Hyperglycemia is commonly detected in hospitalized patients, and it can result in increased mortality and morbidity. One major area of concern is the relationship between hyperglycemia and wound healing. Reduction in wound healing and associated hyperglycemic state is well documented in recent literature. The prevention of delayed, inadequate, and incomplete healing of surgical, accidental, vascular, venous, and/or pressure wounds is the goal of therapy. The relationship between hyperglycemia and delayed wound healing is merely touched upon in the academic setting, leaving the professional nurse to find his or her own way in the care and understanding of the relationship between the two phenomenons. This course reviews the impact of hyperglycemia on wound healing and interventions that may improve the prognosis for these patients.
This course is designed for nurses in all practice settings with a desire to better understand the relationship between diabetes/hyperglycemia and wound management.
The impact of hyperglycemia as a comorbidity in delayed wound healing is well documented. The purpose of this course is to provide nurses with a working knowledge of preventative strategies of skin breakdown, identification of types of skin breakdown, and steps for appropriate care of wounds in patients with diabetes.
Upon completion of this course, you should be able to:
- Identify causes of and risk factors for hyperglycemia in the clinical setting.
- Describe acute and chronic wounds, and discuss the impact of hyperglycemia on both wound types.
- Evaluate the pathologic effects of diabetes as it relates to wound development and healing.
- Differentiate between arterial and venous ulcers.
- Analyze characteristics of sensory-neuropathic ulcers.
- Illustrate differences between pressure injuries of various stages.
- Recognize hyperglycemia's role in delaying surgical wound healing.
- Review significant aspects of wound documentation.
Diane Thompson, RN, MSN, CDE, CLNC, has an extensive history in nursing and nursing education. She possesses a strong background in diabetes and cardiac care, starting her professional career at the cardiac care area of the Cleveland Clinic in Cleveland, Ohio. Ms. Thompson took the knowledge and experience she learned from the Cleveland Clinic and transferred it into the home health arena in rural Ohio, after which she moved to Florida and obtained further knowledge while working as a PRN nurse in all areas, including medical/surgical, intensive care, emergency, critical care, and cardiology. With a desire to have a specific area to concentrate her profession, Ms. Thompson accepted a position as a pneumonia case manager, which led into a diabetes case manager career.
Ms. Thompson has been employed in diabetes care since 2001, when she was hired as a diabetes case manager. After the completion of 1,000 hours of education to diabetes patients, Ms. Thompson earned her certification as a diabetes educator in 2003. From 2006 to 2018, Ms. Thompson was the Director of Diabetes Healthways at Munroe Regional Medical Center in Ocala, Florida. As the director of the diabetes center, Ms. Thompson was responsible for the hospital diabetes clinicians, hospital wound care clinicians, and out-patient education program. Today, she is the nurse manager of a heart, vascular, and pulmonary ambulatory clinic at Metro Health System in Cleveland, Ohio. Ms. Thompson has also lectured at the local, state, and national level regarding diabetes and the hospital management of hyperglycemia. Ms. Thompson is a member of the ADA, AADE, Florida Nurses Association, and the National Alliance of Certified Legal Nurse Consultants.
Ms. Thompson acknowledges her family as her greatest accomplishment. She is a wife of more than 30 years and a mother of a daughter and son, of which she is very proud. Ms. Thompson credits her husband for the support needed to set a goal and achieve it. He has been by her side through nursing school and completion of her Bachelor's degree and Master's degree, which she was awarded in 2015 from Jacksonville University in Florida.
Contributing faculty, Diane Thompson, RN, MSN, CDE, CLNC, has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Mary Franks, MSN, APRN, FNP-C
The division planner has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Sarah Campbell
The Director of Development and Academic Affairs has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
The purpose of NetCE is to provide challenging curricula to assist healthcare professionals to raise their levels of expertise while fulfilling their continuing education requirements, thereby improving the quality of healthcare.
Our contributing faculty members have taken care to ensure that the information and recommendations are accurate and compatible with the standards generally accepted at the time of publication. The publisher disclaims any liability, loss or damage incurred as a consequence, directly or indirectly, of the use and application of any of the contents. Participants are cautioned about the potential risk of using limited knowledge when integrating new techniques into practice.
It is the policy of NetCE not to accept commercial support. Furthermore, commercial interests are prohibited from distributing or providing access to this activity to learners.
Supported browsers for Windows include Microsoft Internet Explorer 9.0 and up, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Supported browsers for Macintosh include Safari, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Other operating systems and browsers that include complete implementations of ECMAScript edition 3 and CSS 2.0 may work, but are not supported. Supported browsers must utilize the TLS encryption protocol v1.1 or v1.2 in order to connect to pages that require a secured HTTPS connection. TLS v1.0 is not supported.
The role of implicit biases on healthcare outcomes has become a concern, as there is some evidence that implicit biases contribute to health disparities, professionals' attitudes toward and interactions with patients, quality of care, diagnoses, and treatment decisions. This may produce differences in help-seeking, diagnoses, and ultimately treatments and interventions. Implicit biases may also unwittingly produce professional behaviors, attitudes, and interactions that reduce patients' trust and comfort with their provider, leading to earlier termination of visits and/or reduced adherence and follow-up. Disadvantaged groups are marginalized in the healthcare system and vulnerable on multiple levels; health professionals' implicit biases can further exacerbate these existing disadvantages.
Interventions or strategies designed to reduce implicit bias may be categorized as change-based or control-based. Change-based interventions focus on reducing or changing cognitive associations underlying implicit biases. These interventions might include challenging stereotypes. Conversely, control-based interventions involve reducing the effects of the implicit bias on the individual's behaviors. These strategies include increasing awareness of biased thoughts and responses. The two types of interventions are not mutually exclusive and may be used synergistically.
#34374: Hyperglycemia and Wound Management
Hyperglycemia, often the result of poorly controlled diabetes, is commonly detected in hospitalized patients and can result in increased mortality and morbidity. The presence of hyperglycemia significantly impacts various aspects of patient care regardless of diagnosis; however, as the number of individuals with type 2 diabetes increases, excessive blood glucose is becoming a significant public health issue. A major area of concern is the relationship between hyperglycemia and wound healing. The reduction in wound healing associated with a hyperglycemic state is well documented in the literature, and it is exceedingly evident in cardiovascular patients. The prevention of delayed, inadequate, and incomplete healing of surgical, accidental, arterial, venous, and/or pressure wounds is the goal of therapy. This course will review the impact of hyperglycemia on wound healing and interventions that may improve the prognosis for these patients.
There are three main causes of hyperglycemia: diabetes, certain medications, and physiologic or psychological stress. Diabetes is by far the most common cause of chronic hyperglycemia and is associated with many adverse health effects, including impaired wound healing and increased risk for lower extremity ulcers and amputations. However, medications and physiologic/psychological stresses are also important contributors to hyperglycemia, particularly in hospital settings. When all of these factors are combined, a patient can be at significantly heightened risk for wound complications and poor outcomes.
Diabetes, known clinically as diabetes mellitus, is a progressive disease process affecting the fuel metabolism functioning within the body [1]. According to the Centers for Disease Control and Prevention (CDC), the prevalence of diabetes has increased 1,100% since 1958, when 0.93% of Americans had diabetes, with the sharpest increase occurring in the 2000s [2]. As of 2022, 11.3% of the United States population, or 37.3 million Americans, had diabetes [3]. Unfortunately, 8.5 million of these individuals are unaware of their diabetes diagnosis. Diabetes has been considered epidemic since the 1970s, and the number of Americans expected to have diagnosed or undiagnosed diabetes is estimated to be 84 million by the year 2060 [4].
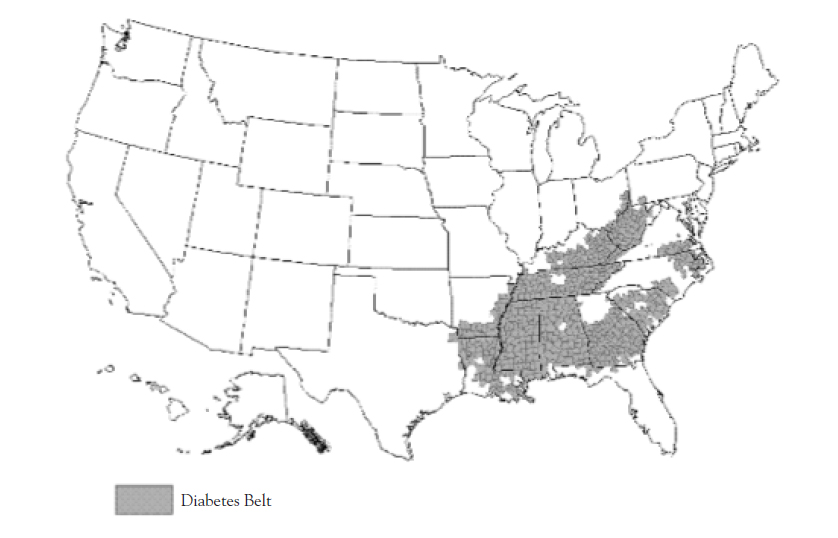
The scope of the problem is vast and diverse, particularly among geographical regions. The CDC identified a "diabetes belt" in the United States in 2011, consisting of 644 counties in 15 states, where 11.7% of the adult population has diagnosed diabetes, compared with an 8.5% average in other counties (Figure 1) [5,73]. Although this represents a particularly dense geographic concentration of disease, there are many other counties and groups of counties outside of this belt with prevalences as high or higher. Many of the counties outside of the "diabetes belt" with especially prevalent diabetes have high American Indian populations; one such example are several counties in northeastern Arizona, northwestern New Mexico, and southern Utah [3].
Genetics, race, age, and lifestyle significantly influence the onset and progression of the disease process [6]. Although all races and ethnicities can develop diabetes, the prevalence is greatest among American Indian/Alaska Natives, people of Hispanic origin, non-Hispanic Black Americans, and Pacific Islander/Native Hawaiians [3,7,8,9]. The incidence of diagnosed diabetes is estimated to be 7.9% among non-Hispanic Black individuals 18 years of age and older, compared with the overall U.S. rate of 6.7% [3]. The prevalence of diabetes among persons of Hispanic descent who are 18 years of age or older is 13.2% [8]. Among the subdivisions of Hispanic groups, diabetes prevalence rates are 8.3% for Central/South Americans, 6.5% for Cubans, 12.4% for Puerto Ricans, and 14.4% for Mexicans. In addition to being at high-risk for diabetes, Mexican Americans are 40% more likely to die from the disease than their non-Hispanic White counterparts [3]. However, American Indians/Alaska Natives and Native Hawaiians present the greatest risk for the development of type 2 diabetes; their risk is nearly three times greater than that of White Americans [9]. It is estimated that approximately 14.7% of American Indians/Alaska Natives 18 years of age and older have type 2 diabetes. Approximately 22.5% of Native Hawaiians have diagnosed diabetes, and another 15% have prediabetes [79]. The highest prevalence of diabetes in the contiguous United States is observed in certain American Indian groups of the Southwest, where an estimated 22.2% of the population has the disease [3]. The highest rate of diabetes for any population (worldwide) has been reported to occur in the Pima Indians of Arizona [10,74].
