It is apparent that psychedelic medicine is now in a renaissance period, and this time could not have come too soon. Many people in the United States and around the world suffer from severe psychiatric disorders, including depression, PTSD, substance use disorders, anxiety disorders, OCD, anorexia nervosa, and multiple other psychiatric disorders that are not readily responsive to treatment with pharmacotherapy and psychotherapy. In the aftermath of the COVID-19 pandemic, depressive disorders are more prevalent, and people are urgently and actively seeking effective treatments. Exploration of novel interventional and psychedelic therapies may be a path to recovery for patients who have not improved on traditional approaches.
- INTRODUCTION
- THE IMPORTANCE OF PSYCHEDELIC AND INTERVENTIONAL MEDICINE
- DEFINITIONS
- PONDERING PSYCHEDELICS
- CONSIDERING PSYCHEDELIC-ASSISTED PSYCHOTHERAPY AS A TREATMENT OPTION
- EMERGING PSYCHEDELIC TREATMENTS
- DIAGNOSES AND PSYCHEDELIC MEDICINE
- INTERVENTIONAL PSYCHIATRY: BRAIN STIMULATION THERAPIES
- CAUTIONS
- CONCLUSION
- Works Cited
- Evidence-Based Practice Recommendations Citations
The course is designed for all members of the interprofessional team, including physicians, physician assistants, nurses, and mental health professionals, involved in caring for patients with mental disorders resistant to traditional treatment approaches.
The purpose of this course is to provide medical and mental health professionals with the knowledge and skills necessary to effectively treat mental disorders using emerging psychedelic and interventional techniques.
Upon completion of this course, you should be able to:
- Outline factors that have contributed to the rise in interest in psychedelic and interventional psychiatry.
- Define terms related to the discussion of psychedelic and interventional psychiatry.
- Discuss the history of psychedelics in medical care.
- Evaluate factors that may impact the provision of psychedelic or interventional psychiatry techniques, including stigma, setting, and culture.
- Outline the role of psilocybin and ketamine in psychiatric care.
- Describe how MDMA and ibogaine may impact mental health.
- Review the clinical effects of kratom, LSD, and mescaline.
- Discuss the potential clinical role of nitrous oxide, ayahuasca, and dimethyltryptamine (DMT).
- Describe how psychedelics may be incorporated into the treatment of mental health disorders, including treatment-resistant depression, post-traumatic stress disorder, and substance use disorders.
- Identify interventional approaches that may be used in the treatment of mental health disorders.
Mark S. Gold, MD, DFASAM, DLFAPA, is a teacher of the year, translational researcher, author, mentor, and inventor best known for his work on the brain systems underlying the effects of opiate drugs, cocaine, and food. Dr. Gold was a Professor, Eminent Scholar, Distinguished Professor, Distinguished Alumni Professor, Chairman, and Emeritus Eminent Scholar during his 25 years at the University of Florida. He was a Founding Director of the McKnight Brain Institute and a pioneering neuroscience-addiction researcher funded by the NIH-NIDA-Pharma, whose work helped to de-stigmatize addictions and mainstream addiction education and treatment. He also developed and taught courses and training programs at the University of Florida for undergraduates and medical students.
He is an author and inventor who has published more than 1,000 peer-reviewed scientific articles, 20 text books, popular-general audience books, and physician practice guidelines. Dr. Gold was co-inventor of the use of clonidine in opioid withdrawal and the dopamine hypothesis for cocaine addiction and anhedonia. Both revolutionized how neuroscientists and physicians thought about drugs of abuse, addiction, and the brain. He pioneered the use of clonidine and lofexidine, which became the first non-opioid medication-assisted therapies. His first academic appointment was at Yale University School of Medicine in 1978. Working with Dr. Herb Kleber, he advanced his noradrenergic hyperactivity theory of opioid withdrawal and the use of clonidine and lofexidine to ameliorate these signs and symptoms. During this time, Dr. Gold and Dr. Kleber also worked on rapid detoxification with naloxone and induction on to naltrexone.
Dr. Gold has been awarded many state and national awards for research and service over his long career. He has been awarded major national awards for his neuroscience research including the annual Foundations Fund Prize for the most important research in Psychiatry, the DEA 30 Years of Service Pin (2014), the American Foundation for Addiction Research’s Lifetime Achievement Award (2014), the McGovern Award for Lifetime Achievement (2015) for the most important contributions to the understanding and treatment of addiction, the National Leadership Award (NAATP) from addiction treatment providers for helping understand that addiction is a disease of the brain, the DARE Lifetime Achievement Award for volunteer and prevention efforts, the Silver Anvil from the PR Society of America for anti-drug prevention ads, the PRIDE and DARE awards for his career in research and prevention (2015), and the PATH Foundation’s Lifetime Achievement Award (2016) as one of the “fathers” of addiction medicine and MAT presented to him by President Obama’s White House Drug Czar Michael Botticelli. He was awarded Distinguished Alumni Awards at Yale University, the University of Florida, and Washington University and the Wall of Fame at the University of Florida College of Medicine. Gold was appointed by the University President to two terms as the University’s overall Distinguished Professor, allowing him to mentor students and faculty from every college and institute. The University of Florida College of Medicine’s White Coat Ceremony for new medical students is named in his honor.
Since his retirement as a full-time academic in 2014, Dr. Gold has continued his teaching, mentoring, research, and writing as an Adjunct Professor in the Department of Psychiatry at Washington University and an active member of the Clinical Council at the Washington University School of Medicine’s Public Health Institute. He regularly lectures at medical schools and grand rounds around the country and at international and national scientific meetings on his career and on bench-to-bedside science in eating disorders, psychiatry, obesity, and addictions. He continues on the Faculty at the University of Florida College of Medicine, Department of Psychiatry as an Emeritus Distinguished Professor. He has traveled extensively to help many states develop prevention, education, and treatment approaches to the opioid crisis.
Contributing faculty, Mark S. Gold, MD, DFASAM, DLFAPA, has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
John M. Leonard, MD
Jane C. Norman, RN, MSN, CNE, PhD
Alice Yick Flanagan, PhD, MSW
James Trent, PhD
The division planners have disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Sarah Campbell
The Director of Development and Academic Affairs has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
The purpose of NetCE is to provide challenging curricula to assist healthcare professionals to raise their levels of expertise while fulfilling their continuing education requirements, thereby improving the quality of healthcare.
Our contributing faculty members have taken care to ensure that the information and recommendations are accurate and compatible with the standards generally accepted at the time of publication. The publisher disclaims any liability, loss or damage incurred as a consequence, directly or indirectly, of the use and application of any of the contents. Participants are cautioned about the potential risk of using limited knowledge when integrating new techniques into practice.
It is the policy of NetCE not to accept commercial support. Furthermore, commercial interests are prohibited from distributing or providing access to this activity to learners.
Supported browsers for Windows include Microsoft Internet Explorer 9.0 and up, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Supported browsers for Macintosh include Safari, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Other operating systems and browsers that include complete implementations of ECMAScript edition 3 and CSS 2.0 may work, but are not supported. Supported browsers must utilize the TLS encryption protocol v1.1 or v1.2 in order to connect to pages that require a secured HTTPS connection. TLS v1.0 is not supported.
The role of implicit biases on healthcare outcomes has become a concern, as there is some evidence that implicit biases contribute to health disparities, professionals' attitudes toward and interactions with patients, quality of care, diagnoses, and treatment decisions. This may produce differences in help-seeking, diagnoses, and ultimately treatments and interventions. Implicit biases may also unwittingly produce professional behaviors, attitudes, and interactions that reduce patients' trust and comfort with their provider, leading to earlier termination of visits and/or reduced adherence and follow-up. Disadvantaged groups are marginalized in the healthcare system and vulnerable on multiple levels; health professionals' implicit biases can further exacerbate these existing disadvantages.
Interventions or strategies designed to reduce implicit bias may be categorized as change-based or control-based. Change-based interventions focus on reducing or changing cognitive associations underlying implicit biases. These interventions might include challenging stereotypes. Conversely, control-based interventions involve reducing the effects of the implicit bias on the individual's behaviors. These strategies include increasing awareness of biased thoughts and responses. The two types of interventions are not mutually exclusive and may be used synergistically.
#96790: Psychedelic Medicine and Interventional Psychiatry
A new and intense interest in psychedelic drugs and interventional medicine is occurring now in the United States and worldwide, as scientists are exploring and discovering innovative ways to treat challenging psychiatric problems, including treatment-resistant depression, suicidal major depressive disorder, post-traumatic stress disorder (PTSD), obsessive-compulsive disorder (OCD), and substance use disorders, as well as multiple other psychiatric problems that have largely been impervious to traditional treatment. Psychedelic medicine refers to the use of drugs that are hallucinogenic and/or anesthetic and that have a unique action on the brain. These approaches may be used only in research situations or may be in current and active use as treatments. In contrast, interventional psychiatry refers to the use of brain-stimulating therapies to treat severe psychiatric disorders. These therapies include electroconvulsive therapy (ECT), repetitive transcranial magnetic stimulation (rTMS), vagus nerve stimulation (VNS), and deep brain stimulation (DBS). As with psychedelic medicine, interventional medicine may be used to provide relief for patients with multiple major and previously unremitting severe psychiatric disorders, although there is still much to learn about these therapies. This course will provide an overview of both of these forms of treatment, with an emphasis on psychedelic medicine.
Today, psychedelics like N,N-dimethyltryptamine (DMT), psilocybin, 3,4-methylenedioxymethamphetamine (MDMA), and lysergic acid diethylamide (LSD) are being explored to treat various psychiatric disorders. Trials of these drugs are in different stages, and the timeline for U.S. Food and Drug Administration (FDA) approval is not always obvious. While ketamine was approved in 2020, most experts believe the first psychedelic approval will come in 2024, likely for PTSD rather than treatment-resistant depression, even though treatment with psilocybin was found to relieve symptoms of major depressive disorder for at least one year for some patients in a 2022 Johns Hopkins study [1]. The safety and efficacy of MDMA-assisted therapy is currently under Phase 3 investigation, but concerns remain regarding efficacy and potential adverse effects. As of 2022, the Multidisciplinary Association of Psychedelic Studies (MAPS) is sponsoring MAPP2, the second of two Phase 3 trials to support FDA approval of MDMA as a breakthrough-designated therapy for the estimated 9 million adults in the United States who experience PTSD each year. In MAPS's first Phase 3 study, 88% of participants with severe PTSD experienced a clinically significant reduction in PTSD diagnostic scores two months after their third session of MDMA-assisted therapy, compared with 60% of placebo participants. Additionally, 67% of participants in the MDMA group no longer met the criteria for PTSD two months after the sessions, compared with 32% of participants in the placebo group [2].
When effective, psychedelic medicine is analogous to a "resetting" of the brain. It is somewhat like when a computer runs awry, and nothing of many actions that the user tries improves the situation. In frustration, the user shuts off the machine, but when the device is turned back on, everything works perfectly. The machine has reset itself. Similarly, psychedelic drugs, when effective, may aid the brain in a sort of resetting. Depending on the individual and the drug, the person may find they have marked improvements in symptoms of depression, PTSD, addiction, or other severe psychiatric problem.
As a result of today's research renaissance on psychedelic drugs, there is a new era of hope for people with major psychiatric disorders who have been largely unresponsive to traditional treatments.
One concern about psychedelic medicine is that many of the drugs may induce hallucinations, even in the low doses used for depression. Mental health professionals who prescribe or administer the drugs will need to ensure patients are monitored adequately. In some cases, the person receiving the drug is hospitalized, but in others, the drug is administered and changes observed in an office setting.
Ketamine's efficacy and protocols to ensure safety have resulted in thousands of patients being treated and reporting excellent responses for treatment-resistant depression. However, the ideal drug would provide the benefits without the hallucinatory side effects. In one unique experiment with mice, researchers effectively blocked 5-HT2A, the serotonin-detecting receptor, and this action appeared to stop mice being administered psilocybin from hallucinating ("tripping"). The antidepressant effects were unaltered in this study, as evidenced by the mice resuming consumption of sugar water, an act they had abandoned while depressed [5]. This is an area of great interest, with the potential that the hallucinations induced by psychedelic drugs could be blocked and increase the acceptability of these agents in the general treatment of depression.
Of course, there are many who believe that the psychedelic trip itself, hallucinations and all, is the crucial experience that allows people to experience psychic relief. These individuals believe that eliminating the crucial experience of hallucination would essentially block the full efficacy of the drug. This issue is likely to continue to be discussed and debated as the science advances.
Psychedelic drugs are often divided into two categories: classic and non-classic or dissociative. The classic psychedelics are usually derived from naturally occurring compounds and include such drugs as psilocybin, LSD, and DMT, an active component of ayahuasca, an increasingly popular sacramental drink originating from South America. The dissociative psychedelics are typically newer analogs and include ketamine, phencyclidine (PCP), MDMA, mescaline, Salvia divinorum, and dextromethorphan (DXM). While considered drugs of abuse, most agents being tested in psychedelic medicine clinical trials are not self-administered by laboratory animals, the usual test for abuse and dependence liability. If anything, hallucinogens tend to lose their ability to produce changes in the person over time and with regular use. These drugs are all variations on tryptamine, and while they may increase dopamine, they tend to do this through an indirect mechanism.
In their 1979 publication, Grinspoon, Grinspoon, and Bakalar define a classic psychedelic drug as [6]:
A drug which, without causing physical addiction, craving, major physiological disturbances, delirium, disorientation, or amnesia, more or less reliably produces thought, mood, and perceptual changes otherwise rarely experienced except in dreams, contemplative and religious exaltation, flashes of vivid involuntary memory, and acute psychosis.
While the classic versus non-classic designation is of interest to researchers, it is likely not an important distinction for prescribers or patients.
There are multiple reasons health and mental health professionals would benefit from education about both psychedelic and interventional medicine. Psychedelic medicine is a multi-billion-dollar industry and is rapidly growing. It is likely that many healthcare professionals will become involved with these approaches as they enter more widespread use.
Many people in the United States suffer from severe depression, and suicide is a public health problem. In 2020, 21,570 people in the United States died from homicide, a significant increase from the number just one year earlier [7]. However, it did not come close to the suicide rate. In 2020, 45,855 people in the United States died from suicide. The annual U.S. suicide rate increased 30% between 2000 and 2020 [7]. As such, depression and suicide are major health problems in the United States today, and approaches to reverse depression rapidly and safely are greatly needed.
It is also important to consider the frustration of many patients with treatment-resistant depression and other disorders, many of whom have turned to cannabis to obtain relief. The majority of states have enacted laws approving medical marijuana, although its efficacy in the treatment of PTSD, depression, and other psychiatric disorders is often lacking [8]. Patients are clearly open to seeking help wherever it may be, whether evidence and healthcare professionals support the approaches. As such, it is vital that clinicians be aware of and knowledgeable regarding novel uses of psychedelic drugs and interventional psychiatry to best serve their patients.
Academic experts, universities, and medical groups continue to research psychedelic medicine, with exciting major breakthroughs in the treatment of depression/anxiety at the end of life and providing relief to patients with treatment-resistant depression, PTSD, and other disorders that most psychiatrists consider difficult to treat. This research will be detailed later in this course.
As noted, the suicide rate in the United States is more than twice as high as the homicide rate [7]. In 2019, suicide was the second leading cause of death for people 10 to 34 years of age and the tenth leading cause of death across all age groups (Table 1). Overall, suicide accounts for 1.7% of all deaths in the United States. Although official national statistics are not compiled on attempted suicide (i.e., nonfatal actions), it is estimated that 1.2 million adults (18 years of age and older) attempted suicide in 2020 [9]. Overall, there are roughly 25 attempts for every death by suicide; this ratio changes to 100 to 200:1 for the young and 4:1 for the elderly [9].
