Anemia may be common in the elderly patient, but it should not be accepted as normal aging. The scientific evidence suggests that consequences of untreated geriatric anemia are significant. Anemia leads to poorer quality of life and increased morbidity and mortality. Even mild anemia is more significant than traditionally thought by clinicians and is associated with increased hospitalizations and mortality. This course will review physical assessment skills, laboratory findings, diagnosis, and initiation of treatment and follow-up plan.
This course is designed for physicians, physician assistants, nurses, and other healthcare professionals involved in the care of elderly patients.
The purpose of this course is to provide primary care health professionals a review of pathophysiology, clinical assessment, and management of anemia in the elderly. The goal is to promote early diagnosis, appropriate treatment, and improved outcomes for the geriatric population.
Upon completion of this course, you should be able to:
- Define anemia and its impact on the geriatric population.
- Describe the pathophysiology of anemias common in the elderly.
- Outline the role of nutrient deficiencies in the development of anemia.
- Compare and contrast other possible causes of anemia in the elderly.
- Discuss standards for the assessment of elders with anemia.
- Develop a management plan for geriatric patients with anemia.
Susan Waterbury, MSN, FNP-BC, ACHPN, entered the medical field in 1985 as a certified medical assistant and basic x-ray operator. She achieved her RN in 1990 and practiced in a variety of settings, including hospital, home health care, and hospice. Ms. Waterbury achieved her BSN in 1996 and her MSN as a Family Nurse Practitioner in 1999. She was board-certified as an FNP-BC in 2000 and has practiced in family practice, geriatrics, corporate leadership, hospice, and palliative care settings. She holds RN and NP licenses in Florida and Arizona.
In addition to her clinical roles, Ms. Waterbury continues to play an active role in educating and mentoring nurses and healthcare professionals. She has been a faculty member of the University of Phoenix since 2015, teaching in the nurse practitioner and MSN programs. She develops and presents educational programs for a variety of healthcare organizations and community groups.
Contributing faculty, Susan Waterbury, MSN, FNP-BC, ACHPN, has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
John M. Leonard, MD
John V. Jurica, MD, MPH
Mary Franks, MSN, APRN, FNP-C
The division planners have disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Sarah Campbell
The Director of Development and Academic Affairs has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
The purpose of NetCE is to provide challenging curricula to assist healthcare professionals to raise their levels of expertise while fulfilling their continuing education requirements, thereby improving the quality of healthcare.
Our contributing faculty members have taken care to ensure that the information and recommendations are accurate and compatible with the standards generally accepted at the time of publication. The publisher disclaims any liability, loss or damage incurred as a consequence, directly or indirectly, of the use and application of any of the contents. Participants are cautioned about the potential risk of using limited knowledge when integrating new techniques into practice.
It is the policy of NetCE not to accept commercial support. Furthermore, commercial interests are prohibited from distributing or providing access to this activity to learners.
Supported browsers for Windows include Microsoft Internet Explorer 9.0 and up, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Supported browsers for Macintosh include Safari, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Other operating systems and browsers that include complete implementations of ECMAScript edition 3 and CSS 2.0 may work, but are not supported. Supported browsers must utilize the TLS encryption protocol v1.1 or v1.2 in order to connect to pages that require a secured HTTPS connection. TLS v1.0 is not supported.
The role of implicit biases on healthcare outcomes has become a concern, as there is some evidence that implicit biases contribute to health disparities, professionals' attitudes toward and interactions with patients, quality of care, diagnoses, and treatment decisions. This may produce differences in help-seeking, diagnoses, and ultimately treatments and interventions. Implicit biases may also unwittingly produce professional behaviors, attitudes, and interactions that reduce patients' trust and comfort with their provider, leading to earlier termination of visits and/or reduced adherence and follow-up. Disadvantaged groups are marginalized in the healthcare system and vulnerable on multiple levels; health professionals' implicit biases can further exacerbate these existing disadvantages.
Interventions or strategies designed to reduce implicit bias may be categorized as change-based or control-based. Change-based interventions focus on reducing or changing cognitive associations underlying implicit biases. These interventions might include challenging stereotypes. Conversely, control-based interventions involve reducing the effects of the implicit bias on the individual's behaviors. These strategies include increasing awareness of biased thoughts and responses. The two types of interventions are not mutually exclusive and may be used synergistically.
#99084: Anemia in the Elderly
Anemia is common in older persons, and the prevalence of anemia increases with age. Overall prevalence is 17% in adults older than 65 years of age, but it is higher among those admitted to hospital (40%) or those residing in nursing homes (47%) [1]. Because rates of premature death and disability have gradually declined in the United States, resulting in unprecedented growth in the number and proportion of older adults in the population, the number of older anemic patients will increase in coming years. The Centers for Disease Control and Prevention (CDC) estimates that two factors—longer life spans and aging baby boomers—will combine to double the population of Americans older than 65 year of age by 2050, to about 72 million [3]. By 2030, older adults will account for 20% of the population. Moreover, a major shift in the leading causes of death has occurred during the past century, from acute illnesses and infectious diseases to chronic illnesses and degenerative diseases. Two out of three adults older than 65 years of age have multiple chronic conditions; medical treatment for this population accounts for 66% of the country's healthcare costs [3].
Although anemia increases with age and is a common clinical condition among elderly patients, anemia should not be viewed as an acceptable feature of the aging process. Growing scientific evidence indicates that untreated anemia is an important risk factor for adverse outcomes, including need for hospitalization, morbidity, and mortality [1,2,64]. Among a cohort of elderly nonanemic participants in a community health surveillance program, the emergence of declining hemoglobin (rather than an absolute level) identified a group of elderly individuals at risk for subsequent adverse outcomes (e.g., cognitive decline) [65]. Untreated anemia in the elderly also has a substantial impact on healthcare expenditures and constitutes a growing burden on the healthcare system. As the geriatric population increases, experts predict that anemia will become a considerable problem [4]. Due to the expected increase in the elderly population, clinicians should become knowledgeable in diseases common in geriatrics. This course will provide healthcare providers the knowledge and tools necessary to identify anemia early and respond appropriately. Health outcomes for the geriatric population should improve if evidence-based clinical practices are incorporated into their care.
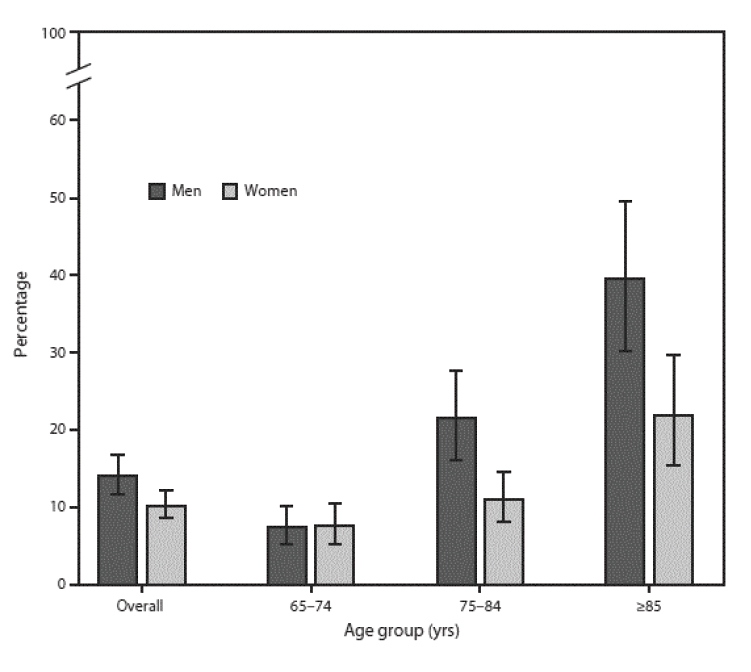
As noted, the risk of developing anemia increases with age. An average of 6.5% of individuals have anemia at 60 to 69 years of age; this increases to 19.4% at 80 to 85 years of age [5]. The prevalence of anemia increases with age and is highest in elderly men 80 to 85 years of age [5]. The prevalence of anemia overall is in the range of 17% to 20%, with little difference between men and women (Figure 1). The prevalence increases as aging advances and does so to a greater extent and more rapid rate in men than women. Data from the National Health and Nutrition Examination Survey (NHANES) show that the prevalence of anemia in men 65 to 74 years of age is 7.6% and increases to 39.5% in men 85 years of age and older [5]. By comparison, the prevalence of anemia in women in the same age categories starts at a similar rate (7.6%) but reaches a rate (21%) only half that of men by 85 years of age. The prevalence of anemia is higher among men than women 75 to 84 years of age and those older than 85 years of age. Rates of anemia in nursing home patients are estimated to be 48% to 63%, and anemic residents of long-term care have a higher associated mortality rate [9,10].
Identifying the underlying cause of the anemia in older adults is often difficult due to prevalence of polypharmacy and multiple medical comorbidities, which complicate evaluation and diagnosis. An analysis of data from the third NHANES (1988–1994) demonstrated a high rate of unexplained anemia in adults older than 65 years of age. The study reported that one-third of cases were attributed to nutrient deficiency, another one-third was associated with chronic inflammation or chronic renal disease, and the remaining one-third was unexplained (no clear etiology) [68]. Even with rigorous clinical assessment protocols, anemia in elderly patients remains unexplained in 15% to 25% of cases. However, the recognition of anemia is important, as it may be the first sign of an underlying illness, such as gastrointestinal malignancy [11]. Goals of the management strategy for anemia in older adults should be to prevent worsening of the condition, slow progression, and promote improvement of outcomes [12].
Most cases of anemia in older adults are mild; however, even slightly lower hemoglobin (Hgb) levels with or without anemia has been found an independent risk factor for excess morbidity and mortality in elderly patients [50,69]. Anemia in community-dwelling older adults is associated with functional decline and decreased mobility, balance, and physical performance (e.g., ability to rise from a chair). A cohort study of 138,670 adults 18 to 93 years of age found that among a subset with anemia (5,510 subjects), anemia in individuals 6o years of age and older was associated with significantly impaired overall survival and health-related quality of life. Anemia had no effect on overall survival and limited impact on health-related quality of life in those younger than 60 years of age [70]. Other studies have shown that anemia in the elderly is an independent risk factor for poor health outcomes and diminished functional capacity, including muscle wasting, cognitive decline, increased risk of falls, depression, and all-cause hospitalization [14,69].
Treatment and care of the elderly is further complicated by physical, psychological, and sociologic changes associated with aging. Physical considerations include comorbid medical conditions, sensory loss, anorexia, reduced thirst, early satiety, and medication side effects. Common psychological manifestations of aging include cognitive impairment, delirium, depression, and psychiatric illness. Socioeconomic issues include poverty, lack of family support, isolation, dependency, substandard living conditions, and elder abuse. To successfully treat anemia in geriatric patients, consideration of these possible issues is essential.
Anemia is generally defined as a medical condition in which the number or function of erythrocytes or red blood cells (RBCs) is inadequate to meet tissue oxygenation needs. The body is unable to compensate for this reduction in the number of circulating erythrocytes.
The World Health Organization (WHO) defines anemia as an Hgb level less than 12 g/dL for women and less than 13 g/dL for men. An Hgb level of 10–11.9 g/dL for women and 11–12.9 g/dL for men is classified as mild anemia [13]. However, it is important to note that the use of Hgb level to define anemia has been controversial. In countries where nutritional deficiencies, infection, or congenital blood disorders are common, it may be difficult to apply a universal Hgb cut point [60]. In addition, there appear to be racial differences in normal Hgb levels and the point at which symptoms of anemia emerge. In general, the Black population has a lower median Hgb and higher prevalence of anemia than the White populations [5]. Further research is needed to determine if a different Hgb level would be appropriate to diagnose and begin treatment for anemia in the Black population [15].