The most rapid increase in diabetes prevalence in the last decade has been among adolescents. Historically, children and adolescents with hyperglycemia have been diagnosed with type 1 diabetes, a result of the body being unable to produce adequate amounts of insulin. However, it is now estimated that as many as 46% of juvenile-onset cases of diabetes are type 2 [11]. Furthermore, it has been predicted that children born in this millennium will have a one-in-three chance of developing diabetes in their lifetime; among high-risk ethnic groups, the estimate is as high as one in two [12].
Individuals with type 1 diabetes typically present to their primary care office or hospital emergency department with a sudden weight loss and severe hyperglycemia [13]. The consequences of the disease can take years to impact the body if optimally controlled. In contrast, an individual with type 2 diabetes may be unaware of the disease process for years prior to being diagnosed. The onset of type 2 diabetes is insidious, with the loss of first-phase insulin response to postprandial levels. The first elevation may be detected at the time of screening as prediabetes or impaired glucose tolerance (IGT) [14]. During this time, the disease progression and hyperglycemia are ravaging the body's micro- and macrovascular systems [1].
In a healthy body, carbohydrate consumption results in an initial swell in serum glucose levels and hyperglycemia. In response to this escalation, the body releases insulin from pancreatic beta cells into the circulatory system to assist with glucose transport into muscle, liver, and adipose tissues; this causes a lowering of the blood glucose levels.
In the case of type 1 diabetes, pancreatic beta cells fail to produce and release insulin, and the only treatment option is exogenous insulin and other appropriate injectables [14].
The pathogenesis of type 2 diabetes is more complex. Virtually all individuals diagnosed with type 2 diabetes have insulin resistance in conjunction with some degree of insulin deficiency [15]. In response, the body's inability to react to the mounting glucose level is magnified by insulin deficiency in the functioning pancreatic beta cells, insulin resistance in the muscular tissue, or both [1]. Unlike the therapy for type 1 diabetes, type 2 diabetes is initially treated with lifestyle modification therapy (e.g., medical nutrition therapy, exercise, behavior change) and frequently with an oral medication, such as a biguanide. Within five years of type 2 diabetes diagnosis, it is estimated that as many as 90% of people will fail this initial therapy and require further oral medications or injectables to achieve adequate glucose control. As a result of this failure rate, medication therapy is initiated early (in the prediabetes stage) in diabetes medical treatment algorithms [16].
As discussed, the most common types of diabetes are type 1 and type 2. However, gestational diabetes is also relatively common and is a source of significant morbidity and mortality. Gestational diabetes is first recognized in pregnancy, usually around the 24th week of gestation, and typically resolves after the birth of the child [13]. Other less common types of diabetes do occur and include [14,15]:
Maturity-onset diabetes of the young (MODY): A genetic, autosomal-dominant defect of the pancreatic beta cells results in insulin deficiency and decreased insulin release without the presence of insulin resistance and obesity. This form of diabetes typically develops in patients younger than 25 years of age. It is a different clinical entity than type 2 diabetes of the adolescent, which presents with insulin resistance.
Diabetes related to diseases of the exocrine pancreas, such as cystic fibrosis, and various endocrine diseases, such as Cushing syndrome, acromegaly, and chromocytoma
Drug-induced diabetes resulting from the use of certain medications, particularly high-dose corticosteroids
All adults older than 45 years of age should be screened for diabetes every three years or every two years if they have any risk factors for type 2 diabetes [15,17]. In addition, individuals of any age who are at risk for or are suspect of having diabetes should be screened. Established risk factors for type 2 diabetes include:
Age older than 45 years
Body mass index (BMI) greater than or equal to 25 kg/m2
Family history of type 2 diabetes
Habitual physical inactivity
Race/ethnicity (e.g., African American, Hispanic American, American Indian, Alaska Native, Pacific Islander)
IGT or elevated fasting glucose
Previous history of gestational diabetes or giving birth to a child weighing more than 9 pounds
Hypertension (i.e., blood pressure greater than 140/90 mm Hg in adults)
Abnormal lipid levels (i.e., high-density lipoprotein [HDL] level <35 mg/dL and/or triglyceride level >250 mg/dL)
Polycystic ovarian syndrome
History of vascular disease
Acanthosis nigricans (most common among individuals of African descent)
The diagnostic criteria for type 2 diabetes are fairly straightforward and are based on fasting plasma glucose and postprandial plasma glucose levels (Table 1). After a diagnosis of type 2 diabetes has been definitively made, education on self-care management is necessary in order to obtain euglycemia and prevent complications related to the detrimental effects of hyperglycemia[13]. As previously stated, it is estimated that as many as 90% of patients with type 2 diabetes will require oral medications to achieve adequate glucose control within five years of diagnosis[13]. When glucose levels cannot be adequately controlled with oral medications, the use of injectable medications is necessary. If elevated blood glucose levels are untreated and continue to rise, the result can be hyperosmolar hyperglycemia syndrome (HHS) and ultimately death[18].
In the past, hyperglycemia was considered an inevitable consequence of acute illness in some patients, and glycemic control was not a focus of hospital care. However, as it has become clear that hyperglycemia is linked to a number of adverse hospital outcomes, controlling inpatients' glucose levels has become a key part of the care of acutely ill patients [20]. A number of features suggest that individuals with newly diagnosed hyperglycemia tend to be more severely ill than individuals with known diabetes [21]. Hospital hyperglycemia, in patients with and without diabetes, has been identified as a marker of poor clinical outcomes, particularly in cardiac surgery patients. However, adverse effects, such as extended length of stay, complications, and increased mortality, have been noted among general and noncardiac surgical patients as well. The impact appears to be greater among patients without a history of diabetes compared to those with a diabetes diagnosis [22].
According to observational studies, 32% to 38% of hospitalized patients are admitted with hyperglycemia or diabetes, and 70% to 80% of patients with diabetes and critical illnesses or undergoing cardiac surgery experience hyperglycemia during their stay [23]. A number of serious complications are associated with long-term diabetes, including macrovascular complications, microvascular complications, neuropathies, alterations of skin integrity, and infection [24]. Stress hyperglycemia is frequently recognized as a complication related to hospitalization for individuals with or without diabetes [14]. Often, hospitalized patients are treated with medications that can affect elevation of blood glucose levels. Most commonly, glucocorticoids can induce hyperglycemia resulting from stimulation of alpha cells, leading to hyperglucagonemia and increased glycogenolysis [14]. Admission hyperglycemia has been associated with increased hospital mortality in critically ill individuals, specifically those with myocardial infarction and cerebrovascular accidents [21,23].
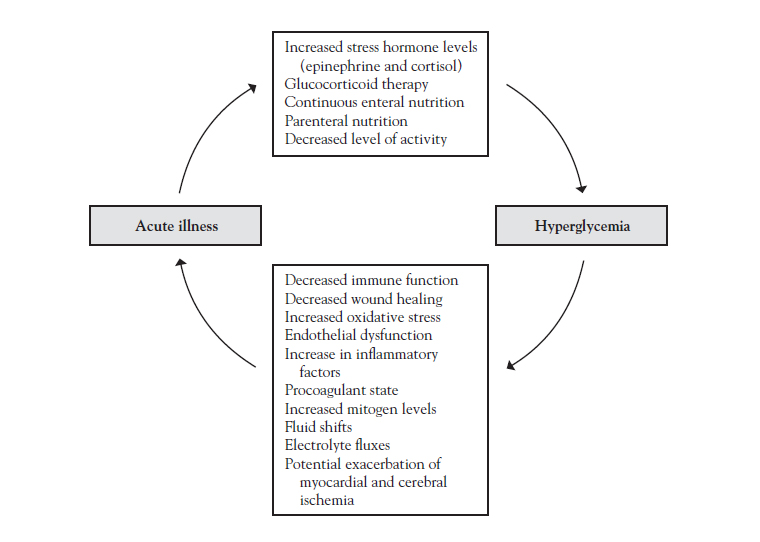
Acute illness can trigger hyperglycemia in susceptible individuals. Furthermore, hyperglycemia can induce changes that allow an acute illness to proliferate (Figure 2).
The endocrine system acts together with the nervous and immune systems to respond to stressors, initiating the stress response [24]. Psychological and physiologic stress have considerable effects on metabolism in individuals without diabetes, resulting in increased counter-regulatory hormones and elevations in blood glucose levels [1]. During stress, the general adaptation syndrome includes the development of three successive stages:
The alarm stage: The central nervous system is aroused, and the body's defenses are mobilized.
Resistance or adaptation: Mobilization contributes to the fight-or-flight response.
Exhaustion: Continuous stress causes the progressive breakdown of compensatory mechanisms and homeostasis.
The alarm stage impacts glucose levels by inducing increased secretion of glucocorticoids and activation of the sympathetic nervous system, including the adrenal medulla gland, with increased release of epinephrine [24].
Critically ill individuals are at risk for a combination of starvation and the physiologic stress resulting from injury, trauma, major surgery, and/or infection. Starvation may occur as a result of the individual being classified as "nothing by mouth," or NPO, in preparation for surgical interventions, being unable to eat due to disease-related factors, and/or being hemodynamically unstable and unable to eat. This physiologic stress results in an increased metabolic rate (hypermetabolism), which in turn causes an increase in oxygen consumption and energy expenditure [26].
The stress response directly influences the immune system through hypothalamic and pituitary peptides and through the sympathetic branch of the autonomic nervous system. There is a direct innervation of the thymus, spleen, lymph nodes, and bone marrow. Endogenous opiates are released during stress and have concentration-dependent enhancing and suppressive effects on various immune cells [24].
Hypermetabolic processes are the result of increased catabolic hormone changes caused by the stressful event. The sympathetic nervous system is stimulated, causing the adrenal medulla to release epinephrine, norepinephrine, and glucocorticosteroids, which increase blood glucose levels[26]. Increased levels of circulating glucocorticosteroids are an important mechanism in the stress-related immune structure alterations and in the suppression of the immune response. The release of immune inflammatory mediators (e.g., interleukin-1, tumor necrosis factor-α[TNF-α], interferon) is triggered by infection, cancer tissue injury, and other insults that in turn initiate a stress response through the hypothalamic-pituitary-adrenal pathway[24]. This results in nutrient substrates, primarily amino acids, moving from peripheral tissue, such as skeletal muscle, to the liver for gluconeogenesis[26]. Enhanced systemic production of cytokines also induces additional central nervous system and behavior changes during an acute infectious episode[24].