LEADING CAUSE OF DEATH IN THE UNITED STATES FOR SELECT AGE GROUPS, 2019
| Rank | Age (in Years) | ||||||
|---|---|---|---|---|---|---|---|
| 10–14 | 15–24 | 25–34 | 35–44 | 45–54 | 55–64 | All Ages | |
| 1 | Unintentional injury (778) | Unintentional injury (11,755) | Unintentional injury (24,516) | Unintentional injury (24,070) | Malignant neoplasms (35,587) | Malignant neoplasms (111,765) | Heart disease (659,041) |
| 2 | Suicide (534) | Suicide (5,954) | Suicide (8,059) | Malignant neoplasms (10,695) | Heart disease (31,138) | Heart disease (80,837) | Malignant neoplasms (599,601) |
| 3 | Malignant neoplasms (404) | Homicide (4,774) | Homicide (5,341) | Heart disease (10,499) | Unintentional injury (23,359) | Unintentional injury (24,892) | Unintentional injury (173,040) |
| 4 | Homicide (191) | Malignant neoplasms (1,388) | Malignant neoplasms (3,577) | Suicide (7,525) | Liver disease (8,098) | CLRD (18,743) | CLRD (156,979) |
| 5 | Congenital anomalies (189) | Heart disease (872) | Heart disease (3,495) | Homicide (3,446) | Suicide (8,012) | Diabetes (15,508) | Stroke (150,005) |
| 6 | Heart disease (87) | Congenital anomalies (390) | Liver disease (1,112) | Liver disease (3,417) | Diabetes (6,348) | Liver disease (14,385) | Alzheimer disease (121,499) |
| 7 | CLRD (81) | Diabetes (248) | Diabetes (887) | Diabetes (2,228) | Stroke (5,153) | Stroke (12,931) | Diabetes (87,647) |
| 8 | Influenza/pneumonia (71) | Influenza/pneumonia (175) | Stroke (585) | Stroke (1,741) | CLRD (3,592) | Suicide (8,238) | Nephritis (51,565) |
| 9 | Stroke (48) | CLRD (168) | Complicated pregnancy (532) | Influenza/pneumonia (951) | Nephritis (2,269) | Nephritis (5,857) | Influenza/pneumonia (49,783) |
| 10 | Benign neoplasms (35) | Stroke (158) | HIV (486) | Septicemia (812) | Septicemia (2,176) | Septicemia (5,672) | Suicide (47,511) |
| CLRD = chronic lower respiratory disease, HIV = human immunodeficiency disease. | |||||||
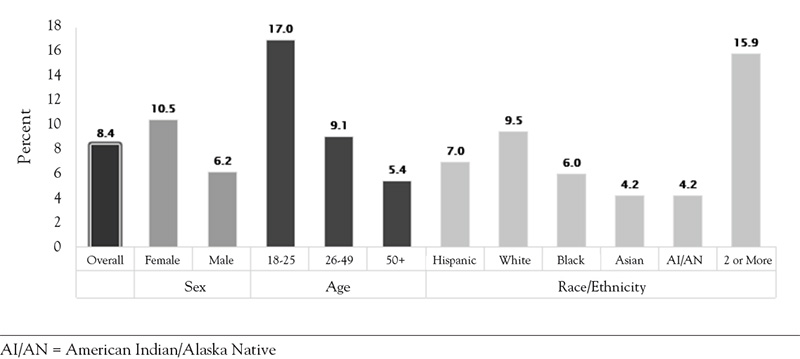
People with depression may experience suicidal ideation and behaviors, which can subsequently lead to suicide completions. As illustrated by Figure 1, in 2020, adults 18 to 25 years of age had the highest risk for a major depressive episode, followed by those 25 to 49 years of age. In addition, individuals of two or more races had the highest risk for depression (15.9%), followed by White individuals (9.5%).
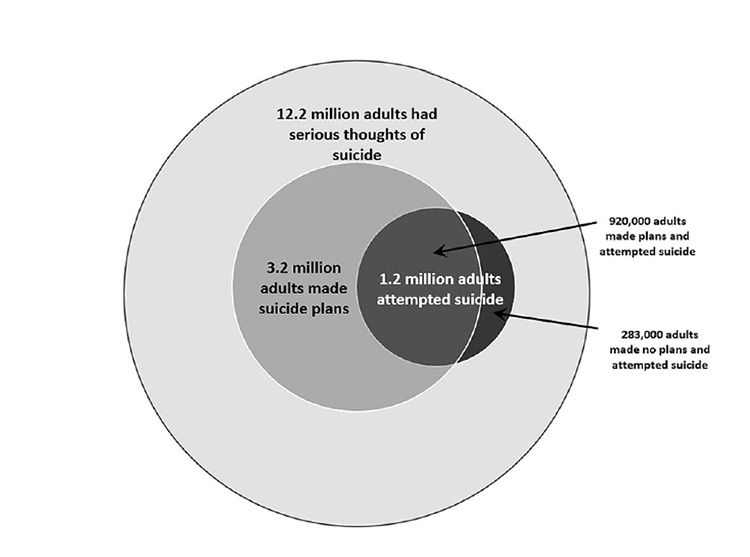
Suicidal behaviors are a major problem in the United States, as depicted in the converging circles shown in Figure 2. This figure demonstrates that 12.2 million adults seriously considered suicide in 2020, represented by the outer circle, while 3.2 million adults made suicide plans, and 1.2 million adults attempted suicide. Of those adults who attempted suicide in 2020, 920,000 had made a suicide plan; 285,000 adults had made no such plan prior to the attempt [10,12].
Clearly, action is needed to help address depression and suicide in the United States, and psychedelic and interventional medicine may have a role.
When they were first introduced, the monoamine oxide (MAO) inhibitors and tricyclic antidepressants were perceived as wonder drugs for depression. However, MAO inhibitors require strict dietary constraints, and both drug classes are associated with multiple troubling side effects. In contrast, when selective serotonin reuptake inhibitors (SSRIs) were introduced, they were much easier to prescribe and expanded treatment approaches to include primary care. Unfortunately, for many patients, SSRIs did not help as much as expected—or indeed at all, in some cases. Today, it is clear that non- or under-response to pharmacotherapy for major depression is far more common than was realized at the time. For example, researchers have found that antidepressants are ineffective for at least one-third of individuals who take them [2]. Suboptimal responses are also common. Many patients for whom the drugs do not work will recalibrate their expectations and accept the treatment response as the best they can hope to achieve. Treatment discontinuation is common among frustrated patients.
It is also important to note that even when antidepressants actually are efficacious, it usually takes at least three or four weeks for the drug to begin to take effect. Tricyclic antidepressants, MAO inhibitors, SSRIs, and serotonin and norepinephrine reuptake inhibitors (SNRIs) all share this issue of a delayed onset of action. Psychiatrists and neuroscientists have been unable to develop faster-acting medications for depression to date. This means that many people with severe depression could take an antidepressant very faithfully for weeks without any relief. These patients may give up hope and halt treatment or try again with another antidepressant or medication combination.
As with any pharmacotherapy, antidepressants have many possible adverse effects, including weight gain, anorgasmia, sluggishness, anxiety, insomnia, and suicidal ideation. As such, a patient may experience no improvements in depression symptoms while also developing adverse drug effects. This is not the end of consequences; discontinuation symptoms are also a concern. Antidepressant discontinuation symptoms can be very challenging. For example, abruptly ending fluoxetine can cause nightmares, vomiting, and irritability. In most cases, patients who no longer wish to take an antidepressant should taper off the drug on a defined schedule [3].
To recap, patients may take antidepressants for months without significant improvements in depression symptoms while also experiencing side effects, and when they stop taking these ineffective drugs, they suffer more side effects unless they carefully taper off. In contrast, some psychedelic drugs have the potential to provide relief in a few sessions, with lasting efficacy over months or even years, although further research is needed. This contrast is the main reason that so many mental health professionals and patients are intrigued about the possibilities of psychedelic medicine, particularly for more difficult cases.
It is not clear why antidepressants work for some patients and not for others. Some have hypothesized it may be related to the size and shape of a person's neurons, which can vary considerably [3]. Another possible contributing factor is the similar mechanisms of action among the different classes of antidepressants. These agents increase blood levels of serotonin, dopamine, or norepinephrine. In contrast, some psychedelic drugs, such as ketamine, are N-methyl-D-aspartate (NMDA)/glutamate receptor antagonists. This represents a completely different target for antidepressant mechanism of action and also a novel approach to treating depression.
There is also some evidence that ketamine can reverse suicidality or depression after a single dose, which suggests that the drug reverses a neurochemical deficit that is close to the problem. Ketamine and psychedelic drugs are effective at promoting plasticity, reconnections, and healing within the brain, a feat beyond the capabilities of traditional antidepressants or most other drugs. Researchers have found that neuroplastic changes, specifically atrophy of neurons in the prefrontal cortex, are an underlying etiology of depression and other mood disorders. The extent to which these drugs, and ketamine in particular, are able to promote structural and functional plasticity in the prefrontal cortex is believed to underlie the fast-acting antidepressant properties [4]. Other drugs, such as LSD and DMT, may stimulate the formulation of synapses [4]. Psychedelic drugs may also create new connections within the brain, although much more research is needed to understand how and why these drugs may be effective in treating serious psychiatric disorders in some who have heretofore not proven responsive to traditionally effective treatments.
Certainly, psychedelic medicine is regarded as a major and burgeoning healthcare market. Data Bridge Market Research has estimated that the market for psychedelic drugs will more than triple, from about $2 billion in 2019 to nearly $7 billion by 2027 [13]. Other estimates are even more favorable; a report from Research and Markets anticipates a market of $10.75 billion in psychedelic drugs by 2027 [13]. In a post-COVID world in which the numbers of people with reported depression have increased by as much as three times, potentially effective treatment options should not be ignored.
It has been estimated that at least 50,000 therapists will be needed by 2031 to provide psychedelic-assisted therapy to patients, and as a result, some organizations have already begun to increase their hiring. The key types of therapies used will be cognitive-behavioral therapy (CBT), acceptance and commitment therapy (ACT), or other types of therapy adapted to psychedelic treatment [15].
The current high interest in psychedelic medicine may stimulate pharmaceutical companies to research and develop novel drug treatments for major psychiatric problems beyond the traditional classes of drugs that solely target serotonin, norepinephrine, and dopamine, which would be yet another positive consequence.
At the same time that the federal government has somewhat loosened its tight reins on psychedelic medicine and researchers and medical professionals have begun to explore the use of these agents, there has been a dramatic increase in interest among consumers in Schedule I drugs, particularly in cannabis, but also in psilocybin and other psychedelic drugs. As of 2022, 37 states as well as the District of Columbia and four U.S. territories allow the medical use of cannabis ("medical marijuana") [16]. (Note that medical use of cannabis is a bit of a misnomer, as prescribers generally have little or no involvement with patients who take the drug and it has not attained FDA approval for any condition.) In addition, the U.S. House of Representatives passed a bill to decriminalize cannabis use in 2022 [17]. In addition, 18 states, the District of Columbia, and 2 U.S. territories have legalized the recreational use of cannabis for adults [18]. This followed several years of decriminalization at the local and state levels. While cannabis is not considered a psychedelic drug, its shift toward decriminalization and medicinal use is a sign that a similar path may be beginning for other Schedule I drugs with potential psychiatric benefit. Further, in states that allow medical or recreational use of cannabis for adults, the federal government has largely backed away from taking any punitive measures against individuals who use the drug, even though cannabis remains illegal at a federal level.
This movement may already be advancing with psychedelic drugs. This began with the decriminalization of psilocybin in Denver, Colorado, in 2019, followed by Oakland and Santa Cruz, California. In 2021, the city of Cambridge, Massachusetts, passed a law decriminalizing all "entheogenic plants," which includes the drugs ayahuasca, ibogaine, and psilocybin [19]. As of 2022, the largest city to decriminalize psilocybin is Seattle, Washington [19]. In 2020, the state of Oregon approved the use of psilocybin by consumers [20]. Also in 2020, the District of Columbia decriminalized the use of psilocybin mushrooms as well as other substances found in peyote and ayahuasca [20]. Other states are considering taking similar actions. In 2021, Health Canada, the premier health agency in Canada, approved trials of MDMA-assisted therapy for the treatment of PTSD [15]. It is important to note that it can be dangerous for psilocybin and other psychedelic drugs to be used by individuals who do not understand its risks. As popularity and interest in the medical use of these agents increases, clinicians have a responsibility to educate themselves and their patients about the safe and appropriate use of psychedelics.
A major factor in the popularity of psychedelic drugs is frustration resulting from unrelenting depression, anxiety, chronic pain, or other health and mental health conditions. Some patients may have already tried cannabis to address these conditions, with varying levels of success.
Although researchers have historically chosen to avoid or been blocked from researching psychedelics because of bans by the federal government, this has changed in the past few decades. For example, in 2006, Johns Hopkins Medicine began their research on psychedelic medicine, subsequently producing more than 80 peer-reviewed clinical studies by 2020 [21]. A new home for the Center for Psychedelic and Consciousness Research was created in 2020, the first such establishment in the United States [21]. Private donors provided funding to launch the Center, and since its opening, the Center has also received federal funding for research. In addition, Yale, Massachusetts General Hospital/Harvard, and other psychiatric and research excellence centers are studying psychedelic medications as treatment options for serious psychiatric disorders.
In addition, training programs focusing on psychedelic psychiatry are being established (Table 2). Johns Hopkins, New York University, and Yale are collaborating to create a psychedelics-psychiatrist program funded by a grant facilitated by Heffter Research Institute [22].
Clear definitions of the concepts related to psychedelic drugs and interventional psychiatry are helpful. The following is a glossary of terms used throughout this course.
Classic psychedelic: Refers to older hallucinogenic drugs, such as psilocybin and LSD. These agents are often derived from natural sources.
Deep brain stimulation: With the use of implanted electrodes, the brain is stimulated to treat such psychiatric problems as treatment-resistant depression.
Electroconvulsive therapy (ECT): Stimulation of the brain causing a seizure. This therapy is administered under sedation and is used to help patients with severe psychiatric diagnoses.
Hallucinogen: Drug that may cause the user to experience visual, auditory, or other types of hallucinations.
Neuromodulation therapy: The use of noninvasive or invasive means to stimulate the brain in order to treat serious psychiatric problems.
Psychedelic medicine: The use of mind-altering (typically but not always hallucinogenic or dissociative) drugs by mental health professionals to improve or even provide remission from severe psychiatric problems, such as depression, PTSD, anxiety, and substance use disorders.
Set: Refers to the patient's mindset. For example, a person who is anxious and fearful is less likely to have a positive experience with psychedelic medicine than a person who has an open and positive outlook.
Setting: Refers to the overall ambiance in which psychedelic medicine is administered. A pleasant atmosphere that makes the individual feel safe is best.
Transcranial magnetic stimulation: A noninvasive form of therapy that uses large magnets external to the patient to stimulate the brain.
Vagus nerve stimulation: Invasive stimulation of the vagus nerve in order to treat serious, treatment-resistant psychiatric diagnoses.
More than 50 years have passed since the federal Controlled Substances Act first criminalized the use of psychedelics in the United States in 1970. The initial use (and misuse) of psychedelic drugs in that era was primarily associated with Timothy Leary, a Harvard professor who promoted the nonmedical use of LSD, a practice subsequently adopted by the amorphous "hippie" counterculture movement of the 1960s and 1970s. Dr. Leary was famously noted as advising his followers to "turn on, tune in, and drop out," scandalizing much of the conservative population of the time. Numerous events led to Leary's loss of reputation, academic standing, and position, but his impact during this period was indisputable. In response to this movement, drugs such as LSD, DMT, psilocybin, and mescaline were all placed in the Schedule I drugs category under the Controlled Substances Act 1970 (Table 3).
The categorization of psychedelics as Schedule I drugs immediately halted intense scientific research on psychedelics, which had begun in the 1950s. This prohibition on psychedelic drug research significantly delayed advances in medical knowledge on the therapeutic uses of these agents. While much of the focus at that time was on Timothy Leary and the counterculture's recreational LSD use, some researchers had demonstrated beneficial effects with psychedelic medicine in end-of-life care as well as in the treatment of addiction and other severe psychiatric problems [24].
This research did not restart in the United States in any meaningful way until the 21st century. In this new wave of research, researchers in Phase 2 and 3 clinical trials of psychedelic medications have found the possibility of remission in diverse psychiatric populations (including in patients with PTSD, depression, eating disorders, and substance use disorders) as well as reduction in end-of-life anxiety and despair in those with terminal diagnoses [25]. At the same time, researchers have explored the use of older drugs (e.g., nitrous oxide, ketamine) to treat unrelenting psychiatric disorders.
Another interesting avenue of research has been in the field of addiction medicine. There is some evidence that certain psychedelic drugs, particularly psilocybin, may act as a sort of "anti-gateway drug." Years ago, there was a belief that some (or all) drugs were "gateway drugs," leading inevitably to taking other drugs; for example, this perspective holds that people who smoked marijuana would eventually progress to using "harder" drugs, injecting heroin or other opioids. This theory has largely been discredited and devalued. In fact, several studies have indicated that persons who use hallucinogens are less likely to progress to harder drugs. In one study, researchers used data from nearly 250,000 respondents from the National Survey on Drug Use and Health over the period 2015–2019. Respondents were asked about their past use of classic psychedelics, and these results were then compared to their later abuse (or non-use) of opioids. Individuals who had used psilocybin ("magic mushrooms") in the past had a significantly lower rate (30% lower than average) of opioid misuse and abuse later. This finding was not replicated with other psychedelic drugs [26]. An earlier study using National Survey on Drug Use and Health data for the period 2008–2013 found that past use of classic psychedelics decreased the risk for past-year opioid dependence by 27% and of opioid abuse by 40% [27].
Both of these studies relied on individuals reporting on their past use of psychedelic drugs, and there are multiple possible issues with this type of retrospective reporting. But the idea that past use of drugs such as psilocybin could be protective against opioid misuse and dependence in the future is promising, given the ongoing opioid epidemic in the United States.
It is unclear how long the various psychedelic substances have been used worldwide, but it is safe to say that some have been used for thousands of years in religious and tribal ceremonies. The earliest known written record of the use of psilocybin mushrooms appeared in the Florentine Codex, a manuscript of ethnographic research of Mesoamerica, particularly of Mexico and the Aztecs, compiled between 1529 and 1579. Psilocybin, mescaline, and ayahuasca (a concoction often brewed in a tea and that includes the psychedelic chemical DMT) have all been used in religious ceremonies in indigenous societies in South and Central America for centuries. The hallucinogenic effects of some plants and fungi also have been known by indigenous cultures and were deliberately exploited by humans for thousands of years. Fungi, particularly some types of mushrooms, are the principal source of naturally occurring psychedelics. Historically, the mushroom extract psilocybin has been used as a psychedelic agent for religious and spiritual ceremonies and as a therapeutic option for neuropsychiatric conditions [28].