In the past, elderly persons with mild anemia (i.e., Hgb >10 g/dL) have not received much attention from the medical community. Clinicians traditionally have not treated mild anemia, as it was considered a natural consequence of aging. However, it is now known that even mild anemia is associated with increased hospitalization and mortality in the elderly [11].
Chronic severe anemia in the elderly may lead to cardiac functional abnormalities. The reduction in tissue oxygen delivery and the compensatory tachycardia that invariably follows may, in time, lead to impaired ventricular systolic function [16]. In persons without heart disease, cardiac function begins to decline when Hgb levels fall below 7–10 g/dL. In the presence of heart disease, the Hgb level should be kept above 10 g/dL [16].
Conceptually, the causes of anemia can be divided into four main categories: blood loss, hemolysis, bone marrow factor deficiency or suppression, and splenic sequestration, each of which may occur alone or concomitantly. Because splenic sequestration occurs most often in children with sickle cell anemia who are between the ages of 2 months and 4 years, it will not be discussed in detail in this course.
Anemia in the elderly is complex, often involving overlapping known etiologic causes from the following categories: chronic inflammation associated with infection, autoimmune disease, or malignancy; nutrient deficiencies such as iron, vitamin B12, and folic acid; chronic kidney disease; and hematologic (bone marrow) dysplasia and malignancy (Table 1) [11,69]. Common risk factors for anemia in older adults include low-grade gastrointestinal bleeding, medication adverse effects, drug/alcohol misuse, and inadequate dietary intake. The anemia associated with gastrointestinal bleeding may be acute or chronic. Acute blood loss can create an emergency requiring immediate attention. In contrast, patients with chronic anemia lose blood over an extended period, and the risks are less immediate. One category remains: unexplained anemia of aging, a recognized disease among older adults, diagnosed by exclusion [69].
Blood loss commonly occurs in the gastrointestinal tract secondary to gastric ulcers, gastritis, colitis, diverticulitis, and cancer. Hemolysis (i.e., destruction of erythrocytes) and shortened RBC life-span (i.e., failure to live the usual 120 days) also leads to anemia. Causes of hemolytic anemia range from genetic disorders to mechanical heart valve effects and medication side effects.
A reduction in RBC production can also result in anemia. This may be related to bone marrow suppression due to medication adverse effects and myelodysplasia, or decreased bone marrow production associated with aging. In some older patients, reduced kidney function will result in decreased erythropoietin production, which in turn causes decreased production of RBCs in the bone marrow.
Anemia of inflammation, also known as anemia of chronic disease (AI/ACD), is the second most common anemia in elderly patients (after iron-deficiency anemia). It is a common feature of diseases that cause prolonged immune activation. Originally, AI/ACD was linked to chronic infections and autoimmune diseases in which laboratory markers of sustained inflammation are easily detected; more recently, the list has grown to include chronic kidney disease, cancer, cirrhosis, congestive heart failure, severe trauma, and obesity [18,66]. Its defining characteristics are a normocytic, normochromic anemia with low serum iron levels despite adequate iron stores and normal or high iron transport mechanisms, the result of blocked delivery of iron to developing RBCs and reduced intestinal absorption [17].
AI/ACD is caused by systemic inflammation that results in immune cell activation and formation of numerous cytokines [66]. Cytokines are chemical messengers of the body that mediate immune or inflammatory response and include tumor necrosis factor, interleukin-1, and the interferons. Over time, the impact of these messengers is to decrease absorption of iron, restrict iron trafficking, and interrupt the release of iron required for RBC production [6]. Additional pathogenic factors related to chronic inflammation include suppression of erythropoietin activity and shortening of RBC lifespan [66]. Independent of defined chronic disease states, the aging process itself is accompanied by an increase in proinflammatory cytokines and reactive oxygen species that may induce a state of low-grade chronic inflammation. In addition, older adults tend to have increased levels of pro-inflammatory cytokines secondary to multiple comorbid illnesses. As a result, AI/ACD is significantly more common in older patients, particularly those with chronic disorders [17].
It is important to note that anemia in elderly patients may be multifactorial—the result of a combination of chronic disease, blood loss, and/or vitamin deficiencies [17]. A varying percentage (25% to 85%) of patients with AI/ACD also suffer from true iron deficiency, which is often the result of concomitant or unrelated gastrointestinal bleeding or blood loss from therapeutic procedures (e.g., hemodialysis) [66]. A confirmed diagnosis requires that other etiologies have been excluded. In difficult cases, bone marrow aspiration/biopsy may be necessary to confirm the diagnosis. In patients with true AI/ACD, the severity of the anemia correlates to the severity of the underlying medical condition. When the underlying condition is treated, AI/ACD usually improves [17].
A prospective, multi-institutional cohort study was performed to characterize anemia in elderly nursing home residents. In healthy young adults, erythropoiesis increases in response to anemia or hypoxia; this study found that compensatory erythropoiesis diminishes with advancing age, attributable in part to unrecognized renal dysfunction [9]. Lower responses were also associated with chronic conditions like rheumatoid arthritis and cancer.
As noted, chronic anemia in the elderly may adversely affect cardiac function [16]. This is significant when establishing a treatment plan, as the presence of heart disease requires careful observation of Hgb levels. Chronic anemia can have other adverse effects on elderly patients, including extreme fatigue, impaired healing, and increased risk of hospitalization and death [1,16].
Nutrient-deficiency anemia is a significant cause of anemia, especially in the elderly. Iron, folate, and vitamin B12 deficiencies may be seen alone, but more often a combination of these deficiencies is present [6].
An estimated 15% to 23% of anemic elders have iron deficiency [11]. Iron-deficiency anemia is characterized by depletion of iron stores and inadequate bone marrow iron deposits required for normal hematopoiesis. Gradually, circulating RBCs become microcytic and hypochromic (smaller and paler). However, the presence of normal RBCs (normocytic anemia) does not exclude iron-deficiency, as microcytosis is a late finding of severe iron deficiency. Elderly individuals may be at increased risk for decreased iron absorption due to medication side effects, chronic illness and inflammation, dietary iron deficiencies, and malabsorption.
Iron deficiency occurs when the rate of iron utilization exceeds the rate of intestinal absorption and iron stores become depleted. This may be caused by inadequate nutritional intake, impaired absorption of iron, or chronic blood loss (e.g., uterine or gastrointestinal bleeding). Insipient onset of iron deficiency occurs commonly in older adults, resulting from unrecognized low-grade gastrointestinal bleeding induced by aspirin or nonsteroidal anti-inflammatory drug (NSAID) use or colorectal cancer. Such patients may present with well-established iron deficiency and exhibit the classic microcytic anemia; however, it is important to bear in mind that early, less severe deficiency states may exhibit a normocytic anemia. In either case, the presence of iron deficiency can be evaluated by obtaining a serum ferritin level. A serum ferritin concentration of 25–45 mg/dL is suspicious for iron deficiency; a level of 45–100 mg/dL makes iron deficiency less likely. Levels greater than 100 mg/dL indicate sufficient iron stores. However, infectious or other immune inflammatory states will increase serum ferritin concentration, making measurements potentially unreliable. In patients with infectious or inflammatory disorders, plasma transferrin receptor concentration may be a more useful measure.
The most common cause of iron-deficiency anemia in the elderly is occult blood loss from the gastrointestinal tract. Bleeding may be chronic or acute depending on the underlying etiology. Common causes include NSAID use, gastric ulcer, colon cancer, diverticulosis, and vascular malformation (angiodysplasia) of the bowel submucosa. In one study, gastrointestinal malignancy was present in 6% of patients with iron-deficiency anemia [19]. Other studies have reported iron-deficiency anemia as the presenting symptom in 15% of colorectal cancers [50]. Endoscopic evaluation is indicated for all patients with iron-deficiency anemia (but particularly those with family histories of gastrointestinal cancers), and colonoscopy is recommended, regardless of age, if upper endoscopy does not reveal a source of bleeding [20]. Stool should be tested for occult blood in the initial anemia work-up. It is important to also consider other potential causes of microcytic anemia during the evaluation of elderly patients with suspected iron-deficiency anemia. For example, lead poisoning, which interferes with the incorporation of iron into hemoglobin, can lead to a hypochromic, microcytic anemia despite adequate iron stores.
The patient with iron-deficiency anemia should always be evaluated for underlying cause. Medical conditions or medications that decrease gastric pH reduce the absorption of iron, which presents as iron-deficiency anemia. In older adults, atrophic gastritis, which causes chronic decreased gastric acid production secondary to damage to the acid producing cells of the stomach, should be considered. Commonly used medications, including antacids, proton pump inhibitors, and H2 histamine blockers, may contribute to the development of anemia.
Vitamin B12 (also called cobalamin) is necessary for DNA synthesis, RBC maturation, and normal functioning of the neurologic system [21]. Older adults are at increased risk for developing vitamin B12 deficiencies for many reasons, including atrophic gastritis, which results in a reduced ability to absorb vitamin B12 from food sources. Vitamin B12 deficiency can also result from inadequate dietary intake and defects in metabolism. In addition to anemia, vitamin B12 deficiency may lead to signs of peripheral neuropathy and early dementia.
Several medications are associated with increased risk of vitamin B deficiencies, and because of the high rate of polypharmacy and chronic conditions among the elderly, drug-induced vitamin B12 deficiency is a serious concern. Long-term use of the antibiotic chloramphenicol may inhibit RBC response to vitamin B12 supplements in some patients [21]. Proton-pump inhibitors, such as omeprazole, and H2 receptor antagonists, such as ranitidine, may be prescribed long term for gastroesophageal reflux disease or peptic ulcer disease; both drug classes slow the release of gastric acid, thereby potentially interfering with the absorption of vitamin B12, particularly in patients with already low stores [22,23,24]. However, studies of older adults have failed to conclusively determine if these acid-lowering agents are a significant cause of vitamin B12 deficiency [23,24,25].
Metformin, a biguanide used to treat diabetes, has also been associated with vitamin B12 deficiencies, and an estimated 10% to 30% of metformin users have reduced vitamin B12 absorption [26,27,28,29]. It is hypothesized that impaired calcium availability due to metformin activity may interfere with the calcium-dependent process of vitamin B12 absorption [29]. Dose and duration of use appear to be most strongly correlated with risk of vitamin B12 deficiency. For this reason, patients taking metformin to control their diabetes should be closely monitored for signs of vitamin deficiency.
Pernicious anemia is the classic term for a subtle autoimmune disorder that causes chronic malabsorption of vitamin B12 in older adults [21]. It is characterized by a decrease in RBCs resulting from impaired intestinal absorption of vitamin B12, caused by autoimmunity against intrinsic factor or gastric parietal cells (which produce intrinsic factor). Intrinsic factor is necessary for the absorption of vitamin B12, and decreased production of intrinsic factor leads to reduced absorption of vitamin B12 [21].
Several other conditions are complicated by vitamin B12 deficiency. Atrophic gastritis, a disorder common in elders, can cause intrinsic factor deficiency and pernicious anemia secondary to loss of functioning parietal cells in the stomach. Vitamin B12 is absorbed in the distal small bowel (terminal ileum), and persons with disease of the terminal ileum (e.g., Crohn disease, Whipple disease, celiac disease) are at risk for malabsorption of vitamin B12 and other nutrients [30].
The primary natural sources of dietary vitamin B12 are animal products, including fish and shellfish, beef, poultry, pork, eggs, and dairy products [21]. However, fortified cereals also usually contain 100% of the recommended daily value of vitamin B12. Strict vegetarians and particularly vegans, who consume no animal products, are at an increased risk of insufficient intake of vitamin B12, and these patients may benefit from vitamin B12-fortified foods, oral vitamin B12 supplements, or vitamin B12 injections.