Neutrophil chemotaxis and phagocytosis are defective in poorly controlled glycemia related to diabetes or stress-induced hyperglycemia. Cell-mediated immunity may also be abnormal. In addition, vascular lesions can hinder blood flow, preventing an inflammatory response in wounds or other possible sources of infection. Therefore, individuals with diabetes are more prone to develop infections and may have more severe infections[27].
Management of critically ill patients requires healthcare professionals to be responsive to the effects of medications and the impact of the effects of hyperglycemia on outcomes [6]. The goals of medical management are to administer vasopressor therapy and corticosteroids and to provide an adequate level of nutrition, including enough protein to maintain optimal cellular and tissue growth [26].
Discontinuation of the offending medication is often necessary in cases of drug-induced hyperglycemia. Epinephrine and norepinephrine are commonly utilized to control hypotension in critically ill individuals. Healthcare professionals should be aware that these medications stimulate gluconeogenesis, increase skeletal muscle and hepatic glycogenolysis, increase lipolysis, suppress insulin secretion, and increase peripheral insulin resistance. All of these actions function to increase the serum glucose levels [26]. Healthcare professionals can correct vasopressor-induced hyperglycemia with an infusion of insulin and dextrose [6].
The incidence of hyperglycemia in the hospital has increased significantly along with increased diagnoses of diabetes, prediabetes, and metabolic syndrome. The more frequent use of steroid therapy for the treatment of other chronic conditions has also contributed to hyperglycemia [28]. Glucocorticoids are the most common cause of drug-induced hyperglycemia [14]. Individuals with decreased insulin secretory reserve and the use of high glucocorticoid doses are risk factors for significant hyperglycemia, including the development of HHS [6]. Mechanisms that contribute to the development of hyperglycemia include a decrease in peripheral insulin sensitivity, increased hepatic glucose production, and inhibition of pancreatic insulin production and secretion [14].
Glucocorticoids that are oxygenated, such as hydrocortisone, and/or have a double bond ring, such as prednisone and prednisolone, have the greatest diabetogenic effect. Glucocorticoids can also produce hyperglycemia through stimulation of alpha cells, leading to hyperglucagonemia and increased glycogenolysis. Mineralocorticoids do not directly influence carbohydrate metabolism, although hypokalemia associated with their use may reduce insulin secretion [14].
Interestingly, both beta-agonists and antagonists are associated with the development of hyperglycemia through the stimulation of an increase in plasma glucose via an increase in glycogenolysis and lipolysis, despite increased insulin secretion. Beta-agonists' diabetogenic potential is dose dependent and more pronounced with intravenous and oral routes than with the subcutaneous route. Multiple mechanisms for this action have been identified, including an increase in hepatic glucose production, peripheral insulin resistance, and plasma glucagon following beta-2 agonist administration [14].
The pathophysiology of acute wound healing has been characterized as a hierarchical progression through four distinct phases. The first phase is coagulation and occurs immediately at the time of tissue injury[29]. The key cell activated in this phase is the platelet, which aggregates at the area of injury and binds with thrombin to form a plug. Vasoconstriction occurs, and cytokines and growth factors, including platelet-driven growth factors and fibroblast growth factors, are released[29]. These reactions initiate healing and act as chemoattractants for circulating polymorphonuclear cells and macrophage[30].
With vasodilatation, cells enter the wound and initiate the inflammation phase. During this phase, the inflammatory cells phagocytose bacteria and macrophages produce a greater amount of cytokines and growth factors such as interleukins-6, fibroblast growth factor, and transforming growth factor-β [29,31]. The inflammatory cells produce matrix metalloproteases, enzymes that digest collagens and elastins, and components of the fibrin clot, also known as the provisional matrix[32]. Through paracrine and autocrine mechanisms, the growth factors serve as a call to action for fibroblasts, which infiltrate the provisional matrix and begin to produce fibronectin and collagen[30].
The matrix metalloproteases also clear the way for endothelial cells and new vessel formation, or angiogenesis. In a sense, they are "drilling for oxygen" to accelerate wound healing. When this process finally occurs, the proliferation phase begins[29,30]. During this phase, fibroblasts and endothelial cells are recruited locally and from circulating precursors from the bone marrow, drawn in by multiple angiogenic growth factors present in the wound[31]. The recently formed matrix, with its rich arcade of new vessels, appears to the naked eye to be fine, red granules termed "granulation"[30]. This granulation tissue provides a supporting lattice to allow the migration of keratinocytes across the surface of the wound to create a new epidermis. After re-epithelialization, endothelial cells undergo apoptosis, and the granulation tissue is replaced by collagen and dermal structure[29].
Simultaneously, scar tissue formation and collagen shortening occur, and fibroblasts are transformed into myofibroblasts, causing the wound to react and contract[30]. When the wound is completely covered with epithelium and not draining, it is considered healed. The entire process typically takes 10 to 14 days to complete in an acute wound[31]. This is followed closely by the remodeling phase, during which collagen fibrils in scar tissue are remodeled in a turnover process resulting from the interplay of degradation of the matrix metalloproteases and the production of matrix components from the fibroblasts. This final phase may continue for the next 6 to 12 months[30].
Unlike the orderly healing process described for acute wounds, chronic wounds are stuck in a disorderly mix of inflammation and failed eruptions of proliferation[30]. Dysfunctional wound healing may occur during any phase of the healing process and may involve insufficient repair, excessive repair, or infection. The causes can be related to a predisposing disorder, such as diabetes, an acquired condition, such as hypoxia, or one of many potential medication interactions or alterations in nutrition[24].
The chronic wound is characterized by several primary defects[30]. Healing may be prolonged if bleeding is not stopped during the acute inflammation phase. A clot increases the quantity of space that granulation tissue may fill and serves as a mechanical barrier to oxygen diffusion. The accumulation of excess blood cells resulting from hemorrhage prolongs the inflammation phase, creating an excellent medium for bacterial growth and infection[24]. Excessive inflammation occurs as a result of overproduction of inflammatory cytokines, such as interleukin-6, TNF-α, and matrix metalloproteinases, particularly matrix metalloproteinase-1, matrix metalloproteinase-8, and matrix metalloproteinase-13. This allows the accumulation of altered matrix substances, such as fibronectin, that have been rendered ineffectual by protein degradation[30].
Hypovolemia also inhibits inflammation, as the physiologic response is vasoconstriction rather than the dilatation required to deliver inflammatory cells to the site of injury. Anti-inflammatory steroids prevent macrophages from migrating to the site of injury and inhibit the release of collagenase and plasminogen activator. Anti-inflammatory steroids also inhibit fibroblast migration into the wound during the reconstructive phase[24].
Optimal nutrition is important during all phases of healing because metabolic needs are increased. The substances most vital to healing include glucose, oxygen, and protein[31]. Because leukocytes require a glucose-rich environment to produce the adenosine-5 triphosphate (ATP) needed for chemotaxis, phagocytosis, and intracellular killing, individuals with poorly controlled diabetes heal poorly, mainly because of infection[33]. Individuals with diabetes are also at an increased risk for ischemic wounds, because they are likely to have both microvascular diseases that impair the microcirculation and altered glycosylated hemoglobin affinity for oxygen, with oxygen not readily released into the tissue. Oxygen delivery is also compromised by the hypoxemia state. Ischemic tissue is susceptible to infection, which in turn prolongs the inflammatory process.
Along with hypoxemia, hypoproteinemia lengthens inflammation by impairing fibroblast proliferation[24]. Most of the factors that interfere with the production of collagen in healing tissues are nutritional[24]. Scurvy, for example, is caused by a lack of ascorbic acid (vitamin C), which is one of the cofactors required for collagen formation by fibroblasts. As a result, patients with scurvy have poorly formed connective tissue and greatly impaired healing. Protein and other nutrients, including iron, oxygen,β-ketoglutarate, manganese, copper, and calcium, are required for collagen synthesis[34]. However, minute amounts of these substances are usually required as cofactors, so deficiencies are not generally clinically significant. Dysfunctional collagen overwhelming the surface of a wound is manifested by a keloid or hypertrophic scar, both of which are related to increased collagen synthesis and decreased collagen lysis[35].
Regulated expression of the gap junction protein connexin 43 plays a pivotal role in wound healing. Connexin 43 is normally down-regulated and protein connexin 26 is upgraded in keratinocytes at the edge of the wound as they adopt a migratory phenotype [36]. However, alterations in this action may result in chronic wounds. Individuals who have diabetes have been found to have decreased protein connexin 43 and 26 communication within the intact epidermis and increased protein connexin 43 communication within the intact dermis. Within 24 hours of injury in patients with diabetes, protein connexin 43 is upregulated in a thickened bulb of keratinocytes at the edge of the wound; in contrast, a healthy individual with normal down-regulation will form a set of thin migratory cells. In the presence of diabetes, protein connexin 43 is delayed for up to 48 hours after re-epithelialization has begun. Furthermore, although protein connexin 26 is upregulated as normal after tissue injury in the skin of an individual with diabetes, the skin at the edge of the wound appears abnormal [36].
The public health impact of wounds is immense. An estimated 2.5 million inpatients develop pressure injuries each year, particularly older and malnourished individuals [37]. Furthermore, as many as 34% of the 37.3 million individuals with diabetes in the United States will develop diabetic ulcers; many more will have venous ulcers or wounds that result from vascular disease [3,38]. The annual cost of treating wounds of this nature is estimated to be more than $15 billion, which has led to specific guidelines based on specialist organization consensus [37,39,40,41,75]. In patients with diabetes, neuropathy and microvascular disease are the greatest contributors to impaired wound healing, both of which can be improved with optimal management of blood glucose levels.
Diabetic neuropathies are the most common form of neuropathy in developed countries, and it is estimated that 60% to 70% of individuals with diabetes have some form of nervous system damage [3,42]. These disorders are among the most widespread complications of diabetes and are a significant source of morbidity and mortality. The most frequent complication is diabetic foot ulcerations, which, if poorly treated, can result in gangrene and ultimately loss of limb [17]. In fact, diabetes is the underlying cause of as many as 85% of all nontraumatic amputations in the United States [76]. In the United States, 170,000 amputations are performed on individuals with diabetes each year; up to 75% of the procedures are considered to be preventable [17,76].
Neuropathy refers to the impaired effectiveness of the nerves in conducting pain, pressure, or movement sensations. In many patients with diabetes, the development of neuropathy in the lower extremities leads to loss of protective sensation, resulting in undetected trauma and infection. The most common type of neuropathy in patients with diabetes is peripheral neuropathy, which affects the ends of the longest nerves first; the toes and feet are the most severely affected. Other types or neuropathy include autonomic neuropathy, mononeuropathy, and diabetic amyotrophy (also referred to as radiculoplexus neuropathy). In patients with peripheral neuropathy, the development of foot ulcers and even foot deformities is common, and the healing of these ulcers can be complicated by the presence of hyperglycemia [42].