Modern pharmaceutical research on psychedelics started in earnest in 1930s Basel, Switzerland, with research chemist Albert Hofmann. Seeking to create a synthetic alkaloid to the ergot fungus, he developed LSD-25 in 1938. The uses of the drug were not immediately obvious, so it sat on a shelf for five years until Hofmann decided to repeat his synthesis of the chemical. Despite his care, Hofmann accidentally contaminated himself with the drug and thereafter experienced highly unusual sensations as well as dizziness. He described his experience as [29]:
I lay down and sank into a not unpleasant intoxicated-like condition, characterized by an extremely stimulated imagination. In a dreamlike state, with eyes closed (I found the daylight to be unpleasantly glaring), I perceived an uninterrupted stream of fantastic pictures, extraordinary shapes with intense, kaleidoscopic play of colors. After some two hours, this condition faded away.
Hofmann decided to experiment on himself with what he believed to be a very low dose of LSD, but the dose was high enough for him to experience what he perceived to be demonic possession and other lurid sensations. His physician was called and only noted that Hofmann had extremely dilated pupils, with normal blood pressure and vital signs. When Hofmann related his experiences to his colleagues, they were dubious that he had measured correctly, but to be safe, they took even lower doses. Each experienced what were later referred to as psychedelic mind "trips" [29].
In 1947, Sandoz began marketing and distributing LSD, under the brand name Delysid, as a possible psychiatric drug to treat neurosis, alcoholism, criminal behavior, and schizophrenia. In addition, LSD-25 was also used to treat autism and verbal misbehavior [28,30]. In his book, Hofmann described how LSD helped provide relief to people who were dying of cancer and in severe pain for whom major analgesics were ineffective. He hypothesized that the analgesic effect was not inherent to the drug but was a result of patients dissociating from their bodies such that physical pain no longer affected them [29].
However, early studies on LSD did not always inform patients about the potential risks. For example, in some cases, patients with schizophrenia were given LSD and not told about the possible risk for a psychotic break [31]. Patients at the Addiction Research Center in Lexington, Kentucky, were often given the drug without being told what it was or the possible effects. Researchers who believed in the importance of "set and setting" (the patient's mindset and the setting where the drug was administered) were more likely to inform patients about possible risks and benefits. The 1962 Kefauver-Harris Amendments required that all patients provide informed consent for therapeutic interventions and research participation. Despite this, the "informed consent" of the 1960s was not as comprehensive as informed consent today. Some have posited that the primary goal was to release researchers from legal responsibility rather than to provide ensure the safety of patients and prospective subjects of clinical trials [31].
For about a decade, Hofmann and Sandoz believed that LSD might provide breakthroughs in psychiatry. However, with the major social change of the 1960s, characterized by protests for social change and against the Vietnam War and increasingly liberal attitudes regarding drugs among young people, the focus shifted to recreational rather than medical use of LSD, and in 1965, Sandoz stopped manufacture and marketing of LSD. In 1966, Sandoz gave their remaining supplies to the National Institute of Mental Health [31].
In 1957, Hofmann received a sample of dried Psilocybe mexicana mushrooms from a mycologist in Huautla de Jiménez in Oaxaca, Mexico. The mycologist, R. Gordon Wasson, had received a sample of the mushrooms and information regarding the sacred rituals of the Mazatec people from a curandera to whom he promised secrecy; this promise was obviously not kept, and Wasson's actions resulted in retaliation against the indigenous woman whom he betrayed [138]. Hofmann used paper chromatography to separate the various components of whole extracts of mushrooms and ingested each separated fraction. The active fraction was then chemically characterized, crystallized, and named psilocybin. In 1958, Hofmann and his colleagues subsequently elucidated the structure and synthesis of psilocybin and psilocin, a minor component of the extract that is a dephosphorylated form of psilocybin. In the 1960s, Sandoz Pharmaceuticals began to distribute Indocybin, a psychotherapeutic drug in pill form, containing 2-mg psilocybin. This period also saw research focusing on psilocybin as a probe for brain function and recidivism and as an entheogen used by religious people (divinity students).
During this era, psilocybin, LSD, mescaline, and other psychedelics were used by some individuals with psychiatric diseases, and they were also used extensively by some psychiatrists to treat patients before the drugs were categorized as Schedule I of the U.N. Convention on Drugs in 1967, which preceded the Controlled Substances Act in the United States. Today, the medical value of hallucinogens is being tested in rigorous trials in settings such as Roland Griffith's Johns Hopkins research program. The experts from the psilocybin research group at Johns Hopkins University have described the importance of trained psychedelic therapists and other components of a psychedelic treatment session to optimize patient safety in hallucinogen research [32].
For most mental health professionals, the idea of psychedelic-assisted psychotherapy is a major paradigm shift and leap from current practices of providing pharmacotherapy or psychotherapy to individuals or groups. At the same time, it may represent a new opportunity to combine the talents and skills of therapists with the proven benefits of a psychedelic drug. Combined psychotherapy/pharmacotherapy is the treatment of choice for most patients with mental health disorders, so interprofessional collaboration is a typical (and vital) part of treatment. Psychedelic medicine requires that diverse disciplines collaborate closely and communicate to clearly ensure that the therapy is safely and effectively administered.
Today, the federal government has provided limited permission or even grants to study Schedule I drugs and their possible role in the treatment of patients. Outside of these limited cases, researchers find it difficult to obtain the needed drug for testing purposes. To avoid legal and regulatory issues, a good amount of research is performed outside of the United States.
Since the 1960s, therapists have noted that the response to psychedelic drugs is impacted by the patient's mindset as well as the setting where the psychedelic drug is administered. For example, if the person feels confident that the experience will be a positive one, then this "set" is considered more conducive to a good experience while under the influence of a psychedelic drug compared with when persons are extremely apprehensive and fearful beforehand. By extension, if patients are in an office setting with a therapist or other practitioner with whom they feel safe, the outcome is generally better than in those who feel unsafe. Research has shown a better outcome with patients receiving psychedelics in a therapeutic setting versus receiving the drug while undergoing a positron emission tomography (PET) scan [33]. These researchers stated [33]:
The finding that the PET environment was strongly associated with anxious reactions could be partially explained by the perceived atmosphere. Whereas non-PET experiments were mostly conducted in laboratory rooms that were furnished in an aesthetically pleasing way, the environment at the PET center was much more clinical and "antiseptic" (i.e., lots of technical equipment, white walls, personnel in white lab coats). Our results are therefore in support of current safety guidelines, which recommend avoiding "cold" and overly clinical environments in human hallucinogen research in order to reduce the risk of anxious reactions.
Another element of setting, and one that is also used to enhance set, is the use of music while the patient undergoes therapy with psychedelic medicine. Johns Hopkins has developed a "psilocybin playlist" lasting nearly eight hours that is used for patients who are undergoing treatment with psilocybin [34].
In many cases, psychedelic therapy is administered after a therapeutic session. Psychotherapy is often also provided during the course of the drug's effects and at integration sessions that occur after the drug was given to help the patient to give meaning and context for the experience [35]. This provision of multiple hours of psychotherapy over a short period of time can translate to higher costs. This scenario might be less appealing to insurance carriers than traditional therapies (e.g., antidepressants or other drugs), but this is yet to be seen.
It should also be noted that in some areas, there are clear manualized approaches to treating patients that carefully consider both set and setting; this is particularly the case for MDMA in the treatment of PTSD. However, these approaches are yet to be developed for most other psychedelic drugs. Again, this field offers burgeoning opportunities for psychiatrists, psychologists, primary care providers, and other mental health practitioners.
Some patients will approach their primary care providers to discuss the possibility of seeking care at a ketamine or MDMA (or other) clinic. It is important not to dismiss these treatment options out of hand. Instead, it may be best to ask the patients the following questions to help assess if the option would be helpful and if the facility is set up to provide optimal care:
Who is the expert or experts running this clinic? What experience(s) make this person or team experts? What outcome data are provided?
Does the patient have a severe and intractable diagnosis, such as treatment-resistant depression, substance use disorder, or PTSD? If not, then conventional medicine is still best.
Does the clinic ensure professional observation after the drug is administered? This is always advisable in case the patient experiences adverse events.
How soon after a drug is administered are patients discharged from the facility? Minimal times (e.g., 15 minutes) are not long enough to ensure safety.
Does the facility offer psychotherapy before, during, and after the drug is administered? Combining psychotherapy with psychedelic medicine is the proven best practice.
Is there a required follow-up?
Are the costs for treatments clearly delineated? If not, patients should request, in writing, an estimate of total costs. Psychedelic medicine is likely not covered by health insurance and may be costly. Also, the cost may fluctuate significantly from one clinic to another.
Has the patient experienced a psychotic break in the past or does the patient have first-degree relatives with a history of psychosis? Psychedelics have the potential to trigger an underlying predisposition for psychosis, although it can be temporary. Still, even a short-term psychotic break is a terrifying experience.
For many people, including some clinicians, the phrase "psychedelic medicine" evokes images of free love, 1960s counterculture, and recreational intoxication. In reality, these therapies typically look much more pedestrian, consisting of a patient sitting or lying on a couch while a clinician guides the person through the experience in order to treat their severe psychiatric disorder. Although many of the drugs described in this course can and do induce hallucinations, subjects have reported that these experiences were integral and allowed them to resolve psychiatric issues that have been resistant to traditional treatments and that have significant impact on their lives. If further studies continue to bear these findings out, it would be unwise to ignore the benefits that may accrue.
The key psychedelic drugs actively being researched and/or currently in use today include psilocybin, ketamine, MDMA, ibogaine, kratom, LSD, mescaline, and ayahuasca (Table 4). In addition, nitrous oxide, a gas used for many years by dentists as both an anesthesia and analgesic for patients undergoing painful procedures, has also been found effective as a treatment for some psychiatric disorders.
MAJOR PSYCHEDELIC RESEARCH CENTERS IN THE UNITED STATES
|
Beginning in the 2010s, psilocybin has been undergoing an era of increased research attention, and this compound remains under active investigation. Psilocybin occurs in nature in hundreds of species of mushrooms as 4-phosphoryloxy-N,N-dimethyltryptamine. However, when used by researchers, the drug is nearly always a chemically synthesized compound to maintain a standard dosage as well as the purity of the drug. In 2020, COMPASS Pathways announced that it had gained a patent in the United States for COMP360, its form of synthetically derived psilocybin [15].
According to a 2022 report from the Associated Press, some states, even in conservative areas (e.g., Utah), have approved studying psilocybin as a treatment. This movement has largely been driven by increasing rates of treatment-resistant PTSD among military veterans [36].
Psilocybin was first studied during the 1960s to establish its psychopharmacologic profile; it was found to be active orally at around 10 mg, with more potent effects at higher doses, with a four- to six-hour duration. Psilocybin is rapidly metabolized to psilocin, a full agonist at serotonin 5-HT1A/2A/2C receptors, with 5-HT2A receptor activation directly correlated with human hallucinogenic activity. Time to onset of effect is usually within 20 to 30 minutes of ingestion. As a drug, it is about 20 times stronger than mescaline but much less potent than LSD [37].
In animal studies of the use of psilocybin, a link has been identified between reduced prefrontal mGluR2 function and both impaired executive function and alcohol craving. Psilocybin also restored healthy mGluR2 expression and reduced relapse behavior in mice [38]. Mice and humans do not always respond equivalently, but this finding may explain why psilocybin is effective in treating induced alcoholism in mice and provides an interesting research avenue in the investigation of psilocybin as a treatment for alcohol use disorder in humans, because relapse is a significant problem; even when a patient has abstained from alcohol for years, the underlying craving remains. If this craving could be reduced or altogether eliminated, this could revolutionize substance use disorder treatment.
In a study at King's College London, researchers studied the effects of psilocybin on the emotional and cognitive functions in healthy subjects in a Phase 1 randomized double-blind controlled study with 89 subjects (average age: 36.1 years). Subjects were randomized to receive placebo or 10 mg or 25 mg of psilocybin. Therapists were available to the subjects throughout the sessions. Six subjects at a time received the drug. The study showed that there were no short- or long-term adverse effects to the emotional processing or cognitive functioning of the subjects [39]. In this study, 70% of the subjects who received 25-mg psilocybin experienced visual hallucinations, compared with 60% of those who received 10-mg psilocybin and 6.9% of those who received placebo. The second most common treatment-emergent adverse event was illusion, which was experienced by 60% of subjects receiving 25-mg psilocybin and 63.3% of those receiving 10-mg psilocybin; 13.8% of those receiving placebo reported experiencing this effect. Other treatment-emergent adverse events reported more commonly among the treatment groups included mood alteration, headache, fatigue, and euphoric mood, all of which were lower or altogether non-existent in the placebo group. Also absent in the placebo group were auditory and tactile hallucinations [39]. The researchers concluded [39]:
This study demonstrated the feasibility of one-to-one psychological support from specially trained therapists during [the] simultaneous administration of psilocybin in a supervised clinical setting in healthy volunteers. A single dose of psilocybin 10 mg or 25 mg elicited no serious adverse effects and did not appear to produce any clinically relevant detrimental short- or long-term effects, compared with placebo, in cognitive or social functioning or emotional regulation in this study in health volunteers.
In studies using psilocybin, the most common adverse reactions were found to be headache, nausea, and hypertension, and events were considered to be equivalent to those found with the use of SSRIs [40]. However, it should also be noted that the subjects in psilocybin clinical trials are usually screened for a family history of schizophrenia, major depression with psychotic features, high risk for suicide, and severe personality disorders before inclusion [40].
Another study at Johns Hopkins evaluated the efficacy and safety of psilocybin for the treatment of major depressive disorder. In this randomized study, 24 patients 21 to 75 years of age with moderate-to-severe unipolar depression were randomized to either immediate or delayed treatment. Subjects were administered two doses of psilocybin along with supportive psychotherapy. Researchers found a greater than 50% reduction in depressive symptoms, as measured by the GRID-Hamilton Depression Rating Scale (GRID-HAMD), in the treatment group. Before initiating psilocybin therapy, subjects first received six to eight hours of preparation with trained facilitators. The psilocybin was administered at doses of 20 mg/70 kg and 30 mg/70 kg, about two weeks apart, while subjects were in a comfortable room supervised by two facilitators. There were also follow-up counseling sessions [1]. The mean scores on the GRID-HAMD decreased from an average of 22.8 at the pretreatment level to 8.7 at 1 week, 8.9 at 4 weeks, 9.3 at 3 months, 7.0 at 6 months, and 7.7 at 12 months. These data indicate that the psilocybin provided persistent relief to many patients [1].
In a 2018 British study, 26 patients, 20 of whom were diagnosed with severe treatment-resistant depression, were administered separate doses of 10- and 25-mg psilocybin one week apart; administration took place in a supportive setting. Nineteen subjects completed the treatment process, including psychological support, and all of the completers reported improved symptoms based on Quick Inventory of Depressive Symptoms (QIDS-SR16) and HAM-D scores. Four patients experienced remission of their depression at week five. Many completers continued to benefit from treatment at three months and six months. Suicidality scores among the patients also significantly fell within the two weeks after treatment [41].
Not all researchers have offered a ringing endorsement of the use of psilocybin. A 2021 study studied 59 patients with moderate-to-severe major depressive disorder. The subjects were administered either two doses of 25-mg psilocybin three weeks apart plus placebo (30 patients) over six weeks, or they were given escitalopram (an SSRI) for six weeks (29 patients). All the patients also received psychological assistance. No significant differences were noted in depression symptoms between the two groups, and the researchers concluded that further studies with larger populations were needed. Even the adverse events in the two groups were somewhat similar; the most common adverse effect in both groups over the course of the study was headache, followed by nausea [42]. Even in this study, psilocybin was about as effective as antidepressant therapy. This is remarkable, in that this new treatment is about as effective as the established criterion standard treatment for major depressive disorder.
Although studies have supported the hypothesis that psilocybin provided under research conditions by physicians has a positive effect on depressive symptoms, until recently, the mechanism by which this improvement has occurred was largely unknown. However, in a study of 16 individuals with treatment-resistant depression, researchers used functional magnetic resonance imaging (fMRI) to assess functional brain changes both at baseline and one day after the study group received 25-mg psilocybin. The researchers found brain network modularity was reduced within just one day after the psilocybin was administered [43]. In a second study by the same researchers, 59 patients with major depressive disorder were randomized to either two doses of 25-mg psilocybin three weeks apart plus six weeks of daily placebo or to six weeks of 10- to 20-mg escitalopram per day plus 1-mg psilocybin (an ineffective dose). In this study, 29 subjects were in the escitalopram arm, although the group ultimately decreased to 21 subjects (28% dropout rate). The 30 patients in the psilocybin group decreased to 22 subjects (27% dropout rate) [43]. The researchers noted that [43]:
It is plausible that this putative liberating effect of psilocybin on cortical activity occurs via its direct agonist action on cortical 5-HT2A receptors, dysregulating activity in regions rich in their expression. We surmise that chronic escitalopram does not have the effect on brain modularity due to its more generalized action on the serotonin system and predominant action on inhibitory postysynaptic 5-HT1A receptors, which are richly expressed in limbic circuity.