Folate (also known as folic acid or vitamin B9) is a B vitamin necessary for RBC production. Folate deficiency usually results from inadequate dietary intake or malabsorption and is more common in the elderly and chronically ill. In adults, it often occurs with alcoholism and during pregnancy and breastfeeding [6]. Less often, folate deficiency may develop in individuals taking medications such as methotrexate, phenytoin, and trimethoprim, which interfere with the absorption of folate [31,32]. Methotrexate is used for a wide variety of conditions (including rheumatoid arthritis, lupus, psoriasis, and asthma) that may be more common in elderly patients. Dialysis patients are also at risk of deficiency, as folate is lost in dialysis fluid [26].
Body stores of folate range from 500–20,000 mcg. It is necessary for humans to absorb 50–100 mcg of folate daily to replenish losses through bile and urine [31]. Food sources of folate include green vegetables, yeast, liver, beans, whole grains, and wheat bran. Many foods are also fortified with folate, including some breakfast cereals, rice, breads, and pasta [31]. Signs and symptoms of folate deficiency (e.g., weakness, fatigue, difficulty concentrating, dyspnea) develop gradually and usually become apparent after about four months.
Older patients may develop folate deficiency for a variety of reasons. Hyperutilization of folic acid by metabolic processes may be caused by malignancy, Crohn disease, rheumatoid arthritis, or medication side effects. Elderly persons may have difficulty chewing and swallowing certain foods, causing various nutrient deficiencies. Food preparation is also a consideration, as folate is destroyed by excessive heat and dilution. As with vitamin B12, polypharmacy and drug-induced deficiency are a greater concern in older patients.
Serum homocysteine is elevated in most patients with folate and B12 deficiencies. Homocysteine is an amino acid in the blood, and studies have shown that elevated homocysteine levels are associated with a higher risk of cardiovascular disease [33]. However, studies of the effect of folic acid and B vitamin supplementation on cardiovascular events found that, despite a significant decrease in serum homocysteine, the incidence of cardiovascular events did not decrease [4,33].
Folate and vitamin B12 deficiency both cause a macrocytic anemia, and one cannot be differentiated from another by laboratory examination of the cells. Some patients may remain asymptomatic despite low Hgb levels due to slow progression of the disease.
While a diet rich in folate has been shown to be protective against certain types of cancer, particularly colorectal cancer, very high levels of the nutrient have also been associated with an increased risk of developing cancer [34,35]. High levels of folate, often the result of oversupplementation, facilitate hyperproliferation, which is present in most dysplastic and malignant neoplasms [34]. If any dysplastic cells are present, even if very small or undetected, high levels of folate may accelerate the disease process and result in more aggressive forms of cancer. This so-called "dual effect" of folate can make recommending a certain level of supplementation difficult. More research is necessary to determine if the bioavailability and action differs between the dietary and pharmaceutical forms of the vitamin and if there are certain populations who may safely take a certain amount of folate supplementation or fortification.
Bone marrow is the hematopoietic organ that produces most cellular components of the blood, including erythrocytes, leukocytes, and platelets. Disorders of hematopoiesis are common in the elderly as functioning bone marrow reserve diminishes with age. Myelodysplastic syndromes (MDS) are one such group of disorders and a cause of anemia in older patients, although it is relatively uncommon. These disorders are characterized by one or more peripheral blood cytopenias resulting from bone marrow dysfunction [32]. According to the French-American-British (FAB) classification system, MDS is further classified according to cellular morphology, etiology, and clinical presentation as [32]:
Refractory anemia
Refractory anemia with ringed sideroblasts
Refractory anemia with excess blasts
Refractory anemia with excess blasts in transformation
Chronic myelomonocytic leukemia
In 2008, the WHO revised its classification system for MDS, which is now based more on the results of genetic testing than previous systems [36]. The main differences include the elimination of the refractory anemia with excess blasts in transformation category, which is considered acute leukemia, and the addition of several subcategories and new categories. The WHO organizes MDS into the following classifications [32,36]:
Refractory anemia
Refractory cytopenia with multilineage dysplasia
Refractory anemia with ringed sideroblasts
Refractory anemia with excess blasts
Myelodysplastic syndrome, unclassifiable
Myelodysplastic syndrome associated with del(5q)
As noted, myelodysplasia is more common in elderly patients, and more than 75% of patients with MDS are older than 60 years of age at diagnosis [32]. Patients may be asymptomatic, and the disease is often found as the result of routine blood tests. When present, signs and symptoms include fatigue, pallor, frequent infections, easy bruising, and petechiae. An estimated 30% of cases will progress to acute leukemia [32].
Anemia dominates the early course of MDS. Other key characteristics include macrocytosis, neutropenia, and thrombocytopenia. A poor prognosis is associated with advanced age, severe thrombocytopenia, and neutropenia.
Kidney function and glomerular filtration rate (GFR) naturally decreases with age, and it may be further decreased in the presence of chronic illnesses such as hypertension and diabetes, the two main causes of chronic kidney disease. Erythropoietin, a hormone-like substance elaborated by the kidney, is responsible for regulation of RBC production in the bone marrow. As kidney function declines, whether from disease or aging, there is a steady decrease in renal production of erythropoietin, and this is the primary etiology of anemia associated with chronic kidney disease. It can be difficult to distinguish the effects of normal aging on the kidneys from chronic kidney disease. Although a diminishing GFR with age is considered normal, the diagnostic criteria for chronic kidney disease are not modified according to a patient's age. Chronic kidney disease is defined as kidney damage or a GFR less than 60 mL/minute/1.73 m2 for more than three months [37]. It is further staged according to severity of GFR impairment and other symptoms (Table 2).
STAGES OF CHRONIC KIDNEY DISEASE
| Stage | Glomerular Filtration Rate (mL/min/1.73 m2) | Description |
|---|---|---|
| 1 | >90 | Normal or increased GFR, with other evidence of kidney damage |
| 2 | 60–89 | Slight decrease in GFR, with other evidence of kidney damage |
| 3 | 30–59 | Moderate decrease in GFR, with or without other evidence of kidney damage |
| 4 | 15–29 | Severe decrease in GFR, with or without other evidence of kidney damage |
| 5 | <15 | Established renal failure |
Although anemia is usually seen as a late manifestation of advanced renal impairment, women, diabetics, and African Americans may develop this complication at an earlier stage of kidney disease [37]. As noted, the primary etiology of anemia in chronic kidney disease is erythropoietin deficiency, but other causes should be considered as there may be a combination of factors contributing to the anemia. Other potential causes include:
Chronic blood loss
Iron deficiency
Vitamin B12 or folate deficiency
Hypothyroidism
Chronic infection/inflammation
Hyperparathyroidism
Aluminum toxicity
Malignancy
Hemolysis
Hemolytic anemias result from inherited or acquired disorders that result in premature destruction or removal of RBCs from the circulation. The normal lifespan of erythrocytes is 120 days; when hemolysis supervenes the rate of destruction or shortening of lifespan may be too severe for bone marrow production to compensate, resulting in anemia [38]. Sickle cell disease and thalassemia are examples of inherited hemolytic anemia; the acquired form is most often a manifestation of an immunologic disease, drug reaction, or infection. Some patients will have no known cause [38].
Sickle cell anemia is an inherited cause of hemolysis in which a genetic abnormality results in alteration of the Hgb structure, known as HgbS. The RBCs have an atypical response to hypoxia, and the cells develop a characteristic rigid sickle shape. The abnormal blood cells deliver less oxygen to the body tissues. Sickle-shaped Hgb can occlude the blood vessels, causing pain, organ damage, or stroke. The sickle cells usually die after 10 to 20 days, and anemia results [39].
Sickle cell disease occurs more often in persons with ancestors from sub-Saharan Africa, the Mediterranean, India, and Saudi Arabia [39,40]. In the United States, the rates are the highest for Black individuals, with 1 in 365 Black or African Americans having sickle cell disease [39,42]. If both parents carry the sickle cell trait, there is a one in four chance of having a child with sickle cell anemia, but carriers of sickle cell trait are generally asymptomatic. The lifespan of sickle cell patients is greatly reduced, so this type anemia is rarely seen in geriatric patients.
Thalassemias are another inherited hemolytic anemia. It is characterized by a genetic defect or deletion that causes an abnormal synthesis of one of the Hgb protein chains (alpha or beta) [43]. The alpha type is more common among African and South Asian persons, while the beta type is endemic to Mediterranean regions [43,44]. Thalassemia major occurs when the gene is inherited from both parents; thalassemia minor occurs if the gene is inherited from one parent. Severe thalassemias can cause a premature death, often between 20 and 30 years of age, but less severe forms have a favorable prognosis. Laboratory tests are usually necessary for diagnosis, but genetic studies can also be helpful [43].
In acquired hemolytic anemia, the body is producing normal RBCs, but the cells are being damaged. Potential causes of hemolysis include mechanical heart valves, immune disorders, infections, hypersplenism, drug or transfusion reactions, chronic liver disease, congestive heart failure, leukemia, and lymphoma.
Aplastic anemia is a life-threatening condition that occurs due to unexplained bone marrow failure. The bone marrow's stem cells are damaged as the result of an inherited condition or may be caused by an acquired condition, including an autoimmune disorder, exposure to toxic chemicals, chemotherapy or radiation exposure, infection, or in rare cases, pregnancy [45]. In some patients the cause of aplastic anemia remains unknown. Hereditary aplastic anemia is very rare; the acquired type is more prevalent. However, only 4 of every 1 million Americans will be diagnosed with any type of aplastic anemia annually [46]. As with other anemias, patients with aplastic anemia are susceptible to bleeding, fatigue, and infections. Pancytopenia is present when there are low counts of RBCs, WBCs, and platelets. The diagnosis of aplastic anemia is confirmed by bone marrow examination.
Unexplained anemia of aging (UAA) is a distinct disease entity, presenting as a normocytic anemia in older adult that cannot be attributed to nutritional deficiency, chronic inflammatory disease, or chronic kidney disease. The prevalence increases with age, and UAA is associated with decreased quality of life and increased mortality [65,69]. In most cases of UAA, the response to erythropoietin is blunted, suggesting an age-associated deficiency in ability to sense erythropoietin or to sustain rates of production required to maintain normal erythrocyte production [69]. In a study of 124 anemic elderly persons older than 65 years of age, 42 (37%) had anemia with these features, including low erythropoietin, lymphopenia, and low inflammatory markers [67]. The diagnosis of UAA rests on exclusion of known causes of anemia. UAA is thought to represent a heterogenous group with diverse causes such as impaired erythropoietin response to anemia, underlying stem cell disorder, or, in men, possibly testosterone deficiency [65,69]. The prevalence of UAA has been estimated at 25% to 44%, independent of whether the study population is community-based, hospital inpatient, or long-term facility residents [69].
Many cases of anemia will be found during routine blood tests done for other reasons. The initial laboratory evaluation of anemia will be determined by the patient's medical history and physical. When completed, the laboratory evaluation should include:
Complete blood count (CBC)
Iron profile
Vitamin profile
Erythropoietin level
Stool for occult blood
The CBC is important for the diagnosis of anemia and for monitoring disease progression and treatment efficacy. When assessing the elderly anemia patient, the most important components of the CBC are [47]:
Erythrocyte (RBC) count: Reports the total number of RBCs per liter of whole blood.
Normal range for men: 4.7–6.1 million cells/mcL
Normal range for women: 4.2–5.4 million cells/mcL
Hgb: Measures the amount of hemoglobin present in the blood. Dehydration may produce a falsely high Hgb.
Normal range for men: 13–17 g/dL
Normal range for women: 12–16 g/dL
Hematocrit (HCT): Packed cell volume in proportion to blood volume.