Foot ulcerations are rarely caused by a single pathology and typically result from two or more contributing factors. Insensitivity resulting from neuropathy combined with either extrinsic factors (e.g., trauma caused by walking barefoot or stepping on a sharp object) or intrinsic factors (e.g., bunions, calluses) ultimately results in ulceration [6]. Neuropathy is the most common cause of lower extremity ulcerations. However, in approximately one-third of all cases, peripheral ischemia resulting from proximal arterial disease is a contributing factor in ulceration. The ischemic foot appears red, dry, and often neuropathic, so it is susceptible to pressure from footwear [30].
Foot deformities, such as claw toes or prominent metatarsal heads, are a proven risk factor for ulceration [26,42]. One cross-sectional study demonstrated that plantar callus accumulation was associated with an 11-fold increase in formation of diabetic foot ulceration [30]. In follow-up with these individuals, plantar ulceration occurred only at sites of callus in neuropathic feet. Other risk factors include a past history of foot ulcers or amputation, the presence of other microvascular complications, long duration of poorly controlled diabetes, increase in plantar foot pressures, and peripheral edema [30,43]. The most common triad leading to breakdown of the diabetic foot is peripheral neuropathy (insensitivity), deformity (claw toe), and trauma (from ill-fitting footwear, repetitive stress, or thermal damage) [30,42,44].
Symmetric polyneuropathy is a potentiating factor in the development of foot ulceration in individuals with diabetes; it manifested at clinical examination as diminished or absent vibratory and cutaneous pressure sensation and absence of ankle reflexes. This type of neuropathy is present in 75% to 90% of patients with current diabetic foot ulcerations [45]. In addition to symmetric polyneuropathy, peripheral vascular disease is an important contributory factor in the development of diabetic foot ulcerations in 10% to 30% of all cases [30]. The location of ulceration on the foot provides indications of the causative factors and assists in the determination of the most effective treatment approach [30].
Diabetic foot ulcers often lead to amputation due to ischemia and impaired immune response related to macrovascular disease (present in 30% to 40% of diabetic foot ulcers) and microvascular disease [27]. Intermittent claudication and pain at rest may be presenting symptoms of major vessel disease. Ulceration is a common feature of the neuroischemic foot [43]. This type of ulceration is usually painless, and there is typically an area of necrosis surrounded by a rim of erythema. Common locations of these ulcerations are the great toe, the medial surface of the first metatarsal head, and the heel [24].
Individuals with diabetes and neuropathy also have significant microvascular defects. Hyperglycemia is associated with a ubiquitous involvement of the microvasculature manifested by capillary sclerosis and dropout, particularly in individuals with type 1 diabetes[30]. This can be seen with capillaroscopy and measurement of elevated capillary pressures. For individuals with type 2 diabetes and neuropathy, functional microvascular abnormalities develop, especially endothelial dysfunction and impaired vasoreactivity[6]. These abnormalities are best demonstrated by laser Doppler imaging of the microcirculation, which demonstrates defective vasodilatory response to heat (generalized defect), acetylcholine (endothelial defect), and sodium nitroprusside (vascular smooth muscle defect). In addition, neuropathy affects the neuron-inflammatory microvascular vasodilatation in response to injury or noxious stimuli[30]. Angiogenesis is also seen in patients with diabetes. Among many contributors to angiogenesis are neural mediators such as acetylcholine, substance P, and neuropeptide Y, all substances involved in the impaired neuron-inflammatory response in patients with diabetes. The presence of peripheral neuropathy may contribute to impaired angiogenesis and wound healing on a cellular basis[31].
Hyperglycemia may slow neutrophil chemotaxis, primarily through a hyperosmotic effect[30]. Studies of fibroblasts in individuals with diabetes demonstrate an alteration in functioning and in response to stimulatory challenges[35]. A vast body of literature suggests that the altered blood glucose levels and free fatty acid metabolism associated with diabetes result in oxidative stress, endothelial dysfunction, and activation of inflammatory cytokines. Additionally, there is an increased expression of TNF-α, transforming growth factor-β, interleukin-6, and other inflammatory factors. These factors are overexpressed in chronic wounds and most likely amplified in the inflammatory milieu of diabetes[30].
Vascular ulcers may develop in the arterial, venous, or lymphatic systems from a variety of causes[33]. Patients with diabetes are at an increased risk for both arterial and venous ulcers. The pathophysiology of venous (or stasis) ulcers is related to venous hypertension that develops as the result of inadequate calf muscle pump action and either primary or secondary valvular incompetence[46]. Arterial ulcers, on the other hand, are wounds that will not heal due to compromised or inadequate arterial blood flow[33]. Arterial calcification is commonly detected in radiographs of diabetic feet and hands. This is related to calcification of the media on muscular arteries[43]. Differentiating between arterial and venous ulcers involves careful patient history and evaluating the appearance of the ulcer and surrounding skin (Table 2)[32].
CHARACTERISTICS OF ARTERIAL AND VENOUS ULCERS
| Aspects | Venous Ulcers | Arterial Ulcers | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location |
|
| ||||||||||||||||
| Surrounding skin |
|
| ||||||||||||||||
| General appearance |
|
| ||||||||||||||||
| Depth | Usually shallow | Deep | ||||||||||||||||
| Margins |
|
| ||||||||||||||||
| Exudate | Moderate to heavy | Minimal | ||||||||||||||||
| Infection | Less common | Frequent, but signs may be subtle |
Studies have proven that individuals with diabetes possess an abnormally thickened vascular basement membrane in the capillaries, but the membrane does not appear to be associated with gangrene [30]. Impairment, calcification, and thickening of these vascular capillaries can lead to impairment of blood flow to the lower extremities, as evidenced by diminished dorsalis pedis and/or posterior tibial pulses [14]. The results include ischemia in the vasculature, skin, and supporting tissues. Symptoms include chronic pooling of blood in the lower extremities and hyperpigmentation of the skin around the feet and ankles. Circulation to the extremities becomes increasingly sluggish, to the point that the metabolic demands of the cells are scarcely met. Any trauma or pressure can therefore result in a further lowering of oxygen supply, leading to cell death and necrosis [24].
In patients with diabetes, venous ulcers predominantly occur on the foot, but may arise on the lower leg or ankle as well. Venous ulcers and venous insufficiency in general often occur in patients with reduced mobility and/or a history of obesity, deep vein thrombosis, traumatic injury, phlebitis, orthopedic procedures, or congestive heart failure [32].
The precipitating events for arterial ulcers vary. Limbs with arterial compromise may have minimal but adequate blood flow to maintain tissue viability. Ischemic lower extremity ulcerations are often precipitated by trauma or infection [33]. Infection can occur as a result of poor circulation impairing the delivery of cells and biochemicals essential for the immune and inflammatory responses [24].
Treatment and management of vascular ulceration include the following approaches [30,31,47]:
Pressure off-loading, utilizing crutches, wheelchairs, therapeutic footwear, half shoes, and removable cast walkers to relieve pressure on the affected area.
Debridement of necrotic tissue from the ulceration to allow for healing. This can be accomplished by surgical or nonsurgical means and allows for viable skin to be produced and adhere to the surface of the ulcer. This therapy is commonly utilized in the mid- or chronic stages of ulceration.
Adjunctive wound therapy, including low-adherent dressings, interactive (occlusive) hydrocolloid dressings, semi-permeable films, hydrogels, and alginate dressings. Synthetic foams work as healing agents, and antimicrobial barriers are commonly utilized in all stages of acute and chronic ulceration.
Growth factor, in combination with a platelet releasate or delivered through recombinant platelet-derived growth factor technology, is utilized in early and involved diabetic ulcerations.
Hyperbaric oxygen therapy. Intermittent inhalation of pure oxygen at a pressure greater than one atmosphere absolute (15 pounds pressure/in2) increases the percentage of oxygen available to produce adequate wound healing. This therapy is utilized in the chronic stages of diabetic ulcerations when other therapies have lost their efficacy. Authors of a 2015 Cochrane review concluded that hyperbaric oxygen therapy is significantly beneficial in the short term but not in the long term.
Electrical stimulation to increase the blood flow. Utilizing asymmetric biphasic stimulation is associated with increased healing of nearly 60%. This form of therapy can be utilized in early or involved stages of diabetic ulceration therapy.
Low-intensity ultrasonic stimulation improves the rate of wound healing.
Topical radiant heat. This therapy optimizes the enzymatic processes involved in wound healing and can be utilized in early or involved stages of diabetic ulceration therapy.
Diabetes may affect both the central and peripheral nerves. The most common cause of lower extremity ulcers in patients with diabetes is chronic sensory-motor neuropathy [43]. Diabetic neuropathy alters the mechanics of the foot, which causes increased shear and pressure on the sole and results in an increased risk for injury and complication [33]. The prevalence of sensory-neuropathic ulcers approaches 30% in patients with diabetes and increases with age and duration of diabetes [43].
Sensory neuropathy contributes to an inability to perceive injury to the foot due to the loss of protective sensation [33]. Individuals with sensory loss are seven times more likely to develop foot ulcers compared to those without neuropathic disorders [30]. Loss of sensation is the result of demyelination of the peripheral nerves (particularly the distal nerve endings) [27]. Presentation of this loss of sensory sensation typically begins with numbness or paresthesia of the toes and progresses upward to create a "stocking" effect to the extremity [48].
With neuropathies of the foot, an individual will initially experience loss of pain and heat perception as a result of the changes in the small and large myelinated nerve fiber. This leads to a loss of touch, vibration, and proprioception sense [43]. Neuropathy can further progress to fragility and wasting of the intrinsic musculature of the lower extremity, with consequential deformities of the foot and toes [33].
Prevention of neuropathic ulcerations of the leg and foot consists of frequent inspection, controlling blood glucose levels, and patient education regarding proper care and maintenance of the foot and lower extremities [48]. It is important to educate the individual with diabetes to remove footwear and socks when at the physician's office [6]. This will allow examination of the foot and identification of any deformities and evidence of neuropathy, vascular disease, plantar calluses, edema, or other risk factors [27]. A simple foot pressure mat can assist in the identification of high pressures under the diabetic foot, and these pressure maps can be utilized to educate patients regarding their risk for development of subsequent ulcerations and the care required to prevent this occurrence [30].
The public health impact of chronic wounds is staggering, with an estimated 3 million individuals in the United States believed to have pressure injuries [49,77]. Treating these wounds is associated with an estimated cost of an estimated $11 billion each year [49,77]. Estimates of the incidence of pressure injuries range from 0.4% to 38% in acute care hospitals, from 2% to 24% in long-term care facilities, and up to 17% in the home care setting, with an overall prevalence in the United States of 13.5% in 2008 and 12.3% in 2009 [49,77]. An analysis of 2018–2019 survey data found that the overall pressure injury prevalence for patients in critical care units was 14.3%, with 5.85% categorized as hospital-acquired [25]. Pressure injury-related hospitalizations are longer and more expensive than many other hospitalizations. The length of hospitalizations for patients with pressure injuries is nearly three times longer than hospitalizations without a diagnosis of pressure injuries [49,77].
