Complementary and alternative medicines are increasing in popularity, and most Americans now report the regular use of at least one supplement. Intact collagens and collagen peptides can be found in various forms, including powders, capsules, tablets, and liquids. They may be found in supplements marketed for skin and joint health, and they may also be found in protein powders marketed for nutritional purposes. Healthcare professionals should be well-informed about these products so that they can provide evidence-based recommendations.
This course is designed for healthcare professionals whose patients are taking or are interested in taking collagen products.
The purpose of this course is to provide healthcare professionals in all practice settings the knowledge necessary to increase their understanding of the various collagen products.
Upon completion of this course, you should be able to:
- Identify and describe the various forms of collagen available on the market.
- Discuss the evidence for the use of oral collagen for common indications.
- Review special considerations related to the use of collagen for certain patients and indications.
Chelsey McIntyre, PharmD, is a clinical pharmacist who specializes in drug information, literature analysis, and medical writing. She earned her Bachelor of Science degree in Genetics from the University of California, Davis. She then went on to complete her PharmD at Creighton University, followed by a clinical residency at the Children’s Hospital of Philadelphia (CHOP). Dr. McIntyre held the position of Drug Information and Policy Development Pharmacist at CHOP until her move to Washington state in 2017, after which she spent the next six years as a clinical editor for Natural Medicines, a clinical reference database focused on natural products and alternative therapies. She continues to create rigorous professional analysis and patient education materials for various publications while also practicing as a hospital pharmacist. Her professional interests include provider and patient education, as well as the application of evidence-based research to patient care.
Contributing faculty, Chelsey McIntyre, PharmD, has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
John M. Leonard, MD
Mary Franks, MSN, APRN, FNP-C
The division planners have disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Sarah Campbell
The Director of Development and Academic Affairs has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
The purpose of NetCE is to provide challenging curricula to assist healthcare professionals to raise their levels of expertise while fulfilling their continuing education requirements, thereby improving the quality of healthcare.
Our contributing faculty members have taken care to ensure that the information and recommendations are accurate and compatible with the standards generally accepted at the time of publication. The publisher disclaims any liability, loss or damage incurred as a consequence, directly or indirectly, of the use and application of any of the contents. Participants are cautioned about the potential risk of using limited knowledge when integrating new techniques into practice.
It is the policy of NetCE not to accept commercial support. Furthermore, commercial interests are prohibited from distributing or providing access to this activity to learners.
Supported browsers for Windows include Microsoft Internet Explorer 9.0 and up, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Supported browsers for Macintosh include Safari, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Other operating systems and browsers that include complete implementations of ECMAScript edition 3 and CSS 2.0 may work, but are not supported. Supported browsers must utilize the TLS encryption protocol v1.1 or v1.2 in order to connect to pages that require a secured HTTPS connection. TLS v1.0 is not supported.
The role of implicit biases on healthcare outcomes has become a concern, as there is some evidence that implicit biases contribute to health disparities, professionals' attitudes toward and interactions with patients, quality of care, diagnoses, and treatment decisions. This may produce differences in help-seeking, diagnoses, and ultimately treatments and interventions. Implicit biases may also unwittingly produce professional behaviors, attitudes, and interactions that reduce patients' trust and comfort with their provider, leading to earlier termination of visits and/or reduced adherence and follow-up. Disadvantaged groups are marginalized in the healthcare system and vulnerable on multiple levels; health professionals' implicit biases can further exacerbate these existing disadvantages.
Interventions or strategies designed to reduce implicit bias may be categorized as change-based or control-based. Change-based interventions focus on reducing or changing cognitive associations underlying implicit biases. These interventions might include challenging stereotypes. Conversely, control-based interventions involve reducing the effects of the implicit bias on the individual's behaviors. These strategies include increasing awareness of biased thoughts and responses. The two types of interventions are not mutually exclusive and may be used synergistically.
#98071: The Scoop on Collagen
Collagen is a hard, insoluble protein that occurs naturally in the human body and is found in the muscles, skin, tendons, ligaments, and connective tissues, including cartilage and bone. It is a structural protein, meaning that it is crucial to the shape and structure of cells and tissues in the body. Collagen typically occurs as long, thin, helical fibrils that are strong and flexible. There are many different types of collagen; however, types I, II, and III are thought to make up 80% to 90% of all collagen in the human body [1].
Collagen type I is the main form of collagen found in skin, tendon, ligament, and bone. It plays a major role as a scaffold in normal wound healing and is found in the extracellular matrix of blood vessels, skin, and other organs. It is found in collagen supplements and has been evaluated in clinical research [1,2].
Collagen type II is the main form of collagen found in cartilage. It is also found in other types of connective tissue but is primarily localized to the cartilage. It is found in collagen supplements and has been evaluated in clinical research [1,3].
Collagen type III is a major structural component of "hollow" organs such as large blood vessels, the uterus, and bowel. It is also thought to be crucial to brain development. Although it is found in collagen supplements, clinical research on its use is lacking [4].
Supplemental collagen products sometimes contain these three forms of collagen in their "intact," or undenatured, state. However, much more often, collagen supplements contain hydrolyzed collagen, or collagen peptides, that are derived from collagen types I, II, and III [1].