Normal range for men: 40% to 52%
Normal range for women: 36% to 48%
Mean cell (corpuscular) volume (MCV): Measures the average size of RBCs, a diagnostic parameter for evaluating anemia, and differentiates microcytic and normocytic anemia in the elderly.
Normal range: 81–100 fL
Macrocytosis: Greater than 100 fL with large RBCs
Microcytosis: Less than 81 fL with small RBCs
Mean cell hemoglobin (MCH): Average amount of Hgb in an RBC.
Normal range: 27–34 Hgb/cell
Mean cell hemoglobin concentration (MCHC): Average concentration of Hgb in an RBC.
Normal range: 30% to 36%
RBC distribution width (RDW-CV): Measures variations in the size of RBCs.
Normal range: 12% to 14%
Leukocyte (white blood cell) count: Reports the number of leukocytes in the blood; the differential includes different types of leukocytes (i.e., neutrophil, eosinophil, basophil, lymphocyte, monocyte).
Normal range: 4,500–10,000 cells/mcL
Thrombocytes/platelet count: Number of platelets present.
Normal range: 150,000–450,000 cells/mcL
Clinicians should remember that laboratory values are not treated, patients are. Any abnormal laboratory results should be correlated with the physical condition of the patient and the patient's goals. Furthermore, different laboratories may use different reference values.
Symptoms of anemia may occur when the Hgb falls below 11 g/dL; when Hgb falls below 8 g/dL, the anemia is life-threatening. The CBC provides valuable insight into the type of anemia. If leukopenia and thrombocytopenia are present along with anemia, a myelodysplastic disorder is suspected. The MCV reveals the size of the cells, leading to the subclassification of anemias as normocytic, microcytic, or macrocytic, which is useful for indicating potential causes (Table 3).
CATEGORIES OF MEAN CORPUSCULAR VOLUME AND ASSOCIATED CAUSES OF ANEMIA
| Category | Mean Corpuscular Volume | Possible Causes of Anemia | |||||
|---|---|---|---|---|---|---|---|
| Microcytosis | <81 fL with small red blood cells |
| |||||
| Normal range | 81–100 fL |
| |||||
| Macrocytosis | >100 fL with large red blood cells |
|
Examination of a peripheral blood smear for morphologic abnormalities of RBCs (and for leukocytes and platelets as well) should be part of any evaluation of anemia. Anisocytosis indicates excessive numbers of RBCs with varying sizes; poikilocytosis denotes variation in shape and contour of RBCs [48]. Reticulocytes, which are young RBCs that mature in the marrow before release into the circulation, will appear in the blood in large numbers when there is accelerated RBC production, as occurs with hemolysis. A normal reticulocyte value is 0.5% to 1.5%; however, the reticulocyte count may be elevated in an anemic patient (reticulocytosis), indicating an erythropoietic response to the anemia [48]. Reticulocytosis may also raise suspicion for hemolytic anemia or increased RBC destruction. A low reticulocyte count (reticulocytopenia) usually indicates decreased RBC production and may point toward aplastic anemia, bone marrow depression, nutritional anemia, or ACI.
The iron profile is a crucial component to anemia evaluation. Before iron replacement therapy is initiated, the patient's iron level should be measured and documented. The serum iron level is an indicator of the amount of iron bound to transferrin in the blood. The iron profile will measure:
Total serum iron
Normal range for men: 60–176 mcg/dL
Normal range for women: 45–170 mcg/dL
Total iron binding capacity
Normal range: 250–450 mcg/dL
Unsaturated iron binding capacity
Normal range: 100–400 mcg/dL
Transferrin saturation
Normal range: 20% to 50%
Serum ferritin
Normal range for men: 12–350 ng/mL
Normal range for women: 12–200 ng/mL
The iron profile, including the ferritin level, will give information about the iron availability, absorption, and iron stores of the body. Serum ferritin levels of 12–100 ng/mL can be present in both iron-deficiency anemia and AI/ACD [6]. Low serum ferritin levels are indicative of iron-deficiency anemia, and these patients should be evaluated for occult gastrointestinal bleeding from a malignancy or other cause.
A vitamin profile may also be necessary, as macrocytic anemia occurs with both folate and vitamin B12 deficiencies. As noted, low levels of folate have been associated with homocysteine accumulation, a risk factor for cardiovascular disease. Although lowering homocysteine levels may not improve outcomes, its presence as a risk factor should be considered. Replacing folate without replacing vitamin B12 may mask a B12 deficiency, leaving the patient at risk for neurologic complications. The vitamin profile may include measurements of:
Folate (vitamin B9)
Normal range: 4–20 mcM
Vitamin B12 (cobalamin)
Normal range: 200–900 pg/mL
Methylmalonic acid
Normal range: 73–271 nM
Homocysteine
Normal range: 5.1–13.9 mcM
Serum methylmalonic acid can be a useful diagnostic tool in identifying vitamin B12 deficiency when the B12 level is in the low-normal range (200–500 pg/mL), which can be particularly helpful for older adults, who experience symptoms at this level with greater frequency than younger patients. Methylmalonic acid begins to rise when the B12 levels fall below 400 pg/mL. The methylmalonic acid is normal in folate deficiency but may be elevated in renal insufficiency. Serum homocysteine and methylmalonic acid levels are high in 90% of patients with vitamin B12 deficiency [26].
Stool specimen for occult blood is another vital test for evaluation of anemia, as gastrointestinal bleeding is often a major contributing factor. Gastrointestinal bleeding may be intermittent, so evaluation of three different bowel movements is recommended. In preparation for the test, patients should stop taking iron supplements for 10 days and avoid red meat and food with red dye for three days prior to testing.
If the patient is positive for blood in the stool, gastroenterology consult is indicated. The patient will need endoscopy or colonoscopy to locate the source of the bleeding; blood in the stool may originate from hemorrhoids or other less serious problems. Alternatively, blood in the stool can be a sign of gastrointestinal malignancy.
Laboratory measurement of erythropoietin is not routine for evaluating anemia but is useful for assessing patients with unexplained polycythemia (abnormally elevated concentration of RBCs). The erythropoietin level is also reduced in chronic kidney disease and myelodysplasia. The normal range is 4.5–21.3 mU/mL. Patients with high erythropoietin levels and anemia will most likely not respond to erythropoietin-stimulating agents (ESAs). Measuring serum erythropoietin levels is of little diagnostic utility in patients with chronic kidney disease [49].
In cases of unexplained anemia, a referral to a hematologist is necessary for bone marrow aspiration and biopsy. Bone marrow aspiration/biopsy is necessary to evaluate cytopenias, leukocytosis, and thrombocytosis. If conducted, this procedure is generally performed in the outpatient setting.
For patients without a definable cause for the anemia, additional laboratory tests may be necessary for follow-up. If an identifiable and treatable etiology of the anemia is not found, a hematology consult is essential. Other laboratory tests that may be ordered in cases of anemia of unknown etiology include:
Serum bilirubin
Liver function profile
Serum levels of heavy metals (e.g., arsenic, lead)
Thyroid-stimulating hormone
Medical records and past medical history help the clinician to determine if the anemia is chronic or acute, hereditary or acquired. If a patient is known to have had a normal CBC in the past, the anemia is most likely not caused by an inherited or congenital disorder. A complete patient history will provide information regarding chronic illness, history of anemia, specialty consults, and history of blood transfusions. Elderly patients with dementia may be unable to provide an accurate medical history. In these cases, caregivers and family may be good sources of information. If certain tests and diagnostics have already been completed, they may not need to be repeated.
The medical history should include an inquiry as to prior history of anemia and whether there is existing laboratory data that could establish prior Hgb levels. This will help to determine if the present condition represents an acute or chronic anemia. One should also assess the patient for:
Chronic medical illness
Chronic kidney disease
Cancer
Use of NSAIDs, aspirin, and/or blood thinners
Gastric surgery
Intestinal disorders
Crohn disease
Celiac disease
Family history of anemia
Thalassemia
Hemoglobinopathy
Autoimmune disorders
Lupus
Rheumatoid arthritis
Exposure to toxic chemicals
Environmental toxins
Radiation/chemotherapy
Advanced age
Loss of functioning bone marrow
Increase in chronic illness
Decreased nutrition
Elderly patients with anemia may have vague, non-specific symptoms; because the symptoms are non-specific, they are often overlooked or of limited help in differentiating between the types of anemia. Older patients are at greater risk for falls, cognitive decline, fatigue, and weakness because of advanced age, making the identification of anemia even more difficult [50]. Patients with mild anemia may remain asymptomatic. New-onset, easy fatigue, increased weakness, and shortness of breath are useful clues. Other signs and symptoms include tachycardia, bradycardia, dyspnea, chest pain, dizziness, headache, cold hands and feet, restless legs syndrome, and tarry stools. On occasion, patients may complain of visible changes in or discomfort of the tongue or lips, indicative of atrophic glottis and cheilitis. The severity of symptoms is dependent on the rapidity of onset, degree of anemia, physical status, and age of the patient [12]. Because anemia may be multifactorial, complete evaluation is necessary.
Pica may develop in some patients with anemia. Pica is a condition whereby the patient has an unusual craving to eat specific non-food item such as dirt, ice, starch, ashes, or clay. Pica is associated with both mineral deficiency (including iron-deficiency anemia) and mental health conditions. Pagophagia, a craving (pica) for ice, is present in about 50% of patients with iron deficiency, even in the absence of frank anemia [51]. Probing for pica is not part of the routine medical history, but it should be included whenever anemia is suspected or newly diagnosed, as it is a powerful clue to iron deficiency.
Patients with symptomatic anemia may present with multiple vague symptoms that do not point to any one diagnosis. To fully evaluate these symptoms, physical assessment and laboratory evaluation are necessary. Healthcare providers who work with the demented elderly should become adept at detecting medical conditions for patients unable to provide any history or symptomatology.
The physical assessment of the elder should focus on differentiating normal aging changes from pathology. The physical assessment of an anemic elder begins with visual evaluation of the patient's general physical and mental condition. This includes signs of malnourishment, pallor, breathing difficulties, and edema or ascites that may indicate chronic liver or kidney disease. Many observations can be made during the review of systems and medical history taking, including an assessment of cognitive function, such as the patient's ability to answer questions appropriately, delayed responses, and signs of compensating for a cognitive deficit.
Basic measurements lay the foundation for the physical assessment. The vital signs, oxygen saturation, height, and weight give valuable clues to the etiology of anemia. If the patient is underweight, with a body mass index of 18.5 or less, suspicion of nutrient-deficiency anemia is increased.
The major clinical signs in the anemic patient are secondary to hemodynamic changes and tissue hypoxia. Acute blood loss causes hypovolemia, hypotension, and if severe enough, signs and symptoms of shock. Over time, decreased blood volume will eventually cause orthostatic hypotension and tachycardia. This change in orthostasis is especially dangerous in elders, as it predisposes them to falls and injury. A bounding or rapid pulse may indicate that the cardiovascular system is compensating for low Hgb. Oxygen saturation may be decreased secondary to low Hgb, and increased respirations may be observed.
In addition to pallor of the skin, the inferior conjunctiva of the eye may appear pale. The tongue may appear smooth and swollen, with loss of papillae (glossitis). Angular stomatitis (cheilitis) causing irritation and fissuring of the corners of the lips may indicate nutritional or iron deficiency. Jaundice could indicate liver failure or hemolytic anemia.
Assessment of the cardiovascular system may reveal tachycardia, heart murmurs, increased peripheral edema, dyspnea, and orthopnea, which will be more pronounced in patients with chronic heart disease. Anemia may precipitate a myocardial infarction in patients with coronary artery disease or the worsening of angina due to the decreased oxygen carrying capacity of Hgb. In patients with peripheral arterial disease, intermittent claudication may develop or worsen. In patients with cerebrovascular disease, severe anemia may lead to transient ischemic attacks and cognitive decline.