Although diabetes is not a cause of pressure injuries, the altered nutritional state, poor circulation, and impaired healing associated with the disease places patients with diabetes at greater risk for pressure injuries. Furthermore, pressure injuries that develop in patients with diabetes tend to be more severe and heal more slowly. It has been hypothesized that the structure of the collagen network in the skin of patients with diabetes may lower its tolerance of external pressure and delay skin reconstruction [50].
Pressure ulcers or injuries primarily develop over a bony prominence when pressure or shearing forces occlude cutaneous and subcutaneous blood flow. In patients with diabetes, pressure injuries often occur on the heel. Cutaneous pressure on the tissue between the bony prominence and a resistant outside surface distorts capillaries and prevents adequate blood from circulating to the area [33]. Unrelieved pressure on the skin constricts capillaries that supply the skin with nutrients and oxygen. This can be exacerbated by existing arterial disease and neuropathy. When skin is starved of nutrients and oxygen for an extended period, the tissue dies and a pressure injury forms. The affected area may feel warmer than surrounding tissue. Skin reddening that disappears after pressure is removed is normal and is not a sign of a pressure injury [51]. If pressure is relieved within a few hours, a brief period of reactive hyperemia may occur with no lasting tissue damage [24].
In addition to diabetes and hyperglycemia, there are many risk factors for the development of pressure injuries, including [24]:
Older age
Neurologic disorders, such as spinal cord injuries, dementia, or cerebrovascular disease, resulting in decreased sensation and/or mobility
Immobilization
Incontinence
Fractures (e.g., hip or femur)
Coarse bed sheets
Reduced tissue perfusion (e.g., as from edema or hypotension)
Obesity
Skin dehydration
Stress
Depression
Low body weight and impaired nutrition
Smoking
Certain medications
There are many scales to validate and predict risk for the development of pressure injuries. The most commonly utilized in the United States is the Braden Scale, which takes into consideration sensory perception, moisture, activity, mobility, nutrition, and friction and shear [33,49]. Each item is scored on a scale between 1 and 4, with the exception of friction and shear, which is scored between 1 and 3. The lower the score, the more severe the impairment and the greater the patient's risk for pressure injury development. Studies have shown scores of 16 to 18 as indicative of increased risk [52]. Although cut-off scores vary, usually a score of 13 to 14 is considered moderate risk, 10 to 12 indicates high risk, and 9 or less is very high risk.
When a patient with diabetes or hyperglycemia is found to be at risk for pressure injury formation, preventive steps should be taken, including an individualized program of skin care. This can include [24,53]:
Scheduled repositioning every one to two hours, depending on the patient's condition
The use of assistive devices to ensure correct position in beds (e.g., floatation mattresses, cushions, suspension boots) or chairs (e.g., cushions, correct size chair)
Maintaining skin cleanliness and moisturizing frequently
Bowel and bladder management
Maintenance of adequate nutrition, oxygenation, and fluid balance
Enhanced patient and caregiver education
Strict control of blood glucose levels
Research supports the findings that undernourishment on admission to a healthcare facility increases the potential of an individual developing a pressure injury [33,54]. In one prospective study, high-risk individuals who were malnourished upon admission had two times greater risk of developing a pressure injury compared to individuals who were adequately nourished [33]. Maintaining an adequate level of nutrition is paramount for successful wound healing [53]. This includes addressing any vitamin and/or mineral deficiencies that may be present (e.g., zinc deficiency in patients with diabetes). Repair of the wound requires proteins, carbohydrates, fats, vitamins, and minerals to ensure the energy demands for cellular proliferation, phagocytosis, and the production of connective, endothelial, and epithelial tissues are met [54]. Each of these components plays a specific and irreplaceable role in the continuum of healing. A delay in, or absence of, any one of these components can result in a prolongation or even a prohibition of healing [34].
Staging is an assessment system that classifies pressure injuries based on anatomic depth of tissue damage. In 2016, the National Pressure Injury Advisory Panel (NPIAP), formerly known as the National Pressure Ulcer Advisory Panel (NPUAP), updated the definitions of pressure injury stages [55]. In the 2016 revision to the NPIAP staging system, the term "pressure injury" replaced "pressure ulcer," to alleviate confusion between injury to intact skin (stages 1 and deep tissue injury) and open ulcers (stages 2–4 and unstageable pressure injury). In addition, the following changes were made [55]:
Arabic numbers replaced Roman numerals in stages 1–4
The term "suspected" was removed from the deep tissue injury diagnostic label
Two additional pressure injury definitions, Medical Device-Related Pressure Injury and Mucosal Membrane Pressure Injury, were added
Staging of a pressure injury can only occur after all necrotic tissue has been removed and it is possible to see the ulcer bed [56]. If this is not possible, the ulcer will be classified as unstageable.
Pressure injury staging is not used to indicate healing; a pressure injury should never be "down staged" or reverse staged (e.g., a pressure ulcer that is healing does not go from a stage 4 to a stage 3). In pressure ulcer healing, there is no regrowth of lost muscle, subcutaneous fat, or dermis; instead, the wound is filled in with scar tissue. Therefore, reverse staging does not accurately reflect the physiologic changes occurring in the pressure ulcer. When a stage 4 ulcer has healed, it should be classified as a healed stage 4 pressure ulcer [33].
Stage 2 pressure ulcers have a healing time that ranges from 8.7 to 38 days. Stage 3 and stage 4 pressure ulcers can take up to 69 days to heal. Healing rates are lower for stage 3 and stage 4 ulcers than for stage 2 ulcers in all healthcare settings [55]. Irreversible tissue damage can happen in as little as two hours in a patient with low tolerance; however, the ulcer may not become apparent for two to five days [57].
Stage 1 pressure injury presents as persistent redness in intact skin. If the area is pressed, it will not lighten in color (non-blanchable) [58]. It usually occurs in a localized area over a bony prominence, and this area can be painful, firm, soft, warmer, or cooler than the surrounding tissue [55]. This area of redness has a clear but possibly irregular boundary [58]. In darker skin tones, blanching may not be visible and the color may differ from the surrounding area [55]. In these instances, it is important to look for the other signs of pressure ulcers, such as pain and changes in skin temperature and/or texture.
Stage 2 pressure injury presents as shallow, open wounds with partial loss of the dermis. The wound bed is pink/red and without slough. A stage 2 pressure ulcer may also present as a serous fluid-filled blister [55]. Fat and deeper tissues are not visible, and slough and eschar are not present. These injuries are most commonly seen on the sacrum due to moisture and shear, and on the heel due to shear [55]. Skin tears, tape burns, incontinence-associated dermatitis, maceration, or excoriation of the skin should not be classified as stage 2 pressure ulcers [33].
Stage 3 is full thickness tissue loss. Subcutaneous fat may be visible and rolled wound edges are often present, but bone, tendon, and muscle are not exposed. There may be slough in the wound, but it does not obscure observation of the wound bed; if slough or eschar obscures the observation, this is an unstageable pressure injury. Tunneling and undermining may be present [55]. It is important to remember that the depth of stage 3 pressure ulcers will differ from one location to another. For example, the bridge of the nose, ear, occiput, and malleolus do not have a subcutaneous layer, and in these areas a stage 3 pressure ulcer can be shallow [55].
Stage 4 ulcers are characterized by full thickness tissue loss with exposed bone, tendon, or muscle. These ulcers often include undermining and tunneling, and rolled wound edges are often seen [55]. Slough and eschar may be present in part of the wound bed; however, if slough or eschar obscures the observation, this is an unstageable pressure injury [58]. In some cases, stage 4 pressure ulcers can affect supporting structures and may lead to osteomyelitis. As with stage 3 ulcers, the depth of the injury will vary by anatomical location.
A wound that is unstageable is defined as having full thickness tissue loss in which the base of the ulcer is covered by slough (yellow, tan, gray, green, or brown) and/or eschar (tan, brown, or black) [55]. Until the base of the wound can be visualized, the wound will remain unstageable. A necrotic wound cannot be staged until the necrotic tissue has been debrided, and it is then that a stage 3 or stage 4 ulcer will be revealed [55]. It is also important to remember that it is clinically inaccurate to stage a granulating wound if it is the first assessment, as visualizing the depth of the actual pressure injury is not possible [33]. In addition, if stable eschar on an ischemic limb or the heel(s) is present, it should not be removed [55].
Deep tissue pressure injury, previously suspected deep tissue injury, is a pressure-related injury, much like a bruise, that is characterized by persistent, non-blanchable, deep red, maroon, or purple discoloration or epidermal separation revealing a dark wound bed or blood-filled blister. Deep tissue pressure injuries can present on either intact or non-intact skin. This type of injury is caused by intense and/or prolonged pressure and/or shear. Deep tissue pressure injury may resolve without tissue loss or may rapidly change to reveal the extent of the tissue injury. If necrotic tissue, subcutaneous tissue, granulation tissue, fascia, muscle, or other underlying structures are visible, this indicates a full thickness pressure injury (unstageable, stage 3, or stage 4). The NPIAP does not recommend using deep pressure tissue injury to describe vascular, traumatic, neuropathic, or dermatologic conditions [55].
Color is the key to differentiating between deep tissue injury and a stage 1 pressure injury. Purple or maroon areas indicate deep tissue injury; non-blanchable redness is characteristic of stage 1 injuries. Deep tissue injury can become a stage 3 or a stage 4 pressure ulcer even with optimal care [55]. In general, the most common areas of involvement are the heels and sacrum [57]. In some patients, particularly those who are debilitated, deep tissue injury can develop rapidly [58].
Medical device-related pressure injuries describe injuries or ulcers that result from the use of devices designed and applied for diagnostic or therapeutic purposes. The injury is generally in the shape or pattern of the device and should be staged using the staging system [55].
Mucosal membrane pressure injury is found on the mucous membranes. This type of injury is typically attributed to use of a medical device on a mucous membrane, and due to the nature of this type of tissue, these injuries cannot be staged [55].
Over the years, the initial care of pressure injuries has commonly involved innovative mattresses, ointments, creams, solutions, dressings, ultrasonography, ultraviolet heat lamps, sugar, and surgery, depending on the degree of ulceration and condition of the ulcer base exposed and the purpose of the treatment (e.g., protection, moisture, removal of necrotic tissue) [59]. Overall, there are four principles for successful medical management of pressure injuries [24,59]:
Debridement of necrotic tissue as needed on initial and subsequent assessments
Cleansing the wound initially and with each dressing change, and using a dressing that keeps the ulcer bed continuously moist and the surrounding intact tissue dry
Prevention, diagnosis, and treatment of infection
Reduction of pressure
Ulcer healing should be assessed at least weekly, with a focus on the efficacy of the basic ulcer care plan. If the ulcer is healing, monitoring for continued progression should continue. If the ulcer is not healing, the treatment plan should be reassessed and the level of adherence to the plan scrutinized [33]. The plan and implementation strategy should be modified as necessary for optimal outcome. Close monitoring of serum blood glucose levels is necessary. Adjunctive therapies may include hyperbaric oxygen therapy, electrical stimulation therapy, electromagnetic therapy, growth factor therapy, and negative-pressure wound therapy; however, these adjuncts may not offer significant benefits and should be used according to clinical protocol [39,53,59]. Protein or amino acid supplementation may accelerate healing in certain patients, particularly those who are malnourished; these patients should receive nutritional support that is consistent with their medical condition and personal desires [39].
