growth chart.
Important information can be gained by the accurate assessment of both anterior and posterior fontanelles. The fontanelle is best palpated with the second or third finger pad when the infant is quiet and in an upright position [77,78].
The anterior fontanelle, or soft spot, is diamond-shaped and demarcated by the coronal and sagittal sutures. Its anteroposterior measurement is approximately 4–5 cm, and it can be palpated midline, above the forehead [77]. The anterior fontanelle normally closes by 18 months of age [77].
The posterior fontanelle can be palpated midline, toward the back of the head, above the occiput. It is triangular in shape and demarcated by the sagittal and lambdoidal sutures. Its posterolateral measurement is approximately 0.5–2 cm [77]. The posterior fontanelle normally closes by 2 months of age; it is possible for a newborn to be born with a posterior fontanelle already closed [79].
A normal fontanelle should feel soft, yet spongy, and very slightly depressed [80]. A bulging fontanelle appears as a convex shape that feels firm but not spongy. The presence of a bulging fontanelle is indicative of increased intracranial pressure (ICP). Although there are numerous causes, the most common are hydrocephalus, trauma, intracranial hemorrhage, and infections, such as meningitis and encephalitis [74,80]. Accurate diagnosis of the cause of increased ICP may require imaging techniques, such as magnetic resonance imaging, computed tomography, and/or cranial ultrasound [74,79,81]. Crying, lying down, or vomiting can also cause slight bulging of the fontanelle. If the fontanelle returns to normal when the infant is returned to an upright position, it not considered a true bulging fontanelle.
A sunken fontanelle presents as a concave area that feels spongy but depressed. Sunken fontanelles are associated with dehydration and decreased ICP. Decreased peripheral perfusion, poor skin turgor, and sunken eyes may also be present [74]. During fluid resuscitation for dehydration, frequent assessments of the fontanelle can aid in preventing overload.
There are four suture lines that can be palpated: the frontal, coronal, sagittal, and lambdoid sutures. The frontal suture can be felt midline above the eyes running up the forehead and ending at the anterior fontanelle. The coronal suture can be felt from the anterior fontanelle running down the side of the head along the forehead line towards the ears. The sagittal suture can be palpated running midline between the anterior and posterior fontanelle. The lambdoid suture can be felt from the posterior fontanelle running down the head above the occiput towards the area behind the ears [35].
Overriding sutures are a normal finding resulting from birth trauma and molding and usually resolve spontaneously. However, they should be followed closely, and in the event that they do not spontaneously resolve, intervention should be taken to correct the problem. Widely spaced sutures may occur with a bulging anterior fontanelle and are a red flag for increased ICP. In more severe cases of increased ICP, the veins over the scalp may appear enlarged. These infants should receive an infectious disease and metabolic work-up, a standard eye exam, and imaging techniques similar to those used for diagnosing a bulging fontanelle [81].
During the head assessment, warning signs that warrant further investigation and/or immediate intervention include [35,74,81]:
Abnormally large fontanelles
Abnormally small fontanelles
Suture lines that do not override or are widely spaced
Bulging fontanelles
Sunken or depressed fontanelles
Enlarged veins over the scalp
According to the American Academy of Ophthalmology, neonatal conditions that prove to be the most severe and threatening to vision are congenital cataract, retinopathy of prematurity, congenital glaucoma, retinoblastoma, and cerebral visual impairment [82]. There are many risk factors for eye conditions in the newborn, including systemic conditions, neurologic disorders, perinatal complications, and a family history of eye or vision problems [82]. Special attention to eye health should be given to infants who are born prematurely and/or have multiple conditions [82,83].
Following an examination of the general size and appearance of the head, the nurse should assess the eyes. Warning signs should be noted followed by an orderly assessment of eye size, shape, placement, sclera color, and reflexes. Observations of any anomalies should be reported immediately [82].
All regions of the eye should be examined thoroughly for dysmorphic features. Determining the symmetry and completeness of brows and lashes with intact lid margins is important [47]. Initial observations should assess that the eyes are equal in size and placed symmetrically on the face. The outer corner of the eye should be at the same height as the top of the ear, if one were to draw an imaginary line between the two. Low-set ears may be associated with other signs of trisomy 18 or trisomy 21, such as Brushfield spots, a speckling of the iris [75]. Short palpebral fissures are associated with fetal alcohol and other syndromes [84].
When assessing for dysmorphic features, any missing or defective ocular tissue or incomplete development of portions of the eye should be noted as a possible coloboma. Colobomas may involve the eyelid margin or the iris and retina and are associated with several syndromes [85]. The iris may be absent altogether, referred to as aniridia; this most often occurs bilaterally [85].
The upper lid should cover only the top part of the eye. Drooping of the eyelid, or ptosis, may signal neuromuscular weakness [35]. "Doll's eyes" are characterized by a lag in eye movement [86]. This is a normal finding in newborns with muscular immaturity [87].
Epicanthal folds are vertical folds of skin covering the inner canthus of the eye. Epicanthal folds commonly occur among some races (i.e., Asian, Native American) and in newborns of any race prior to the elevation of the bridge of the nose [88]. However, presence of epicanthal folds has also been associated with fetal alcohol syndrome, Down syndrome, and Turner syndrome [88].
Examination of the internal parts of the eye should follow the peripheral examination. Lifting the infant's head while he or she is in the supine position encourages the infant to open its eyes [34]. Term infants are myopic, with a visual acuity of 20/200. Their optimal visual field is approximately 8–12 inches, or about as far away as their mother's face would be during feeding. The lids should unfuse by 28 weeks' gestation, but infants do not have full muscle control of the eyelids at birth. It is also important to assess newborns' tears and exudate. Exudate that is copious, greenish-yellow, or persists or appears after 24 hours of age is a sign of underlying infection [35,85].
The sclera may be white or bluish-white. A yellow appearance of the sclera is indicative of jaundice. Subconjunctival hemorrhage may be present from the pressure of birth. Any irregular shape or unequal size of the iris or pupil should be noted. A white pupil, or cat's eye reflex, indicates abnormalities [85,89]. Downward deviation of the irises exposing the sclera, or sun-setting sign, may be caused by hydrocephalus [90]. When assessing the infant's pupils the terms "equal," "round," "reactive," and "accommodating" can be helpful.
The red reflex is characterized by an equal and round red area of light at the pupil. If a red reflex is absent, white, dull, opaque, or asymmetric, the infant should be further examined for congenital cataracts, and dysmorphia related to chromosomal abnormalities should be considered [35,47,82,85].
There are several eye reflexes that should be examined. The blinking reflex can be tested either by bright light or a light touch. The infant should demonstrate an immediate blink when the eyes are stimulated. The corneal reflex is tested by a light pressure applied to the cornea with a piece of cotton, which should induce an instinctive blink. This reflex is not generally examined unless brain or eye damage is suspected [1]. The pupillary reflex may be determined by shining a light into the eye. The pupil should constrict instantly. Both eyes should be examined in the same manner, with a comparison made between the two. They should have equal size constriction in the same amount of time.
Examination of the ears should include size, shape, and location. Ear cartilage becomes firmer as the fetus ages, so preterm infants may have more pliable ears. The placement of the ear should be assessed as it relates to the inner canthus of the eye. A normal ear will at least touch the imaginary horizontal line. If the top of the ear falls below the line, then the ear is considered low set. A low-set ear may indicate chromosomal abnormalities (e.g., Down Syndrome, Turner Syndrome) and may be associated with renal complications [91,92]. The ears can be measured at their longest axis and compared to standardized charts for determination of dysmorphia [47]. Ears are considered small if less than 2.5 cm in the term infant [47].
In addition to size and position, appearance should also be analyzed for malformations, including absent pinna, abnormal folds, discharge, reddening, or preauricular tags [93]. Abnormal structure may be indicative of other conditions [94,95].
The National Institutes of Health (NIH) and the Joint Committee on Infant Hearing recommend the implementation of universal newborn hearing screening. Since the NIH first endorsed screening all newborns for hearing loss, the number of infants identified as hearing-impaired has increased dramatically [96]. According to the Joint Committee on Infant Hearing, more than 95% of newborns are assessed for hearing loss in the United States. However, nearly one-third of newborns who do not pass initial screening do not have appropriate follow-up to either confirm hearing loss and/or initiate appropriate early intervention services [97]. Technologies used to screen for hearing impairment in this population are auditory brainstem response and otoacoustic emission [97,98].
Infants with normal hearing should have some response to sounds and voices. In newborns, risk factors for hearing loss include a family history of hearing loss, neonatal intensive care stay of greater than five days, aminoglycoside administration of greater than five days, assisted ventilation, ototoxic medications, hyperbilirubinemia, in utero infections, craniofacial malformations, congenital microcephaly, temporal bone abnormalities, and ear anomalies [97]. However, as many as one-half of infants born with hearing loss in the United States have no known risk factors [92].
The nose should be assessed for placement, shape, patency, and the presence of drainage. The nose should be midline on the face. Nares should be symmetrical in placement and size. The assessment for dysmorphic features, such as asymmetric nares or a notched or broad nasal tip, is necessary [47].
A small amount of clear or white discharge from the nose may be noted as a normal finding in the newborn [35,77]. However, copious or discolored nasal discharge may be a sign of congenital syphilis or respiratory problems [77]. Patency may be determined by closing the infant's mouth and assessing the quality of respiratory effort. Obstructing one nare at a time can be useful in determining choanal atresia, which is a blockage in the posterior nasal passage. One can assess the movement of air in and out of the nares by placing a finger under the nares to feel air movement. Obstructed nasal passages are an important finding as newborns are obligatory nose breathers and usually cannot breathe orally even when compromised.
When examining the mouth of a newborn, symmetry, completeness, size, and color are considerations. The mouth should be midline and symmetrical [77]. Asymmetrical movement may be caused by nerve injury from birth trauma and may include other parts of the face.
Inspect the mouth, lips, tongue, and gums for deformities. If the lighting is poor, a pen light may be used to thoroughly visualize the palate. Gentle assistance should be employed to open the mouth while a second team member completes the assessment. The intactness of the palate may be determined by both visual and tactile strategies. The palate should be palpated for intactness by inserting a gloved finger, soft side up, and feeling the roof of the mouth. Alterations in the shape of the palate may cause breathing or feeding problems. Epstein's pearls, small calcium deposits that form midline on the hard palate, may be present. They will reabsorb within approximately one week [77].
The infant's tongue may appear large for its mouth and shorter than either a child's or an adult's [34,77]. Dysmorphic findings concerning the mouth, tongue, and chin include a flattened or elongated philtrum, disproportionate tongue, small chin, short frenulum ("tongue tie"), and cleft palates and lips [47]. If the infant's mouth remains open and the tongue protrudes, this may be a sign of an existing condition, such as Down syndrome, and should be noted [92].
The color of mucous membranes and lips should be monitored. The colors may be described as dark, purple, dusky, pink, or pale. Using a referenced color gauge or having two nurses assess the infant's color can help with the accuracy. Mucous membranes should be pink and well-hydrated, and a small amount of saliva should be present [77]. If there is a large amount of saliva and the infant is "blowing bubbles," the mouth should be suctioned with a bulb syringe [77]. If this does not resolve the issue, further assessment should be conducted to determine the patency of the esophagus before the infant is allowed to eat.
Observe for natal teeth. While natal teeth occur in normal infants, they are more likely to occur with cleft palate [85]. If teeth are present, they should be noted and reported. Natal teeth are generally removed to prevent a choking hazard, especially if they are loose [77].
There are several reflexes that should be assessed while examining the mouth. The intensity of the sucking reflex may be described as strong or weak and may be assessed either by placing a finger in the infant's mouth or monitoring feeding. For the critically ill infant who is being fed through a tube, the assessment of the strength of his or her sucking reflex may be determined as the infant sucks on either the feeding tube or the endotracheal tube. A strong suck response occurs when the infant is capable of forming a tight seal around the finger, nipple, or bottle. A weak suck occurs if the infant is either unable to form a seal or unable to suck because of fatigue or deformity. If an alert term infant is unable to suck after many attempts, the infant should be evaluated further.