The researchers found that the antidepressant effect of the psilocybin was sustained and rapid and that it also corresponded with decreases in fMRI brain network modularity. This indicates that the antidepressant effect of psilocybin, when it works, is linked with a global increase in brain network integration. In contrast, the response to the escitalopram was mild and caused no changes to the brain network [43].
Ketamine is a derivative of phencyclidine (PCP), which itself was originally developed as an anesthetic. However, the major adverse effects of PCP, such as aggression, psychosis, and dysphoria, made it an undesirable and unacceptable anesthetic choice [44]. In contrast, ketamine was effective as an anesthetic and had few adverse effects. PCP subsequently became a drug of abuse.
While ketamine has been used in operative analgesia for decades, it has also become a drug of abuse and misuse [45]. Most notoriously, ketamine became known as a "date-rape drug," because it was administered in drinks to unknowing victims who were subsequently sexually assaulted by their predators. Because ketamine causes amnesia, victims have little or no memory of what occurred to them, although they often experienced after-effects, such as pain. As a result of this growing criminal use, Congress passed the Drug-Induced Rape Prevention and Punishment Act of 1996. During this period and the decade following, there was increased awareness of the dangers of ketamine and other drugs that were used in a similar manner, such as flunitrazepam (Rohypnol) and gamma hydroxybutyric acid (GHB) [46]. As a result, ketamine developed a stigma, and this negative view may persist in many minds.
Ketamine is a Schedule III drug that is a combination of s-ketamine (esketamine) and r-ketamine (arketamine). In 2019, the use of esketamine as a nasal spray (brand name Spravato) was approved by the FDA for the treatment of treatment-resistant depression. Since then, it has also been approved to treat suicidal depression. However, it should be noted that this nasal spray formulation is not available at most pharmacies; instead, it is provided solely through a restricted distribution system. The FDA also requires that patients be overseen for a minimum of two hours after treatment, in order to allow sufficient time to identify and address and adverse reactions that develop in patients. (It is not clear if all ketamine clinics adhere to this provision.)
After treatment with ketamine, patients should not leave the facility until they are cleared to do so by a healthcare provider and they should also be cautioned to avoid driving or using heavy equipment until the following day. In addition, patients are not allowed to take the nasal spray home, because it may only be used in the medical office while under the supervision of qualified staff members [47].
Intravenous ketamine has been used off-label for treatment-resistant depression by some clinicians, and ketamine clinics are established in many parts of the United States, although their fees vary widely. The effects of intravenously administered ketamine may last for hours, days, or even weeks in some patients. Some believe that intravenous ketamine is significantly more effective than its intranasal form because it includes both the s and r forms of the drug.
Some researchers have found that the mental state of the patient (set) prior to receiving treatment with ketamine may affect the outcome of treatment. In a 2019 study, 31 patients with major depressive disorder were treated with ketamine infusions. Researchers used multiple instruments to measure the mental state of subjects prior to and after receiving treatment, including the Montgomery-Asberg Depression Rating Scale (MADRS) and the Beck Hopelessness Scale. In this study, 17 subjects (55%) responded to the ketamine, while 14 (45%) had no response [48]. Non-responders had significantly higher rates on anxiety scales than responders. The researchers stated [48]:
The present study showed for the first time that non-responders had more anxiety-related experiences induced by the first ketamine infusion than responders confirming our initial hypothesis of significantly different subjective experiences as a function of treatment response. Specifically, we found that it was the extent of ketamine-induced anxiety that was negatively predictive of a treatment response after a series of six infusions on average.
They also noted that providing a calm treatment environment to patients might be sufficient to reduce anxiety levels in patients to improve outcomes. This is the goal of treatment providers as well as researchers who emphasize the importance of set (mindset) and setting, as discussed. In this study, there was no follow-up after the last infusion, which may also have improved efficacy [48].
In another study of 30 individuals with PTSD of a median duration of 15 years, half of subjects were randomized to a ketamine group and half were assigned to a midazolam (a benzodiazepine) group. The subjects received six infusions over the course of two weeks of either ketamine (0.5 mg/kg) or midazolam (0.045 mg/kg). The subjects were evaluated with the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) at baseline and also at the end of treatment [49].
The average CAPS-5 total scores following the infusions were 11.88 points lower among the subjects in the ketamine group compared with the midazolam group. About two-thirds of the ketamine subjects (67%) responded to the treatment, versus only 20% of treatment responders in the midazolam group. The median time to loss of treatment following the two-week ketamine treatment period was 27.5 days. However, in outlier cases, two subjects still had not lost their response; improvements continued at 50 days and 102 days since the last infusion. The ketamine group experienced a major reduction in symptoms of depression as well as in clinical ratings of global psychiatric illness severity. The researchers concluded that the findings from this study support the assertion that "repeated ketamine infusions are safe and generally well tolerated among individuals with chronic PTSD, with only transient emergence of psychoactive and hemodynamic side effects" [49].
In a French study, ketamine was explored as a treatment for individuals with severe suicidal ideation in a double-blind randomized clinical trial. In this six-study report, published in 2022, 156 patients were given either a 40-minute infusion of ketamine or placebo (saline solution). The administration was repeated 24 hours later. The groups were also divided into subjects with bipolar disorder, depressive disorder, and other diagnoses. Of patients in the ketamine group, 93.1% had a past history of the commission of a suicidal act, as did 86.6% of the subjects in the placebo arm [50].
On day 3, nearly two-thirds (63%) of the patients in the ketamine group achieved full remission from suicidal thoughts. In contrast, 31.6% of the patients in the placebo group were in remission. In nearly 44% of the ketamine subjects, remission occurred within two hours after the first infusion, compared with 7.3% of the placebo group. Ketamine was particularly effective in the bipolar group, while its effect was not significant in the group with major depressive or other psychiatric disorders. The researchers speculated that ketamine might provide an analgesic kind of effect to mental pain [50].
In the past and even to date, MDMA (also referred to as "Ecstasy" or "Molly") has been largely a drug of abuse. According to the National Institute on Drug Abuse, about 2.6 million people in the United States 12 years of age and older reported past-year use of MDMA in 2020 [51]. The drug was originally developed by Merck in 1912, and in the 1970s, it was found to be useful in combination with psychotherapy [52]. However, because of considerable active abuse of the drug in the United States, in 1985, MDMA was categorized as a Schedule I drug under the Controlled Substances Act in an emergency ban, and consequently research on this drug largely halted until the 2010s [53].
Today, researchers have demonstrated the efficacy of combination psychotherapy and MDMA in treating PTSD. The FDA has granted "breakthrough therapy" permission for MDMA therapeutic treatment, largely as a result of the findings of several small studies. Clinicians who use MDMA-assisted psychotherapy to treat individuals with PTSD have access to a manual outlining best practices for this therapeutic use. In the 2017 revision of this manual, the following explanation is given [54]:
The basic premise of this treatment approach is that the therapeutic effect is not due simply to the physiological effects of the medicine; rather, it is the result of an interaction between the effects of the medicine, the therapeutic setting, and the mindsets of the participant and the therapists. MDMA produces an experience that appears to temporarily reduce fear, increase the range of positive emotions toward self and others, and increase interpersonal trust without clouding the sensorium or inhibiting access to emotions. MDMA may catalyze therapeutic processing by allowing participants to stay emotionally engaged while revisiting traumatic experiences without being overwhelmed by anxiety or other painful emotions. Frequently, participants are able to experience and express fear, anger, and grief as part of the therapeutic process with less likelihood of either feeling overwhelmed by these emotions or of avoiding them by dissociation or emotional numbing. In addition, MDMA can enable a heightened state of empathic rapport that facilitates the therapeutic process and allows for a corrective experience of secure attachment and collaboration with the therapists.
In six double-blind, randomized clinical studies conducted between 2004 and 2017, 72 subjects are administered 75–125 mg of MDMA in two or three sessions, comparing these results with 31 patients who received placebo; all the patients had diagnosed PTSD. The drug was administered following 90-minute sessions of psychotherapy and three to four therapy sessions were also provided during follow-up after MDMA therapy [55].
Members of the treatment group reported significantly reduced scores on the CAPS-5 compared with the control group. In addition, after two sessions, 54.2% of those who received MDMA no longer met the criteria for PTSD—they were in remission. In contrast, only 22.6% of the control group experienced remission. The researchers noted that "MDMA-assisted psychotherapy was efficacious and well tolerated in a large sample of adults with PTSD" [55].
In another randomized, double-blind, placebo-controlled phase 3 clinical trial with 90 individuals with severe PTSD, the subjects received manualized therapy with either MDMA or placebo. Three preparatory sessions occurred before the administration of the drug, and there were nine integrative therapy sessions afterwards. Subjects in the MDMA treatment group experienced a significant decrease in CAPS-5 (-24.4) scores compared with placebo subjects (-13.9). Scores on the Sheehan Disability Scale (SDS) also significantly improved in the MDMA subjects compared with the placebo subjects [56]. The researchers noted [56]:
Given that PTSD is a strong predictor of disability in both veterans and community populations, it is promising to note that the robust reduction in PTSD and depressive symptoms identified here is complemented by a significant improvement in SDS score (for example, work and/or school, social and family functioning). Approximately 4.7 million U.S. veterans report a service-related disability, costing the U.S. government approximately $73 billion per year. Identification of a PTSD treatment that could improve social and family functioning and ameliorate impairment across a broad range of environmental contexts could provide major medical cost savings, in addition to improving the quality of life for veterans and others affected by this disorder.
Because major problems with sleep quality are common among patients with PTSD, some researchers have studied the effects of MDMA-assisted psychotherapy to determine its effects on sleep disorder. In a series of four studies with 63 subjects at sites in the United States, Canada, and Israel, subjects were randomized to two or three sessions of MDMA-assisted psychotherapy or to a control group. PTSD symptoms were assessed with the CAPS-IV, and the Pittsburgh Sleep Quality Index (PSQI) was used to measure changes in sleep quality. At the conclusion of the study, the CAPS-IV severity scores had decreased by 34 points in the MDMA group, compared with a decrease of 12.4 points for the control group. In addition, sleep quality improved significantly in the experimental group compared with the control group. In the treatment group, 53.2% of subjects reported a PSQI score drop of 3 or more points, compared with 12.5% in the control group [57].
Although there appears to be a benefit for MDMA therapy in the management of PTSD, especially for patients who have failed other therapies, the durability of this affect has been questioned. One study indicated improvement may be persistent for a considerable period of time for some subjects. In a study involving 107 subjects with PTSD, individuals were administered either two or three doses of MDMA (75–125 mg) during blinded or open-label therapy sessions. The subject's PTSD symptoms were evaluated 1 to 2 months after the last MDMA session and again after 12 months. The researchers reported that at the 12-month follow-up time, nearly all (97.6%) of the subjects said they had benefited from the treatment, and 53.2% reported large benefits that had lasted or even increased. A minority of subjects reported unfavorable results; 8.4% reported harms. However, in 86% of these cases (six of seven subjects), the harms were rated as a 3 or less on a 5-point scale. There were no reports of severe harm, and all the subjects who reported harm also reported one or more benefits. The most common harm reported was worsened mood (3.6%) [58]. The researchers noted that, "Overall findings from the present analyses support MDMA-assisted psychotherapy as an efficacious treatment for PTSD with symptom improvements that were sustained at 1 to 3.8 years post-treatment. These findings corroborate and expand preliminary results from the first phase 2 trial of this treatment" [58].
Largely derived from the Western African shrub Tabernanthe iboga, ibogaine has been explored as a possible treatment for opioid use disorder, although there are many caveats to be considered, including the fact that ibogaine is a Schedule I drug. Given the current climate surrounding opioid misuse and use disorder in the United States, possible treatment options are a major focus. According to the Centers for Disease Control and Prevention, more than 70% of drug overdoses in the United States in 2019 were related to opioid use [59]. Ibogaine apparently acts to eliminate craving for opioids and rapidly detoxifies individuals with opioid dependence, although much further study with larger populations is needed. Most people who seek treatment with ibogaine have opioid use disorder, but some have been dependent on stimulants such as cocaine.
The anti-addictive capabilities of ibogaine were first noted by Howard Lotsof in 1962 as a result of his own experience with the drug as well as reports from others. Lotsof, a man in recovery from heroin use disorder from New York City who unexpectedly found relief and remission with ibogaine, subsequently actively and tirelessly lobbied researchers to study the drug. He eventually succeeded, and multiple researchers using both animal and human studies have demonstrated ibogaine's apparent ability to induce recovery in some persons struggling with substance use disorders [60,61].
Metabolism of ibogaine is purportedly mediated by the p450 cytochrome enzyme CY2D6. Because of genetic differences, an estimated 10% of persons of European heritage (predominantly White Americans in the United States) lack the necessary gene to synthesize this enzyme. Among this group, including the many individuals who do not realize they lack this gene, administration of ibogaine can result in plasma levels as much as twice as high as those in persons with the gene. As a precaution, a test dose of the drug may be given to subjects to assess the response. Another option is genotype screening of subjects who seek treatment with ibogaine, to ensure safety and to aid in treatment decisions [62].
Although it provides insufficient data from which to draw major conclusions, a study of the use of ibogaine in two adults with opioid use disorder is interesting. The experiences of one of the patients are described here, although it should be noted that both patients have remained abstinent for several years [62]. The first patient developed an opioid use disorder secondary to pain from chronic pancreatitis. His physician was concerned about potential misuse and weaned the patient off opioids; however, the patient began taking large quantities of oxycodone tablets he purchased illegally. As the substance use disorder progressed, this patient was actively resistant to conventional treatment despite clear physical and psychosocial consequences. Eventually, he agreed to experimental treatment with ibogaine.
The patient was screened with an electrocardiogram prior to treatment and administered a test dose of ibogaine. During the first four days of treatment, he was administered oxycodone (legally obtained via prescription). The opioid doses were steadily titrated down and on day 4, all opioid medications stopped. During this same period, the patient was given increasing doses of ibogaine. On day 4, the patient was given a "flood dose" of both iboga and ibogaine (variations of the same drug). Between treatments, diazepam was given to support sleep and assuage anxiety. Treatment lasted for six days, and the patient remained at the clinic for a total of eight days. At three-year follow-up, the patient had remained abstinent from opioids, as indicated by negative drug screens. Interestingly, after the flood dose of ibogaine, the client also reported that his chronic pain issues ended, and they have not recurred [62]. The reasons for this finding are unknown.
In a study of 14 individuals with opioid use disorder, subjects were given staggered doses of 200-mg ibogaine capsules at two different clinics. Because ibogaine is a stimulant, most patients were given benzodiazepines or sleep aids so they could attain sufficient hours of sleep. The first dose administered was a test dose given when the patient was in a withdrawal state from opioids; then, a larger dose of up to 600 mg of ibogaine was given one to four hours later. This was followed by smaller dosages of 200 mg given at 20-minute intervals until ended by the provider. The subjects were interviewed pretreatment, immediately post-treatment, and 12 months later. The outcome was that 12 of the 14 subjects (85.7%) had either a marked reduction in opioid use or ended use of the drug altogether [61].
In a larger study of 191 adults wishing to detoxify from opioids or cocaine, a single dose of ibogaine was administered during a medically supervised period of detoxification. According to the researchers, the goals of the study were to safely detoxify the subjects from opioids or cocaine, to provide motivational counseling, and to refer the patients to aftercare and 12-step programs [63]. All subjects received a physical examination, and a medical history was taken. Laboratory tests were administered, as were electrocardiograms. The subjects were drug tested at the beginning of the program, and all tested positive for either opioids or cocaine. A licensed therapist worked with the subjects during and after ibogaine was administered. The average age of subjects was 36 years, and all were habitual users. The subjects were given one dose oral (gel capsule) ibogaine 8–12 mg/kg. In this study, the most common adverse effect was headache, reported by 7% of the subjects; orthostatic hypotension occurred in 5% of the subjects. About 2% of adverse events were considered to be moderately severe.
After the ibogaine was administered, its effects began about 30 to 45 minutes later. According to the researchers [63]:
Sensory and perceptual changes included reports of visual images, changes in the quality and rate of thinking, and heightened sensitivity to sound. Most subjects reported a dream-like experience lasting between four and eight hours, after which there was an abrupt change in the sensory experience to a more quiet period of deep introspection.
Approximately 92% of subjects reported benefits from the experience. They also reported that both drug craving and depression symptoms improved with doses of 500–1,000 mg. One shortcoming of this study, however, was a lack of follow-up. It would be especially helpful to know if these individuals remained abstinent 6 to 12 months later. Unfortunately, this was not among the goals of the researchers [63].
Ibogaine is difficult to obtain in the United States, and travel to other countries to obtain treatment has been reported, which can be very costly. Assuming that ibogaine were to be equal in efficacy to clonidine or lofexidine for detoxification from opioids or acute discontinuation, it is still unclear what long-term effects or level of continued abstinence can be expected. Naltrexone (Vivitrol) following detoxification might be facilitated. But, data supporting the use of suboxone and methadone in reducing overdoses, deaths, and emergency department visits are clear, including both short- and long-term outcomes. It is important to compare ibogaine to buprenorphine or methadone treatment, just as psilocybin was compared to SSRI therapy [64].