In cases in which an ulcer lacks progression in the healing process, operative repair may be an option for selected patients [24,53]. Information regarding available operative procedures and the anticipated benefits and harms of each should be part of patient counseling and the decision-making process. In the presence of obesity, longer surgical times (more than five hours) can adversely impact the patient's skin integrity, increasing the risk for deep tissue injury and necrosis. Furthermore, the presence of excess adipose tissue can prolong surgical time and increase the need for forceful retraction, potentially leading to tissue trauma [60]. In general, patients who are poor surgical candidates should not undergo pressure ulcer reconstruction due to the extremely high risk of complication [59]. Vigilance in regard to postoperative follow-up care is crucial to a successful operative outcome [33]. Educate patients and caregivers that measures to promote healing and prevent recurrence are lifelong [53].
For appropriate healing of any acute or chronic wound, infections should be prevented or treated aggressively. All pressure ulcers will become colonized with both aerobic and anaerobic bacteria; they are not sterile [33]. Infections within the wound must be addressed before healing is possible, but it can be challenging to distinguish an infected wound from one that is not [38]. Potential signs and symptoms of pressure ulcer infection include [24]:
Erythema
Localized pain
Periulcer edema
Increased skin temperature surrounding the ulcer
Leukocytosis
Purulent and/or foul-smelling exudate
As discussed, individuals with diabetes are at a greater risk for infection, with greater severity in relation to the level of blood glucose control [27]. This increased potential for infection in patients with diabetes can be attributed to physiologic alterations precipitated by inadequate glucose control [61].
Several observational studies point to a strong association between hyperglycemia and poor surgical outcomes, including on measures of length of stay, infection rates, posthospitalization disability, and mortality [62]. One well-studied population is cardiovascular surgery patients, partially because individuals with diabetes are at increased risk for cardiovascular disease and frequently require coronary artery bypass and grafting. Hyperglycemia, whether related to diabetes or stress, is associated with suboptimal outcomes when experienced on the first post-operative day after cardiovascular surgery [63]. However, the impact of diabetes on the risk of cardiothoracic surgical site infection and other poor outcomes may not simply be related to alterations in glucose control. Patients with diabetes are more likely to possess other factors associated with poor surgical outcomes, including obesity [61].
When hyperglycemia is present, there is an impact on fibroblast function during the period of granulation tissue formation and maturation. Decreased levels and cross-linking of collagen may impair wound healing and strength [14]. For these reasons, patients with diabetes preparing for a surgical intervention should maintain as close to normal metabolic functioning as possible. Therapeutic goals should include avoidance of hypoglycemia, hyperglycemia, lipolysis, ketogenesis, proteolysis, dehydration, and electrolyte imbalance [14].
There is significant research regarding the benefit of glycemic control after surgery, specifically after cardiac surgical interventions. One study revealed that improvements in postsurgical cardiac surgery glucose control were linked to a reduction in mediastinitis [60]. Some reviews recommend target presurgical glucose levels in the 100–180 mg/dL range because this level of glycemia has been shown to prevent infection, risk of hypoglycemia, and dehydration as a result of osmotic diuresis [14]. Although hyperglycemia is associated with adverse patient outcomes, controversy remains as to the appropriate levels required to prevent adverse outcomes while also avoiding potentially detrimental hypoglycemia [62,64]. A 2017 study of 300 patients with hyperglycemia (50% with diagnosed diabetes) who underwent cardiac surgery, found that intensive (100–140 mg/dL) and conservative (141–180 mg/dL) glucose control regimens produced similar circulating markers of acute inflammatory and oxidative stress response [63].
In addition to hyperglycemia, obese patients with or without diabetes have a significantly increased risk of impaired wound healing than those of normal weight [60]. Severe obesity is associated with venous stasis disease, pitting pretibial edema, bronze edema (a result of extravasation of red blood cells into the skin), cellulitis, and obesity hypoventilation [65]. Furthermore, obesity is a known risk factor for surgical-site infection. Patients with diabetes have been shown to have increased carriage rates for Staphylococcus aureus colonization, a leading cause of surgical-site infection [61]. These conditions impact the body's ability to heal an incision, potentially leading to wound dehiscence, rhabdomyolysis, and complications of the skin and underlying tissue [60].
Wound dehiscence is a serious complication for all obese patients whether or not they have diabetes. However, as previously stated, when hyperglycemia is present, wound healing and strength is impaired; when both are present, the risk of delayed healing is even greater [14]. In the early postoperative period, wounds stay approximated due to the strength of the sutures or the normal healing process as the muscle regains its strength. Consequently, obesity, heavy coughing or retching, and ascites can strain the wound and result in dehiscence [33].
A wound is at greatest risk of dehiscence in the first two weeks following surgery, when the wound is still fresh and very fragile. When dehiscence does occur, it can be mild (e.g., a small area of the incision begins to pull apart and leave a gap) or severe, causing the sutures, staples, or surgical glue to completely give way and the entire incision to open. In severe cases, the open incision is a surgical emergency and medical attention should be obtained immediately [66].
When wound dehiscence occurs, swift detection and action is critical. Interventions include antibiotic therapy and analgesics [67]. When appropriate, exposing the wound to air may accelerate healing by allowing growth of new tissue from below and preventing infection. Surgical removal of contaminated and dead tissue may also be necessary. A temporary or permanent piece of mesh may be placed to bridge the gap in the incision or wound. Vacuum-assisted wound closure may be used to increase granulation tissue and remove excess drainage. When appropriate, frequent changes in the incisional dressing are required to prevent infection.
Documentation is an imperative part of caring for the patient with wounds. Not only are nurses caring for patients, identifying adverse reactions, and taking appropriate actions, they are also responsible for documenting all findings, actions, treatments, and response to any interventions [26]. Wound assessments are required to be extremely detailed and complete. The well-documented history of pressure injuries and other wounds is a valuable communication tool between disciplines and a historical document to evaluate past care and efficacy and guide future care [33]. A factual record may be utilized in lawsuits to determine the quality of care rendered to serve as evidence of physical harm. Experts have suggested using the Nine Cs of Wound Assessment as a guide to the assessment and documentation process. This consists of [33]:
Cause(s) of the wound
Clear picture of what the wound looks like
Comprehensive picture of the patient
Contributing factors
Communication to other healthcare practitioners
Continuity of care
Centralized location for wound care information
Components of the wound care plan
Complications from the wound
The etiology of a lesion must be correctly identified in order to provide the most appropriate and effective care and treatment [33]. A thorough assessment of the ulcer involves evaluating its appearance, the wound location and size, the wound bed tissue, the wound edges, the periwound skin, and wound exudate [68]. It is important to document the location of ulcers and wounds, as this will assist in identifying the etiology [32]. Pressure injuries are typically located over bony prominences (particularly the heel in patients with diabetes) and should be documented as occipital, sacral, scapular, or malleolus [32]. Unlike pressure injuries, venous stasis and neuropathic ulcers are located primarily on the lower extremities [33]. Incisions are obviously in the operative area, but it is important to document the exact location (e.g., distal, lateral, mediastinal) [24].
The size of an ulcer or wound is important to assess and document as it will provide vital information regarding the efficacy of the treatment and advancement of infection or necrosis [32]. Documentation of the sizes of any ulcers should include [68]:
An accurate length in centimeters, typically measured from head to toe or from twelve o'clock to six o'clock from wound edge to wound edge
An accurate width in centimeters, typically measured from left to right or from nine o'clock to three o'clock from wound edge to wound edge
An accurate depth in centimeters in the center of the wound or the deepest area within the wound
The location and extent of any undermining and tunneling, measured using a cotton probe
The optimal time interval between wound measurements is approximately seven days. More frequent measurement is unlikely to demonstrate clinically relevant differences in wound dimension [24]. Weekly documentation should minimally include a reassessment of wound appearance. The NPIAP recommends the use of digital photography in documenting changes in dimension and base of wounds over time. These photographs may be used to supplement written documentation, but they should never be used as a replacement for clearly written documentation [69,70]. As noted, the face of a clock can be used to provide additional accuracy to the description of wound characteristics [71]. The terms used in documentation should represent objective data rather than subjective findings [24]. When documenting wounds, descriptive words such as "huge" or "deep" and diagnostic terms such as "infected" should be avoided. Instead, the objective descriptors such as "perierythema" or "induration" should be used [68].
Clean wound beds may or may not have granulation tissue. An agranular, friable wound bed (that may bleed easily) can indicate a high bacterial load that must be controlled before granulation occurs. Granulation tissue is composed of new capillary buds that should be supported by moist wound healing techniques [33]. Documentation should include the presentation of abnormal growth, such as slough and eschar. Slough is necrotic tissue and may be identified as a moist, stringy, yellow tissue in the base of the wound. In addition to slough, it is essential to document the presence of eschar tissue [71]. Thick, black, and leathery eschar indicates that wound bed has become dehydrated [33]. It is also essential to document the percentage of each type of tissue on the wound bed, such as "50% yellow slough" [68].
The skin surrounding the wound also provides a wealth of information to the practitioner and is an important piece of the documentation process [33]. Wound edges should be open to allow for epithelial migration (not rolled or hardened). Erythema and induration of the periwound skin is common in the inflammatory phase but can indicate infection if it is accompanied by increased drainage, size, pain, or odor [68]. Erythema and warmth may denote inflammation or infection and should be communicated in the documentation record and monitored closely [33]. Interruption of the periwound area may indicate an allergy or sensitivity to dressing adhesives or tape products [24]. Maceration or desiccation may be an indication that the dressing is too moist or too dry for the amount of exudate [33]. Maceration present in periwound tissue is a significant indication of poorly controlled exudate, which could impair wound healing [32].
Assessment of the wound bed is completed with visualization and devices such as wound rulers and cotton swabs [32]. These devices can be utilized for assessment of the periwound areas as well, although palpitation is often a significant part of the assessment process. Palpitation can reveal induration or fluctuance, an abnormal accumulation indicative of further tissue damage or an abscess [33].