Assess for a gag reflex by gently stimulating the posterior oral cavity. The infant should have a strong coughing response to the stimulation. If the response is weak, this should also be noted and the appropriate intervention completed. Absence of a gag reflex should be considered an emergency situation because newborns cannot protect their airway without a gag reflex. The extrusion reflex occurs when the infant responds to foreign objects in the mouth by pushing them outward with the tongue.
The rooting reflex is present at birth and assists the feeding process in newborns. To elicit this reflex, the infant's cheek is gently stroked. The normal response is for the infant to turn the mouth in the same direction of the cheek that was stroked and initiate a sucking motion. Variants of this reflex include stroking the upper lip, which should cause the infant to flex his or her head back, and stroking the bottom lip, which should cause the infant to drop his or her jaw. This reflex may be slowed as a result of maternal sedation or a recent feeding. The rooting reflex lasts until approximately 4 to 6 months of age [92,99,100].
The neck of the normal newborn is noticeably shorter and more flexible than that of an adult or child. The normal newborn will exhibit creasing and skinfolds on the neck. Infants are generally not capable of supporting their heads at birth and will experience head lag when they are moved to a sitting position from a lying one [33]. Assess lymph nodes and monitor for webbing and any masses [33]. Webbing of the neck, generally noticed from the back of the neck, may be indicative of chromosomal abnormalities [77].
Palpation of each clavicle may help determine intactness. A fractured clavicle may present with an elevation of the bone, or a grating sensation may be felt when manipulated [33]. The newborn should be able to demonstrate free range of motion that is symmetrical.
The newborn neurologic assessment takes into consideration the immaturity of the neurologic system. As the infant grows and develops, a more certain presentation of the intactness of the neurologic system will surface. When assessing neurologic status, one should be alert for warning signs and then proceed by assessing the newborn's reflexes.
Warning signs of the neurologic assessment, which would warrant further investigation and/or immediate intervention, include [35,92]:
Lack of reflex/response to stimuli
Hypertonic or hypotonic position
General lethargy
Pupillary changes
Assessing primitive reflexes in newborns gives one great insight into the newborn's neurologic status. Reflexes are key to the survival of the newborn and may be the single greatest indicator of neurologic health. Some of the most common reflexes include the Moro, rooting, tonic-neck, stepping, palmar grasp, plantar grasp, and Babinski[92,99,101](Table 3).
PRIMITIVE REFLEXES IN THE NEWBORN
| Reflex | Appears | Disappears (Approximate) | Brief Description |
|---|---|---|---|
| Rooting | At birth | Generally becomes voluntary after 3 weeks of age | Turns mouth to the same side of the cheek that is being stroked |
| Gag | At birth | Continues into adulthood | Strong coughs in response to stimulation of the posterior oral cavity |
| Extrusion | At birth | 3 to 4 months of age | Uses tongue to push foreign objects out of mouth |
| Moro (startle) | As early as 32 weeks' gestation | 6 months of age | When dropped slightly, quickly abducts extremities and forms the index finger and thumb into a "c" shape |
| Tonic neck (fencing) | Between birth and 2 months of age | 4 to 6 months of age | When the infant's head is turned to one side, with the jaw over the shoulder, the arm and the leg on the infant's same side extend while the opposite arm and leg flex |
| Stepping | At birth | 2 months of age | Simulates walking when held in an upright position and the sole of the foot touches a flat surface |
| Palmar grasp | As early as 28 weeks' gestation | 4 to 6 months of age | Wraps fingers around the examiner's finger when it is placed into the infant's palm |
| Plantar grasp | At birth | 8 months of age | Curls toes downward in response to pressure applied to the sole of the foot at the base of the toes |
| Babinski | At birth | 2 years of age | Flexes the big toe when an object is dragged along the sole of the foot from the heel to the head of the 5th metatarsal |
During the assessment of all newborn reflexes, considerations of symmetry and the strength of the infant's response should be taken into account. It is important to note that gestational age, but not weight, will directly impact the rate by which the infant responds [103,104]. Infants delivered via cesarean section may have a reduced response to reflex evaluations [105].
Because the assessment of the neurologic system can be rigorous for the infant, it may be recommended to assess the infant's glucose level and special oxygen demands as well as any signs of fatigue before beginning the assessment. The infant should be assessed in an alert state.
The Moro reflex, also known as the startle reflex, is generally present from 32 weeks' gestation to six months after birth. The Moro reflex can be assessed by holding the infant in a supine position and, with the head, neck, and spine appropriately supported, allowing the infant to drop slightly. The infant should respond by extending and abducting extremities. The infant should also fan his or her fingers and form a "c" shape with the index finger and thumb. Subsequently, the arms should adduct and the infant should return to a relaxed position. Absence of the Moro reflex may indicate damage to the brain or spinal cord. Absence on only one side of the body may indicate either a broken humerus or scapula or a nerve injury [99].
The tonic neck reflex, commonly referred to as fencing, appears between birth and 2 months of age and disappears at approximately 4 to 6 months of age [99]. To obtain this response, place the infant in a supine position. Rotate the infant's head to one side so the jaw is over the shoulder. The arm and leg on the same side should extend and the arm and leg of the opposite side should flex. Turn the head to the other side and the extremities should mirror this posture.
The stepping reflex is generally present from birth to 2 months of age. This reflex is elicited by holding the infant upright and placing his or her sole of the foot against a hard, flat surface [99]. The infant should respond by alternately flexing and extending the legs to simulate walking.
Palmar grasp reflex is present from 28 weeks' gestation until four to six months after birth. Pressing the examiner's finger into the palm of the infant's hand may elicit this response [99]. The infant's fingers should flex around the examiner's finger. The plantar grasp is present from birth to 8 months of age. The examiner applies pressure to the bottom of the foot at the base of the toes. The toes should curl downward.
Babinski reflex is generally present from birth to 2 years of age [106]. Babinski reflex is simple yet extremely important in the determination of pathology [102]. The infant should be awake with the head in a midline position for testing. The examiner may drag the wooden or plastic end of a cotton-tipped applicator along the lateral aspect of the sole from the heel to the head of the 5th metatarsal. One study rated the response based on flexion or extension of the great toe and did not gauge the response based on fanning of the other toes [102]. Using the described method, researchers found a 90% response of extension of the great toe. They also found that a small percentage of infants may demonstrate extension at a later age and suggested that other infants may demonstrate extension when in a more awake state. Other studies found similar responses [107,108]. An inappropriate response to this test indicates the need for further neurologic evaluation [106].
Pain is an important consideration when assessing an infant's neurologic status. A failure to respond appropriately to painful stimuli can be an indicator of altered neurologic status. The newborn should demonstrate chemical, behavioral, and physiologic responses to pain. Chemical responses are caused from the release of catecholamines, glucagon, growth hormone, cortisol, and aldosterone. This, in turn, results in an increase in metabolic demand, possibly leading to metabolic instability.
Behavioral responses such as facial expression, crying, and body movements are appropriate areas of assessment of pain response in the newborn. Research has shown that body movements such as tremors, twitches, startles, arching, and squirming are not accurate pain indicators; however, limb extensions and finger splays have been shown to be more accurate indicators of pain, especially in preterm infants [109,110,111]. Factors that affect pain response include exposure to multiple painful procedures, lower Apgar scores, earlier gestational age, and illness [109,110]. When assessing behaviors associated with pain in the newborn, these factors should be considered. Physiologic responses also include changes in heart rate, respiratory rate, blood pressure, color, oxygen saturation, vagal tone, and ICP.
A visual inspection of the chest should be completed initially for size, shape, symmetry of movement, and presence of identifying features. The chest should be round and 1–2 cm smaller than the circumference of the infant's head [92]. Chest circumference is obtained by measuring around the infant's chest at the nipple line midway between inspiration and expiration. Measuring the distance between the nipples divided by the chest circumference determines a narrow versus broad chest. In a narrow chest, the internipple distance will be less than 25% of the chest circumference. In a broad chest, the difference will be more than 33% of the chest circumference [47].
Assessment of the infant's breasts should include size, shape, and nipple formation, placement, and number. Breast bud size is an important indicator of gestational age. Some infants are born with supernumerary nipples, usually located vertically, below the normal nipple [92]. These are very rarely associated with any adverse effects or conditions [92,112]. As discussed, they are often mistaken for nevi [67].
Gynecomastia is common in either gender and may be noted as late as the second or third day of life. It is caused by high levels of maternal estrogen that have passed through the placenta, and should resolve spontaneously [1]. Neonatal gynecomastia is often accompanied by galactorrhea, also called pseudomenses milk or "witch's milk" [113]. In infants with galactorrhea, the discharge is usually bilateral. Unilateral, bloody, serous, or purulent discharge should be noted as a sign of an underlying pathologic cause [113]. According to the American Academy of Family Physicians, galactorrhea should also be documented as [113]:
Scant or abundant
Expressed or spontaneous
Intermittent or persistent
If no underlying disease exists, galactorrhea will resolve spontaneously.
Throughout pregnancy, the fetus receives its oxygen through the umbilical cord from maternal oxygen stores. Oxygenated blood flows from the mother through the uterine artery into the intervillous spaces of the placenta, where gas exchange occurs [33]. Fetal circulation begins with the umbilical vein picking up the oxygen stores from the intervillous spaces of the placenta and transporting them to the fetal heart. Most of the blood bypasses the fetal lungs as they do not have a respiratory gas exchange function [33]. When an infant is born, therefore, a sequence of events must occur in order for the infant to breathe.
In utero, the pulmonary circulation has elevated pressures and low flow states because of high pulmonary vascular resistance [114]. The newborn's pulmonary vascular pressure is partially related to a higher CO2 from passive maternal oxygenation and is partially related to the pressure applied by amniotic fluid inside the lung [114]. Also, pulmonary vascular pressures are consistently higher than systemic resistance prior to birth [114]. The result is that only 5% to 10% of the entire cardiac output is used for perfusion of the fetal lung [114].
Pressure increases on the fetal chest as the infant is expelled from the uterus during a vaginal delivery. The amniotic fluid inside the lung compartment is pushed out through the airway. Once the infant's head and chest are delivered, the chest re-expands, causing an intake of air to fill the void where amniotic fluid once was [115]. The first few breaths taken by the infant are critical to changes in pressures and functioning of the newborn's pulmonary function. The most common cause of spontaneous pneumothorax in infants is the degree of transpulmonary pressure generated by the first few breaths [116].
The exact stimulant for breathing may not become evident because the newborn is exposed to so many new factors at once, including pain, acidosis, light stimulation, and sensory factors, among others [114,117]. Physical (mechanical) factors that stimulate breathing include the compression of the fetal chest as it moves through the birth canal, and the chest wall recoil, which occurs as the newborn's trunk emerges. Chemical factors, such as the decreases in oxygen concentration and lack of placental blood flow, cause the medulla to stimulate the respiratory center to begin functioning [117]. Once the lungs are open to airflow, the pressure within the capillaries decreases and an increase in the amount of blood to the pulmonary bed occurs [117]. The change in temperature from the intrauterine environment to the extrauterine environment (a decrease of more than 20°F) also works to produce active respiratory effort. An increase in the blood flow to the pulmonary bed occurs as a result of pulmonary vascular relaxation. The pulmonary vascular bed relaxes due to the increase in oxygen saturation that the newborn is able to obtain using his or her circulatory system [114,117].
Due to the difference in fetal hemoglobin and exposure to lower PO2 levels, laboratory data obtained from the newborn are considerably different than that of an older child or adult. The normal hemoglobin level in the newborn is 17–18 g/dL [33,34]. The newborn is born with up to 90% of fetal hemoglobin. Hematocrit elevation is a normal finding [34].
Leukocytosis of the newborn, caused by increased neutrophil production in the first days of life, is related to the stress of birth [33]. This phenomenon self-corrects in about two weeks.
A thorough evaluation of the infant's respiratory system includes two components. Inspection of the newborn's breathing effort and chest movement begins the process, followed by auscultation of the infant's lung fields. The infant's lungs are positioned anteriorly from above the middle third of the clavicles to the sixth rib at the midclavicular line and to the eighth rib at the midaxillary line. A thorough posterior assessment is warranted as the infant's lower lobes are primarily located posteriorly to the eleventh rib at the vertebral line [50].