Evaluation of the fingernails may reveal splitting and fraying associated with folate deficiency. Spooning of the nails (nails that grow upward) can indicate vitamin B12 deficiency.
Abdominal scars may be present due to gastrectomy or ileal resection, which gives information about possible malabsorption or gastrointestinal disorders. Older patients may give inaccurate medical histories, so these clues can be quite useful. There may be symptoms of anorexia, weight loss, constipation, and nausea.
Neurologic examination may reveal memory deficits, paresthesias, loss of position sense, and unsteady gait in patients with B12 deficiency anemia. Vitamin B12 deficiency should be considered for patients with even minimal neurologic symptoms. In later stages of vitamin B12 deficiency, the neurologic symptoms worsen and may become severe.
Close monitoring of pertinent laboratory values is necessary to evaluate patients' response to treatment. If a patient does not respond to the prescribed treatment, further evaluations and diagnostics may be needed to detect anemia of multiple etiologies.
Management of elderly patients with anemia is guided by the underlying diagnosis, severity of disease, and comorbidities. Treatment of the anemia is largely dependent upon the etiology and whether clinical signs and symptoms can be linked specifically to the anemia. Therapy directed to the underlying cause, if effective, should result in improvements in Hgb levels. Less certain, even controversial, is whether unexplained anemia of aging is amenable to specific therapy. Treatment decisions should be individualized and based on a variety of factors, including patient goals, functional status, and presence of comorbidities [60]. Although treatment with ESAs may result in significant improvements in Hgb levels, this approach has not been fully studied in patients with unexplained anemia and there is little evidence of pharmacologic treatment improving elderly patients' quality of life, physical function, or disability [52]. One small placebo-controlled study, involving 66 elderly patients with unexplained anemia, reported that epoetin alpha (administered weekly for 16 weeks) was associated with significant rise in Hgb levels (>2g/dL) and concomitant improvement in fatigue and quality of life [56]. Due to the low risk of adverse effects, lifestyle interventions are often a first approach, including improvements in diet and vitamin supplementation. In most patients with mild disease, this may be sufficient.
As discussed, in cases of AI/ACD, the stabilization of the underlying chronic illness should improve Hgb and symptoms of anemia. This should be the first approach and should include assessment and treatment of other complicating factors, including iron deficiency, malignancies, or blood loss [18]. However, if the patient is experiencing impaired quality of life because of anemia symptoms or if the presence of anemia could complicate other age-related disorders, treatment with an ESA, such as epoetin alfa, and/or blood transfusion should be considered.
ESAs are used in the treatment of many anemias, regardless of etiology, and they have been found to be effective in improving Hgb levels in elderly patients. The overall goal of ESA treatment is to improve the Hgb level to the point that blood transfusion can be avoided [54,55]. Usually, ESAs are considered when the Hgb is less than 10 g/dL. However, more research in elderly patients is necessary to determine if this applies similarly to older adults. A randomized, double-blind, placebo-controlled study was conducted to evaluate the efficacy of epoetin alfa in the treatment of anemia in women older than 65 years of age [56]. Among this group, epoetin alfa was well tolerated, and the treatment group experienced an increase of 2 g/dL or more in their Hgb levels. Researchers found a direct correlation between Hgb levels and improvements in measures of fatigue and quality of life [56]. Review of mortality information related to Hgb levels indicates that levels greater than 13 g/dL are associated with poor outcomes, while Hgb levels of 9.5–11.5 g/dL are associated with better outcomes [57].
ESAs are associated with significant adverse effects, particularly in patients with cancer or chronic kidney disease. In all patients, ESAs are associated with an increased risk for serious cardiovascular events, thromboembolic events, stroke, and mortality when administered to target hemoglobin levels greater than 11 g/dL [55]. Due to the risk of deep vein thrombosis, all patients who require surgery should be given prophylaxis prior to the procedure. Prescribing of ESAs should be restricted to clinicians experienced in use and monitoring of the treatment. Because there is an increased risk of death, serious cardiovascular events, hypertension, thrombosis, and progression of certain cancers, patients should be closely monitored. In addition, patients must understand the risks associated with this treatment. Quality of life measures should be considered.
The recommended dosage of epoetin alfa is 100–150 U/kg subcutaneously three times per week along with supplemental oral iron [18]. If no improvement is seen in six to eight weeks, this dose may be increased to daily administration or to 300 U/Kg three times weekly. A once-weekly dose of 30,000–40,000 U subcutaneously is also available. If the patient has no response to treatment after 12 weeks, it is unlikely to be clinically useful [18].
In order for treatment with ESAs to be effective, iron supplementation to ensure adequate stores is necessary. Supplementation should maintain a transferrin saturation of 20% or greater and a serum ferritin level of 100 ng/mL or greater [18].
Because the major cause of iron deficiency is overt or occult blood loss, the first step should be to identify and treat the source of the iron or blood loss, which should improve Hgb levels and symptoms of anemia. Any medications that may be blocking iron absorption should be discontinued. In cases of malnutrition or non-drug-induced impaired gastrointestinal absorption of iron, oral supplementation may be indicated. The recommended oral supplement for the treatment of iron deficiency in older adults has not been established. The adult dosage regimen is usually 325 mg of ferrous sulfate three times per day to provide 150–200 mg/day of elemental iron; however, this regimen is associated with high rates of adverse effects, including black stools, abdominal discomfort, diarrhea, nausea/vomiting, and constipation [50]. one study of patients older than 80 years of age found that daily doses of 15 mg or 50 mg liquid ferrous gluconate or 150 mg ferrous calcium citrate tablets was better tolerated and resulted in similar outcomes, with significant increases in Hgb evidenced in all three treatment groups [58]. Liquid formulations of ferrous sulfate administered in small doses (15 mg) less frequently and with orange juice to facilitate absorption may be more effective and better-tolerated (50). The lowest possible dose should be used first, with gradual titration, if necessary. If a patient is unable to take even the smallest dose of oral iron due to adverse effects (e.g., nausea, constipation, vomiting) or if improvement in Hgb cannot be obtained by oral supplementation (e.g., continued bleeding), parenteral iron therapy may be appropriate. After hemoglobin and serum ferritin levels have normalized, it is usually advisable to continue therapy for three to six months to allow restoration of tissue iron stores.
A ferric carboxymaltose injection (Injectafer) is approved by the U.S. Food and Drug Administration for the treatment of iron-deficiency anemia in patients who have intolerance to oral iron or have had an unsatisfactory response to oral iron [59]. This parenteral iron replacement product is given as a single dose of up to 750 mg of iron via an IV push injection or over a 15-minute infusion followed by a second dose seven days later for a total treatment of up to 1,500 mg of iron [55,59].
Improvements in patient functioning, including less fatigue, resolution of pica, and increased participation in activities of daily living, should be seen within the first week. The Hgb level will rise more slowly, with full resolution expected within six to eight weeks [60].
For patients low in vitamin B12 or folate, replacement therapy can be started immediately. Vitamin B12 supplements are available in oral, sublingual, intranasal, and injectable formulations. Traditionally, vitamin B12 replacement consisted of a series of injections of cyanocobalamin. However, a meta-analysis found that high daily doses (1,000 mcg) of oral vitamin B12 were as effective as intramuscular injections [4].
Folate and vitamin B12 cannot be synthesized by the body and require daily intake. There is some concern that folate supplementation may "mask" a vitamin B12 deficiency; therefore, clinicians should screen older patients for vitamin B12 deficiency prior to initiating folic acid supplementation [61]. Folate deficiency is mainly treated using supplementation of the vitamin. Most multivitamin preparations contain 400 mcg of folate, but this dosage may not be sufficient in cases of malabsorption or drug-induced deficiencies. Intake of folate should not exceed 1,000 mcg/day.
Treatment of MDS generally consists of supportive care, including transfusion of RBCs, which temporarily corrects the low blood counts. Platelet infusions become less effective over time and are associated with a risk of alloimmunization [32]. Frequent transfusions can cause an iron overload, which can damage the liver and other organs; iron overload may become a problem after as few as 10 transfusions. When the serum ferritin level is between 1,000 and 2,000 ng/mL, the patient may require iron chelation therapy with subcutaneous or oral deferasirox or subcutaneous deferoxamine and vitamin C [32,55].
An ESA (e.g., epoetin alpha 150–300 mcg/kg/day) may be used in patients with a low erythropoietin level and is most useful in patients who do not yet require transfusion and patients who have a serum erythropoietin level less than 200 mU/mL. Other possible treatments of MDS include growth factor, chemotherapy, and bone marrow or stem cell transplantation.
The mainstays of treatment of anemia in chronic kidney disease patients are ESAs and iron supplementation [54]. In 1989, the FDA approved the use of epoetin alfa for use in anemia of chronic kidney disease. Response to treatment with ESAs is inhibited in patients with kidney disease, and increased doses may be necessary to obtain an adequate response. The recommended initial dose is 50–100 U/kg per week or 10,000 U subcutaneously once weekly, with adjustment of the dose based on subsequent Hgb levels, response to treatment, and development of adverse side effects [62]. As discussed, for ESA treatment to be effective, concurrent iron supplementation is also necessary.
However, the severe adverse effects of epoetin alpha and other ESAs have made their use complicated [63]. Studies of have shown increased thromboembolic events, tumor progression, and cardiovascular events when Hgb levels are greater than 12 g/dL [51,53]. The FDA has issued a black box warning for epoetin alfa regarding the increased risk of death, serious cardiovascular events, and stroke in patients with chronic kidney disease with Hgb levels of 11 g/dL or greater [55]. Although no optimal dose or Hgb target has been established for patients with kidney disease to prevent these adverse events, an Hgb level that raises more than 1 g/dL in one week may indicate an increased risk and should initiate a reduction in ESA dose [55]. The lowest possible dose of ESA to prevent blood transfusion should be used.
Treatment of sickle cell anemia involves the use of hydroxyurea therapy, amelioration of pain, and other complications and supportive care. Blood transfusions are not used to treat normal anemia and pain associated with sickle cell disease, but it may be used during pregnancy or in cases of severe acute anemia or acute chest syndrome [41]. However, patients who receive multiple transfusions are at risk for alloimmunization and iron overload. Iron chelation therapy may be necessary to prevent damage to the organs due to high iron levels. Allogeneic bone marrow transplant may produce a cure in a small number of patients [41].
Treatment of thalassemia consists of blood transfusions, iron chelation therapy, folic acid supplements, and bone marrow and stem cell transplant, depending on the type of thalassemia diagnosed [43]. Some will require splenectomy.
The goal of treatment of aplastic anemia is to control symptoms and prevent complications. Initial treatment includes blood and platelet transfusions and IV antibiotics. While immunosuppressive drugs are effective in treating aplastic anemia, these medications further weaken the immune response, leaving the patient at risk of complications. ESAs and colony-stimulating factors are also used [45]. For severe cases, an allogeneic bone marrow transplant may be advised. However, it is important to note that elderly patients may be unable to tolerate the preparation for transplant and are at an increased risk for complications. If left untreated, death will usually occur rapidly.
For cases of acute blood loss, as in trauma or surgery, a blood transfusion is the standard treatment for patients willing to receive blood and blood products. In addition, blood transfusions are indicated in the treatment of a wide variety of anemias in elderly patients. For chronic anemia, which may be caused by myelosuppression, inflammation, infection, or malignancy, a blood transfusion is not recommended. In patients with chronic anemia, blood transfusion causes a temporary increase in Hgb but fails to treat underlying condition(s) [7]. Transfusion of blood products is generally reserved for patients with an Hgb level of 8 g/dL or less, due to risks of infection, volume overload, and transfusion reactions [11].