Kratom is a drug derived from Mitragyna speciosa, an evergreen tree native to Southeast Asia, where it has been used for generations, largely by locals who chew on the leaves or brew it into a tea and reportedly use the drug for an energizing purpose (e.g., to facilitate longer work periods), much as Americans use caffeine. Kratom is used by consumers in the United States as a drug of abuse and, less commonly, to manage depression. As of 2022, the drug is not scheduled by the U.S. Drug Enforcement Administration (DEA), although the DEA did consider categorizing kratom constituents mitragynine and 7-hydroxymitragynine under Schedule I in 2016. This effort was met with considerable resistance and was abandoned. As such, the product remains available locally in smoke and "head" shops, although many purchase the drug over the Internet. Kratom is banned in six states, including Arkansas, Indiana, Tennessee, Vermont, Wisconsin, and most recently in Alabama [65].
Experts exploring the potential psychiatric uses of kratom have expressed optimism. According to McCurdy, kratom "seems to have mood lifting and elevating properties in addition to its ability to seem to move people off of hardcore opiates" [66]. Although the drug is traditionally used as a stimulant, it has a sedative or opioid-like effects in very high doses. It has been hypothesized that kratom might have a role in the treatment of opioid use disorder, although much more study is needed.
It is important to note that kratom products available in the United States are very different from those that are used by people in their native environments. For example, the kratom used in Southeast Asia is almost always derived from fresh leaves, while in the United States, the products are freeze-dried leaves, concentrated extracts, or liquid "energy shots." As a result of these differences, concentrations and adulteration are concerns. Some individuals in the West who consume kratom products have displayed blood serum levels of mitragynine (the key alkaloid in kratom) 100 to 1,000 times higher than in those found in consumers in Southeast Asia [67].
Another issue is one of purity. In an analysis of eight samples of the drug, researchers found that all the samples tested positive for varying levels of Mitragyna, ranging from 3.9–62.1 mg/g, which is a wide range that could significantly alter efficacy and toxicity [68]. In addition, six of the samples tested positive for fungi and bacteria. Most (seven) of the samples were positive for significant levels of toxic heavy metals, including nickel, lead, and chromium. The presence of lead was particularly troubling, as lead has many potentially toxic effects, particularly in terms of potential problematic neurologic effects in children and young adults as well as a variety of cognitive, developmental, immunologic, renal, and cardiovascular effects [68]. Although this study did not find evidence of Salmonella contamination, in 2018, a Salmonella outbreak originating from kratom products was reported to affect 199 people spanning 41 states [69]. It is clear that the purity of kratom purchased in the United States is highly questionable, largely because there are no federal constraints on its production by the FDA or other federal agencies. Healthcare professionals who know or suspect that their patients are using kratom may wish to warn them about these findings.
As discussed, LSD is a compound synthesized from ergot. It is usually administered as an oral solution. LSD takes effect within 20 to 40 minutes after ingestion, and its effects may last for up to 12 hours. Flashbacks may also occur with this drug, defined as a feeling of re-experiencing an event or emotion that occurred during the course of the LSD "trip." LSD is about 2,000 times more potent than mescaline [37].
Prior to the Controlled Substances Act passage in 1970, there were numerous research studies on LSD as a treatment for depression, substance use disorder, and other psychiatric diagnoses, although some of these studies were not scientifically rigorous by today's standards. Fewer studies on LSD are published today, but several merit some attention. For example, a 2022 study assessed the impact of LSD on stressed mice [70]. Anxious mice were administered low doses of LSD for seven days, during which their anxiety levels decreased. In addition, researchers found that the mice given LSD showed signs of increased production of new dendritic spines, a sign of brain plasticity. The researchers also found that the LSD increased the production of serotonin in the treated mice, in a somewhat similar manner to SSRI antidepressants [70].
In an earlier study of the effects of LSD on humans with life-threatening diseases, 8 of the 12 subjects were given 200 mg of LSD and a control group was given 20 mg, an insufficient dose to generate significant response. After the initial blinded study was unmasked, the control group subjects were also given 200 mg of LSD. All subjects had a score of higher than 40 on the state or trait scale of the Spielberger State-Trait Anxiety Inventory before the study. In addition, half the subjects had diagnosed generalized anxiety disorder. A therapist was present for two sessions conducted two to three weeks apart. The experimental sessions lasted eight hours, and patients left only to use the restroom [71]. Subjects who received the 200-mg dose of LSD displayed a decrease in anxiety as measured by multiple instruments, and this decrease persisted at the 12-month follow-up evaluation. Overall, the subjects experienced a 78% drop in anxiety scores and a 67% increase in quality of life scores after one year. They also reported better access to and control of their own emotions [72].
While this research is interesting and points to areas for future research, it remains to be seen if LSD (or a similar compound) will ever be in clinical use for anxiety and depression. In addition to overcoming stigma and issues with adverse effects, significant additional research on efficacy is necessary.
3,4,5-trimethoxyphenethylamine, also known as mescaline, is a psychedelic drug that is mainly found in Lophophora williamsii, or the peyote cactus. Its effects upon ingestion are similar to the effects found with LSD or psilocybin, including hallucinations and euphoria [37]. The drug is known to have been used for thousands of years for these and perceived spiritual or medical effects; archaeologists have found evidence of this drug in Texas dating back 5,700 years [73]. Today, it is a Schedule I drug, but it may be used legally in religious ceremonies of the Native American Church. Mescaline has been suggested as a potentially effective treatment for a variety of mental health conditions, including depression, OCD, anxiety, and substance use disorder; however, research has yet to be conducted to support these claims.
The average dose of mescaline ranges from 20–500 mg, and the duration of action is about 10 to 12 hours. Individuals suffering from mescaline toxicity (typically seen with doses of 20 mg/kg or greater) may experience tachycardia, hypertension, seizures, hyperthermia, respiratory depression, and rarely death [73]. Concomitant use of mescaline with stimulant drugs (e.g., nicotine, cocaine, ephedrine, amphetamines) may increase the risk of adverse central nervous system effects.
In a survey of 452 individuals who reported using mescaline, researchers found that the drug was usually used once per year or less frequently, and only 9% of users reported a craving for mescaline. About 50% of users reported established psychiatric diagnoses, including anxiety and depression, and of this group, more than 65% reported that these problems improved after taking mescaline [74]. Clinical studies are necessary to confirm or refute these findings.
In another analysis of these data, nearly 50% of respondents reported their experience with mescaline was either the most meaningful experience of their lives or in the top five most meaningful experiences. Respondents who said they had experienced improvement in psychiatric problems were significantly more likely to also report experiencing mystical/spiritual experiences and psychological insight [75].
Nitrous oxide (chemical formula N2O) is a component familiar to many, as it is commonly used today to facilitate comfort and address anxiety in dental settings. Historically, it has been used in both dental and medical interventions. The origins of nitrous oxide are attributed to Joseph Priestley's discovery in 1772, who referred to it as "dephlogisticated nitrous air" [76]. Anesthetic use of nitrous oxide was discovered by a dentist in 1844, and it was used for this purpose almost solely until the 1980s. The first research into the use of nitrous oxide for neuropsychiatric purposes was published between 1920 and 1950, and in the early 1980s, low-dose titration of nitrous oxide was introduced into medical practice as a possible adjunct to the treatment of psychiatric disorders, including substance use disorders [77]. Before then, it was limited to use as an anesthetic or for analgesia during childbirth. In 1994, the term psychotropic analgesic nitrous oxide was introduced in order to better distinguish anesthetic and nonanesthetic preparations [77].
The anxiolytic action of nitrous oxide is believed to be due to binding at select gamma-aminobutyric acid (GABA) receptors, an action similar to the benzodiazepines [78]. The mild analgesic effect appears to be linked to the endogenous opioid receptor system, as experimental studies have shown that the introduction of opioid receptor antagonists to the brain decreases the analgesic efficacy of nitrous oxide [79].
The route of administration is inhalation via a mask secured to the patient's nose. In the dental setting, the concentration of nitrous oxide is 25% to 50% (usually 30% to 40%) nitrous oxide with oxygen. When utilized in obstetrics, a fixed 50% concentration with oxygen is used [77]. Onset of action can occur in as quickly as 30 seconds, with the peak effects seen in five minutes or less. Unlike the benzodiazepine medications, nitrous oxide is not metabolized in the body. It is eliminated via respiration within minutes after 100% oxygen is inhaled at the conclusion of the intervention [78]. Repeated doses could be problematic, as extended use of nitrous oxide has been linked to vitamin B12 deficiency [76]. As such, serum vitamin B12 level may need to be measured before and after treatment.
Nitrous oxide has been demonstrated to improve the condition of individuals with treatment-resistant depression. A study of 20 subjects with treatment-resistant depression were randomly placed in either a nitrous oxide treatment group (10 subjects) or placebo group (10 subjects). The nitrous oxide group inhaled 50% nitrous oxide/50% oxygen, and the placebo group received 50% nitrogen/50% oxygen. There were two sessions one week apart. At the end of the study, four patients (40%) had a decrease in symptoms of depression and three patients (30%) experienced full remission. In contrast, one patient improved after receiving the placebo (10%) and none of the placebo patients remitted from their depression. The improvements in the nitrous oxide group were rapid, occurring in some cases within as little as two hours of receiving the drug [80]. Adverse events were mild and included nausea and vomiting, headache, and dizziness/lightheadedness. At the time of the second session, some patients in the treatment group experienced a carryover effect from the first week's treatment, as evidenced by sustained improvements in their scores on the Hamilton Depression Rating Scale (HDRS-21).
A separate study was undertaken to determine whether a single solution of 25% nitrous oxide would be as beneficial as a 50% solution. This study included 24 subjects with treatment-resistant depression who were randomly placed in one of three groups. Each group received either 50% nitrous oxide therapy, 25% nitrous oxide therapy, or placebo each month; each patient had the opportunity to receive all three treatments. At the end of the study, 55% of the subjects reported improvement in at least half of their symptoms, while 40% reported full remission [81]. Of interest, the 25% nitrous oxide solution had about the same level of efficacy in reducing depression as the 50% solution; however, there were significantly lower levels of adverse events in the 25% group. For example, 21% of those who had received 50% nitrous oxide concentration reported nausea; this decreased to 5% in the group that received 25% concentration. Further, the incidences of headache and dizziness were 17% and 13%, respectively, in the 50% concentration group, while the rates were 10% and 0% in the 25% group [82]. The study made it clear that with nitrous oxide, a 25% solution administered over one hour could improve treatment-resistant depression. Most of the study patients had failed an average of 4.5 antidepressants before the study, so the results were significant for a group in need of additional treatment options.
Ayahuasca is a brew derived from the leaves of Psychotria viridis, a shrub found in Amazonian South America, and which contains DMT, a hallucinogenic alkaloid. The brew is also made with the Banisteriopsis caapi vine, the bark of which contains ingredients that act as MAO inhibitors.
In a Brazilian study involving 29 subjects with treatment-resistant depression, patients were randomized to receive a dose of either ayahuasca or placebo. Subjects were evaluated on the MADRS at the following points: baseline, day 1, day 2, and day 7 after dosing. They found MADRS scores were significantly lower in the ayahuasca group at all points and all individuals in this group experienced improvements. In contrast, 27% of patients in the placebo group developed worse depression symptoms. However, ayahuasca sickens many people, and most of the subjects who were given this substance felt nauseous and 57% vomited [83].
In another small Brazilian study, six subjects with recurrent major depressive disorder (without psychotic symptoms) were assessed for response to ayahuasca therapy. All individuals were inpatients at a psychiatric unit and were not taking any psychiatric or recreational drugs. The ayahuasca used by the volunteers was plant-based and refrigerated before the study, and each person drank 120–200 mg [84]. All subjects experienced decreases in depression symptoms on days 1 and day 7 of treatment. There were significant decreases in the Brief Psychiatric Rating Scale (BPRS), indicating improvements in both depression and anxiety. There were also statistically significant decreases in scores on the HAM-D and the MADRS. For example, on day 1, there was a 62% decrease on the HAM-D, and a 72% decrease by day 7. On day 14, however, depression symptoms increased. Similar changes were seen with the MADRS scores [84]. About half the volunteers did vomit; however, vomiting did not appear to impact the efficacy of the drug [84]. If ayahuasca is to be considered as a therapeutic option, a way to counteract the emetic effects and make the drug more tolerable to patients is necessary. To date, experts have hypothesized that antiemetic drugs might interfere with the action of ayahuasca.
Another problem with the scientific study of ayahuasca is that the effects of the drug depend on the concoction and there are no standardized dosages. If the drug could be provided in a synthesized form, it would become easier to evaluate and study in patients with depression and other disorders. In Barker's report on DMT, he states [85]:
While ayahuasca obviously holds promise in many social, cultural, and therapeutic paradigms, including treatment of addiction, anxiety, and depression in psychiatry and many other possible applications, it is, nonetheless, a complex mixture of perhaps thousands of compounds.
DMT has been identified in additional substances. The Sonoran Desert toad (Bufo alvarius), native to Texas, California, and Mexico, excretes a venom when threatened that contains a naturally occurring form of DMT. This venom, which can be made into crystals and smoked, is popular for inducing psychedelic trips among recreational users. However, this venom is unsafe, and some have died after smoking it. Further, harvesting this venom has reduced the population of the toad in some areas. Overall, experts recommend that people not attempt to capture the toads or harvest the venom [86].
This section will outline the possible role of psychedelics in the management of specific psychiatric diagnoses, including diagnoses not previously discussed. It is important to remember that most of these uses are investigational.
Depression and suicidal depression are major problems in the United States. As noted, at least 30% of persons with depression do not respond to psychotherapy and/or medication. Psilocybin has proven effective at providing breakthroughs with treatment-resistant depression as well as in treating suicidal depression [41,42]. Nasal spray esketamine (Spravato) is FDA-approved as an adjunct treatment in addition to a conventional antidepressant for treatment-resistant depression and/or major depressive disorder with suicidal ideation or behavior [87]. The nasal spray formulation of esketamine is administered in two sprays (28 mg) per device. The recommended dosage for adults with treatment-resistant depression is 56 mg on day 1, then 56–84 mg twice per week for four weeks, reducing to once per week for the next four weeks, and then once weekly or once every two weeks thereafter. This drug is only administered under medical supervision, and patients should remain under observation for at least two hours following administration.
There are concerns regarding misuse, excessive sedation, and diversion, and a Risk Evaluation and Mitigation Strategy (REMS) has been established. The full document is available online at https://www.accessdata.fda.gov/drugsatfda_docs/rems/Spravato_2022_01_03_REMS_Document.pdf.
MDMA and ketamine are well on their way to being proven safe and effective in the treatment of PTSD, and further studies on other psychedelics are likely to provide even more breakthrough information. According to the National Center for PTSD, an estimated 12 million adults in the United States have PTSD in a given year; 8% of women and 4% of men develop PTSD in their lifetime [88]. However, PTSD is very difficult to treat with medications and psychotherapy.
The usual dosage of ketamine for the treatment of persistent PTSD is 0.5 mg/kg given via a 40-minute IV infusion. The regimen typically consists of multiple sessions per week for two to four weeks [89].
In the research setting, MDMA for PTSD is typically given during or immediately preceding a psychotherapy session. The usual dose is 75–125 mg in a single dose [90]. As a Schedule I drug, MDMA is only used in clinical trials and research settings.
To date, psychedelic drugs such as ibogaine have not been proven effective in treating opioid use disorder and may not compare well to existing and approved treatments. However, limited studies have shown decreased substance use after administration of psilocybin and ketamine. A 2014 open-label pilot study married a 15-week smoking cessation program with several doses of psilocybin. This study included 15 smokers who were considered psychiatrically healthy adults who had smoked an average of 19 cigarettes per day for an average of 31 years [91]. Psilocybin was administered during the 5th, 7th, and 13th week of the study. During the first four weekly meetings, cognitive-behavioral therapy was provided as was preparation for receiving psilocybin. A target quit date was set to occur with the first dosage of psilocybin during week five, when the subjects were given 20 mg/70 kg of psilocybin. Weekly meetings continued, and then on the seventh week, a higher dose of 30 mg/70 kg was given. During the 13th week, the higher dose of psilocybin was made optional for the subjects. Before the psilocybin was administered, subjects noted their motivational statement for smoking cessation. The subjects also participated in a guided imagery exercise at the end of the first psilocybin session [91]. At six-month follow-up, 80% of the former smokers (12 of 15) were abstinent from tobacco, as verified by breath and urine tests. This was a much higher abstinence rate than seen with traditional smoking cessation programs [91].
The researchers returned to their subjects later, reporting on smoking abstinence at 12 months and over the long term, with an average of 30 months after the study. They found that at the 12-month point, 67% were abstinent from smoking. At the long-term point, 60% were still smoking-abstinent, an excellent success rate [92].