Warning signs of the respiratory assessment, which would warrant further investigation and/or immediate intervention, include [33,35,118]:
Respiration rate less than 30 or greater than 60 breaths per minute. In some cases, respiratory rates up to 70 breaths per minute in the first few hours of life are acceptable. When assessing rate, a full minute count should be taken to compensate for irregularities in pattern.
Apnea lasting 20 seconds or longer
Presence of central cyanosis. Acrocyanosis may be a normal finding in the newborn, but central cyanosis, or the blue or gray coloring of the mucous membranes, trunk, or entire body, is never a normal finding and requires immediate intervention.
Inspection of the infant's breathing effort and chest movements is ideally done while the infant is at rest. Many newborns experience a breathing pattern commonly known as periodic breathing of the newborn. The infant's breathing pattern may be irregular and include pauses lasting 5 to 15 seconds [119]. Respiratory patterns change in the newborn depending upon the infant's state. If in a deep sleep, the pattern is usually regular; however, it may become irregular with increased motor activity [33]. The work of breathing should be assessed and noted to be unlabored. The infant should appear to be breathing easily. The chest should rise and fall with each breath and be symmetrical in its movement. Chest movement should be synchronized and smooth. Infants in distress may present with lag or seesaw movements of the chest. The assessment of the infant's skin color is necessary as changes in color offer information regarding air exchange [119].
When auscultating neonatal lung fields, it is recommended that a diaphragm stethoscope of 2.5 cm diameter or less be used [50]. When listening with a stethoscope, it is beneficial to first assess one position and then, immediately after, assess the mirror position on the opposite side of the body to determine even the slightest differences in quality. The method of moving the stethoscope in an "S" shaped pattern over the chest is optimal but not always possible if the infant is squirming or has bandages that preclude this systematic manner. Listening along the axilla for resonant sounds can also provide vital information.
The normal newborn may have very noisy or wet-sounding lungs, especially in the first 24 hours of life [119]. In general, there is vast improvement in the noise of the newborn's lungs in the first several hours of life. Abnormal sounds include [119]:
Stridor: An inspiratory, high-pitched piercing sound usually indicative of an upper airway blockage
Rales: Low, coarse sounds heard on inspiration or expiration
Crackles: A cracking or popping sound that occurs when the air that passes through the small airways in the lung is somewhat obstructed by fluid, mucus, or pus at different cycles of inspiration
Wheezing: A continuous, high-pitched sound heard on inspiration
The three cardinal signs of respiratory distress in newborns are grunting, nasal flaring, and intercostal retractions. Expiratory grunts occur when the infant attempts to expel trapped air or fetal lung fluid [91]. They may be audible with or without the use of a stethoscope. As they become more severe, grunts can be heard with the unaided ear. Dilatation of the nares, or nasal flaring, may be an early symptom in cases of mild distress and may progress as the infant continues to experience problems with air movement. Flaring occurs when the newborn attempts to bring more air into the lungs. Intercostal retractions are noted as the ribs become visible on inspiration. Intercostal, or xiphoid, retractions may occur and have the appearance of a strong tug at the area of the xiphoid. Retractions are generally described as mild, moderate, or marked.
Respiratory distress syndrome (RDS) is caused by surfactant deficiency and structural immaturity of the infant's lungs [120]. RDS has also been referred to as hyaline membrane syndrome due to the formation of hyaline membranes concurrent with this condition; however, hyaline membranes have also been associated with other respiratory conditions [120]. RDS is the recommended term to distinguish the specific symptoms characteristic of this condition.
The incidence and acuteness of RDS is directly related to gestational age. It is most prevalent in newborns younger than 28 weeks' gestation and/or weighing less than 1,200 g [120]. RDS affects more white infants than infants of other races [121]. Risk factors include maternal diabetes, caesarean delivery devoid of labor, latter born of twins, and familiality [120].
RDS presents either at birth or within the first eight hours of life [120]. If symptoms occur after the first eight hours, then it is unlikely that RDS is the cause. The most common signs of RDS are [120]:
Tachypnea
Dyspnea
Grunting with expiration
Intercostal retractions with inspiration
Nasal flaring
Increasing cyanosis
Oxygen and surfactant therapy are used for support and treatment. Minimizing disturbances, handling the infant gently, and maintaining an ideal body temperature will help reduce the infant's oxygen needs. Infants with RDS need careful fluid management and should be carefully monitored for signs of infection [120].
Transient tachypnea of the newborn (TTN) is a relatively common condition in near-term infants. It is thought to be caused by residual fluid or debris in the lungs and is more common in infants delivered via cesarean section [33]. TTN occurs more frequently with infant sedation via maternal medication, prolonged labor, fetal macrosomia, and maternal asthma [122].
The condition generally presents as mild respiratory distress with marked tachypnea, in some cases with rates up to 100 to 140 breaths per minute [33]. Though a thorough workup should be done to rule out other causes of respiratory distress, TTN is generally benign and will resolve within 48 to 72 hours [75,122]. Oxygen support may be required in severe cases [33].
The presenting sign of sepsis in many newborns is respiratory distress. Therefore, a thorough septic workup is in order in a newborn with signs of respiratory compromise.
Neonatal sepsis is categorized as early onset when it presents within the first seven days of life; most cases present within the first 24 hours [123]. The leading cause of early-onset neonatal sepsis is GBS, followed by Escherichia coli[124]. Events that increase the risk of neonatal sepsis include [122]:
Maternal infection with GBS during pregnancy
Premature delivery
Membrane rupture lasting longer than 24 hours
Chorioamnionitis
Frequent vaginal examinations during labor
In addition to respiratory distress, signs of sepsis may include behavioral changes (e.g., lethargy, temperature instability, feeding difficulty), jaundice, and cardiac changes, such as tachycardia or bradycardia. The following manifestations may be observed in neonatal sepsis [1]:
General Signs
Infant generally "not doing well"
Poor temperature control (i.e., hypothermia, hyperthermia [rare])
Circulatory System
Pallor, cyanosis, or mottling
Cold, clammy skin
Hypotension
Edema
Abnormal heartbeat (i.e., bradycardia, tachycardia)
Respiratory System
Irregular respirations (i.e., apnea, tachypnea)
Cyanosis
Grunting
Dyspnea
Retractions
Central Nervous System
Diminished activity (e.g., lethargy, hyporeflexia, coma)
Increased activity (e.g., irritability, tremors, seizures)
Bulging fontanelle
Increased or decreased tone
Abnormal eye movements
Gastrointestinal System
Poor feeding
Vomiting
Diarrhea or decreased stooling
Abdominal distension
Hepatomegaly
Hemocult (i.e., positive stools)
Hematopoietic System
Jaundice
Pallor
Petechiae, ecchymosis
Splenomegaly
Although some studies have shown that the use of certain antibiotics may lead to antibiotic resistance and possible damage to the intestine, kidneys, liver, or hearing, antimicrobial therapy is still commonly used to treat sepsis [125]. The American College of Obstetricians and Gynecologists recommends that if the mother is receiving intrapartum antibacterial prophylaxis (IAP) for suspected GBS or chorioamnionitis and the newborn exhibits signs of sepsis, empiric therapy should be administered along with a complete diagnostic evaluation [24,126]. If the infant does not exhibit signs of sepsis but maternal chorioamnionitis is present, empiric therapy and a limited evaluation (i.e., blood culture and complete blood count) are indicated. If the gestational age is less than 37 weeks or the duration of maternal IAP has been less than four hours, a limited evaluation and close observation for a minimum of 48 hours are recommended [24,126]. If neonatal sepsis is suspected, empiric therapy should be administered along with a complete diagnostic evaluation. If there are no signs of sepsis, maternal IAP duration exceeds four hours, and the infant is older than 37 weeks' gestation, no evaluation or therapy is required [24,126]. However, the infant should be closely observed for a minimum of 48 hours. Penicillin is considered the most effective treatment [24,126]. Extreme cases may require assisted ventilation. As sepsis can have an extremely fast onset and cause rapid deterioration, it is imperative that treatment begin immediately.
Meconium aspiration syndrome (MAS) is another cause of respiratory distress in newborns and occurs in approximately 5% of those born with meconium-stained amniotic fluid [127,128]. It is caused by the aspiration of meconium-stained amniotic fluid into the trachea. Meconium staining may be noted by assessing the infant's skin, nails, and umbilical cord and can affect the infant's Apgar score [35]. Presence of meconium may clue the practitioner to assess for tachypnea, hypoxia, and hyper- or hypoventilation [86]. The presence of rales, rhonchi, wheezing, wet sounds with possible retractions, and labored breathing are possible, depending upon the extent of aspiration. Risk factors for MAS include [128]:
Fetal distress, long labor, or difficult delivery
Maternal diabetes
Maternal hypertension
Low oxygen supply to the infant in utero
Meconium aspiration is associated with asphyxia, but routine intubation and intrapartum suctioning are no longer recommended, regardless of the infant's activity level [127]. Gently clearing the nose and mouth of meconium with a bulb syringe may be warranted. A neonatal advanced life support team should be notified and available to treat a newborn with meconium-stained amniotic fluid [127]. Other treatments for MAS include oxygen therapy, mechanical ventilation, fluid support, a warmer to control temperature, and antibiotics [128,129].
The fetal cardiovascular system is markedly different from that of the newborn. Because the fetus does not rely upon its lungs for oxygenation, very little blood flows through the pulmonary vasculature. Fetal circulation begins with oxygenated blood traveling to the fetus through the umbilical vein. Note the unusual circumstance of a vein carrying richly oxygenated blood. The blood enters the fetus at the site of the umbilicus and divides into two branches. One branch carries blood to the fetal liver, while the other, larger branch carries blood through the ductus venosus into the fetal vena cava [33]. The oxygen-rich blood then travels to the fetal heart through the right atrium. In an adult, the blood passes into the right ventricle for distribution into the pulmonary vasculature, which would then oxygenate the blood. Because the blood in the fetus is already oxygenated, the majority then passes through the foramen ovale to the left atrium. A small amount of blood does pass into the right ventricle, which pumps the blood into the pulmonary artery to oxygenate the lung tissue. However, the majority of this blood also passes through another duct, the ductus arteriosus, into the aorta for distribution to the body [33]. The deoxygenated blood then leaves the fetus via two umbilical arteries for nutrient and gas exchange in the intervillous spaces of the placenta.
Once the newborn is breathing and maternal blood flow from the umbilicus stops, changes in blood flow, pressures, and volume occur within the heart, causing structural changes. The foramen ovale, which is a naturally occurring atrial septal defect, closes, as does the ductus venosus, due to the changes in pressure and flow [37]. The increase in arterial oxygen tension causes the ductus arteriosus to begin to close at a later time, approximately 12 hours after birth. It is completely closed no later than 21 days after birth [37,130]. The size and shape of the heart changes over a period of time as the left ventricle assumes the primary pumping role.
After the newborn's structural cardiovascular changes occur and the infant is breathing, the pressures in the heart and the volume of blood during flow also shift because of the new route. When the lungs begin receiving a higher concentration of oxygen, the pulmonary vascular bed relaxes, allowing blood to flow through the lungs [130]. Blood through the ductus venosus is halted following the clamping of the umbilical cord. The ductus venosus usually completely closes seven days after birth, though there is no flow through it after the umbilical cord is clamped. When the cord is clamped, it separates the newborn from maternal blood flow, causing systemic vascular resistance to rise. This is due to the increase in pressure of the newborn's circulatory system as opposed to the low pressures in the placenta [130].
It takes time for the fetal right ventricular muscle to remodel itself into the lesser pump side and decrease work [114]. Though the left side of the heart is functioning as in an adult, the muscle mass may not achieve complete dominance over the right ventricle until the sixth month of life [114].
The cardiovascular assessment is composed of auscultation, electrocardiogram (ECG) analysis, blood pressure monitoring, pulse quality, and capillary refill time. However, in the healthy term infant, many practitioners will do little more than auscultation if there are no other signs of problems. Cardiac abnormalities generally present themselves quite obtrusively.