As noted, iron overload and clinical signs of hemochromatosis may occur with repeated blood transfusions. The excessive iron builds up in organs and tissues, especially the liver. Signs and symptoms of hemochromatosis may include joint paint, fatigue, generalized weakness, weight loss, and abdominal pain. Due to the potential hazards of iron overload on the body organs and tissues, iron should never be prescribed prior to laboratory assessment of iron stores of the body.
In patients with terminal disease, blood transfusions may be used to palliate the symptoms associated with anemia. Blood transfusions have been found to provide some relief from anemia symptoms (e.g., fatigue, dyspnea, weakness) and improved quality of life for patients suffering from cancer or terminal illness [8]. Again, this is a palliative care technique and will not be curative or a long-term solution. In fact, it is important to remember that a blood transfusion has the potential to prolong pain and suffering at the end of life and to give patients and/or their families false hope. A risk and benefit analysis should be performed prior to initiating transfusions for these patients.
Language and cultural barriers have the potential for far-reaching effect, given the growing percentages of racial/ethnic populations. As noted, patient understanding of the causes and available treatments is an essential component of caring for the geriatric patient with anemia, and it should be assured that all patients have a clear understanding of the concepts discussed. When there is an obvious disconnect in the communication process between the practitioner and patient due to the patient's lack of proficiency in the English language, an interpreter is required.
Clinicians should use plain language in their discussions with their patients who have low literacy or limited English proficiency. They should ask them to repeat pertinent information in their own words to confirm understanding, and reinforcement with the use of low-literacy or translated educational materials may be helpful.
Patient H is a White woman, 89 years of age, who resides in a skilled nursing facility. She is being evaluated due to an Hgb level of 8.1 g/dL. She is ambulatory with a rolling walker, generally alert, and oriented with some mild cognitive impairment. She is compliant with medical treatments and takes medications as prescribed. Her medical history is positive for congestive heart failure, chronic obstructive pulmonary disease (COPD), chronic kidney disease, and osteoarthritis. She is oxygen dependent on 2 L/minute per nasal cannula. She is bright and outgoing and verbalizes multiple vague physical complaints.
During her last hospitalization, one year ago for pneumonia, the nephrologist and pulmonologist told Patient H there was not much else that could be done for her. Despite the poor prognosis, her multiple medical conditions stabilized and she completed a rehabilitation program. She enjoys participating in activities and has developed friendships with some other residents. Over the past year, she has been treated for multiple infections, including bronchitis and multiple urinary tract infections.
Patient H's chief complaint is of feeling tired and short of breath at times. She also complains of arthritic pains in her neck and hands. Review of systems is notable for hearing loss, dentures, glasses, and dyspnea, mostly with exertion. She has occasional palpitations of the heart and orthopnea at times. Her bowel movements are regular, and she has not noticed any blood in the stool. She has 1+ chronic edema of the legs, which is about usual for her. She has not had a mammogram for five years, and she has not had a dual energy x-ray absorptiometry scan, colonoscopy, or other preventive care recently.
Patient H's record indicates an allergy to sulfa drugs and penicillin. She also has completed advance directives (a do not resuscitate order and a living will). She is taking the following medications:
Amlodipine besylate (Norvasc): 5 mg/day
Calcium carbonate (OsCal) with vitamin D twice daily
Polyethylene glycol powder (Miralax): 17 g in 8 oz liquid daily
Furosemide (Lasix): 40 mg/day
Escitalopram (Lexapro): 20 mg/day
Prednisone: 10 mg/day
Omeprazole (Prilosec): 20 mg/day
Cinacalcet (Sensipar): 30 mg/day
Simvastatin (Zocor): 20 mg at bedtime
Tiotropium oral inhalation (Spiriva): 1 cap per inhalation device daily
Vitamin B12: 1,000 mcg twice daily
Enteric-coated aspirin: 81 g/day
Upon physical examination, Patient H appears well-nourished and groomed. She is mildly short of breath at rest but in no apparent pain or distress. She is 5 feet 6 inches tall and weighs 156 pounds. Her vital signs indicate a blood pressure of 132/84 mm Hg; pulse 72 beats/minute; temperature 97.4 degrees F; respirations 20 breaths/minute; and oxygen saturation 94% on 2 L/minute. Her oropharynx is clear, and her neck is supple. There is no lymphadenopathy. The patient is hard of hearing and wears glasses for distance and reading.
Patient H's heart rate is slightly irregular, with a soft systolic ejection murmur. Evaluation of her lungs indicates diminished breath sounds in the bases with no adventitious sounds. The abdomen is soft and non-tender, with active bowel sounds and no signs of hepatosplenomegaly. As noted, Patient H has 1+ pitting chronic edema and vascular changes to lower extremities. There are no active skin lesions. Neurologic assessment shows no focal deficit. Extremity strength is rated 4 out of 5. A mini-mental status exam is administered, and the patient scores 22/30, indicating mild cognitive impairment.
Blood is drawn and sent to the laboratory for CBC and a basic metabolic panel. The results are:
Leukocytes: 5,700 cells/mcL
RBC: 3.02 million cells/mcL
Hgb: 8.1 g/dL
HCT: 25.2%
MCV: 83 fL
MCH: 26.5 Hgb/cell
MCHC: 32%
RDW-CV: 15.8%
Platelets: 150,000 cells/mcL
Glucose: 82 mg/dL
Blood urea nitrogen (BUN): 34 mg/dL
Creatinine: 1.4 mg/dL
GFR: 38 mL/minute/1.73 m2
Patient H is in no apparent distress at present, but she appears to have anemia, as evidenced by the low Hgb. She has chronic kidney disease (stage 3), which may be contributing to the anemia. Further laboratory evaluation is necessary to determine the etiology of the anemia and to determine if specialty referral to gastroenterologist or hematologist is necessary. The clinician orders an iron profile, vitamin B12 and folate levels, reticulocyte count, and stool for occult blood. The results of this testing are:
Vitamin B12: 1,996 pg/mL
Folate: 9.9 mcM
Ferritin: 20 ng/mL
Serum iron: 26 mcg/dL
Unsaturated iron binding capacity: 216 mcg/dL
Total iron binding capacity: 242 mcg/dL
Transferrin saturation: 11%
Reticulocyte count: 1%
Stool for occult blood: Negative (three samples)
Vitamin B12 is a water-soluble vitamin that is excreted in urine, so a high level is generally not significant. The folate level is sufficient, while the ferritin level is considered low-to-normal. The iron profile shows a low level of iron in the blood; this may be caused by gastrointestinal bleeding or by inadequate absorption of iron by the body. Patient H has medical conditions that can cause elevated cytokines, which would interfere with iron absorption. If her ferritin level was high, which it is not, it would suggest AI/ACD. Therefore, the patient appears to have anemia secondary to chronic kidney disease and possibly inadequate iron absorption and processing.
Prior to initiating treatment with an ESA, the patient is evaluated for a history of cancer, as these agents may cause progression/recurrence of cancer. Before writing the prescription for darbepoetin alfa, the clinician signs the ESA APPRISE Oncology Patient and Healthcare Professional Acknowledgement Form to document discussing the risks associated with darbepoetin alfa with the patient. The lowest dose that will prevent blood transfusion is prescribed.
The multidisciplinary team works with Patient H to develop a treatment plan. It is determined that treating the anemia will improve the patient's quality of life. The patient is prescribed ferrous sulfate 325 mg twice daily. Because vitamin C facilitates iron absorption, the iron can be given with a glass of orange juice or other citrus juice (not grapefruit). Iron must not be given with calcium, milk products, and certain medications as they can interfere with absorption. The patient should be monitored for the development of constipation and the need for stool softeners. In addition, darbepoetin alfa 40 mcg is prescribed, to be administered subcutaneously every week. This requires significant monitoring. Hgb and HCT should be measured on the day patient is to receive the injection, and the drug should be held if the Hgb is greater than 11.5 g/dL. If a current Hgb level is unavailable, the drug should not be given.
Blood pressure should be measured twice daily after treatment with darbepoetin alfa is initiated. Staff must also monitor for symptoms of a deep vein thrombosis and pulmonary embolus (e.g., unilateral edema, cough, and/or hemoptysis). Daily exercise is encouraged to help reduce the risk of a blood clot.
After one month, Patient H has received darbepoetin alfa weekly for four weeks. She is also taking the ferrous sulfate and a stool softener. The review of systems is unchanged from the previous evaluation. The physical examination is also unchanged aside from a 1-pound weight loss. No new complaints or problems are reported. A review of the patient's vital signs shows a blood pressure of 130/80 mm Hg; pulse 78 beats/minute; temperature 97.8 degrees F; and oxygen saturation 97 on 2 L/minute. Her Hgb levels over the last month have improved:
Week 1: 8.4 g/dL
Week 2: 9.2 g/dL
Week 3: 9.6 g/dL
Week 4: 10.1 g/dL
No side effects from darbepoetin alfa have been observed. Patient H's blood pressure remains stable, with no signs or symptoms of a blood clot.
The clinician orders the weekly monitoring of Hgb and HCT to continue with the darbepoetin alfa held if the Hgb is greater than 11.5 g/dL. If the medication is held more than once, the clinician will re-evaluate the dosage and frequency. The patient may only need the injection once or twice a month after the anemia is stabilized. The clinician also reduces the patient's vitamin B12 supplement to daily (rather than twice daily) and reduces her prednisone dose to 5 mg/day.
Discussion
Patient H's initial complaint was of shortness of breath and fatigue. These symptoms may have been related to the anemia or may have been a chronic complaint secondary to her congestive heart failure and COPD. The decreased hematocrit level is likely contributing to the patient's symptomatic heart failure. By exploring her condition further, it was determined that treating the anemia might improve the patient's quality of life and prevent the necessity for more extreme interventions (e.g., blood transfusion). Patient H was informed of the potential side effects of darbepoetin alfa and agreed to take it to prevent transfer to the hospital for blood transfusion.
The patient's condition was monitored closely, with weekly blood draws for Hgb. Her blood pressure remained stable, and no signs of complications were detected. Her shortness of breath and fatigue improved as the treatment progressed and her Hgb level rose above 10 g/dL. This allowed her to be more active.
For patients with terminal or end-stage conditions, treatment of anemia can restore or preserve their quality of life. Treating problems that could potentially cause suffering, while avoiding futile care, is the goal for patients with a life-limiting condition. Anemia affects the quality of life in elders by causing fatigue, poor endurance, and shortness of breath, and alleviating these symptoms can allow for more activity and comfort.
Patient P is a White man, 92 years of age, who resides in an assisted living facility. He uses a motorized scooter for mobility but can ambulate short distances with his rolling walker. He requires assistance with medication administration and attends community meals three times a day. His daughter visits once a week and assists with laundry and transports to medical appointments.
Patient P is brought to the primary care clinic by his daughter. She states that he is very fatigued and is not performing his usual daily tasks. He sleeps more than is usual and must be coaxed to attend the community meals. At times, he has refused to take medications that the staff attempt to administer. The patient minimizes his symptoms saying, "What do you expect? I'm 92 years old." Patient P's medical history is positive for Parkinson disease, hypertension, COPD, osteoporosis, compression fracture to the lumbosacral spine, and weight loss. He is a widower and reports no tobacco use and rarely drinking beer or wine. His chief presenting complaints are fatigue, shortness of breath, chronic back and leg pain, and poor appetite.