In an older study of single versus repeated sessions of ketamine-assisted psychotherapy in 59 subjects who had detoxified from heroin, subjects were divided into two groups. The subjects in the first group received two addiction counseling sessions with ketamine, followed by two ketamine-assisted psychotherapy sessions, with sessions held at monthly intervals. The subjects in the second group received two addiction counseling sessions without ketamine and one ketamine therapy session. At the one-year follow-up point, 50% of subjects in the first group were still abstinent from heroin, versus 22.2% of subjects in the second group. The researchers concluded that three sessions in the ketamine-assisted psychotherapy program was more effective in promoting abstinence from heroin than one session followed by counseling [93]. There are also emerging data showing positive effects in alcohol use disorders and other substance use disorders.
It is important to keep in mind comparable efficacy. For opioid use disorder, it is vital to know both short- and long-term safety and efficacy comparisons to the standard of care (medication-assisted treatment plus therapy). Also consider that psychedelics will not be proved safe and effective by a professional consensus but rather by the FDA. It may be that psychoactive substances are legalized much in the same fashion cannabis has, but whether they are approved for clinical use will depend on the outcomes of Phase 2 and 3 FDA-qualifying clinical trials and safety and comparable efficacy trials. As of 2022, these trials are ongoing.
As discussed, research has demonstrated that psilocybin can be effective in improving mood and quality of life of patients with terminal cancer diagnoses. This aspect of cancer care has been largely overlooked and undertreated. Agrawal notes that, "Oncologists are well-equipped to fight the physical threats of cancer with powerful, yet sometimes imperfect tools including chemotherapy, radiation, and surgery, but they often feel helpless when it comes to treating the intense psychological agony many patients experience" [94]. A seminal study published in 2016 explored the use of a modest dose of psilocybin given to patients with terminal cancer under the supervision of trained therapists. The findings demonstrated that more than 80% of 51 patients who had received life-threatening cancer diagnoses and who subsequently developed depression or anxiety experienced significant and sustained improvements in mood and quality of life six months after taking psilocybin. In addition to feeling calmer and happier, the participants reported forging a closer connection with their friends and family [95]. This study demonstrated the careful and controlled use of psilocybin might be a safe and effective treatment for existential anxiety and despair that often accompany advanced-stage cancers. In addition, in limited studies, LSD has been found to significantly decrease anxiety levels in patients with life-threatening diseases.
Oncology and palliative care specialties have been associated with relatively high burnout rates, at least in part from seeing the psychological distress of patients with potentially terminal diagnoses. In this setting, any therapy that can improve patients' experiences and mood would be beneficial, and initial results of research incorporating psilocybin, LSD, and other psychedelics has been positive [94]. Agrawal further states [94]:
I have never witnessed the sort of dramatic response to any medical intervention as I have with some patients through psychedelic-assisted therapy. It is not a magic bullet or cure for a cancer patient's suffering—and it won't change their prognosis or life expectancy. But it could be a spark that begins their healing journey, helping them come to terms with their most difficult fears.
The use of psychedelic medications in end-of-life care is logical and should be tested compared to the standard treatment (counseling) in randomized, blind clinical trials and other investigations to facilitate FDA approval.
OCD can be an extremely debilitating disorder that is often difficult to treat. In a 2006 study of nine subjects with treatment-resistant OCD who were treated with psilocybin, the subjects experienced a significant decrease (range 23% to 100%) in OCD symptoms. One of the subjects experienced an issue with temporary hypertension. These are positive findings; however, it is obviously a very small study and additional research would be needed to replicate findings in a larger and more diverse group [96].
Other researchers have discussed the potential for the use of ketamine and esketamine in treating OCD [97]. In a 2013 randomized, double-blind, placebo-controlled, crossover study of drug-free adults with OCD, subjects were given two 40-minute intravenous infusions, one of saline and one of ketamine (0.5 mg/kg), spaced at least one week apart [98]. Individuals who received ketamine reported significant improvement in obsessions (measured by OCD visual analog scale) during the infusion compared with those given placebo. One-week post-infusion, 50% of those who had received ketamine met the criteria for treatment response (defined as a 35% or greater reduction in Yale-Brown Obsessive-Compulsive Scale scores); no subjects receiving placebo displayed treatment response after one week. The authors of this study concluded that "rapid anti-OCD effects from a single intravenous dose of ketamine can persist for at least one week in some patients with constant intrusive thoughts" [98]. However, other studies have found no effect on OCD symptoms [99]. Solid evidence is lacking and requires greater and more rigorous research.
In a study of 12 adults with autism and issues with severe social anxiety, subjects were randomized to receive either MDMA (75 mg or 125 mg) or placebo during the course of two 8-hour psychotherapy sessions. The MDMA was administered after a guided progressive muscle relaxation exercise. The experimental sessions were held one month apart and separated by three nondrug sessions of psychotherapy. The patients were provided with as few sensory interruptions as possible, such as soft lights, noise abatement, and fidget objects to help them with self-regulation through repeated actions (i.e., "stimming") [100]. On the Leibowitz Social Anxiety Scale, the MDMA group experienced a significantly greater improvement in social anxiety scores compared with the placebo group. Improvements persisted at six-month follow-up. The researchers said of the follow-up, "social anxiety remained the same or continued to improve slightly for most participants in the MDMA group after completing the active treatment phase" [100].
Social anxiety disorder is relatively common among the general population; about 12% suffer from this disorder at some point in their lives [101]. If it is determined to be an effective treatment, MDMA-assisted psychotherapy could be an option for these patients who have not responded to traditional psychotherapy or pharmacotherapy.
Anorexia nervosa is a severe eating disorder characterized by restriction of energy intake relative to an individual's requirements, typically resulting in low body weight and malnutrition. It is notoriously difficult to treat and has a high mortality rate. Experts have continued to search for more effective treatment options for this population.
In one study, the authors treated 15 patients (23 to 42 years of age) with treatment-resistant anorexia nervosa with infusions of 20 mg/hour of ketamine over 10 hours. The subjects were also given 20 mg twice per day of nalmefene. The subjects showed a marked decreased in scores on compulsion. Before the ketamine was administered, the average scores were 44.0; after treatment, mean compulsion scores dropped to 27.0. Nine of the subjects (60%) showed remission after two to nine ketamine infusions over the course of five days to three weeks [102]. The authors reported the following details on three specific patients [102]:
Patient 4 increased her weight after three treatments but agreed to more in the hope that her compulsion score would come down further. After a year in follow-up with a normal weight, she then started work and remained in a stable state while followed-up for nine months.
Patient 5 was a married woman and reached a normal weight after five treatments. As an outpatient, her periods returned and she had a successful pregnancy. Patient 6 had a long history of alternating anorexia and bulimia. After four treatments and despite only a small fall in compulsion score, she became able to control her eating and her weight. She held a responsible job with no relapse during two years of follow-up.
In a 2020 study with only one subject, the researchers treated a patient, 29 years of age, who had developed anorexia nervosa at 14.5 years of age and had been unable to attain remission. The researchers prescribed a ketogenic diet along with intravenous ketamine infusions. (A ketogenic diet was chosen because it had proven in the past to prevent starvation, a real risk with anorexia.) The patient sustained complete recovery and continued her ketogenic diet while maintaining a normal weight [103]. After three months, the woman remained on the ketogenic diet and reported feeling significantly better but still suffered from anorexic compulsions. At that time, she was sent for ketamine infusions. The patient reported that within one hour of her first infusion the "anorexic voice" inside her was decreasing and she felt more like herself. The patient had three more infusions over the next 14 days. After the fourth infusion, the patient stated [103]:
I know this sounds ridiculous, but I am no longer anorexic. I had so many rules I didn't even know them. But they are gone. I can exercise because it feels good. It isn't that I have to. I can stop when I want to.
Because this study had two potentially essential factors (ketamine and the ketogenic diet), it is unclear if either or both are responsible for the single patient's improvements. As is the case for many of these novel treatments, additional research is warranted.
Cluster headaches, which affect less than 1% of adults, are considered to be the most painful of all headaches and can last for a week or longer, potentially becoming a chronic health issue [104]. Traditional treatment approaches include triptan medications and oxygen therapy. Understandably, most sufferers seek quick relief and would prefer to never experience another attack.
In one report, the authors interviewed 53 people with cluster headaches who had self-medicated with psilocybin or LSD. (This is not recommended or considered safe.) Of 26 patients who used psilocybin, 22 said the drug successfully aborted their headache attacks. Of five people who said they used LSD to treat their headaches, four reported experiencing remission [105]. Based on these findings, the authors recommend further study of psychedelics as a possible treatment for cluster headaches. It is important to remember that self-reports are no basis for concluding that psilocybin or LSD is effective at improving a cluster headache condition. There is a current clinical trial underway examining the role of LSD as a possible treatment for cluster headaches [106].
In another study of 77 patients with treatment-resistant migraines or new daily headaches, all of whom had failed aggressive outpatient and inpatient treatment, patients were infused with ketamine. According to the researchers, the mean headache pain rating at the start of the study was 7.1; this fell to 3.8 upon discharge. Most of the patients responded well to the ketamine. Researchers concluded [107]:
Pending higher level evidence and given that ketamine is generally well-tolerated, ketamine may be considered a reasonable acute treatment for well-selected headache patients for whom standard therapies are either ineffective or medically contraindicated.
Some psychiatric disorders, particularly those with psychotic features such as schizophrenia, schizophreniform disorder, brief psychotic disorder, schizoaffective disorder, and delusional disorder, should certainly not be treated with psychedelic drugs. It is unclear if other psychiatric conditions would be amenable to psychedelic treatment. This can only be determined by clinical trials that administer these drugs under scientific rigor and with a sufficiently high number of patients. Many of the studies published to date have included very small numbers of patients, though this is largely because of necessity. It may have been that few individuals with the disorder could be recruited into a trial consisting of experimental treatment with a psychedelic drug. As the knowledge base grows based on clinical trials, it is hoped that it will become increasingly more feasible to test psychedelics on patients with a multitude of psychiatric disorders, particularly for those individuals whose conditions have been challenging to treat.
Electroconvulsive therapy has been in use for nearly a century and continues to be used in psychiatric treatment today. Newer forms of brain stimulation are increasing popular options for patients—or likely will be soon at major medical centers, including rTMS, VNS, and DBS. New brain mapping techniques may help eliminate the need for more invasive procedures. Interventional psychiatry represents an opportunity to help patients who otherwise have found no relief from pharmacotherapy and standard treatments [108].
For health professionals interested in the latest techniques on neuromodulation to aid patients with refractory psychiatric disorders, interventional psychiatry may be the answer. In order for physicians to effectively enter this field, experts recommend an additional year of training with an emphasis on interventional psychiatry.
ECT has been used to treat depression, bipolar disorder, schizophrenia, and other psychiatric diagnoses for many years, starting in the first half of the 20th century. The goal of ECT is to induce a seizure through applied electric shocks. The procedure was initially introduced in the late 1930s in Italy, and in the 1940s through the 1960s, ECT became popular in the United States as a mainstream treatment [109]. However, early treatments did not provide anesthesia and sometimes led to physical and psychological trauma [110]. Physicians later learned that significantly milder shocks could achieve the same goals.
Today, the procedure is used rarely for treatment-resistant depression and major depression with suicidal ideation or behaviors, as well as for schizophrenia and schizoaffective disorder. A team of professionals are involved, including a psychiatrist, a neurologist, an anesthesiologist, and a nurse [110]. Some believe that ECT should be used before psychedelics or newer brain intervention therapies are attempted, although agreement on this subject is not universal. It should also be noted that there is some residual fear/concern of ECT itself that persists among many patients (and some healthcare professionals), largely because ECT was historically traumatic. However, ECT has proven highly effective at treating both major depressive disorder and suicidal depression. About 100,000 patients receive ECT each year, and most of them are residents in psychiatric hospitals or psychiatric units of hospitals [111].
The modern use of ECT consists of [112]:
induction of brief general anesthesia (typically lasting less than 10 minutes), pharmacologic muscle relaxation, and continuous monitoring of oxygen saturation, blood pressure, and heart rate, and rhythm. An electrical charge is delivered to the brain through scalp electrodes, which results in a generalized seizure typically lasting for 20 to 60 seconds. Most patients receive between 6 and 12 treatments spaced over a period of 2 to 4 weeks as an initial course of treatment.
Patients who receive ECT may have mild-to-moderate cognitive side effects that generally resolve within days or weeks after the course of treatment has ended [112]. Improvement in depressive symptoms is apparent as soon as the third treatment, and remission rates may be as high as 60% among patients with treatment-resistant depression [113].
In a study of 31 patients with major depressive disorder who received ECT treatment, neurocognitive function was assessed with multiple tests, such as the MATRICS Consensus Cognitive Battery, the Everyday Memory Questionnaire, and the MADRS. These instruments were used before ECT, six weeks after ECT, and six months after the procedure. There was a significant decrease in depression scores six weeks and six months after ECT. Patients also exhibited significantly improved neurocognitive abilities six weeks subsequent to the ECT; these improvements were maintained at six months. The researchers concluded that improvements in depression and stability of subjectively reported memory function indicate that the antidepressant effects of ECT do not occur at the expense of cognitive function [114].
A Swedish analysis of 254,906 sessions of ECT conducted with 16,681 individuals between 2012 and 2019 found that fewer than 1% of individuals suffered broken teeth incurred as a result of their treatment. More specifically, the rate was 0.3% per individual, and there were no differences found between patients by age, gender, or diagnosis, although the dental fracture group had a greater number of treatments. Despite the low rate, bite guards and muscle relaxants are recommended to be used as a safety precaution during treatment with ECT [115].
In a 2021 survey of 192 ECT physician practitioners in the United States, 30% of the survey respondents had graduated from one of 12 residency programs in the United States. Several barriers to ECT programs were identified, stigma against ECT on the part of patients and problems with patient transportation, because patients cannot drive themselves home after treatment [116]. With regard to starting a new ECT program, barriers included lack of well-trained ECT practitioners, lack of institutional support or interest in leading the initiative, and insufficient physical space at the facility. The highest concentration of ECT providers were based in New England, and the lowest concentration was in the southern central region of the United States. Overall, the researchers were able to identify a variety of institution-related barriers (e.g., finances, bureaucracy, stigma, lack of understanding) that prevent enthusiastic adoption of this intervention. As a result, although ECT potentially could provide relief to many patients with treatment-resistant depression and other disorders, it may not be an option for many patients who live remotely from centers that offer this service.
In a 2018 study, a MarketScan database of more than 47 million patients was analyzed to determine the incidence of ECT. Of about 1 million patients with a mood disorder, 2,471 (0.25%) had received ECT. Individuals who had received ECT were five times more likely to have additional comorbid psychiatric disorders and twice as likely to have comorbid substance use disorder [117]. Whether ECT should be used more frequently is beyond the scope of this course, but it is important to understand that is can be an effective treatment even though it remains rarely used.
TMS, a noninvasive form of neural modulation, was initially developed in the 1980s. Later, it was discovered that repeated sessions of TMS (rTMS) were more effective than a single treatment. In 2008, the FDA approved rTMS to treat major depressive disorder; in 2018, it was approved to treat OCD [118]. Trials are also investigating the efficacy of rTMS in the treatment of substance use disorders with alcohol, opioids, cannabis, tobacco, methamphetamine, and cocaine [119]. The procedure is also used to treat patients with neurologic disorders, including Parkinson disease, multiple sclerosis, and stroke [120].
An increasingly popular procedure in the United States and other Western countries, rTMS is available at major medical centers throughout the country. This procedure uses large magnets to stimulate the neurons in the prefrontal cortex of the brain. An electromagnetic coil is placed on the patient's forehead at the site of the left prefrontal cortex, an area of the brain that often displays reduced activity in persons with severe and refractory depression. Nonpainful electromagnetic pulses pass through the skin and to the brain. There is no anesthesia needed or given with this procedure, and the only potential adverse effects are headache and minor discomfort in the scalp.
In a U.S. study involving 247 adults with severe treatment-resistant depression, the efficacy of rTMS in improving psychiatric symptoms was evaluated. The average age of the subjects was 43 years, and the average Patient Health Questionnaire-9 score was 21.7. The subjects received single 37-minute sessions over six weeks, up to a maximum of 30 total sessions [121]. Following rTMS therapy, there was a remission rate of 72% after three weeks, with no differences in response by sex of the subject, but age was a factor, with older individuals taking a longer time to achieve remission of their depression. In addition, remission correlated with past suicide attempts, previous psychiatric hospitalizations, and substance use disorder, illustrating that the procedure was highly effective for individuals with severe and/or comorbid disease. In this study, there was a higher efficacy with the MagVenture device compared with the NeuroStar device.
A Dutch study randomized 14 patients with alcohol use disorder to 10 days of rTMS therapy and 16 patients to sham rTMS. The patients were subsequently evaluated for alcohol craving and alcohol use. For a period of time, subjects in the rTMS treatment group reported lower levels of alcohol craving and use than those in the control group. Differences in alcohol craving in the study group were most prevalent 3 months after treatment; at the 12-month point, there were no differences between the two groups, indicating the beneficial effects of rTMS may fade over time [122].
Because rTMS is a safe and effective FDA-approved treatment for depression, some experts have recommended turning the treatment algorithm for depression upside down, putting TMS in a first-choice position. Rather than requiring patients to undergo months of potentially ineffective antidepressant trials, starting with TMS (with an artificial intelligence component to ensure the right dose and optimal targeting) may be a better option [123]. Additional studies are underway to examine TMS and expand evidence-based access to this treatment [123].