One should note the regularity or irregularity of the rhythm when assessing heart sounds. Noting changes as they associate with breathing patterns will be helpful in identifying the need for further evaluation. Normal heart tones are a result of the heart's valves opening and closing.
Auscultation of heart sounds may be performed in the following sequence [50,131]:
The aortic area, which is defined as the second intercostal space at the right of the sternum
The pulmonic area, which is located at the second intercostal space to the left of the sternum
Erb's point, which is located at the third intercostal space to the left of the sternum
The tricuspid area, which is located in the fifth intercostal space to both the left and right of the sternum
The apical area, which is found at the fourth intercostal space at the left of the left midclavicular line
The normal heart rate range of an awakened newborn is between 100 to 150 bpm [132]. Bradyarrhythmias occur when the heart rate is less than 60 bpm [133]. If the slow rate is associated with poor systemic perfusion, it should be treated [63]. Tachycardias vary in origin; they may originate from the sinus, supraventricular, or ventricular areas. In infants with tachycardia, the rate is greater than normal for age and is typically greater than 220 bpm [133]. Both bradycardia and tachycardia should be considered as signs of cardiac compromise, noted, and referred for appropriate treatment.
Monitoring the infant's ECG can provide valuable assessment information. The basic ECG cycle consists of a P wave, a QRS, and a T wave. The P wave represents depolarization of both atria. The QRS represents depolarization of the ventricles, and the T wave represents repolarization of the ventricles. Abnormal rhythms may be classified as fast, slow, or pulseless [133]. Rhythm disturbances should be treated if they compromise cardiac output or have the potential to degenerate to a lethal rhythm [133].
Blood pressure monitoring is usually performed to identify low perfusion states associated with decreased vascular volume or decreased cardiac output and also to identify hypertensive states. In healthy term newborns, blood pressure monitoring is rarely performed unless there is an indication of a problem. In the healthy newborn, blood pressure is maintained at a fairly constant level by inter-related changes in resistance and cardiac output. Regional blood flow is determined by metabolic needs and adjusted by changes in resistance rather than pressure. Cardiac output adjusts flow to maintain a fairly constant blood pressure. In cases of severe asphyxia, septic shock, or blood loss, a decrease in blood pressure is a late sign of compromise [134,135]. In the first week of life, blood pressure may be slightly higher in the lower extremities than in the upper extremities [3]. Pulse pressure is obtained by subtracting the diastolic pressure from the systolic pressure. For the term infant, a wide pulse pressure is 25–30 mm Hg, and in the preterm infant, it is 15–25 mm Hg [3]. Multiple readings should be obtained and averaged for the best results [134].
Murmurs occur as blood moves through a highly turbulent area in the heart. Heart murmurs are very common in newborns as their cardiopulmonary systems adjust to extrauterine life [92,136]. These benign murmurs in newborns are usually transient in nature and caused by the foramen ovale not being closed completely. Murmurs are usually benign and not necessarily indicative of heart disease [85]. They generally resolve spontaneously.
Murmurs are classified by intensity (i.e., loudness) from grades 1 to 6. A grade 1 murmur is barely audible and not heard in all positions with the use of a stethoscope. A grade 6 murmur is audible with the stethoscope off the chest [137,138]. The location of the murmur identifies the type of defect. Apical murmurs are indicative of mitral insufficiency, mitral stenosis, subaortic stenosis, aortic insufficiency, aortic ejection click of aortic stenosis, or click or late systolic murmur of mitral valve prolapse [137]. Tricuspid murmurs are indicative of tricuspid insufficiency or stenosis, pulmonary insufficiency, ventricular septal defect, or aortic insufficiency [137]. Aortic murmurs are indicative of aortic insufficiency or aortic stenosis [137]. Pulmonic murmurs are generally caused by pulmonary stenosis or insufficiency, atrial septal defect, pulmonary ejection click, or patent ductus arteriosus [137].
In the United States, approximately 1%, or 40,000, of infants born annually have congenital heart defects (CHDs), which are considered the most common type of birth defect [139]. Survival rates of infants born with CHDs have improved over the past several decades. Approximately 75% of infants born with CHDs are expected to survive to 1 year of age and 69% are expected to survive to 18 years of age [140,141]. Family history, maternal diabetes, maternal overweight, maternal obesity, maternal smoking during pregnancy, genetic or chromosomal abnormalities, and maternal exposure to certain drugs or organic solvents may all contribute to the development of CHDs [140,141,142]. Genetic defects are also associated with a higher risk of CHDs. For example, 50% of infants born with Down syndrome also have CHDs [139]. Acute CHD symptoms occur within the first few days of life in approximately one-third of infants [141].
Feeding difficulties are often a first sign, and they may be evident as early as 6 to 12 hours prior to symptoms of heart failure [144]. Because feeding difficulty may be attributed to many different causes, it should exceed 30 minutes and be accompanied by tachypnea, sweating, and subcostal retraction to be considered related to a CHD or heart failure [139,141].
CHDs are classified as acyanotic or cyanotic. Acyanotic defects are usually associated with left-to-right shunting and have symptoms similar to congestive heart failure. Cyanotic defects are characterized by a mixing of oxygenated and unoxygenated blood resulting from the various defects. These defects cause low oxygen-saturated blood to be pumped through the circulatory system.
Patent Ductus Arteriosus
Patent ductus arteriosus (PDA) is an acyanotic cardiac lesion that occurs when the ductus arteriosus that is present in fetal circulation fails to close completely within 12 hours of birth. It allows for a mixing of oxygenated and deoxygenated blood at the level of the aorta, causing the body and brain to receive blood with a lower PO2[139].
PDA has a higher incidence in premature and low birth weight infants, females, exposure to rubella during the first trimester of gestation, and high-altitude births [139,141]. Signs associated with PDA include a grade 2 or 3 murmur and an increased difference between systolic and diastolic pulse pressure [33]. Untreated, PDA can lead to right ventricular failure and pulmonary congestion. Treatment includes medications such as indomethacin or ibuprofen, transcatheter placement, or surgery [139].
Atrial Septal Defect
Atrial septal defect (ASD), another acyanotic cardiac lesion, occurs when the foramen ovale fails to close, leaving an opening between the right and left atrium. This generally results in a left-to-right shunting of blood and is generally asymptomatic initially.
The signs of ASD include a murmur and, eventually, failure to thrive, poor exercise tolerance, and upper respiratory infections [33]. Approximately 50% of ASDs close spontaneously, and approximately 20% resolve within the first year of life [139,140]. Treatment includes catheter and surgical procedures [139].
Ventricular Septal Defect
Ventricular septal defect (VSD) is an acyanotic lesion that occurs due to an abnormal opening between the left and right ventricles. It is more common in male infants than female infants. VSDs vary in size and can lead to left-to-right shunting of blood. These defects are often asymptomatic initially, followed by a degenerative course over the first several weeks to months.
The signs of VSD include a loud murmur, tachypnea, growth failure, feeding difficulties, and eventual heart failure [33]. VSDs can exist without symptoms of heart failure, and as with ASDs, more than 50% close spontaneously. If no signs of heart failure are evident, surgical intervention may be delayed with close follow-up [146]. Because infants with VSDs are often premature and/or tire easily during feedings, nutrition therapy is recommended. Nutrition therapy is considered temporary treatment and may be administered in the form of high-calorie formula, breast milk supplements, and in some cases, tube feeding. If VSD symptoms persist or worsen, surgical intervention is required [146].
Coarctation of the Aorta
Coarctation of the aorta is an acyanotic cardiac lesion that occurs when there is a narrowing of the aorta. It accounts for 5% to 8% of all CHDs [147]. This stricture may cause an obstruction to the blood flow, resulting in increased left ventricular pressure [33]. Signs of coarctation include absent or diminished femoral pulses, murmur, and degeneration into heart failure within the first 7 to 21 days of life [33]. Treatment is surgical [148,149].
Transposition of the Great Arteries
Transposition of the great arteries (TGA) affects approximately 1 in 3,400 newborns annually and is often accompanied by heart failure [141,150,151]. The aorta and pulmonary artery are switched when they are formed in the embryonic stage. Signs of transposition include cyanosis, hyperpnea, difficulty feeding, and clubbing of the fingers or toes [150,151].
Transposition has been associated with exposure to rubella or other viral illnesses, poor prenatal nutrition, alcoholism, maternal diabetes, and advanced maternal age (i.e., older than 40 years of age) [150,151]. Prostaglandin E is administered intravenously, which maintains a patent ductus arteriosus until transposition is repaired via surgery [150].
Tetralogy of Fallot
The tetralogy of Fallot is the most common cyanotic CHD, occurring in approximately 1 in every 2,500 newborns, although it may not be evident as early as other defects [152,153]. It is characterized by four components: pulmonary stenosis, overriding aorta, right ventricular hypertrophy, and VSD [152,153]. Tetralogy of Fallot has been associated with the same conditions as TGA; however, chromosomal disorders are also common in this population [152].
Infants with tetralogy of Fallot eventually develop what are commonly known as "tet spells," which include hyperpnea, irritability, decreased murmur intensity, and extreme cyanosis in the extremities and mouth during crying, feeding, or exertion [141,152,153]. Infants may also have feeding difficulties, failure to thrive, syncope, clubbing of fingers, or sudden death [152]. Some immediate relief during a spell may be achieved by squatting. For newborns, this maneuver may be accomplished by placing infants on their sides and putting their knees up to their chests. Other interventions that are helpful are slower feedings, smaller and more frequent meals, and comfort measures to minimize anxiety [152].
The tetralogy of Fallot is the most likely CHD to remain untreated past infancy [139,141]. In the past, a palliative surgery was completed to improve blood flow, with complete corrective surgery often postponed until the child reached 3 years of age [141]. Complete surgical repair is now performed during infancy, and infants who have surgery usually do well [139,153]. However, infants too weak or too small to have full surgical repair may still receive the palliative surgery first and the complete surgical repair later [139]. Without surgery, death usually occurs before 20 years of age [153].
Peripheral pulses should be assessed for quality and equality. The pulse rate and rhythm should match that of the apical rate and rhythm. Assessing these at the same time can assure mechanical conduction.
Capillary refill time determination is usually performed by applying enough pressure to cause blanching to the dorsum of the foot or palm for approximately five seconds and then tracking the length of time until color uniformity returns. This technique is sometimes used to help assess the newborn cardiovascular status. However, it is considered controversial due to the large variation in the results of studies. Many factors affect refill time, including skin temperature of the examiner's hand; axillary temperature of the newborn; environmental temperature; length of time the examiner applies pressure (i.e., accuracy/ability of the examiner to precisely measure five seconds); body site used; and the firmness of applied pressure [155]. Studies indicate that more research is necessary regarding parameters and diagnostic efficacy before capillary refill time can be recommended as a clinical tool; however, one systematic review suggests that a finger capillary refill time greater than three seconds should be considered abnormal if the test was performed correctly [155,156,157].
Assessing the infant's abdomen includes inspection, palpation, and auscultation. Inspection will include looking at the shape, contour, and movement of the abdomen as well as assessing the umbilical stump. Auscultation should be done for bowel sounds and bruits. Palpation for abdominal masses, as well as for major organs, is an important aspect of the abdominal exam, followed lastly by assessment of the infant's stool.
Assessing stool quantity and quality is an important part of the gastrointestinal assessment. Meconium should be passed within 24 hours [75]. If the infant has not passed meconium within the first 24 hours, a further assessment is warranted to rule out ileus or obstruction. By the second or third day, the infant should begin to have transitional stool, which is green or yellowish and may have a seedy appearance. Within several days to a week, breastfed infants' stools take on the appearance of mustard.
The first step in assessing the abdomen is inspection. The shape, contour, and movement should be evaluated. The shape should be domed or cylindrical because of immature musculature. There should be no bulging or distention. Distention is one of the first signs of problems. Movement should be fluid and synchronous with chest movement. The presence of localized edema or discoloration is a finding of peritoneal disease [3]. The umbilical stump initially is white and gelatinous in appearance and begins to dry within the first few hours. The umbilical stump should be inspected for the presence of two umbilical arteries and one vein. They make the appearance of a "smiley face," as the musculature in the arteries makes them appear round, like the eyes of a face, and the vein tends to collapse due to lack of musculature. The anus should be inspected for patency and absence of fissures. A digital examination can assist in ruling out complications, if necessary.