On review of systems, the patient is noted to be hard of hearing and wearing a hearing aid. Vision is adequate with correction. He wears dentures and denies pain or difficulty with mastication. He has difficulty swallowing large pills, and his caregivers crush his medications. He states his appetite is not good; he has lost 15 pounds over the last year. Patient P denies chest pain, but complains of shortness of breath. This occurs when he exerts himself and often when he lays down. He has a regular bowel movement every one to two days and denies nausea, vomiting, or diarrhea. He has not had dyspepsia but does state he does not seem to be able to eat as much as he used to (early satiety). He complains of nonradiating pain in his lower back. All other review of systems is non-contributory. Patient P has previously been tried on antiparkinsonian medications and osteoporosis medications that were discontinued due to unacceptable side effects. He is currently taking the following medications:
Atenolol (Tenormin): 25 mg twice daily
Vitamin D3: 1,000 units/day
Calcium carbonate: 500 mg three times every day
Vitamin B12: 1,000 mcg/day
Amlodipine (Norvasc): 10 mg/day
Omeprazole: 20 mg/day
Multivitamin daily
Tiotropium oral inhalation (Spiriva): 1 cap per inhalation device daily
Acetaminophen (Tylenol): 650 mg three times daily routinely
Stool softener twice daily
Tramadol (Ultram): 50 mg every six hours as needed
Upon physical examination, Patient P appears frail but well groomed; he is in no apparent distress. He is 5 feet 5 inches tall and weighs 134 pounds. His vital signs indicate a blood pressure of 110/64 mm Hg; pulse 82 beats/minute (regular rate and rhythm); temperature 97.8 degrees F; respirations 22 breaths/minute; and oxygen saturation 91% on room air.
As noted, the patient is hard of hearing with hearing aid and wears glasses. There is no evidence of lymphadenopathy or carotid bruit. His oropharynx is clear. His lungs are clear to auscultations, with diminished air flow in lung bases. The abdomen is soft and non-tender, not distended, with bowel sounds active in four quadrants. There is no hepatosplenomegaly. Extremities are clear of edema. Vascular changes are noted on both legs, and the toenails are thickened. The patient displays dry, flaky skin of lower extremities. Tenderness is noted over the lumbar spine with palpation. Disuse atrophy is noted to the extremities. Chronic skin lesions are also present, with actinic keratoses on the nose and forehead. Neurologic assessment reveals fine resting tremor of both hands and flat affect. Strength is equal bilaterally with strong hand grasps. Gait and balance are unsteady.
The clinician orders Patient P's medical records and diagnostics from previous care providers. She also requests a CBC with differential, a complete metabolic profile, and thyroid studies. A home health care evaluation is also recommended to determine if rehabilitation services would be appropriate. Patient P's laboratory studies indicate:
Leukocytes: 5,400 cells/mcL
RBC: 2.95 million cells/mcL
Hgb: 8.7 g/dL
HCT: 26%
MCV: 88 fL
MCH: 29.4 Hgb/cell
MCHC: 33.3%
RDW-CV: 13.7%
Platelets: 289,000 cells/mcL
Glucose: 77 mg/dL
BUN: 20 mg/dL
Sodium: 139 mEq/L
Potassium: 4.5 mEq/L
Chloride: 104 mmol/L
Carbon dioxide: 27 mmol/L
Creatinine: 0.9 mg/dL
Calcium: 8.8 mg/dL
GFR: Greater than 60 mL/minute/1.73 m2
Thyroid-stimulating hormone: 2.88 mIU/L
The low RBC, Hgb, and HCT indicate that the patient has a normocytic, normochromic anemia. However, blood-loss anemia is not ruled out by lack of microcytosis. If the blood loss is recent, changes to the cells may not yet be evident. The RDW-CV is normal, which may indicate a lack of erythropoietic response. Further laboratory evaluation is indicated.
The clinician requests an iron profile, ferritin level, folate level, vitamin B12 level, reticulocyte count, and stool for occult blood. The results of this testing are:
Vitamin B12: 1,006 pg/mL
Folate: 17.2 mcM
Ferritin: 246 ng/mL
Serum iron: 38 mcg/dL
Total iron binding capacity: 197 mcg/dL
Transferrin saturation: 27%
Reticulocyte count: 1.2%
Stool for occult blood: Negative on two samples, mildly positive on third sample
These findings indicate the patient is not deficient in vitamin B12 or folate. The serum iron is low while the ferritin level is high, suggesting adequate iron stores that are not being utilized by the body. This is a diagnostic indicator of anemia of chronic inflammation. The total iron binding capacity is low, showing that the blood's ability to bind transferrin with iron is reduced. Patient P has signs of a "mixed" anemia, the result of both ACI and gastrointestinal blood loss. Elderly patients often have more than one etiology contributing to the anemia, and all the possible causes should be thoroughly evaluated.
One of the stools for occult blood is positive, which may be representative of intermittent gastrointestinal bleeding. The clinician refers the patient to a gastroenterologist for further diagnostic evaluation. An endoscopy and colonoscopy should be performed if the patient and healthcare surrogate are willing. He is also prescribed a 5% lidocaine patch to be applied to his lower back in the morning and to be removed at bedtime.
Patient P visits a gastroenterologist, who performs an endoscopy and a colonoscopy. He is found to have several polyps in the large intestine, which are removed and biopsied during the colonoscopy. He tolerates the procedures well and has no adverse effects. The polyps are found to be benign.
One month later, Patient P returns to the clinic for follow-up. His review of systems is unchanged, although he reports improvement in his lower back pain. His vital signs are all stable. A CBC is completed to monitor the anemia, and the results are:
Leukocytes: 5,600 cells/mcL
RBC: 3.36 million cells/mcL
Hgb: 9.6 g/dL
HCT: 29.7%
MCV: 88.0 fL
MCH: 28.7 Hgb/cell
MCHC: 32.5%
RDW-CV: 14.9%
Platelets: 262,000 cells/mcL
Patient P's RBC, Hgb, and HCT have improved slightly. The RDW-CV has also increased, indicating an improved erythropoietic response. The removal of the colon polyps, which may have been causing some intermittent bleeding contributing to the anemia, appears to have improved the patient's condition. Patient P is instructed to return in one month for additional follow-up.
Discussion
Patient P is a frail, elderly patient with multiple medical problems. From a primary care perspective, it is important to identify any new or existing medical problems that can be treated and improved. Elderly persons often present with multiple vague complaints that do not point to any one disorder. Laboratory evaluation is crucial to narrow down the diagnostic differential.
Because there is an upward trend in the CBC values, the necessity of a blood transfusion is reduced. However, if the anemia worsens, a hematology consult may be necessary. At 92 years of age, the patient is at risk for myelodysplasia, but if present, lower values of leukocytes, RBCs, and platelets would be expected.
While the use of an ESA might result in an improvement in elderly patients, there are multiple side effects and precautions associated with their use. For Patient P, they should be avoided unless truly necessary to prevent the need for transfusions.
Anemia is common in men and women older than 65 years of age and becomes more prevalent with aging. Varying degrees of anemia may be present in the elderly patient. It may be common, but it should not be accepted as normal aging. Anemia in the elderly is associated with diminished functional capacity and waning quality of life and increased risk of death from all causes. As the geriatric population grows, the incidence of anemia and its complications in this population will become a significant healthcare burden.
Geriatric patients have psychological, sociologic, and physical changes resulting from aging that complicate treatment. Due to multiple possible etiologies of anemia in the elderly, a thorough evaluation is necessary. Anemia may be the first sign of a gastrointestinal malignancy or another underlying condition.
It is useful to classify anemia as acute or chronic, divided into three main categories: blood loss, hemolytic, and bone marrow suppression. The main types of anemia seen in elders are AI/ACD, anemia of chronic kidney disease, unexplained anemia of aging, nutrient-deficiency anemia, hemolytic anemia, and myelodysplasia. The genetic disorders causing anemia are usually not seen in the elderly as they are associated with mortality at a younger age. Aplastic anemia is a rare form of anemia that may be seen in the elderly and can be fatal if left untreated.
Primary care providers should be proficient in the initial work-up of the anemic elder. Often, there are multiple medical comorbidities requiring specialist referral. A good working relationship with gastroenterologists and hematologists is important, and nephrology referral may be indicated for patients with chronic kidney disease. It is up to the primary care clinician to accumulate all pertinent information from specialists and diagnostics and to establish a plan of care that will benefit the patient. Careful oversight will help prevent redundant tests or treatments and help to reduce unnecessary polypharmacy.
The risk/benefit ratio is such that blood transfusion has little place in the management of most chronic anemias. However, in cases of an Hgb level less than 8 g/dL, a blood transfusion may be necessary and can be given while the work-up for anemia is being conducted. Dangers of repeated blood transfusions include iron overload, hemochromatosis, alloimmunization, and other adverse reactions.
Anemia can impair older patients' quality of life by causing fatigue, dyspnea, and other vague but troubling symptoms. Improving anemia will thereby improve the quality of life for these patients, including those who may have life-limiting illnesses.
Anisocytosis: Excessive numbers of cells with varying sizes.
Actinic keratoses: Skin lesions that are rough and dry, considered pre-cancerous. Usually occurring in sun-exposed areas of skin.
Atrophic gastritis: Loss of gastric glandular cells secondary to inflammation of the stomach mucosa.
Atrophic glossitis: Swollen and smooth-appearing tongue.
Cheilitis: Condition of inflammation, redness, ulcerations, or cracks in the lips. Also known as angular stomatitis.
Chelation therapy: Used to remove iron and heavy metals from the body.
Cobalamin: Vitamin B12, a water-soluble vitamin responsible for normal function of the neurologic and hematopoietic systems.
Cytopenia: Deficiency of RBCs, leukocytes, or platelets.
Erythrocyte: Red blood cell.
Erythropoiesis: Production of red blood cells from the bone marrow, usually in the pelvis, vertebrae, sternum, ribs, and proximal femur.
Erythropoietin: Recombinant hematopoietic growth factor produced by the kidneys.
Glomerular filtration rate (GFR): Measure of kidney function and determination of stage of chronic kidney disease. A GFR less than 30 mL/minute/1.73 m2 usually requires evaluation by a nephrologist.
Hemochromatosis: A condition of iron overload caused by the body's inability to regulate iron absorption and distribution, leading to accumulation of iron in the tissues.
Hematopoiesis: The production of blood elements.
Hemolysis: Destruction of blood cells.
Homocysteine: An amino acid in the blood. High levels are associated with cardiovascular disease.
Hypochromic: RBC that is pale due to low amounts of Hgb in the cell.
Inflammatory cytokine: Chemical messenger of the body that produces increased inflammation.
Intermittent claudication: A condition usually secondary to peripheral arterial disease causing cramps, aching, numbness, and fatigue to the calf muscle. Usually relieved by rest.
Leukocytosis: Elevated leukocyte count.
Leukopenia: Deficiency of circulating leukocytes.
Macrocytosis: Condition of having larger than normal RBCs (i.e., MCV greater than 100 fL).
Methylmalonic acid: A substance produced by the body, a byproduct of the breakdown of amino acids. Elevated in vitamin B12 deficiency.
Microcytosis: Condition of having smaller than normal RBCs (i.e., MCV less than 80 fL)
Myelodysplasia: Defective production of blood cells by the bone marrow.
Myelosuppression: Decreased production of blood components by the bone marrow.
Neutropenia: Deficiency of circulating granulocytic leukocytes.
Normochromic: RBCs that have the normal concentration of Hgb.
Normocytic: Normal sized RBCs.
Orthopnea: Shortness of breath that comes on while lying flat and is prevented or relieved by propping up.
Orthostatic hypotension: An exaggerated fall in blood pressure when a person stands.
Pancytopenia: A reduction below normal range in the number of RBCs, leukocytes, and platelets.
Paresthesia: A transient or chronic numbness and/or tingling of the extremities.
Platelet (thrombocyte): Non-nucleated cellular element in circulating blood active in thrombus formation (clotting).