Another form of TMS, Stanford accelerated intelligent neuromodulation therapy (also known as Stanford neuromodulation therapy or SAINT), has been associated with an extremely high success rate in patients with treatment-resistant depression. In a 2022 study, nearly 80% of 29 subjects who had been depressed for a mean period of nine years experienced remission in just four weeks. This is a much quicker response time than traditional antidepressant therapy. The difference between SAINT and other TMS procedures lay with a greater number of treatments for a shorter time frame, such as 10-minute sessions 10 times per day. These treatments are also more targeted to the patient's brain circuitry [124].
VNS is an invasive form of neuromodulation consisting of implantation of a device that sends electrical pulses to the vagus nerve of the brain. The vagus nerve (also referred to as cranial nerve X) is very long and extends from the brain into the neck, chest, and abdomen. This nerve has many effects and impacts such diverse functions as mood, digestion, blood pressure, heart rate, immune function, saliva production, and taste [125].
The first VNS event occurred in the 1880s in New York, when James Corning applied an electrical current to a carotid compression fork, believing this approach would prevent or end seizures [126]. The procedure has evolved drastically to become the sophisticated procedure used today.
In 2005, the FDA approved VNS for the management of treatment-resistant depression [127]. Since then, a transcutaneous form of VNS has been developed, eliminating the need for surgery. However, this approach was not approved by the FDA as of 2022.
Some researchers have noted that cognitive dysfunction may accompany depression and be a factor in the associated reduced work productivity. A Canadian study analyzed the cognitive performance of individuals with treatment-resistant depression subsequent to their treatment with VNS. In 14 subjects, both the learning capabilities and memory of the subjects improved significantly after one month of receiving VNS. These cognitive improvements persisted for years subsequent to treatment with VNS. After VNS, 29% of the subjects experienced remission from treatment-resistant depression after 1 month, 50% after 3 months, 57% at 12 months, and 64% at 24 months. As such, at the end of the study, nearly two-thirds of patients had recovered with VNS therapy [128]. The researchers stated [128]:
Improvements were observed in measures of psychomotor speed, verbal fluency, attention, and executive functioning, as well as verbal and visual memory. We observed clear differences in improvement rate between cognitive measure. Memory measures, such as recall of a complex figure, as well as learning and recall of a word list, show more than 25% improvement after two months of treatment.
An invasive form of therapy that is used infrequently, DBS has proven effective at treating severe depression and OCD. DBS is also approved to treat some patients with severe, refractory neurologic disorders, such as epilepsy and Parkinson disease. DBS is also under investigation for the treatment of schizophrenia, Alzheimer disease, substance use disorder, and other challenging psychiatric disorders [129].
The first documented use of DBS occurred in 1948, when neurosurgeon J. Lawrence Pool implanted an electrode into the brain of a women with anorexia and depression. Results were initially positive, until the wire broke several weeks later [130]. Today, DBS involves the permanent implantation of electrodes that send regular and continuous electrical impulses to stimulate a specific part of the brain. Some describe DBS as a sort of brain pacemaker to correct imbalances, comparable to a heart pacemaker that corrects cardiac abnormalities. It should be noted that DBS is an invasive and expensive procedure that is only available to very few individuals, and it is not approved for the treatment of depression by the FDA as of 2022.
The electrodes used in DBS are made of platinum-iridium wires and nickel alloy connectors, which are enclosed in a polyurethane sheath [129]. Some patients may worry about the potential for hacking into a DBS system in today's connected world and the possibility of control over individuals, referred to as "brainjacking." This does not appear to be a problem at this time of very limited use of DBS, but it is a subject worthy of consideration in the future.
In a nationwide database of 116,890 hospitalized patients in the United States with major depressive disorder, patients receiving DBS represented 0.03% [131]. The average age of participants was 49.1 years; all were White, and 88% were female. Patients stayed in the hospital for 1 to 1.6 days. The highest rate of DBS use occurred in the southern United States, followed by the northeast and west. Patients receiving DBS either had private insurance or they were self-pay patients [131].
In a study of five patients with severe OCD who received DBS over the period 2015–2019, not only did the patients experience improvement in their OCD symptoms after DBS, but they also experienced a 53% improvement in their levels of depression (on the MADRS scale) and a 34.9% improvement on the Hamilton Anxiety Rating scales. In addition, patients also improved on the Quality of Life Enjoyment and Satisfaction Questionnaire [132]. The researchers reported anecdotal evidence of improvement as well, such as this report from one of the five patients [132]:
Despite persistent low body mass index [BMI] of 14, she has remained out of the hospital for 29 months, the longest time period since onset of OCD and anorexia. She is working part-time as a research assistant, is active in her church, and though she wishes for further reduction in symptoms, she notes her quality of life and mood is better than prior to DBS. In addition, she no longer engages in self-injurious behaviors and no longer experiences suicidal ideation.
In another study, DBS was used to treat seven patients with treatment-resistant depression [133]. Researchers specifically targeted the bilateral habenula, which is the seat of the anti-reward system [133]. After one month, depression and anxiety symptoms had decreased by 49%, and the patients reported a dramatic improvement in their quality of life.
In a one-person study of an individual treated with DBS for treatment-resistant depression, the patient experienced continuous improvement until depressive symptoms remitted by the 22nd week. At 37 weeks, the subject was randomized to continuous treatment or discontinuation. When treatment was stopped, the patient reported increasingly worse depression and anxiety until he met rescue criteria, resulting in the resumption of treatment. The depression symptoms rapidly abated when treatment restarted [134].
Although the news about both psychedelics and brain stimulation techniques is generally positive, caution is important, particularly in the case of psychedelic drugs. Patients should be actively discouraged from trying psychedelic drugs on their own, because these drugs can trigger an underlying psychosis in individuals who would otherwise likely have remained healthy, particularly because dosage and purity of the illicit drug is unpredictable. In addition, FDA-approval processes, regulated pharmaceutical drugs rather than street drugs, and comparable efficacy can help identify the safest and most effective medication or interventional treatment for a particular patient at a particular time. In essence, buying MDMA and taking it is not the same as being administered MDMA in a PTSD clinical trial at a research institution. Today, adulteration of street drugs is of great concern, particularly with potentially lethal doses of fentanyl [135].
Patients have no idea what dosage is in a street drug and could take a suboptimal dose (to no effect) or take an excessively high dose of the drug, which could cause inadvertent harm. Importantly, patients under the influence of such drugs require supervision, lest they take actions that might be potentially dangerous to themselves or others.
For patients considered for psychedelic or interventional psychiatric options who are not proficient in English, it is important that information regarding the risks associated with the use of psychedelics and/or interventional procedures and available resources be provided in their native language, if possible. When there is an obvious disconnect in the communication process between the practitioner and patient due to the patient's lack of proficiency in the English language, an interpreter is required. Interpreters can be a valuable resource to help bridge the communication and cultural gap between patients and practitioners. Interpreters are more than passive agents who translate and transmit information back and forth from party to party. When they are enlisted and treated as part of the interdisciplinary clinical team, they serve as cultural brokers who ultimately enhance the clinical encounter. In any case in which information regarding treatment options and medication/treatment measures are being provided, the use of an interpreter should be considered. Print materials are also available in many languages, and these should be offered whenever necessary.
It is apparent that psychedelic medicine is now in a renaissance period, and this time could not have come too soon. Many people in the United States and around the world suffer from severe psychiatric disorders, including depression, PTSD, substance use disorders, anxiety disorders, OCD, anorexia nervosa, and multiple other psychiatric disorders that are not readily responsive to treatment with pharmacotherapy and/or psychotherapy [136]. In the aftermath of the COVID-19 pandemic, depressive disorders are more prevalent, and people are urgently and actively seeking effective treatments. Exploration of novel interventional and psychedelic therapies may be a path to recovery for patients with mental health disorders who have not improved on traditional approaches [137].
1. Gukasyan N, Davis AK, Barrett FS, et al. Efficacy and safety of psilocybin-assisted treatment for major depressive disorder: prospective 12-month follow-up. J Psychopharmacol. 2022;36(2):151-158.
2. Gander K. Why Don't Antidepressants Work for Some People? Scientists Offer New Answer. Available at https://www.newsweek.com/why-dont-antidepressants-work-some-people-scientists-offer-new-answer-1372051. Last accessed June 13, 2022.
3. Huizen J. What To Know About Prozac Withdrawal Symptoms. Available at https://www.medicalnewstoday.com/articles/prozac-withdrawal. Last accessed June 13, 2022.
4. Ly C, Greb AC, Cameron LP, et al. Psychedelics promote structural and functional neural plasticity. Cell Rep. 2018;23(11):3170-3182.
5. Hesselgrave N, Troppoli TA, Wulff AB, Cole AB, Thompson SM. Harnessing psilocybin: antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice. Proc Natl Acad Sci U S A. 2021;118(17):e2022489118.
6. Grinspoon DH, Grinspoon L, Bakalar JB. Psychedelic Drugs Reconsidered. New York, NY: Basic Books; 1979.
7. Lucas R. FBI Data Shows an Unprecedented Spike in Murders Nationwide in 2020. Available at https://www.npr.org/2021/09/27/1040904770/fbi-data-murder-increase-2020. Last accessed June 13, 2022.
8. Gold M. Medicinal marijuana, stress, anxiety, and depression: primum non nocere. Mo Med. 2020;117(5):406-411.
9. American Association of Suicidology. Facts and Statistics. Available at https://suicidology.org/facts-and-statistics. Last accessed June 15, 2022.
10. National Institute of Mental Health. Suicide. Available at https://www.nimh.nih.gov/health/statistics/suicide. Last accessed June 21, 2022.
11. National Institute of Mental Health. Major Depression. Available at https://www.nimh.nih.gov/health/statistics/major-depression. Last accessed June 21, 2022.
12. Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results from the 2020 National Survey on Drug Use and Health. Available at https://www.samhsa.gov/data/report/2020-annual-national-report. Last accessed June 21, 2022.
13. PR Newswire. Psychedelic Drugs Market Size is Projected to Reach $10.75 Billion by 2027. Available at https://www.prnewswire.com/news-releases/psychedelic-drugs-market-size-is-projected-to-reach-10-75-billion-by-2027--301273405.html. Last accessed June 13, 2022.
14. Curtin SC, Hedegaard H, Ahmad FB. Provisional numbers and rates of suicide by month and demographic characteristics: United States, 2020. Natl Vital Stat Rep. 2021;16.
15. Psych. The Psychedelics as Medicine Report: Third Edition. Available at https://psych.global/report. Last accessed June 14, 2022.
16. National Conference of State Legislatures. State Medical Cannabis Laws. Available at https:www.ncsl.org/research/health/state-medical-marijuana-laws.aspx. Last accessed June 13, 2022.
17. Yakowicz W. U.S. House of Representatives Passes Federal Cannabis Legalization Bill MORE Act. Available at https://www.forbes.com/sites/willyakowicz/2022/04/01/us-house-of-representatives-pass-federal-cannabis-legalization-bill-more-act/?sh=4bfd858766d7. Last accessed June 13, 2022.
18. Hartman M. Cannabis Overview. Available at https://www.ncsl.org/research/civil-and-criminal-justice/marijuana-overview.aspx. Last accessed June 14, 2022.
19. Gilman IY, Shields KI. At Harvard, Psychedelic Drugs' Tentative Renaissance. Available at https://www.thecrimson.com/article/2022/2/19/psychedelics-tentative-renaissance/. Last accessed June 14, 2022.
20. Feuer W. Oregon Becomes First State to Legalize Magic Mushrooms as More States Ease Drug Laws in "Psychedelic Renaissance." Available at https://www.cnbc.com/2020/11/04/oregon-becomes-first-state-to-legalize-magic-mushrooms-as-more-states-ease-drug-laws.html. Last accessed June 14, 2022.
21. Lowe H, Toyang N, Steele B, et al. The therapeutic potential of psilocybin. Molecules. 2021;26(10):2948.
22. Yale School of Medicine. Grant Supports Development of Training for Psychiatrists in Psychedelic Medicine. Available at https://medicine.yale.edu/news-article/grant-supports-development-of-training-for-psychiatrists-in-psychedelic-medicine. Last accessed June 21, 2022.
23. U.S. Drug Enforcement Administration. List of Controlled Substances. Available at https://www.deadiversion.usdoj.gov/schedules. Last accessed June 21, 2022.
24. Breitbart W, Rosenfeld B, Pessin H, et al. Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA. 2000;284(22):2907-2911.
25. Grob CS, Danforth AL, Chopra GS, et al. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry. 2011;68(1):71-78.
26. Jones G, Ricard JA, Lipson J, Nock MK. Associations between classic psychedelics and opioid use disorder in a nationally representative U.S. adult sample. Sci Rep. 2022;12(1):4099.
27. Pisano VD, Putnam NP, Kramer HM, Franciotti KJ, Halpern JH, Holden SC. The association of psychedelic use and opioid use disorders among illicit users in the United States. J Psychopharmacol. 2017;31(5):606-613.
28. Rucker JJH, Iliff J, Nutt DJ. Psychiatry and the psychedelic drugs: past, present and future. Neuropharmacology. 2018;142:200-218.
29. Hoffman A. LSD My Problem Child: Reflections on Sacred Drugs, Mysticism, and Science. 4th ed. Santa Cruz, CA: Multidisciplinary Association for Psychedelic Studies; 2017.
30. Belouin SJ, Henningfield JE. Psychedelics: where we are now, why we got here, what we must do. Neuropharmacology. 2018;142:7-19.
31. Bonson KR. Regulation of human research with LSD in the United States (1949–1987). Psychopharmacology (Berl). 2018;235(2):591-604.
32. Johnson MW, Richards WA, Griffiths RR. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22(6):603-620.
33. Studerus E, Gamma A, Kometer M, Vollenweider FX. Prediction of psilocybin response in healthy volunteers. PLoS One. 2012;7(2):e30800.
34. Shapiro M. Inside the Johns Hopkins Psilocybin Playlist. Available at https://www.hopkinsmedicine.org/news/articles/inside-the-johns-hopkins-psilocybin-playlist. Last accessed June 14, 2022.
35. Schenberg EE. Psychedelic-assisted psychotherapy: a paradigm shift in psychiatric research and development. Front Pharmacol. 2018;9:733.
36. Whitehurst L. "Magic Mushrooms" for Therapy? Veterans Help Sway Conservatives. Available at https://www.sltrib.com/news/2022/04/17/magic-mushrooms-therapy. Last accessed June 14, 2022.
37. Dinis-Oliveira RJ, Pereira CL, da Silva DD. Pharmacokinetic and pharmacodynamic aspects of peyote and mescaline: clinical and forensic repercussions. Curr Mol Pharmacol. 2019;12(3):184-194.
38. Meinhardt MW, Pfarr S, Fouquet G, et al. Psilocybin targets a common molecular mechanism for cognitive impairment and increased craving in alcoholism. Sci Adv. 2021;7(47):eabh2399.
39. Rucker JJ, Marwood L, Ajantaival RLJ, et al. The effects of psilocybin on cognitive and emotional functions in healthy participants: results from a phase 1, randomised, placebo-controlled trial involving simultaneous psilocybin administration and preparation.J Psychopharmacol. 2022;36(1):114-125.
40. Zeiss R, Gahr M, Graf H. Rediscovering psilocybin as an antidepressive treatment strategy. Pharmaceuticals (Basel). 2021;14(10):985.
41. Carhart-Harris RL, Bolstridge M, Day CMJ, et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology (Berl). 2018;235(2):399-408.
42. Carhart-Harris R, Giribaldi B, Watts R, et al. Trial of psilocybin versus escitalopram for depression. N Engl J Med. 2021;384(15):1402-1411.
43. Daws RE, Timmermann C, Giribaldi B, et al. Increased global integration in the brain after psilocybin therapy for depression.Nat Med. 2022;28(4):844-851.
45. Sassano-Higgins S, Baron D, Juarez G, Esmaili N, Gold M. A review of ketamine abuse and diversion. Depress Anxiety. 2016;33(8):718-27.
46. Cleveland Clinic. Date rape drugs: what parents should know. Cleve Clin J Med. 2001;68(6):551-552.
47. Carboni E, Carta AR, Carboni E, Novelli A. Repurposing ketamine in depression and related disorders: can this enigmatic drug achieve success? Front Neurosci. 2021;15:657714.
48. Aust S, Gärtner M, Basso L, et al. Anxiety during ketamine infusions is associated with negative treatment responses in major depressive disorder. Eur Neuropsychopharmacol. 2019;29(4):529-538.
49. Feder A, Costi S, Rutter SB, et al. A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. Am J Psychiatry. 2021;178(2):193-202.
50. Abbar M, Demattei C, El-Hage W, et al. Ketamine for the acute treatment of severe suicidal ideation: double blind, randomised placebo-controlled trial. BMJ. 2022;376:e067194.
51. National Institute on Drug Abuse. MDMA (Ecstasy) Abuse Research Report: Introduction. Available at https://nida.nih.gov/publications/research-reports/mdma-ecstasy-abuse/Introduction. Last accessed June 14, 2022.