Auscultation should be completed before palpation. Listen for the presence of bowel sounds. Bowel sounds should be present approximately one to two hours after birth. In the older newborn, absence of bowel sounds is a significant finding. Auscultation over the kidneys for bruits from renal artery stenosis is important. Auscultation over the liver for bruits may reveal arteriovenous fistula [3].
Palpate and percuss beginning below the umbilicus and proceeding upward. The abdomen should feel soft. Infants may draw their legs up or cry if in pain during palpation of the abdomen. The liver is palpable 1–3 cm below the right costal margin. The most reliable measurement of the liver via palpation and percussion may be obtained in the midlines and below the right costal margin [158,159]. The first step is percussion of the upper and lower borders. In this step, the upper border is defined by percussion in a downward manner while the lower borders are palpated with the fingers in a perpendicular position to the midclavicular line. The second step includes percussion of both upper and lower borders at the position of the midclavicular line. The kidneys are moderately firm and lobulated. The bladder can be assessed for distension by palpating for a firm dome shape midline, in the lower portion of the abdomen.
Necrotizing enterocolitis (NEC) occurs most often in very low birth weight and low birth weight infants. In term infants, NEC is associated with the use of concentrated formulas, blood transfusions, NEC outbreak in the healthcare facility, and early use of certain medicines, such as indomethacin and dexamethasone. Early gastrointestinal signs may be ambiguous. Signs of NEC include lethargy, difficulty feeding, bilious emesis, temperature instability, abdominal distention and rigidity, abdominal tenderness, fecal occult blood, and palpable loops in the stool. In the later stages of NEC, a discoloration of the abdominal wall, the labia, or the scrotum may be seen. Because NEC symptoms are similar to other conditions, such as neonatal sepsis, care should be taken to provide immediate and accurate intervention [160,161,162,163].
Various types of hernias may be seen in newborns, including congenital diaphragmatic hernia, gastroschisis, omphalocele, and umbilical hernia. In some cases, hernias may be diagnosed in utero via ultrasonography. Gastroschisis, omphalocele, and umbilical hernias are apparent through inspection and palpation postnatally.
Congenital diaphragmatic hernia is an anomaly in which the muscular or tendinous parts of the diaphragm do not develop in the fetus and, consequently, abdominal contents protrude into the chest cavity [75]. This intrusion affects the development of other organs, mainly the lungs. It is important to note that congenital diaphragmatic hernia is not readily detectable through inspection and palpation. Signs of diaphragmatic hernia include a flat or scaphoid abdomen, barrel chest, difficult intubation, cyanosis, tachypnea, tachycardia, and bowel sounds that may be auscultated in the thoracic cavity [33,164]. Mortality rates and prognosis are generally poor and have been related to the presence of associated anomalies, prenatal diagnosis, prematurity, low birth weight, and early pneumothorax. Pulmonary hypertension and pulmonary hypoplasia are the recognized pathophysiologic cornerstones [164,165].
Gastroschisis is evident as the infant's intestines protrude from the abdominal wall through a rupture at the umbilical cord, usually on the right side [166,167]. With gastroschisis, intestine loops or other abdominal contents that protrude outside the body are not covered with any membranes and are clearly visible [166]. The risk factors for this type of hernia include young maternal age, low parity, and maternal use of selected drugs. It occurs concomitantly with NEC in approximately 20% of cases [167]. Prognosis is positive, with overall survival rates between 83% and 97% [167].
Omphalocele is similar to gastroschisis, but the protruding contents of the abdomen are usually covered by a very thin layer of tissue and protrude directly through the center of the belly button [168]. An omphalocele appears as a firm mass and, dependent on the size of the rupture, may include the intestines only or other organs, such as the bowel, liver, or spleen [168]. Omphalocele is associated with other conditions, including chromosomal anomalies, congenital diaphragmatic hernia, and cardiac anomalies [168]. Prognosis is linked to associated conditions; for example, prognosis is poor with concomitant cardiac defects, but positive if no associated conditions exist [169].
Umbilical hernias occur as a result of the incomplete closure of the umbilical ring. They present as a bulging of the area around the umbilical stump [169]. The hernia may be more visible when the infant is in an upright position or cries, subsiding when the infant is calm and in the supine position. Cutaneous rupture or damage is rare. It is possible for some umbilical hernias to close spontaneously by 4 years of age, if the rupture is relatively small and no other associated conditions are present [75].
Surgical intervention is necessary for treatment of hernias in most cases and should ensue as early as possible. However, surgical repair may be delayed if more critical concomitant conditions must be managed first, such as cardiac events. Antibiotic therapy may also be used in case of infection or risk of infection. Supportive therapy is often necessary [169].
Warning signs of the abdominal assessment, which would warrant further investigation and/or immediate intervention, include [35,170]:
Obvious defects in the abdominal wall, possibly hernia
Single umbilical artery, associated with congenital, especially renal, anomalies
Meconium-stained or shriveled umbilical cord, associated with intrauterine growth retardation or perinatal asphyxia
Imperforate anus, which may be associated with a tracheoesophageal fistula or esophageal atresia
Hepatosplenomegaly, associated with congenital infections and hemolysis
Flat or scaphoid-shaped abdomen, which may be associated with a diaphragmatic hernia
Failure to void within the first 24 hours
Failure to pass meconium stool within 12 hours
Observe for color, odor, frequency, and amount of void. Failure to void with in the first 24 hours is considered a warning sign and warrants further evaluation. The normal urine output for an infant is at least 1–2 cc/kg/hour. Output may be as high as 4 cc/kg/hour in the first few days of life [50]. Urine output should be made with a stream of urine under an adequate amount of pressure. Urine is normally straw colored, though variations in color do exist, ranging from clear to amber, and may or may not have sediment.
During the initial head-to-toe assessment of all infants, a thorough examination of genitalia should be completed. The infant's genitalia should be assessed initially for appropriate development and function. Unusual appearance of the genitalia is more often a structural abnormality and not ambiguous genitalia [171]. Accurate diagnosis is vital in order to avoid adverse consequences and undue stress for the family [172,176]. However, a true case of ambiguous genitalia, or disorder of sex development (DSD), may be disturbing to parents and carries lifelong therapeutic and psychosocial implications [172,173]. Immediate intervention is required in newborns with a DSD in order to evaluate adrenal and pituitary function, as certain underlying conditions, such as congenital adrenal hyperplasia, may be life-threatening [154,170,174]. DSDs have variable etiology, ranging from chromosomal abnormalities to maternal ingestion of androgenic steroids or other drugs [175,176].
An expeditious assessment and decision on gender assignment is necessary for improved patient outcome and to ease parental anxiety [154,172,173]. Gender assignment is generally influenced by a variety of aspects, including diagnosis, structure and appearance of the genitalia, surgical alternatives, possible requirement of lifelong therapeutic measures, fertility, and family and cultural views and beliefs [154,172]. Due to the psychosocial implications of DSDs, the AAP issued a policy statement that addresses these implications, including revision and standardization of nomenclature and care [172]. The AAP recommendations for the clinical care of infants with DSDs include [172]:
Evaluation by an expert should be completed before gender is assigned.
A center with an experienced, multidisciplinary team is ideal for evaluation and long-term management.
A gender should be assigned to all individuals with a DSD.
Family should be encouraged to participate in the decision-making process; open communication is crucial.
Family concerns should always be respected and addressed with the highest level of confidentiality.
When examining a male infant, the penis should be midline and straight, with the urethral opening midline at the tip of the penis. Hypospadias occurs when the meatus is located on the ventral surface of the glans, penile shaft, or the perineal area [91]. It may be identified by a groove that extends from the usual area of the meatus interiorly. Epispadias occurs when the urethral meatus occurs on the dorsal surface of the penis [75]. The length of the nonerect penis is 2–3 cm at birth [91]. Until 3 to 4 years of age, the foreskin is usually tight but should not affect the stream of urinary output [91]. The urinary stream should be neither highly pressurized nor of reduced force.
The scrotum should appear loose and pendulous, and each side should be manually assessed to determine the presence of testes. The testes usually descend in the third trimester and are approximately 1 cm in diameter at birth [3,91]. Containing the testes with the index finger and the thumb of one hand at the upper part of the scrotal sac may prevent retraction of the testes during assessment. Undescended testes may or may not be palpable in the inguinal canal. If the testicle cannot be pushed into the scrotum manually, then it is considered undescended. Bilateral undescended testes, micropenis, and/or bifid scrotum should prompt further evaluation [185]. The appearance of rugae may assist in providing information concerning the maturity of the infant. Absence of rugae may be a sign of prematurity [3,91]. Edema of the genital area following birth may be present, especially in breech births, but should resolve in a few days. Hydrocele is the collection of fluid around the testes in the scrotum and a relatively common finding. It can appear quite large and feel tight; diagnosis is made by transilluminating the scrotum. Hydrocele generally resolves on its own and requires no intervention.
Appearance of the female genitalia is dependent upon the general nutritional status of the newborn as well as gestational age. The genitalia should be inspected for placement of labia and hymen. The placement of the urinary meatus as well as position of the rectum and length of the perineum should be noted [35]. If the newborn is undernourished, the genital area will appear less developed than it should, with the exception of the clitoris, which may appear to be large because of the underdeveloped state of the surrounding tissues [3,170]. In a healthy term infant, the labia cover the clitoris without fusion. Due to maternal hormone involvement, labia may appear swollen and darker than surrounding tissue [35]. Mucus and possible blood-tinged vaginal discharge called pseudomenstruation may be present for several days [170]. Hymenal tags are common and usually resolve spontaneously within a few weeks [93].
The final portion of the head-to-toe assessment of the newborn is examining the extremities and back. A thorough examination of the arms and legs begins with noting the presence of any warning signs. Attitude, or resting posture, is an important piece of the neonatal evaluation, as is the evaluation of reflexes. The infant should be observed closely for the presence of tremors, and neuromuscular maturity and symmetry should be noted.
Even in sleep states, the normal resting posture for an infant is flexion. Tone should be assessed in the alert infant. Both decreases and increases in muscle tone can be symptoms of underlying problems and should be further evaluated. Hypotonia, sometimes referred to as low tone, is noted in the "floppy" infant with poor head control and limp extremities. Hypertonia is noted in the infant with tightly flexed upper extremities and extended lower extremities.
Tremors are not considered a normal finding in the newborn; periodic jerking or brief twitching is considered normal [33,35]. If not yet completed, reflexes should also be evaluated, as discussed in the neurologic assessment section of this course. Neuromuscular maturity should be evaluated with the use of age determination scales. Symmetry should be addressed in the alert infant. Bilateral assessment of the infant's reflexes and resting movement can provide a picture of muscular symmetry.
The arms and hands should be evaluated for symmetry, webbing, range of motion, and the number of digits [33]. Fingernails are generally long and in need of trimming in the term newborn. The palms should be examined for the presence of a single palmar crease (previously referred to as a "simian" crease) that extends all the way across the hand. A single palmar crease is associated with Down syndrome [177].
The newborn's legs should be assessed for flexion, symmetry, and length. In some cases, the uterine position can make an infant's foot appear clubbed, such as in the case of metatarsus adductus. The feet should be examined for the presence of metatarsus adductus and club foot [33,170]. With metatarsus adductus, only the front of the foot is turned inward; the back of the foot and ankle remain straight [178]. In cases of true club foot, both the foot and the ankle are turned inward and offer resistance [178].