Poikilocytosis: The presence of abnormally shaped RBCs in the blood.
Polycythemia: Abnormal increase in the circulating RBC mass, indicated by an abnormally high HCT.
Reticulocyte: Immature RBC. Will eventually become a mature RBC in one to four days.
Thrombocytopenia: Deficiency of circulating platelets.
Thrombocytosis: Abnormal increase in the amount of platelets in the blood.
Transferrin: A glycoprotein that binds iron.
1. Stauder R, Valent P, Theurl I. Anemia at older age: etiologies, clinical implications, and management. Blood. 2018;131:505-514.
2. Lefebvre P, Duh MS, Buteau S, Bookhart B, Mody SH. Medical costs of untreated anemia in elderly patients with pre-dialysis chronic kidney disease. J Am Soc Nerphrol. 2006;17(12):3497-3502.
3. Centers for Disease Control and Prevention. The State of Aging and Health in America 2013. Available at https://www.cdc.gov/aging/pdf/State-Aging-Health-in-America-2013.pdf. Last accessed January 8, 2024.
4. Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299(17):2027-2036.
5. Centers for Disease Control and Prevention. QuickStats: The prevalence of anemia among adults aged >/=65 years, by sex and age group—National Health and Nutrition Examination Survey, 2013–2016. MMWR. 2018;67:1198.
6. Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104(8):2263-2268.
7. Bisbe E, Castillo J, Sáez M, Santiveri X, Ruíz A, Munoz M. Prevalence of preoperative anemia and hematinic deficiencies in patients scheduled for elective major orthopedic surgery. Transfusion Alter Transfusion Med. 2008;10(4):166-173.
8. Brown E, Bennett M. Survey of blood transfusion practice for palliative care patients in Yorkshire: implications for clinical care. J Palliat Med. 2007;10(4):919-922.
9. Artz AS, Fergusson D, Drinka PJ, et al. Mechanisms of unexplained anemia in the nursing home. J Am Geriatr Soc. 2004;52(3):423-427.
10. Steensma DP, Tefferi A. Anemia in the elderly: how should we define it, when does it matter, and what can be done? Mayo Clin Proc. 2007;82(8):958-966.
11. Artz AS. Anemia in Elderly Persons. Available at https://emedicine.medscape.com/article/1339998-overview. Last accessed January 8, 2024.
12. Lipschitz D. Medical and functional consequences of anemia in the elderly. J Am Geriatr Soc. 2003;51(3 Suppl):S10-S13.
13. World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Geneva: World Health Organization; 2011.
14. Gado K, Khodier M, Virag A, et al. Anemia of geriatric patients. Physiol Internat. 2022;109:119-134.
15. Artz A. Racial impact of anemia and mortality in the elderly: abstract and commentary. Clinical Oncology Alert. 2007;23(7):53-54.
16. Aessopos A, Deftereos S, Farmakis D, et al. Cardiovascular adaptation to chronic anemia in the elderly: an echocardiographic study. Clin Invest Med. 2004;27(5):265-273.
17. National Hematologic Diseases Information Service. Anemia of Inflammation and Chronic Disease. Available at https://www.niddk.nih.gov/health-information/blood-diseases/anemia-inflammation-chronic-disease. Last accessed January 8, 2024.
18. Camaschella C, Weiss G. Anemia of Chronic Disease (Anemia of Chronic Inflammation). Available at https://www.uptodate.com/contents/anemia-of-chronic-disease-inflammation. Last accessed January 8, 2024.
19. Killip S, Bennett JM, Chambers MD. Iron deficiency anemia. Am Fam Physician. 2007;75(5):671-678.
20. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323.
21. National Institutes of Health, Office of Dietary Supplements. Dietary Supplement Fact Sheet: Vitamin B12. Available at https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional. Last accessed January 8, 2024.
22. Coté GA, Howden CW. Potential adverse effects of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10(3):208-214.
23. Cotter PE, O'Keeffe ST. Use of proton pump inhibitors is not associated with vitamin B12 deficiency and in older hospital patients: a case control study. Eur Geriatr Med. 2011;2(4):253-255.
24. Valuck RJ, Ruscin JM. A case-control study on adverse effects: H2 blocker or proton pump inhibitor use and risk of vitamin B12 deficiency in older adults. J Clin Epidemiol. 2004;57(4):422-428.
25. Dharmarajan TS, Kanagala MR, Murakonda P, Lebelt AS, Norkus EP. Do acid-lowering agents affect vitamin B12 status in older adults? J Am Med Dir Assoc. 2008;9(3):162-167.
26. Nagalla S. Megaloblastic Anemia. Available at https://emedicine.medscape.com/article/204066-overview. Last accessed January 8, 2024.
27. Liu KW, Dai LK, Jean W. Metformin-related vitamin B12 deficiency. Age Ageing. 2006;35(2):200-201.
28. de Jager J, Kooy A, Lehert P, et al. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial. BMJ. 2010;340:c2181.
29. Ting RZ, Szeto CC, Chan MH, Ma KK, Chow KM. Risk factors of vitamin B(12) deficiency in patients receiving metformin. Arch Intern Med. 2006;166(18):1975-1979.
31. National Institutes of Health, Office of Dietary Supplements. Dietary Supplement Fact Sheet: Folate. Available at https://ods.od.nih.gov/factsheets/Folate-HealthProfessional. Last accessed January 8, 2024.
32. National Cancer Institute. Myelodysplastic Syndromes Treatment (PDQ). Available at https://www.cancer.gov/types/myeloproliferative/hp/myelodysplastic-treatment-pdq. Last accessed January 8, 2024.
33. Clarke R, Halsey J, Lewington S, et al. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: meta-analysis of 8 randomized trials involving 37,485 individuals. Ann Intern Med. 2010;170(18):1622-1631.
34. Mason JB. Folate, cancer risk, and the Greek god, Proteus: a tale of two chameleons. Nutr Rev. 2009;67(4):206-212.
35. Sanjoaquin MA, Allen N, Couto E, Roddam AW, Key TJ. Folate intake and colorectal cancer risk: a meta-analytical approach. Int J Cancer. 2005;113(5):825-828.
36. Valent P, Wimazal F, Sperr WR, Horny H-P. Diagnostic criteria and classification of myelodysplastic syndromes. In: Várkonyi J (ed). The Myelodysplastic Syndromes. New York, NY: Springer; 2011: 43-54.
37. Johnson CA, Levey AS, Coresh J, Levin A, Lau J, Eknoyan G. Clinical practice guidelines for chronic kidney disease in adults: part I. Definition, disease stages, evaluation, treatment, and risk factors. Am Fam Physician. 2004;70(5):869-876.
38. National Heart, Lung, and Blood Institute. Hemolytic Anemia. Available at https://www.nhlbi.nih.gov/health/anemia/hemolytic-anemia. Last accessed January 8, 2024.
39. National Heart, Lung, and Blood Institute. Sickle Cell Disease. Available at https://www.nhlbi.nih.gov/health/sickle-cell-disease. Last accessed January 8, 2024.
40. World Health Organization. Sickle Cell Anaemia: Report by the Secretariat. Available at https://apps.who.int/gb/archive/pdf_files/WHA59/A59_9-en.pdf. Last accessed January 8, 2024.
41. Maakaron JE. Sickle Cell Anemia. Available at https://emedicine.medscape.com/article/205926-overview. Last accessed January 8, 2024.
42. Centers for Disease Control and Prevention. Sickle Cell Disease (SCD): Data and Statistics. Available at https://www.cdc.gov/NCBDDD/sicklecell/data.html. Last accessed January 8, 2024.
43. National Heart, Lung and Blood Institute. Thalassemias. Available at https://www.nhlbi.nih.gov/health/thalassemia. Last accessed January 8, 2024.
45. National Heart, Lung and Blood Institute. Aplastic Anemia. Available at https://www.nhlbi.nih.gov/health/anemia/aplastic-anemia. Last accessed January 8, 2024.
46. National Institute of Diabetes and Digestive and Kidney Diseases. Aplastic Anemia and Myelodysplastic Syndromes. Available at https://www.niddk.nih.gov/health-information/blood-diseases/aplastic-anemia-myelodysplastic-syndromes. Last accessed January 8, 2024.
48. Taber's Online. Available at https://www.tabers.com/tabersonline. Last accessed January 8, 2024.
49. Colbert GB. Anemia of Chronic Disease and Renal Failure: Diagnosis. Available at https://emedicine.medscape.com/article/1389854-overview#aw2aab6b6. Last accessed January 8, 2024.
52. Makipour S, Kanapuru B, Ershler WB. Unexplained anemia in the elderly. Semin Hematol. 2008;45(4):250-254.
53. McKinney M, Arcasoy MO. Erythropoietin for oncology supportive care. Exp Cell Res. 2011;317(9):1246-1254.
54. National Kidney Foundation. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. Available at https://www.kidney.org/sites/default/files/docs/anemiainckd.pdf. Last accessed January 8, 2024.
55. Lexicomp Online. Available at https://online.lexi.com. Last accessed January 8, 2024.
56. Agnihotri P, Telfer M, Butt Z, et al. Chronic anemia and fatigue in elderly patients: results of a randomized, double-blind, placebo-controlled, crossover exploratory study with epoetin alfa. J Am Geratr Soc. 2007;55(10):1557-1565.
57. Paltiel O, Clarfield AM. Anemia in elderly people: risk marker or risk factor? CMAJ. 2009;181(3-4):129-130.
58. Rimon E, Kagansky N, Kagansky M, et al. Are we giving too much iron? Low-dose iron therapy is effective in octogenarians. Am J Med. 2005;118(10):1142-1147.
59. Brooks M. FDA Approves Injectafer for Iron Deficiency Anemia. Available at https://www.medscape.com/viewarticle/808800. Last accessed January 8, 2024.
60. Auerbach M, DeLoughery TG. Treatment of the Adult with Iron Deficiency Anemia. Available at https://www.uptodate.com/contents/treatment-of-anemia-due-to-iron-deficiency. Last accessed January 8, 2024.
61. Kim YI. Will mandatory folic acid fortification prevent or promote cancer? Am J Clin Nutr. 2004;80(5):1123-1128.
62. Rosenberg M. Overview of the Management of Chronic Kidney Disease in Adults. Available at https://www.uptodate.com/contents/overview-of-the-management-of-chronic-kidney-disease-in-adults. Last accessed January 8, 2024.
63. Rizzo JD, Lichtin AE, Woolf SH, et al. Use of epoetin in patients with cancer: evidence-based clinical practice guidelines of the American Society of Clinical Oncology and the American Society of Hematology. J Clin Oncol. 2002;20(19):4083-4107.
64. Goodnough LT, Schrier SL. Evaluation and management of anemia in the elderly. Am J Hematol. 2014;89:88-96.
65. 1zzakai NA, French B, Arnold AM, et al. Hemoglobin decline, function, and mortality in the elderly: The Cardiovascular Health Study. Am J Hematol. 2013;88:5-9.
67. Ferrucci L, Guralnik JM, Bandinelli S, et al. Unexplained anemia in older persons is characterized by low erythropoietin and low levels of pro-inflammatory markers. Br J Hematol. 2007;136(6):849-855.
68. Guralnik JM, Eisenstaedt RS, Ferrucci L, et al. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263-2268.
1. Kidney Disease: Improving Global Outcomes. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Gut. 2021;70(11):2030-2051. Available at https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf. Last accessed January 11, 2024.
2. Snook J, Bhala N, Beales ILP, et al. British Society of Gastroenterology guidelines for the management of iron deficiency anaemia in adults. Ann Intern Med. 2013;159(11):770-779. Available at https://gut.bmj.com/content/70/11/2030.long. Last accessed January 11, 2024.
Mention of commercial products does not indicate endorsement.