Drainage amount and quality should also be noted. If drainage strikes through the dressing, dressing absorption is inadequate. Moisture seepage can facilitate bacterial spread from either the wound or the environment [68]. Observance of yellow purulent drainage may indicate staphylococcal involvement within the wound or ulcer. Green drainage may indicate the presence of pseudomonal involvement. Estimating the amount of drainage present can help determine increases or decreases on subsequent assessments [71]. The type of exudates, such as serous, serosanguineous, sanguineous, or purulent, is an essential aspect of documenting a wound and should be reflected consistently and accurately [68,71]. The odor of the wound and drainage can offer clues as to potential infections in the wound. An acrid, fruity odor is indicative of staphylococcal organisms; a musty odor is indicative of Pseudomonas aeruginosa; a foul (fecal-like) odor is consistent with other gram-negative bacteria [68,71].
Healthcare professionals may be following several ulcers simultaneously, making it difficult to recall individual wound progression. Accurate, objective documentation provides a timeline of the wound progression on which to base changes in ulcer care [33]. An accurate depiction of the wound is also important legal protection. Ambiguity can be risky [24]. Documentation is legal evidence of the quality of wound care that has been provided [68]. When present, diabetes may cause profound difficulties in wound healing. Identifying and understanding these comorbidities will guide more effective interventions and assist in establishing realistic expectations for the healing process.
Patient M is a White man, 67 years of age, with type 2 diabetes who is admitted to the hospital with a fever, two-day history of lethargy, and a rapid decrease in level of consciousness. His vital signs on admission are: temperature, 39.7 degrees Celsius; heart rate, 112 beats per minute, regular rate and rhythm; 26 respirations per minute and shallow; blood pressure, 88/40 mm Hg; blood glucose level, 329 mg/dL. The patient is complaining of a severe pain, reported at a 7 on a scale from 1 to 10. Physical assessment indicates:
Height: 5 feet 10 inches
Weight: 239 pounds without shoes
Body mass index: 34.4 kg/m2
Lungs: Crackles upon auscultation
Heart sounds: Clear without rubs or murmurs auscultated
Abdomen: Soft and non-tender all quadrants
Peripheral pulses: Present upon Doppler assessment at the lower extremities
Feet and lower extremities: Cool to touch, hairless and shiny, taut, and thin bilaterally. Toes mottled.
Capillary refill: Absent
Two lesions are present on the left lateral lower extremity, approximately 4 cm distal to the malleolus. The lesions are 5 cm in length, 3 cm in width, superficial depth, with yellowed edges, pale red base, and weepy.
Laboratory values reveal:
Blood cultures positive forS. aureus
White blood cell count: 21,000/mcL
Glycated hemoglobin (HbA1c): 8.5%
pH: 7.29
Patient M is admitted to the hospital with a diagnosis of septicemia secondary to a venous stasis ulcer, peripheral vascular disease, and uncontrolled hyperglycemia related to infection. He is started on antibiotic therapy, pain management, and dressing changes.
On day three of therapy, Patient M's temperature has been stabilized for the past 24 hours. Laboratory values are normalizing. His blood glucose levels remain elevated, with measurements between 276 mg/dL and 310 mg/dL. His pain is fluctuating between a 1 and a 5 and is being controlled with analgesics. Patient M's wound drainage is scant and yellow in color, with a slightly foul odor. His treatment plan consists of a moist, non-adherent dressing, and improvements have been noted. After three weeks of treatment, the wound assessment documentation indicates one lesion present on the left lateral lower extremity, approximately 4 cm distal to the malleolus. It is 4.6 cm in length, 2.8 cm in width, and superficial depth. The lesion is dry with irregular, yellowed edges and a reddened base. The surrounding tissue remains shiny and ruddy in color.
Patient M is discharged with orders for home health care for wound assessment and antibiotic therapy via a midline catheter.
Patient J is a White female patient, 54 years of age, with a history of uncontrolled insulin-dependent diabetes for the past 10 years. She is obese, with a body mass index of 41 kg/m2. She reports a history of shortness of breath with minimal exertion and severe bilateral osteoarthritis of the knees. She was a two-pack-per-day smoker but has been cigarette-free for the past six months. She is scheduled for gastric bypass surgery in two days. Presurgical teaching includes:
Incisional support and care to prevent infection
Techniques for turning, coughing, and deep breathing after surgery
The need to turn/change position at least every two hours to prevent pressure injuries
The surgery is completed without incident, and Patient J appears to be recovering as expected. However, two weeks postoperative she begins to cough uncontrollably, neglecting to support her incision. This occurs a total of four times throughout the night. At approximately 4:00 a.m., Patient J feels her incision "let go" and begins to bleed profusely; her incision has dehisced. She is rushed into surgery to resecure the incision and stop the bleeding. Although the bleeding can be cauterized, the periwound is too friable and further suturing will not adhere.
Patient J's incision measures 15 cm from twelve o'clock to six o'clock, 10 cm from three o'clock to nine o'clock, and 6 cm in depth. It is beefy red in color and appears to be healthy, with granulation tissue present. The surgeon prescribes treatment of the dehisced incision with a vacuum-assisted closure. She is premedicated for pain prior to dressing initiation and for subsequent dressing changes every 48 to 72 hours. After three weeks of vacuum-assisted closure therapy, Patient J's incision measures 12 cm from twelve o'clock to six o'clock, 9 cm from three o'clock to nine o'clock, and 4 cm in depth. It continues to look healthy and show signs of granulation. No odor is present within the wound. Wound edges are healthy, without maceration or rolling. The vacuum-assisted closure therapy is continued. At Patient J's next appointment eight weeks after the surgical procedure, her incision is assessed to be 8 cm from twelve o'clock to six o'clock, 5 cm from three o'clock to nine o'clock, and 2 cm in depth, with healthy color and edges. Vacuum-assisted closure therapy is discontinued, and a saline dressing is applied. Patient J's surgery is a success for both weight loss and the healing of her dehisced wound.
The impact of hyperglycemia as a comorbidity in delayed wound healing is well documented, and it can impact a wide variety of wounds, including pressure injuries. The Centers for Medicare and Medicaid Services identifies nosocomial pressure injuries as a "never" event [72]. As a result, hospital staff is required to have a working knowledge of preventative strategies of skin breakdown, identification of types of skin breakdown, and steps for appropriate care. This course has provided a thorough explanation of the pathophysiology of hyperglycemia and its impact on healing and wound management. With an understanding of these points, healthcare providers should be in a position to ensure that their patients receive optimal care, resulting in decreased healthcare costs and improved quality of life.
1. Cornell S, Halstenson C, Miller DK (eds). The Art and Science of Diabetes Care and Education. 5th ed. Chicago, IL: Association of Diabetes Care and Education Specialists; 2020.
2. Cowie CC, Casagrande SS, Geiss LS. Prevalence and incidence of type 2 diabetes and prediabetes. In: Cowie CC, Casagrande SS, Menke A, et al. (eds). Diabetes in America. 3rd ed. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2018.
3. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2022. Available at https://www.cdc.gov/diabetes/data/statistics-report/index.html. Last accessed September 14, 2023.
4. Lin J, Thompson TJ, Cheng YJ, et al. Projection of the future diabetes burden in the United States through 2060. Pop Health Met. 2018;16(9):1-9.
5. Centers for Disease Control and Prevention. Appalachian Diabetes Control and Translation Project. Available at https://www.cdc.gov/diabetes/health-equity/appalachian.html. Last accessed September 14, 2023.
6. Westerfield J, Holcomb S, Jensen S (eds). Current Trends in Diabetes Management: A Guide for the Healthcare Professional. 7th ed. Nashville, TN: Healthways; 2008.
7. American Diabetes Association. Statistics about Diabetes. Available at https://www.diabetes.org/resources/statistics/statistics-about-diabetes. Last accessed September 18, 2023.
8. U.S. Department of Health and Human Services Office of Minority Health. Diabetes and Hispanic Americans. Available at https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=63. Last accessed September 18, 2023.
9. U.S. Department of Health and Human Services Office of Minority Health. Diabetes and American Indians/Alaska Natives. Available at https://www.minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=33. Last accessed September 18, 2023.
10. Acton KJ, Burrows NR, Moore K, Querec L, Geiss LS, Engelgau MM. Trends in diabetes prevalence among American Indian and Alaska Native children, adolescents, and young adults. Am J Public Health. 2002;92(9):1485-1490.
11. U.S. Department of Health and Human Services. Rates of New Diagnosed Cases of Type 1 and Type 2 Diabetes on the Rise Among Children, Teens. Available at https://www.nih.gov/news-events/news-releases/rates-new-diagnosed-cases-type-1-type-2-diabetes-rise-among-children-teens. Last accessed September 18, 2023.
12. Colberg S, Friesz M. Diabetes Free Kids: A Take-Charge Plan for Preventing and Treating Type 2 Diabetes in Children. New York, NY: Avery; 2005.
13. American Diabetes Association. Standards of medical care in diabetes—2023. Diabetes Care. 2023;46(suppl 1):S1-S282.
14. Goldstein BJ, Müller-Wieland D (eds). Type 2 Diabetes: Principles and Practice. 2nd ed. New York, NY: Informa Healthcare; 2008.
15. Childs BP, Cypress M, Spollett G (eds). Complete Nurse's Guide to Diabetes Care. 3rd ed. Alexandria, VA: American Diabetes Association; 2017.
16. Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677-1686.
17. Johnson EL, Vinik AI, Vinik EJ. Diabetic neuropathies. In: Cornell S, Halstenson C, Miller DK (eds). The Art and Science of Diabetes Self-ManagementCare and Education. 5th ed. Chicago, IL: Association of Diabetes Care and Education Specialists; 2020: 867-904.
18. Edelman SV, Henry RR. Diagnosis and Management of Type 2 Diabetes. 13th ed. New York, NY: Professional Communications, Inc.; 2017.
19. American Diabetes Association. Diagnosis. Available at https://www.diabetes.org/a1c/diagnosis. Last accessed September 18, 2023.
20. Inzucchi SE. Management of hyperglycemia in the hospital setting. N Engl J Med. 2006;355(18):1903-1911.
21. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978-982.
22. Frisch A, Chandra P, Smiley D, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative in non-cardiac surgery. Diabetes Care. 2010;33(8):1783-1788.
23. Corsino L, Dhatariya K, Umpierrez G. Management of Diabetes and Hyperglycemia in Hospitalized Patients. Available at https://www.ncbi.nlm.nih.gov/books/NBK279093/. Last accessed September 18, 2023.
24. Rogers JL, Brashers VL. McCance and Huether's Pathophysiology. 9th ed. St. Louis, MO: Mosby; 2023.
25. Cox J, Edsberg LE, Koloms K, VanGilder CA. Pressure injuries in critical care patients in US hospitals: results of the International Pressure Ulcer Prevalence Survey. J Wound Ostomy Continence Nurs. 2022;49(1):21-28.
26. Urden L, Stacy K, Lough M. Critical Care Nursing: Diagnosis and Management. 9th ed. Maryland Heights, MO: Esevier; 2021.