Formerly known as congenital hip dislocation, developmental dysplasia of the hip (DDH) is an uncommon finding. However, according to the AAP, ease and efficacy of treatment is directly influenced by how early this condition is diagnosed [179,180]. Diagnosis of DDH is based upon the presence of unstable, subluxated, or dislocated hips or acetabula malformations. The majority (76%) of DDH cases occur in female infants. Other conditions that predispose infants to DDH include [179,180]:
Breech birth
Maternal oligohydramnios
Family history
The Ortolani and Barlow tests are recommended to assist in determining hip instability. Both tests are best performed with an infant relaxed and in the supine position [181]. The Ortolani test begins by holding the infant's thigh with a thumb. Place the index finger of the same hand over the greater trochanter area. Lift and abduct the hip gently and simultaneously push down on the knee. If the hip is not stable, a "clunk" sound will be heard. This sound should not be confused with a click type sound that is sometimes audible from the knee area during this examination [181]. The Barlow test also begins by holding the infant's thigh with a thumb. Use the palm of the same hand to press down on the knee. While applying this gentle pressure, feel for dislocation with the middle finger of the same hand. If the hip is not stable, one should feel the hip dislocate [181]. If findings of the clinical evaluation are unclear, the infant should be re-evaluated at follow-up examinations [179].
Imaging techniques have limited value in newborns. Newborn screening with ultrasonography has led to a high frequency of re-examination and a large number of hips being unnecessarily treated. Ultrasonography is, therefore, recommended only as an adjunct to clinical evaluation "either to clarify suspicious findings on physical examination after 3 to 4 weeks of age or to detect clinically silent DDH in the high-risk infant from 6 weeks to 4 to 6 months of age" [179]. The AAP recommends referral to an orthopedist if limited hip abduction or asymmetric hip abduction is present after four weeks [179]. Infants with risk factors for DDH, a questionable examination, and pediatrician or parental concern should also be referred.
The back is generally the last part of the newborn that undergoes examination. Skin intactness over the spinal area should be noted as well as curvatures and asymmetry. Assess for hip and shoulder symmetry. Trunk incurvation can be elicited by stroking the infant's back along the spine. The hips should move toward the stimulated side. Gluteal folds should also be symmetrical and may have small dimples within the gluteal crease [170].
When nevi appear midline on the back, they are considered possible cutaneous markers for spinal dysraphism. Other signs include lipomas, dimples (especially if they are connected to the spinal cord), dermal sinuses, tails or skin tags, hypertrichosis, and signs of drainage or local infection. If two or more of these anomalies appear concomitantly, the risk of spinal dysraphism is considered higher. Due to the serious neurologic implications of missed diagnoses of spinal dysraphism, it is important that anomalies be noted and referred appropriately [33,67,182].
Warning signs of the extremities assessment, which would warrant further investigation and/or immediate intervention, include [35,92]:
Absence of limbs or digits, usually isolated
Deformities of digits, including fusion (i.e., syndactyly) and extra digit(s) (i.e., polydactyly), also usually isolated deformities
Single palmar line associated with Down syndrome
Lack of movement of limb, possibly from brachial nerve palsy due to excessive traction and flexion of the neck during delivery (arm held adducted and internally rotated) or fracture
Limited abduction and unequal femur length
Asymmetrical thigh creases or positive Ortolani maneuver (i.e., clicks indicating developmental dysplasia of the hip)
A thorough neonatal assessment should be performed on all newborns in the first hours after birth to ensure an appropriate transition to extrauterine life. This assessment should be done systematically so early intervention may be initiated in the event that an abnormality is discovered. The prenatal record should be reviewed whenever possible to be aware of newborn and/or maternal risk factors that may complicate care. Maternal and environmental factors, such as smoking, drug or alcohol use, gestational diabetes, and GBS, are important considerations when assessing newborns. The placenta, examined by either a nurse or the delivering physician, may also give important clues regarding the health of the infant.
Infants should receive expert postbirth care by a person competent in neonatal resuscitation standards. The formal assessment and documentation of the newborn assessment in a timely fashion is essential. Most institutions use standardized forms and charts based on accepted assessment tools, such as the Apgar and the New Ballard scoring methods. Incomplete or incorrect documentation, as well as a lack of follow-up, may have hazardous implications [183]. Apgar scores should be assigned and may indicate future wellness; however, necessary treatment in critical situations should not wait until a score has been established. Any abnormalities should be reported immediately to the physician and appropriate measures should be taken. By following the same consistent approach with each newborn assessment, the nurse can be confident that the highest level of care has been provided for each infant.
1. Hockenberry MJ, Duffy EA, Gibbs K. Wong's Nursing Care of Infants and Children. 12th ed. St Louis, MO: Elsevier Mosby; 2023.
3. Narvey M, MacDonald MG. Physical assessment and classification. In: MacDonald MG, Seshia MMK (eds). Avery's Neonatology: Pathophysiology and Management of the Newborn. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2015; 242-264.
4. Lowdermilk DL, Perry SE, Cashion MC. Maternity Nursing. 8th ed (revised). St Louis, MO: Mosby, Inc.; 2013.
5. Centers for Disease Control and Prevention. Smoking During Pregnancy. Available at https://www.cdc.gov/tobacco/basic_information/health_effects/pregnancy/index.htm. Last accessed July 15, 2023.
6. National Institute on Drug Abuse. Tobacco, Nicotine, and E-Cigarettes Research Report. Available at https://nida.nih.gov/publications/research-reports/tobacco-nicotine-e-cigarettes/introduction#maternal. Last accessed July 15, 2023.
7. U.S. Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006.
8. National Institute on Drug Abuse. Heroin. Available at https://nida.nih.gov/research-topics/heroin. Last accessed July 15, 2023.
9. Kandall ST, Consensus Panel. Improving Treatment for Drug-Exposed Infants: Treatment Improvement Protocol (TIP) Series 5. Rockville, MD: U.S. Department of Health and Human Services; 1993.
10. Wang M. Perinatal Drug Abuse and Neonatal Drug Withdrawal. Available at https://emedicine.medscape.com/article/978492-overview#a0199. Last accessed July 15, 2023.
11. Gray TR, Eiden RD, Leonard KE, Connors GJ, Shisler S, Huestis MA. Identifying prenatal cannabis exposure and effects of concurrent tobacco exposure on neonatal growth. Clin Chem. 2010;56(9):1442-1450.
12. DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007–2010. Prev Chronic Dis. 2014;11:E104.
13. American Diabetes Association. Gestational Diabetes. Available at https://www.diabetes.org/diabetes/gestational-diabetes. Last accessed July 15, 2023.
14. Mayo Clinic. Gestational Diabetes: Symptoms and Causes. Available at https://www.mayoclinic.org/diseases-conditions/gestational-diabetes/symptoms-causes/syc-20355339. Last accessed July 15, 2023.
15. MedlinePlus. Low Blood Sugar: Newborns. Available at https://medlineplus.gov/ency/article/007306.htm. Last accessed July 15, 2023.
16. Mayo Clinic. Preeclampsia. Available at https://www.mayoclinic.org/diseases-conditions/preeclampsia/symptoms-causes/syc-20355745. Last accessed July 15, 2023.
17. Centers for Disease Control and Prevention. Perinatal group B streptococcal disease after universal screening recommendations—United States, 2003–2005. MMWR. 2007;56(28):701-705.
18. Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR. 2010;59(RR10):1-36.
19. Mahlmeister L. Perinatal group B streptococcal infections: the nurse's role in identification and prophylaxis. J Perinat Neonatal Nurs. 1996;10(2):1-16.
20. Centers for Disease Control and Prevention. About Group B Strep. Available at https://www.cdc.gov/groupbstrep/about/index.html. Last accessed July 15, 2023.
21. Borghesi A, Stronati M, Fellay J. Neonatal group B streptococcal disease in otherwise healthy infants: failure of specific neonatal immune responses. Front Immunol. 2017;8:215.
22. Sweet RL, Gibbs RS. Infectious Diseases of the Female Genital Tract. 5th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2009.
23. Cincinnati Children's Hospital Medical Center. Evidence-Based Care Guideline for Fever of Uncertain Source in Infants 60 Days of Age or Less. Cincinnati, OH: Cincinnati Children's Hospital Medical Center; 2019.
24. American Congress of Obstetricians and Gynecologists. Prevention of Group B Streptococcal Early Onset Disease in Newborns. Available at https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2020/02/prevention-of-group-b-streptococcal-early-onset-disease-in-newborns. Last accessed July 15, 2023.
25. Jackson B, Mid Essex Hospital Services, National Health Service Trust. Examination of the Placenta: Clinical Guideline. Available at https://www.meht.nhs.uk/EasysiteWeb/getresource.axd?AssetID=18709&type=full&servicetype=Attachment. Last accessed July 15, 2023.
27. Beall MH. Umbilical Cord Complications. Available at https://emedicine.medscape.com/article/262470-overview#aw2aab6b3. Last accessed July 15, 2023.
28. American Academy of Pediatrics. Textbook of Neonatal Resuscitation. 8th ed. Dallas, TX: American Academy of Pediatrics; 2021.
29. Orshan SA. Maternity, Newborn, and Women's Health Nursing: Comprehensive Care Across the Life Span. Philadelphia, PA: Lippincott Williams & Wilkins; 2008.
30. LeFevre ML, U.S. Preventive Services Task Force. Screening for chlamydia and gonorrhea: recommendation statement. Ann Intern Med. 2014;161(12):902-910.
31. American Academy of Pediatrics. Gonococcal infections. In: Kimberlin DW, Brady MT, Jackson MA, Long SS (eds). Red Book 2018: 2018 Report of the Committee on Infectious Diseases. 30th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2018: 355-365.
32. American Academy of Pediatrics. Prevention of neonatal ophthalmia. In: Kimberlin DW, Brady MT, Jackson MA, Long SS (eds). Red Book 2018: 2018 Report of the Committee on Infectious Diseases. 30th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2018: 1046.
33. Olds SB, London ML, Ladewig P. Maternal-Newborn Nursing: A Family and Community-Based Approach. 6th ed. Upper Saddle River, NJ: Prentice Hall Health; 2000.
34. Silbert-Flagg J, Pillitteri A. Maternal and Child Health Nursing: Care of the Childbearing and Childrearing Family. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2017.
35. Dickason EJ, Schultz MO (eds). Maternal and Infant Nursing Care. 3rd ed. St Louis, MO: Mosby; 1998.
36. MedlinePlus. Apgar Score. Available at https://medlineplus.gov/ency/article/003402.htm. Last accessed July 15, 2023.
37. Mattson S, Smith J (eds). Core Curriculum for Maternal-Newborn Nursing. 5th ed. St. Louis, MO: Mosby; 2015.
38. Weinberger B, Anwar M, Hegyi T, Hiatt M, Koons A, Paneth N. Antecedents of neonatal consequences of low Apgar scores in preterm newborns: a population study. Arch Pediatr Adolesc Med. 2000;154(3):294-300.
39. Lagatta J, Yan K, Hoffmann R. The association between 5-min Apgar score and mortality disappears after 24 h at the borderline of viability. Acta Paediatr. 2012;101(6):e243-e247.
40. AAP Newborn Screening Task Force. Serving the family from birth to the medical home: newborn screening: a blueprint for the future: a call for a national agenda on state newborn screening programs. Pediatrics. 2000;106:389.
41. Baby's First Test. Newborn Screening 101. Available at https://www.babysfirsttest.org/newborn-screening/screening-101. Last accessed July 15, 2023.
42. Centers for Disease Control and Prevention. Impact of expanded newborn screening—United States, 2006. MMWR. 2008;57(37): 1012-1015.
43. Rose NC, Wick M. Carrier screening for single gene disorders. Semin Fetal Neonatal Med. 2017;23(2):78-84.
44. U.S. Department of Health and Human Services. Recommended Uniform Screening Panel. Available at https://www.hrsa.gov/advisory-committees/heritable-disorders/rusp. Last accessed July 15, 2023.
45. Price DL, Gwin JF. Pediatric Nursing: An Introductory Text. 11th ed. St Louis, MO: Saunders Elsevier; 2012.
46. Lowman LB, Stone LL, Cole JG. Using developmental assessments in the NICU to empower families. Neonatal Netw. 2006;25(3):177-186.
47. Bodurtha J. Assessment of the newborn with dysmorphic features. Neonatal Network. 1999;18(2):27-30.
48. Brazelton TB, Nugent JK. Neonatal Behavioral Assessment Scale. 4th ed. London: MacKeith Press; 2011.
49. Tedder JL. Using the Brazelton Neonatal Assessment Scale to facilitate the parent-infant relationship in a primary care setting. Nurse Pract. 1991;16(3):26-30, 35-36.