52. Vermetten E, Yehuda R. MDMA-assisted psychotherapy for posttraumatic stress disorder: a promising novel approach to treatment. Neuropsychopharmacology. 2020;45(1):231-232.
53. National Institute on Drug Abuse. MDMA (Ecstasy) Abuse Research Report: What is the History of MDMA? Available at https://nida.nih.gov/publications/research-reports/mdma-ecstasy-abuse/what-is-the-history-of-mdma. Last accessed June 14, 2022.
54. Mithoefer MC. A Manual for MDMA-Assisted Psychotherapy in the Treatment of Posttraumatic Stress Disorder. Version 8.1. Available at https://maps.org/2014/01/27/a-manual-for-mdma-assisted-therapy-in-the-treatment-of-ptsd/. Last accessed June 14, 2022.
55. Mithoefer MC, Feduccia AA, Jerome L, et al. MDMA-assisted psychotherapy for treatment of PTSD: study design and rationale for phase 3 trials based on pooled analysis of six phase 2 randomized controlled trials. Psychopharmacology (Berl). 2019;236(9): 2735-2745.
56. Mitchell JM, Bogenschutz M, Lilienstein A, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2021;27:1025-1033.
57. Ponte L, Jerome L, Hamilton S, et al. Sleep quality improvements after MDMA-assisted psychotherapy for the treatment of posttraumatic stress disorder. J Trauma Stress. 2021;34(4):851-863.
58. Jerome L, Feduccia AA, Wang JB, et al. Long-term follow-up outcomes of MDMA-assisted psychotherapy for treatment of PTSD: a longitudinal pooled analysis of six phase 2 trials. Psychopharmacology (Berl). 2020;237(8):2485-2497.
59. Centers for Disease Control and Prevention. Death Rate Maps and Graphs. Available at https://www.cdc.gov/drugoverdose/deaths/index.html. Last accessed June 14, 2022.
60. Hevesi D. Howard Lotsof Dies at 66; Saw Drug Cure in a Plant. Available at https://www.nytimes.com/2010/02/17/us/17lotsof.html. Last accessed June 14, 2022.
61. Noller GE, Frampton CM, Yazar-Klosinski B. Ibogaine treatment outcomes for opioid dependence from a twelve-month follow-up observational study. Am J Drug Alcohol Abuse. 2018;44(1):37-46.
62. Wilson C, Millar T, Matieschyn Z. Novel treatment of opioid use disorder using ibogaine and iboga in two adults. J Psychedelic Stud. 2021;4(3):149-155.
63. Mash DC, Duque L, Page B, Allen-Ferdinand K. Ibogaine detoxification transitions opioid and cocaine abusers between dependence and abstinence: clinical observations and treatment outcomes. Front Pharmacol. 2018;9:529.
64. Oesterle TS, Thusius NJ, Rummans TA, Gold MS. Medication-assisted treatment for opioid-use disorder. Mayo Clin Proc. 2019;94(10):2072-2086.
65. Partnership to End Addiction. Six States Ban Kratom Over Concerns About Addiction Potential. Available at https://drugfree.org/drug-and-alcohol-news/six-states-ban-kratom-concerns-addiction-potential. Last accessed June 15, 2022.
67. Ramanathan S, McCurdy CR. Kratom (Mitragyna speciosa): worldwide issues. Curr Opin Psychiatry. 2020;33(4):312-318.
68. Prozialeck WC, Edwards JR, Lamar PC, et al. Evaluation of the mitragynine content, levels of toxic metals and the presence of microbes in kratom products purchased in the western suburbs of Chicago. Int J Environ Res Public Health. 2020;17(15):5512.
69. Centers for Disease Control and Prevention. Multistate Outbreak of Salmonella Infections Linked to Kratom (Final Update). Available at https://www.cdc.gov/salmonella/kratom-02-18/index.html. Last accessed June 17, 2022.
70. McGill University. Press Release: LSD, a Future Anti-Anxiety Pill? Available at https://www.mcgill.ca/newsroom/channels/news/lsd-future-anti-anxiety-pill-338481. Last accessed June 15, 2022.
71. Gasser P, Holstein D, Michel Y, et al. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis. 2014;202(7):513-520.
72. Gasser P, Kirchner K, Passie T. LSD-assisted psychotherapy for anxiety associated with a life-threatening disease: a qualitative study of acute and sustained subjective effects. J Psychopharmacol. 2015;29(1):57-68.
73. Bruhn JG, De Smet PAGM, El-Seedi HR, Beck O. Mescaline use for 5700 years. Lancet. 2002;359(9320):1866.
74. Uthaug MV, Davis AK, Haas TF, et al. The epidemiology of mescaline use: pattern of use, motivations for consumption, and perceived consequences, benefits, and acute and enduring subjective effects. J Psychopharmacol. 2022;36(3):309-320.
75. Agin-Liebes G, Haas TF, Lancelotta R, Uthaug MV, Ramaekers JG, David AK. Naturalistic use of mescaline is associated with self-reported psychiatric improvements and enduring positive life changes. ACS Pharmacol Transl Sci. 2021;4(2):543-552.
76. Whizar-Lugo VM, Heredia-Mota C, Camacho A. Back to the future: ketamine and nitrous oxide in major refractory depression.J Anesth Crit Care. 2017;9(3):1-5.
77. Gillman MA. Mini-review: a brief history of nitrous oxide (N2O) use in neuropsychiatry. Curr Drug Res Rev. 2019;11(1):12-20.
78. Silverman M, Hexem J. Nitrous oxide/oxygen sedation and the single-dose sedative. Inside Dentistry. 2011;42-54.
79. Quach DF, de Leon VC, Conway CR. Nitrous oxide: an emerging novel treatment for treatment-resistant depression. J Neurol Sci. 2022;434:120092.
80. Nagele P, Duma A, Kopec M, et al. Nitrous oxide for treatment-resistant major depression: a proof-of-concept trial. Biol Psychiatry. 2015;78(1):10-18.
81. Dryden J. Press Release: Laughing Gas Relieves Symptoms in People with Treatment-Resistant Depression. Available at https://medicine.wustl.edu/news/laughing-gas-relieves-symptoms-in-people-with-treatment-resistant-depression. Last accessed June 15, 2022.
82. Nagele P, Palanca BJ, Gott B, et al. A phase 2 trial of inhaled nitrous oxide for treatment-resistant major depression. Sci Transl Med. 2021;13(597):eabe1376.
83. Palhano-Fontes F, Barreto D, Onias H, et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychol Med. 2019;49(4):655-663.
84. Osório Fde L, Sanches RF, Macedo LR, et al. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a preliminary report. Braz J Psychiatry. 2015;37(1):13-20.
85. Barker SA. Administration of N,N-dimethyltryptamine (DMT) in psychedelic therapeutics and research and the study of endogenous DMT. Psychopharmacology (Berl). 2022;239(6):1749-1763.
86. Romero S. Demand for This Toad's Psychedelic Toxin Is Booming. Some Warn That's Bad for the Toad. Available at https://www.nytimes.com/2022/03/20/us/toad-venom-psychedelic.html#:~:text=Toxin%20Is%20Booming.-,Some%20Warn%20That%27s%20Bad%20for%20the%20Toad.,at%20risk%20of%20population%20collapse. Last accessed June 15, 2022.
87. U.S. Food and Drug Administration. FDA Approves New Nasal Spray Medication for Treatment-Resistant Depression; Available Only at a Certified Doctor's Office or Clinic. Available at https://www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified. Last accessed June 21, 2022.
88. National Center for PTSD. How Common Is PTSD in Adults? Available at https://www.ptsd.va.gov/understand/common/common_adults.asp. Last accessed June 15, 2022.
89. Liriano F, Hatten C, Schwartz TL. Ketamine as treatment for post-traumatic stress disorder: a review. Drugs Context. 2019;8:212305.
90. Jerome L, Feduccia AA, Wang JB, et al. Long-term follow-up outcomes of MDMA-assisted psychotherapy for treatment of PTSD: a longitudinal pooled analysis of six phase 2 trials. Psychopharmacology (Berl). 2020;237(8):2485-2497.
91. Johnson MW, Garcia-Romeu A, Cosimano MP, Griffiths RR. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol. 2014;28(11):983-992.
92. Johnson MW, Garcia-Romeu A, Griffiths RR. Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse. 2017;43(1):55-60.
93. Krupitsky EM, Burakov AM, Dunaevsky IV, Romanova TN, Slavina TY, Grinenko AY. Single versus repeated sessions of ketamine-assisted psychotherapy for people with heroin dependence. J Psychoactive Drugs. 2007;39(1):13-19.
94. Agrawal M. Psychedelics May Ease Cancer Patients' Depression, Anxiety. Available at https://www.washingtonpost.com/health/2022/04/02/cancer-psychedelics-psilocybin-anxiety-depression/. Last accessed June 15, 2022.
95. Griffiths RR, Johnson MW, Carducci MA, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol. 2016;30(12):1181-1197.
96. Moreno FA, Wiegand CB, Taitano EK, Delgado PL. Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J Clin Psychiatry. 2006;67(11):1735-1740.
97. Martinotti G, Chiappini S, Pettorruso M, et al. Therapeutic potentials of ketamine and esketamine in obsessive-compulsive disorder (OCD), substance use disorders (SUD) and eating disorders (ED): a review of the current literature. Brain Sci. 2021;11(7):856.
98. Rodriguez CI, Kegeles LS, Levinson A, et al. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology. 2013;38(12):2475-2483.
99. Bloch MH, Wasylink S, Landeros-Weisenberger A, et al. Effects of ketamine in treatment-refractory obsessive-compulsive disorder. Biol Psychiatry. 2012;72(11):964-970.
100. Danforth AL, Grob CS, Struble C, et al. Reduction in social anxiety after MDMA-assisted psychotherapy with autistic adults: a randomized, double-blind, placebo-controlled pilot study. Psychopharmacology (Berl). 2018;235(11):3137-3148.
101. National Institute of Mental Health. Social Anxiety Disorder. Available at https://www.nimh.nih.gov/health/statistics/social-anxiety-disorder. Last accessed June 15, 2022.
102. Mills IH, Park GR, Manara AR, Merriman RJ. Treatment of compulsive behaviour in eating disorders with intermittent ketamine infusions. QJM. 1998;91(7):493-503.
103. Scolnick B, Zupec-Kania B, Calabrese L, Aoki C, Hildebrandt T. Remission from chronic anorexia nervosa with ketogenic diet and ketamine: case report. Front Psychiatry. 2020;11:763.
104. International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808.
105. Sewell RA, Halpern JH, Pope HG Jr. Response of cluster headache to psilocybin and LSD. Neurology. 2006;66(12):1920-1922.
106. U.S. National Library of Medicine. Lysergic Acid Diethylamide (LSD) as Treatment for Cluster Headache (LCH). Available at https://clinicaltrials.gov/ct2/show/NCT03781128. Last accessed June 20, 2022.
107. Pomeroy JL, Marmura MJ, Nahas SJ, Viscusi ER. Ketamine infusions for treatment refractory headache. Headache. 2017;57(2):276-282.
108. Nikayin S, Taylor JJ, Ostroff RB. Advanced training in interventional psychiatry. J Neurol Sci. 2022;434:120093.
109. Gazdag G, Ungvari GS. Electroconvulsive therapy: 80 years old and still going strong. World J Psychiatry. 2019;9(1)1-6.
110. Salik I, Marwaha R. Electroconvulsive Therapy. Available at https://www.ncbi.nlm.nih.gov/books/NBK538266. Last accessed June 15, 2022.
111. Mental Health America. Electroconvulsive Therapy (ECT). Available at https://www.mhanational.org/ect. Last accessed June 15, 2022.
113. Subramanian S, Lopez R, Zorumski CF, Cristancho Pilar. Electroconvulsive therapy in treatment resistant depression. J Neurol Sci. 2022;434:120095.
114. Mohn C, Rund BR. Maintained improvement of neurocognitive function in major depressive disorders 6 months after ECT. Front Psychiatry. 2016;7:200.
115. Göterfelt L, Ekman CJ, Hammar A, et al. The incidence of dental fracturing in electroconvulsive therapy in Sweden. J ECT. 2020;36(3):168-171.
116. Wilkinson ST, Kitay BM, Harper A, et al. Barriers to the implementation of electroconvulsive therapy (ECT): results from a nationwide survey of ECT practitioners. Psychiatr Serv. 2021;72(7):752-757.
117. Wilkinson ST, Agbese E, Leslie DL, Rosenheck RA. Identifying recipients of electroconvulsive therapy: data from privately insured Americans. Psychiatr Serv. 2018;69(5):542-548.
118. U.S. Food and Drug Administration. FDA Permits Marketing of Transcranial Magnetic Stimulation for Treatment of Obsessive Compulsive Disorder. Available at https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-transcranial-magnetic-stimulation-treatment-obsessive-compulsive-disorder. Last accessed June 21, 2022.
119. Mahoney JJ III, Hanlon CA, Marshalek PJ, Rezai AR, Krinke L. Transcranial magnetic stimulation, deep brain stimulation, and other forms of neuromodulation for substance use disorders: review of modalities and implications for treatment. J Neurol Sci. 2020;418:117149.
120. Lefaucheur JP, Aleman A, Baeken C, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018). Clin Neurophysiol. 2020;131(2):474-528.
121. Davila MC, Ely B, Manzardo AM. Repetitive transcranial magnetic stimulation (rTMS) using different TMS instruments for major depressive disorder at a suburban tertiary clinic. Ment Illn. 2019;11(1):7947.
122. Belgers M, Van Eijndhoven P, Markus W, Schene AH, Schellekens A. rTMS reduces craving and alcohol use in patients with alcohol use disorder: results of a randomized, sham-controlled clinical trial. J Clin Med. 2022;11(4):951.
123. LaFee S. Major Contract Fuels Three-University Study of TMS for Treating Depression. Available at https://health.ucsd.edu/news/releases/Pages/2022-02-22-major-contract-fuels-three-university-study-of-tms-for-treating-depression.aspx. Last accessed June 15, 2022.
124. Hageman M. This New Treatment Cured Depression in 80 Percent of People, Study Says. Available at https://bestlifeonline.com/depression-treatment-news. Last accessed June 15, 2022.
125. Cleveland Clinic. Vagus Nerve. Available at https://my.clevelandclinic.org/health/body/22279-vagus-nerve. Last accessed June 15, 2022.
126. Thompson SL, O'Leary GH, Austelle CW, et al. A review of parameter settings for invasive and non-invasive vagus nerve stimulation (VNS) applied in neurological and psychiatric disorders. Front Neurosci. 2021;15:709436.
127. U.S. Food and Drug Administration. FDA Approves First-of-Its-Kind Stroke Rehabilitation System. Available at https://www.fda.gov/news-events/press-announcements/fda-approves-first-its-kind-stroke-rehabilitation-system. Last accessed June 21, 2022.
128. Desbeaumes Jodoin V, Richer F, Miron JP, Fournier-Gosselin MP, Lespérance P. Long-term sustained cognitive benefits of vagus nerve stimulation in refractory depression. J ECT. 2018;34(4):283-290.
129. Krauss JK, Lipsman N, Aziz T, et al. Technology of deep brain stimulation: current status and future directions. Nat Rev Neurol. 2021;17(2):75-87.
131. Youssef NA, Dela Cruz SAMF, Riva-Posse P, Patel RS. Characteristics of patients who had deep brain stimulation for treatment-resistant depression from among 116,890 inpatients with major depressive disorder. Ann Clin Psychiatry. 2021;33(4):251-257.
132. Kahn L, Sutton B, Winston HR, Abosch A, Thompson JA, Davis RA. Deep brain stimulation for obsessive-compulsive disorder: real world experience post-FDA-humanitarian use device approval. Front Psychiatry. 2021;12:568932.
133. Zhang C, Zhang Y, Luo H, et al. Bilateral habenula deep brain stimulation for treatment-resistant depression: clinical findings and electrophysiological features. Transl Psychiatry. 2022;12(1):52.
134. Sheth SA, Bijanki KR, Metzger B, et al. Deep brain stimulation for depression informed by intracranial recordings. Biol Psychiatry. 2021; [Epub ahead of print].
135. U.S. Drug Enforcement Administration. Press Release: DEA Issues Public Safety Alert on Sharp Increase in Fake Prescription Pills Containing Fentanyl and Meth. Available at https://www.dea.gov/press-releases/2021/09/27/dea-issues-public-safety-alert. Last accessed June 15, 2022.
136. Correa da Costa S, Oesterle T, Rummans TA, Richelson E, Gold M. Psychedelic drugs for psychiatric disorders. Journal of the Neurological Sciences. 2022;440:120332.
1. U.S. Department of Veterans Affairs. VA/DoD Clinical Practice Guideline for the Management of Major Depressive Disorder. Washington, DC: U.S. Government Printing Office; 2022. Available at https://www.healthquality.va.gov/guidelines/MH/mdd/VADoDMDDCPGFinal508.pdf. Last accessed July 8, 2022.
2. National Collaborating Centre for Mental Health. Depression in Adults: Treatment and Management. London: National Institute for Health and Care Excellence; 2022. Available at https://www.nice.org.uk/guidance/ng222. Last accessed July 8, 2022.
Mention of commercial products does not indicate endorsement.