27. Frankel TL, Sonnenday CJ. Disorders of the endocrine pancreas. In: Hammer GD, McPhee SJ (eds). Pathophysiology of Disease: An Introduction to Clinical Medicine. 8th ed. New York, NY: Lang, McGraw Hill; 2019.
28. Custer ML. Outcomes of clinical nurse specialist-initiated system-level standardized glucose management. Clin Nurse Spec. 2010;24(3):132-139.
29. Demidova-Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 1: normal and chronic wounds: biology, causes, and approaches to care. Adv Skin Wound Care. 2012;25(7):304-314.
30. Armstrong DG, Lavery LA (eds). Clinical Care of the Diabetic Foot. 3rd ed. Alexandria, VA: American Diabetes Association; 2016.
31. Gabriel A, Rosenberg LZ, de la Torre JI. Wound Healing and Growth Factors. Available at https://emedicine.medscape.com/article/1298196-overview. Last accessed September 18, 2023.
32. Wound, Ostomy, and Continence Nurses Society. Clinical Fact Sheet: Quick Assessment of Leg Ulcers. Available at state.in.us/isdh/files/Clinical_Fact_Sheet_Quick_Assessment_of_Leg_Ulcers.pdf. Last accessed September 18, 2023.
33. Baranoski S, Ayello EA (eds). Wound Care Essentials: Practice Principles. 5th ed. Philadelphia, PA: Lippincott Williams Wilkins; 2020.
34. Quain AM, Khardori NM. Nutrition in wound care management: a comprehensive overview. Wounds. 2015;27(12):327-335.
35. Christianakis S, Nguyen MC, Panush RS. Musculoskeletal complications of diabetes mellitus. In: Shaw KM, Cummings MH (eds). Diabetes: Chronic Complications. 3rd ed. Portsmouth: Wiley; 2012.
36. Wang CM, Lincoln J, Cook JE, Becker DL. Abnormal connexin expression underlies delayed wound healing in diabetic skin. Diabetes. 2007;56(11):2809-2817.
37. Bauer K, Rock K, Nazzal M, Jones O, Qu W. Pressure ulcers in the United States' inpatient population from 2008 to 2012: results of a retrospective nationwide study. Ostomy Wound Manage. 2016;62(11):30-38.
38. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. NEJM. 2017;376:2367-2375.
39. Gould L, Stuntz M, Giovannelli M, et al. Wound Healing Society 2015 update on guidelines for pressure ulcers. Wound Repair Regen. 2016;24(1):145-162.
40. Lyder CH, Ayello EA. Pressure ulcers: a patient safety issue. In: Hughes RG (ed). Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
41. Reddy M, Gill S, Rochon P. Preventing pressure ulcers: a systematic review. JAMA. 2006;296(8):974-984.
42. National Institute of Diabetes and Digestive and Kidney Diseases. Diabetic Neuropathy. Available at https://www.niddk.nih.gov/health-information/diabetes/overview/preventing-problems/nerve-damage-diabetic-neuropathies. Last accessed September 18, 2023.
43. Marsden K, Tuck S, Meeking D. Diabetes and foot disease. In: Shaw KM, Cummings MH (eds). Diabetes: Chronic Complications. 3rd ed. Hoboken, NJ: Wiley; 2012: 101-118.
44. Al-Rubeaan K, Al Derwish M, Ouizi S, et al. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS One. 2015;10(5):e0124446.
45. O'Loughlin A, McIntosh C, Dinneen SF, O'Brien T. Basic concepts to novel therapies: a review of the diabetic foot. Int J Low Extrem Wounds. 2010;9(2):90-102.
46. Gabriel A, Camp MC, Paletta CE, Massey B. Vascular Ulcers. Available at https://emedicine.medscape.com/article/1298345. Last accessed September 18, 2023.
47. Kranke P, Bennett MH, Martyn-St James M, Schnabel A, Debus SE, Weibel S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2015;6:CD004123.
48. Sperl-Hillen J. Cardiovascular complications of diabetes. In: Cornell S, Halstenson C, Miller DK (eds). The Art and Science of Diabetes Care and Education. 5th ed. Chicago, IL: Association of Diabetes Care and Education Specialists; 2020: 783-816.
49. Agency for Healthcare Research and Quality. Pressure Ulcer Risk Assessment and Prevention: A Comparative Effectiveness Review. Available at https://effectivehealthcare.ahrq.gov/topics/pressure-ulcer-prevention/research-protocol. Last accessed September 18, 2023.
50. Huang L, Nakagami G, Minematsu T, et al. Ulceration and delayed healing following pressure loading in hyperglycemic rats with an immature dermal collagen fiber network. Wounds. 2010;22(9):237-244.
51. Agency for Healthcare Research and Quality. AHCPR Supported Clinical Practice Guidelines. Available at https://www.ncbi.nlm.nih.gov/books/NBK63863. Last accessed September 18, 2023.
52. Emory University School of Medicine Wound, Ostomy and Continence Nursing Education Center. Skin and Wound Module. 6th ed. Atlanta, GA: Emory University WOCNEC; 2006.
53. Wound, Ostomy, and Continence Nurses Society. Guideline for Prevention and Management of Pressure Ulcers. Mount Laurel, NJ: WOCN; 2016.
54. Rudolph DM. Why won't this wound heal? Understand the causes of and interventions for chronic wounds. Am J Nurs. 2002;102(2):24DD-24HH.
55. National Pressure Injury Advisory Panel. NPIAP Pressure Injury Stages. Available at https://npiap.com/page/PressureInjuryStages. Last accessed September 18, 2023.
56. Bryant RA, Nix DP. Acute and Chronic Wounds: Intraprofessionals from Novice to Expert. 6th ed. St. Louis, MO: Elsevier-Mosby; 2023.
57. Wound, Ostomy and Continence Nurses Society. WOCN Society Response to NPUAP White Papers: Deep Tissue Injury, Stage I Pressure Ulcers, and Stage II Pressure Ulcers. Ninth National NPUAP Conference. February 2005.
58. Medline Industries, Inc. Pressure Ulcer Prevention Program. Mundelein, IL: Medline Industries, Inc.; 2008.
59. Kirman CN. Pressure Ulcers and Wound Care Treatment and Management. Available at https://emedicine.medscape.com/article/190115-overview. Last accessed September 18, 2023.
60. Baugh N, Zuelzer H, Meador J, Blankenship J. Wound wise: wounds in surgical patients who are obese. Am J Nurs. 2007;107(6):40-50.
61. Talbot TR. Diabetes mellitus and cardiothoracic surgical site infections. Am J Infect Control. 2005;33(6):353-359.
62. Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119-1131.
63. Reyes-Umpierrez D, Davis G, Cardona S, et al. Inflammation and oxidative stress in cardiac surgery patients treated to intensive versus conservative glucose targets. J Clin Endocrinol Metab. 2017;102(1):309-315.
64. Korytkowski MT. In-patient management of diabetes: controversies and guidelines. Indian J Encrocrinol Metab. 2013;17(suppl 3): S630-S635.
65. Sugerman HJ, Sugerman EL, Wolfe L, Kellum JM, Schweitzer MA, DeMaria EJ. Risks and benefits of gastric bypass in morbidly obese patients with severe venous stasis disease. Ann Surg. 2001;234(1):41-46.
66. Bruns TB, Worthington JM. Using tissue adhesive for wound repair: a practical guide to Dermabond. Am Fam Phys. 2000;61(5):1383-1388.
67. Sandy-Hodgetts K, Ousey K, Howse E. Ten top tips: management of surgical wound dehiscence. Wounds Asia. 2018;1(1):16-19.
69. Wound Care Advisor. Wound Photography – How it Benefits Clinical Documentation. Available at https://woundcareadvisor.com/wound-photography-benefits-clinical-documentation. Last accessed September 18, 2023.
70. Dryden B. Photography in Wound Documentation: Fact Sheet. Available at https://cdn.ymaws.com/member.wocn.org/resource/resmgr/document_library/Photography_Wound_Documentat.pdf. Last accessed September 18, 2023.
71. Freedline A, Fishman TD. Wound Documentation Tips. Available at https://www.woundsource.com/blog/wound-documentation-dos-don-ts-10-tips-success. Last accessed September 18, 2023.
72. Centers for Medicare and Medicaid Services. Letter to State Medicaid Directors: SMDL #08-004. Available at http://downloads.cms.gov/cmsgov/archived-downloads/SMDL/downloads/SMD073108.pdf. Last accessed September 18, 2023.
73. Lòpez-DeFede A, Stewart JE. Diagnosed diabetes prevalence and risk factor rankings, by state, 2014–2016: a ring map visualization. Prev Chronic Dis. 2019;16:180470.
74. National Indian Council on Aging. Diabetes Still Highest Among AI/AN. Available at https://www.nicoa.org/diabetes-still-highest-among-ai-an. Last accessed September 18, 2023.
75. Rice JB, Desai U, Cummings AKG, et al. Burden of diabetic foot ulcers for Medicare and private insurers. Diabetes Care. 2014;37(3): 651-658.
76. Geiss LS, Li Y, Hora I, et al. Resurgence of diabetes-related nontraumatic lower-extremity amputation in the young and middle-aged adult U.S. population. Diabetes Care. 2019;42(1):50-54.
77. Agency for Healthcare Research and Quality. Preventing Pressure Ulcers in Hospitals. Available at https://www.ahrq.gov/patient-safety/settings/hospital/resource/pressureulcer/tool/pu1.html. Last accessed September 18, 2023.
78. Barker LE, Kirtland KA, Gregg EW, Geiss LS, Thompson TJ. Geographic distribution of diagnosed diabetes in the U.S.: a diabetes belt. Am J Prev Med. 2011;40(4):434-439.
79. Diabetes Research Center, John A Burns School of Medicine. Diabetes in Hawaii. Available at https://drc.jabsom.hawaii.edu/diabetes-in-hi. Last accessed September 14, 2023.
1. Senneville É, Albalawi Z, van Asten SA, et al. Guidelines on the Diagnosis and Treatment of Foot Infection in Persons with Diabetes. Available at https://iwgdfguidelines.org/wp-content/uploads/2023/07/IWGDF-2023-04-Infection-Guideline.pdf. Last accessed September 20, 2023.
2. Qaseem A, Mir TP, Starkey M, Denberg TD, Clinical Guidelines Committee of the American College of Physicians. Risk assessment and prevention of pressure ulcers: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2015;162(5):359-369. Available at https://www.acpjournals.org/doi/10.7326/M14-1567. Last accessed September 20, 2023.
3. Card R, Sawyer M, Degnan B, et al. Perioperative Protocol: Health Care Protocol. Bloomington, MN: Institute for Clinical Systems Improvement; 2014. Available at https://www.icsi.org/wp-content/uploads/2019/01/Periop.pdf. Last accessed September 20, 2023.
Mention of commercial products does not indicate endorsement.