50. Bruno JP. Systematic neonatal assessment and intervention. MCN Am J Maternal Child Nurs. 1994;20(1):21-28.
51. Subramanian S. Extremely Low Birth Weight Infant. Available at https://emedicine.medscape.com/article/979717-overview. Last accessed July 15, 2023.
52. National Institute of Child Health and Human Development. Preterm Labor and Birth. Available at https://www.nichd.nih.gov/health/topics/preterm. Last accessed July 15, 2023.
53. MedlinePlus. Appropriate for Gestational Age (AGA). Available at https://medlineplus.gov/ency/article/002225.htm. Last accessed July 15, 2023.
54. MedlinePlus. Large for Gestational Age (LGA). Available at https://medlineplus.gov/ency/article/002248.htm. Last accessed July 15, 2023.
55. MedlinePlus. Premature Infant. Available at https://medlineplus.gov/ency/article/001562.htm. Last accessed July 15, 2023.
56. MedlinePlus. Small for Gestational Age (SGA). Available at https://medlineplus.gov/ency/article/002302.htm. Last accessed July 15, 2023.
57. Kyle T, Carman S. Essentials of Pediatric Nursing. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2020.
58. Centers for Disease Control and Prevention. Growth Charts. Available at https://www.cdc.gov/growthcharts. Last accessed July 15, 2022.
59. Centers for Disease Control and Prevention. WHO Growth Standards are Recommended for Use in the U.S. for Infants and Children 0 to 2 Years of Age. Available at https://www.cdc.gov/growthcharts/who_charts.htm. Last accessed July 15, 2023.
60. Centers for Disease Control and Prevention. Clinical Growth Charts. Available at https://www.cdc.gov/growthcharts/clinical_charts.htm. Last accessed July 15, 2023.
61. The New Ballard Score. New Ballard Score Maturational Assessment of Gestational Age. Available at https://www.ballardscore.com. Last accessed July 15, 2023.
62. MedlinePlus. Skin Turgor. Available at https://medlineplus.gov/ency/article/003281.htm. Last accessed July 15, 2023.
63. Ralston M, Hazinski MF, Zaritsky AL, et al. PALS Provider Manual. Dallas, TX: American Academy of Pediatrics, American Heart Association; 2006.
64. Stanford Medicine. Acrocyanosis. Available at https://med.stanford.edu/newborns/professional-education/photo-gallery/general.html#acrocyanosis. Last accessed July 15, 2023.
65. MedlinePlus. Newborn Jaundice. Available at https://medlineplus.gov/ency/article/001559.htm. Last accessed July 15, 2023.
66. Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician. 2014;89(11):873-878.
67. McLaughlin MR, O'Connor NR, Ham P. Newborn skin: part II: birthmarks. Am Fam Physician. 2008;77(1):56-60.
68. MedlinePlus. Red Birthmarks. Available at https://medlineplus.gov/ency/article/001440.htm. Last accessed July 15, 2023.
69. MedlinePlus. Port-Wine Stain. Available at https://medlineplus.gov/ency/article/001475.htm. Last accessed July 15, 2023.
70. MedlinePlus. Stork Bite. Available at https://medlineplus.gov/ency/article/001388.htm. Last accessed July 15, 2023.
71. MedlinePlus. Dermal Melanocytosis. Available at https://medlineplus.gov/ency/article/001472.htm. Last accessed July 15, 2023.
72. MedlinePlus. Erythema Toxicum. Available at https://medlineplus.gov/ency/article/001458.htm. Last accessed July 15, 2023.
73. O'Connor NR, McLaughlin MR, Ham P. Newborn skin: part I: common rashes. Am Fam Physician. 2008;77(1):47-52.
75. Taber's Online. Available at https://www.tabers.com/tabersonline. Last accessed July 15, 2023.
76. Kersten CM, Moellering CM, Mato S. Spontaneous drainage of neonatal cephalohematoma: a delayed complication of scalp abscess. Clin Pediatr. 2008;47(2):183-185.
77. Simpson KR, Creehan PA, O'Brien-Abel N, et al. AWHONN's Perinatal Nursing. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2020.
78. Potts NL, Mandleco BL. Pediatric Nursing: Caring for Children and Their Families. 3rd ed. Florence, KY: Thomson Delmar Learning; 2012.
79. MedlinePlus. Cranial Sutures. Available at https://medlineplus.gov/ency/article/002320.htm. Last accessed July 15, 2023.
80. MedlinePlus. Fontanelles—Bulging. Available at https://medlineplus.gov/ency/article/003310.htm. Last accessed July 15, 2023.
81. MedlinePlus. Sutures—Separated. Available at https://medlineplus.gov/ency/article/003307.htm. Last accessed July 15, 2023.
82. AAO Pediatric Ophthalmology/Strabismus PPP Panel, Hoskins Center for Quality Eye Care. Pediatric Eye Evaluations PPP: 2022. Available at https://www.aao.org/education/preferred-practice-pattern/pediatric-eye-evaluations-ppp-2022. Last accessed July 15, 2023.
83. Healthychildren.org. Warning Signs of Vision Problems in Infants and Children. Available at https://www.healthychildren.org/English/health-issues/conditions/eyes/Pages/Warning-Signs-of-Vison-Problems-in-Children.aspx. Last accessed July 15, 2023.
84. Bertrand J, Floyd LL, Weber MK. Guidelines for identifying and referring persons with fetal alcohol syndrome. MMWR. 2005;54(RR11):1-14.
85. Fuloria M, Kreiter S. The newborn examination: part 1: emergencies and common abnormalities involving the skin, head, neck, chest, and respiratory and cardiovascular systems. Am Fam Physician. 2002;65(1):61-68.
86. Hockenberry MJ, Wilson D, Rodgers CC. Wong's Clinical Manual of Pediatric Nursing. 10th ed. St. Louis, MO: Mosby; 2017.
87. Snir M, Hasanreisoqlue M, Goldenberg-Cohen N, et al. Suppression of the oculocephalic reflex (doll's eyes phenomenon) in normal full-term babies. Curr Eye Res. 2010;35(5):370-374.
88. MedlinePlus. Epicanthal Folds. Available at https://medlineplus.gov/ency/article/003030.htm. Last accessed July 15, 2023.
89. MedlinePlus. White Pupil. Available at https://medlineplus.gov/ency/imagepages/9313.htm. Last accessed July 15, 2023.
90. Penn Medicine. Hydrocephalus. Available at https://www.pennmedicine.org/for-patients-and-visitors/patient-information/conditions-treated-a-to-z/hydrocephalus. Last accessed July 15, 2023.
91. Ball JB, Dains JE, Flynn JA, Solomon BS, Stewart RW. Seidel's Guide to Physical Examination. 9th ed. St Louis, MO: Mosby; 2018.
92. Ricci SS. Essentials of Maternity, Newborn, and Women's Health Nursing. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2020.
93. Vermont Department of Health. Vermont Newborn Screening Program. Available at https://www.healthvermont.gov/family/health-care-children-youth/vermont-newborn-screening-program. Last accessed July 15, 2023.
94. MedlinePlus. Ear Tag. Available at https://medlineplus.gov/ency/article/003304.htm. Last accessed June 13, 2023.
95. MedlinePlus. Newborn Ear Anatomy. Available at https://medlineplus.gov/ency/imagepages/9529.htm. Last accessed July 15, 2023.
96. National Institutes of Health. NIDCD Fact Sheet: Your Baby's Hearing Screening and Next Steps. Available at https://www.nidcd.nih.gov/sites/default/files/your-babys-hearing-screening-and-next-steps.pdf. Last accessed July 15, 2023.
97. The Joint Committee on Infant Hearing. Year 2019 position statement: principles and guidelines for early hearing detection and intervention programs. JEHDI. 2019;4(2):1-44.
98. National Institutes of Health, National Institute on Deafness and Other Communication Disorders. Recommendations of the NIDCD Working Group on Early Identification of Hearing Impairment on Acceptable Protocols for Use in Statewide Universal Newborn Hearing Screening Programs. Available at https://www.nidcd.nih.gov/research/workshops/working-group-early-identification-hearing-impairment/1997. Last accessed July 15, 2023.
99. MedlinePlus. Infant Reflexes. Available at https://medlineplus.gov/ency/article/003292.htm. Last accessed July 15, 2023.
100. Upledger JE. A Brain is Born: Exploring the Birth and Development of the Central Nervous System. Berkeley, CA: North Atlantic Books; 1996.
102. Jaynes ME, Gingold MK, Hupp A, Mullett MD, Bodensteiner JB. The plantar response in normal newborn infants. Clin Pediatr. 1997;36(11):649-651.
103. Eisengart MA. Reflex-arc latency measurements in newborn infants and children. Pediatrics. 1970;46(1):28-35.
104. Pedroso FS, Rotta NT. Neurological examination in the healthy term newborn. Arq Neuropsiquiatr. 2003;61(2A):165-169.
105. Carratalá F, Moya M. Neonatal reflexes variability in the normal full term neonate during the neurological exam. Rev Neurol. 2002;34(5):481-485.
106. MedlinePlus. Babinski Reflex. Available at https://medlineplus.gov/ency/article/003294.htm. Last accessed July 15, 2023.
107. Kumhar GD, Dua T, Gupta P. Plantar response in infancy. Eur J Paediatr Neurol. 2002;6(6):321-325.
108. Gupta A, Gupta P. Neonatal plantar response revisited. N Paediatr Child Health. 2003;39(5):349-351.
109. Morison SJ, Holsti L, Grunau RE, et al. Are there developmentally distinct motor indicators of pain in preterm infants? Early Hum Dev. 2003;72(2):131-146.
110. Holsti L, Grunau RE, Oberlander TF, Whitfield MF. Specific Newborn Individualized Developmental Care and Assessment Program movements are associated with acute pain in preterm infants in the neonatal intensive care unit. Pediatrics. 2004;114(1):65-72.
111. Grunau RE, Holsti L, Whitfield MF, Ling E. Are twitches, startles, and body movements pain indicators in extremely low birth weight infants? Clin J Pain. 2000;16(1):37-45.
112. MedlinePlus. Supernumerary Nipples. Available at https://medlineplus.gov/ency/article/003110.htm. Last accessed July 15, 2023.
113. Leung AK, Pacaud D. Diagnosis and management of galactorrhea. Am Fam Physician. 2004;70(3):543-550.
114. Alvaro RE. Cardiorespiratory adjustments at birth. In: MacDonald MG, Seshia MMK (eds). Avery's Neonatology: Pathophysiology and Management of the Newborn. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2015: 212-226.
115. Ward JPT, Ward J, Leach RM. The Respiratory System at a Glance. 5th ed. Hoboken, NJ: John Wiley & Sons Ltd.; 2022.
116. Brenner JS, Karlowicz MG. Nonfatal symptomatic spontaneous pneumothorax in neonates: association with white ethnicity and lack of association with major urinary tract malformations. Clin Pediatr. 1997;36(4):241-243.
117. White L. Foundations of Nursing: Caring for the Whole Person. Corpus Christi, TX: Delmar Cengage Learning; 2000.
118. American Academy of Pediatrics Committee on Fetus and Newborn. Apnea, sudden infant death syndrome, and home monitoring. Pediatrics. 2003;111(4 pt 1):914-917.
119. Bickley LS. Bates Guide to Physical Examination and History Taking. 13th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2020.
120. MedlinePlus. Neonatal Respiratory Distress Syndrome. Available at https://medlineplus.gov/ency/article/001563.htm. Last accessed July 15, 2023.
121. Pramanik AK. Respiratory Distress Syndrome. Available at https://emedicine.medscape.com/article/976034-overview#a0199. Last accessed July 15, 2023.
122. Islam O. Imaging in Transient Tachypnea of the Newborn. Available at https://emedicine.medscape.com/article/414608-overview. Last accessed July 15, 2023.
123. MedlinePlus. Neonatal Sepsis. Available at https://medlineplus.gov/ency/article/007303.htm. Last accessed July 15, 2023.
124. Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR. 2010;59(RR10):1-36.
125. Mtitimila EI, Cooke RWI. Antibiotic regimens for suspected early neonatal sepsis. Cochrane Database Syst Rev. 2004;3:CD004495.
126. Centers for Disease Control and Prevention. Group B Strep (GBS): Clinical Overview. Available at https://www.cdc.gov/groupbstrep/clinicians/index.html. Last accessed July 15, 2023.
127. Committee on Obstetric Practice. Committee opinion no 689: delivery of a newborn with meconium-stained amniotic fluid. Obstet Gynecol. 2017;129(3):e33-e34.
128. MedlinePlus. Meconium Aspiration Syndrome. Available at https://medlineplus.gov/ency/article/001596.htm. Last accessed July 15, 2023.
129. Goldsmith JP. Continuous positive airway pressure and conventional mechanical ventilation in the treatment of meconium aspiration syndrome. J Perinatol. 2008;28(suppl 3):S49-S55.
130. McCance KL, Huether SE. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 9th ed. St Louis, MO: Mosby, Inc.; 2022.
131. Springhouse. Nursing Know-How: Evaluating Heart and Breath Sounds. Ambler, PA: Lippincott Williams & Wilkins; 2009.
132. MedlinePlus. Pulse. Available at https://medlineplus.gov/ency/article/003399.htm. Last accessed July 15, 2023.
133. Topjian AA, Raymond TT, Atkins D, et al. Pediatric basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020;142(16):Suppl_2.
134. Darnall RA. Noninvasive blood pressure measurement in the neonate. Clin Perinatol. 1985;12(1):31-49.
135. Puchalski ML. Should Blood Pressure Be Measured in Newborn Infants? Available at https://www.medscape.com/viewarticle/741563. Last accessed July 15, 2023.
136. Noponen AL, Lukkarinen S, Angerla A, Sepponen R. Phono-spectrographic analysis of heart murmur in children. BMC Pediatr. 2007;7:23.
137. Slota MC (ed). AACNCore Curriculum for Pediatric High Acuity, Progressive, and Critical Care Nursing. 3rd ed. New York, NY: Springer; 2019.
138. McConnell ME, Adkins SB III, Hannon DW. Heart murmurs in pediatric patients: when do you refer? Am Fam Physician. 1999;60:558-565.
139. National Heart, Lung and Blood Institute. Congenital Heart Defects. https://www.nhlbi.nih.gov/health /congenital-heart-defects. Last accessed July 15, 2023.
140. Centers for Disease Control and Prevention. Data and Statistics on Congenital Heart Defects. Available at https://www.cdc.gov/ncbddd/heartdefects/data.html. Last accessed July 15, 2023.
141. Saenz RB, Beebe DK, Triplett LC. Caring for infants with congenital heart disease and their families. Am Fam Physician. 1999;59(7):1857-1866.
142. American Heart Association. About Congenital Heart Defects. Available at https://www.heart.org/en/health-topics/congenital-heart-defects/about-congenital-heart-defects. Last accessed July 15, 2023.
143. American Society for Microbiology. Guidelines for the Detection and Identification of Group B Streptococcus. Available at https://asm.org/Guideline/Guidelines-for-the-Detection-and-Identification-of. Last accessed July 15, 2023.
144. Satou GM. Pediatric Congestive Heart Failure. Available at https://emedicine.medscape.com/article/901307. Last accessed July 15, 2023.
145. Spong CY. Defining "term" pregnancy: recommendations from the Defining "Term" Pregnancy Workgroup. JAMA. 2013;309:2445-2446.
146. Centers for Disease Control and Prevention. Facts about Ventricular Septal Defect. Available at https://www.cdc.gov/ncbddd/heartdefects/ventricularseptaldefect.html. Last accessed July 15, 2023.
147. Patnana SR. Coarctation of the Aorta. Available at https://emedicine.medscape.com/article/895502. Last accessed July 15, 2023.
148. MedlinePlus. Coarctation of the Aorta. Available at https://medlineplus.gov/ency/article/000191.htm. Last accessed July 15, 2023.
149. Centers for Disease Control and Prevention. Facts about Coarctation of the Aorta. Available at https://www.cdc.gov/ncbddd/heartdefects/coarctationofaorta.html. Last accessed July 15, 2023.
150. MedlinePlus. Transposition of the Great Arteries. Available at https://medlineplus.gov/ency/article/001568.htm. Last accessed July 15, 2023.
151. Centers for Disease Control and Prevention. Facts about Dextro-Transposition of the Great Arteries (d-TGA). Available at https://www.cdc.gov/ncbddd/heartdefects/d-tga.html. Last accessed July 15, 2023.
152. MedlinePlus. Tetralogy of Fallot. Available at https://medlineplus.gov/ency/article/001567.htm. Last accessed July 15, 2023.
153. Centers for Disease Control and Prevention. Facts about Tetralogy of Fallot. Available at https://www.cdc.gov/ncbddd/heartdefects/tetralogyoffallot.html. Last accessed July 15, 2023.
154. Indyk JA. Disorders/differences of sex development (DSDs) for primary care: the approach to the infant with ambiguous genitalia. Transl Pediatr. 2017;6(4):323-334.
155. LeFlore JL, Engle WD. Capillary refill time is an unreliable indicator of cardiovascular status in term neonates. Adv Neonatal Care. 2005;5(3):147-154.
156. Raichur DV, Aralihond AP, Kasturi AV, Patil DH. Capillary refill time in term neonates: bedside assessment. Indian J Pediatr. 2001;68(7):613-615.
157. Fleming S, Gill P, Jones C, Taylor JA, Van den Bruel A, Heneghan C, Thompson M. Validity and reliability of measurement of capillary refill time in children: a systematic review. Arch Dis Child. 2015;100(3):239-249.
158. Michie C, Alu S, Wild K, Hampsheir R, Chabonnaud P, Harvey D. Should we estimate liver span in the right mid-clavicular line or the midline? J Paediatr Child Health. 1995;31(3):241-244.
159. McMillan JA, Feigin RD, DeAngelis C, Jones MD (eds). Oski's Pediatrics: Principles and Practice. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
160. MedlinePlus. Necrotizing Enterocolitis. Available at https://medlineplus.gov/ency/article/001148.htm. Last accessed July 15, 2023.
161. Pietz J, Achanti B, Lilien L, Stepka EC, Mehta SK. Prevention of necrotizing enterocolitis in preterm infants: a 20-year experience. Pediatrics. 2007;119(1):e164-e170.
162. Wise BV, McKenna C, Garvin G, Harmon BJ (eds). Nursing Care of the General Pediatric Surgical Patient. Gaithersburg, MD: Aspen Publishers, Inc.; 2000.
163. Alfa MJ, Robson D, Davi M, Bernard K, Van Caeseele P, Harding GK. An outbreak of necrotizing enterocolitis associated with a novel Clostridium species in a neonatal intensive care unit. Clin Infect Dis. 2002;35(suppl 1):S101-S105.
164. Steinhorn RH. Pediatric Congenital Diaphragmatic Hernia. Available at https://emedicine.medscape.com/article/978118-overview. Last accessed July 15, 2023.
165. Kipfmueller F, Heindel K, Schroeder L, et al. Early postnatal echocardiographic assessment of pulmonary blood flow in newborns with congenital diaphragmatic hernia. J Perinat Med. 2018;46(7):735-743.
166. Centers for Disease Control and Prevention. Facts about Gastroschisis. Available at https://www.cdc.gov/ncbddd/birthdefects/gastroschisis.html. Last accessed July 15, 2023.
167. Williams T, Butler R, Sundem T. Management of the Infant with Gastroschisis: A Comprehensive Review of the Literature. Available at https://www.medscape.com/viewarticle/458475. Last accessed July 15, 2023.
168. Centers for Disease Control and Prevention. Facts about Omphalocele. Available at https://www.cdc.gov/ncbddd/birthdefects/Omphalocele.html. Last accessed July 15, 2023.
169. Glasser JG. Pediatric Omphalocele and Gastroschisis (Abdominal Wall Defects). Available at https://emedicine.medscape.com/article/975583-overview. Last accessed July 15, 2023.
170. Fuloria M, Kreiter S. The newborn examination: part II: emergencies and common abnormalities involving the abdomen, pelvis, extremities, genitalia, and spine. Am Fam Physician. 2002;65(2):265-270.
171. American Academy of Pediatrics Committee on Genetics. Evaluation of the newborn with developmental anomalies of the external genitalia. Pediatrics. 2000;106:138-142.
172. Lee PA, Houk CP, Ahmed SF, Hughes IA. Consensus statement on management of intersex disorders. Pediatrics. 2006;118(2):e488-e500.
174. Al-Mutair A, Iqbal MA, Sakati N, Ashwal A. Cytogenetics and etiology of ambiguous genitalia in 120 pediatric patients. Ann Saudi Med. 2004;24(5):368-372.
175. Nimkarn S, Likitmaskul S, Sangacharoenkit P, et al. Ambiguous genitalia: an overview of 22 years' experience and the diagnostic approach in the Pediatric Department, Siriraj Hospital. J Med Assoc Thai. 2002;85(suppl 2):S496-S505.
176. MedlinePlus. Ambiguous Genitalia. Available at https://medlineplus.gov/ency/article/003269.htm. Last accessed July 15, 2023.
177. MedlinePlus. Single Palmar Crease. Available at https://medlineplus.gov/ency/article/003290.htm. Last accessed July 15, 2023.
178. MedlinePlus. Metatarsus Adductus. Available at https://medlineplus.gov/ency/article/001601.htm. Last accessed July 15, 2023.
179. Shaw BA, Segal LS; Section on Orthopaedics. Evaluation and referral for developmental dysplasia of the hip in infants. Pediatrics. 2016;138(6).
180. MedlinePlus. Developmental Dysplasia of the Hip. Available at https://medlineplus.gov/ency/article/000971.htm. Last accessed July 15, 2023.
181. Curry LC, Gibson LY. Congenital hip dislocation: the importance of early detection and comprehensive treatment. Nurse Pract. 1992;17(5):49-55.
183. Kumar P. Physician documentation of neonatal risk assessment for perinatal infections. J Pediatr. 2006;149(2):265-267.
184. Lewis ML. A comprehensive newborn examination: part I. General, head and neck, cardiopulmonary. Am Fam Physician. 2014;90(5):289-296.
1. U.S. Preventive Services Task Force. Tobacco Smoking Cessation in Adults, Including Pregnant Women: Interventions. Available at https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Last accessed August 4, 2023.
2. Vanguri S, Rogers-McQuade H, Sriraman NK, Academy of Breastfeeding Medicine. ABM clinical protocol #14: breastfeeding-friendly physician's office: optimizing care for infants and children, revised 2021. Breastfeed Med. 2021;16(3):175-184. Available at https://www.bfmed.org/assets/DOCUMENTS/PROTOCOLS/Protocol%20%2314%20-%20English%20Translation.pdf. Last accessed August 4, 2023.
3. U.S. Preventive Services Task Force. Screening for speech and language delay and disorders in children aged 5 years or younger: U.S. Preventive Services Task Force recommendation statement. Pediatrics. 2015;136(2):e474-e481. Available at https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/speech-and-language-delay-and-disorders-in-children-age-5-and-younger-screening. Last accessed August 4, 2023.
4. ACOG Committee on Obstetric Practice. ACOG Committee Opinion 797: Prevention of Group B Streptococcal Early-Onset Disease in Newborns. Available at https://www.acog.org/-/media/project/acog/acogorg/clinical/files/committee-opinion/articles/2020/02/prevention-of-group-b-streptococcal-early-onset-disease-in-newborns.pdf. Last accessed August 4, 2023.
5. Fallon EM, Nehra D, Potemkin AK, et al. A.S.P.E.N. clinical guidelines: nutrition support of neonatal patients at risk for necrotizing enterocolitis. JPEN J Parenter Enteral Nutr. 2012;36(5):506-523. Available at https://onlinelibrary.wiley.com/doi/pdf/10.1177/0148607112449651. Last accessed August 4, 2023.
Mention of commercial products does not indicate endorsement.