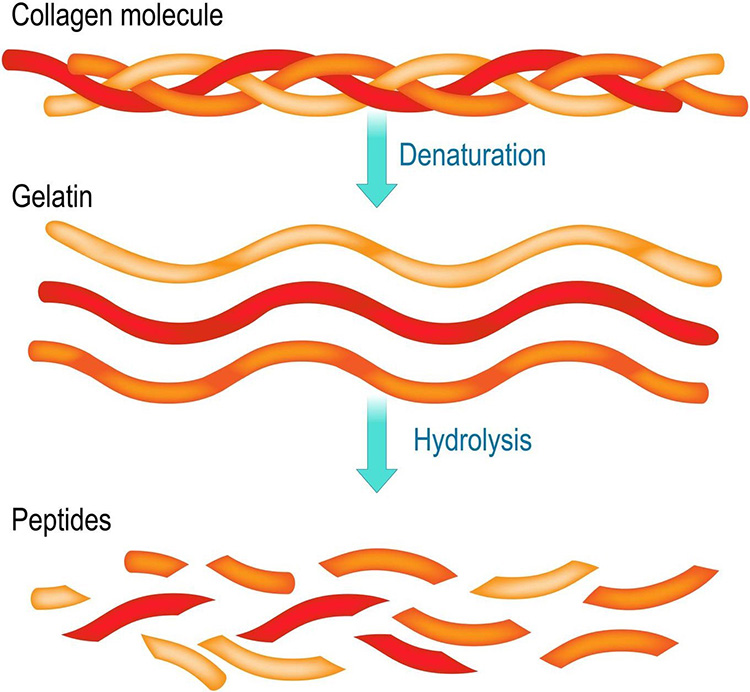
Gelatin is a purified protein substance that is formed by the partial hydrolysis of collagen. Gelatin is most commonly used as an inactive ingredient in the manufacturing of capsules, cosmetics, ointments, and suppositories. It is also used as a thickener in many foods, including jellied meats and marshmallows. A specific form of gelatin derived from donkey hide, called colla corii asini, has been used as a treatment in traditional Chinese medicine. However, due to its very different properties and limited medicinal use, gelatin will not be discussed further in this course [1].
Collagen peptides are created by enzymatic degradation of gelatin, via use of specific proteinases (Figure 1). This produces shorter peptides with an average size of 3.3 kilodaltons. Unlike gelatin, collagen peptides are soluble in water and do not form a gel [1]. Collagen peptides, which are also commonly referred to as hydrolyzed collagen or collagen hydrolysate, comprise the majority of collagen supplement products available on the market.
One of the reasons for the increasing prevalence of collagen peptide products is the fact that collagen peptides are up to 90% absorbed when taken by mouth. After absorption, they are distributed to target organs, including the dermis, muscle, bone, liver, kidney, and brain. It is thought that higher concentrations of these peptides at target locations will increase the production of collagen at those sites [1].
Intact collagens and collagen peptides can be found in various forms, including powders, capsules, tablets, and liquids. They may be found in supplements marketed for skin and joint health, and they may also be found in protein powders marketed for nutritional purposes. Collagens may be provided alone as a single ingredient, or in combination with other ingredients that are thought to benefit joint and skin health, such as chondroitin sulfate and hyaluronic acid [1].
Collagens may also be found in topical products. As collagen is an important structural component of the skin, there has been interest in applying it directly to the skin as a component of a cream, lotion, or gel. However, topical collagen does not appear to penetrate into the dermis and is not thought to provide benefit when applied topically [1].
Collagen products are derived from animal sources; there are no vegetarian sources of collagen protein. The most common sources are the bones, cartilage, and hides of pigs, donkeys, and cows. The bones and cartilage of chickens, as well as eggshell membranes, are another common source. Marine collagen is sourced from fish scales and skin. These sources can all be used to provide intact, or undenatured, collagen, as well as the source material for collagen peptides [1].
Be aware that some products may be marketed as "vegan collagen" or "plant-based collagen." These products do not contain collagen. Instead, they typically contain ingredients that are intended to boost the production of collagen in the body. Most of these products contain vitamin C, along with various other botanical ingredients. However, there is currently a lack of evidence supporting the use of these ingredients for the promotion of collagen production [1].
Collagen types I and II, as well as collagen peptides, have been evaluated for use in knee osteoarthritis, with studies suggesting the potential for modest benefit with certain formulations [2,3].
Collagen Type I
One clinical study of 58 patients with knee osteoarthritis shows that taking a specific bovine collagen type I (Colafit), 8 mg daily for three months, reduces pain by 41% and improves function by 38%, compared to changes of 10% and 11%, respectively, in patients taking placebo. These benefits persisted for one month after supplementation ended [2].
Larger, higher-quality studies are needed to determine if collagen type I is truly beneficial, and how it compares with other treatment options [2].
Collagen Type II
Oral collagen type II has shown modest benefit for knee osteoarthritis. Clinical research shows that taking a specific form of collagen type II (UC-II, InterHealth Nutraceuticals, Inc.) 40 mg daily for up to 6 months improves stiffness, pain, and function by about 20% when compared to baseline, and may improve pain and stiffness when compared with taking a combination of glucosamine 1,500 mg and chondroitin 1,200 mg. Also, a small clinical study shows that taking a different collagen type II product (UC II, SEMNL Biotechnology) 40 mg daily for three months improves pain by 36%, compared with an improvement of only 5% with placebo. It is unclear how collagen type II compares with other therapies for knee osteoarthritis [3].
Collagen Peptides
Some clinical studies in adults with knee osteoarthritis suggest that collagen peptides 10 grams, taken for three to six months, may modestly reduce pain. However, any improvements appear to be small, and not all research has yielded positive findings [1].
One clinical study shows that taking oral collagen peptides (Colnatur) 10 grams daily for five months reduces pain by at least 30% in 42% more patients when compared with placebo. However, there was no improvement when the Western Ontario and McMaster Universities (WOMAC) scale, a self-administered questionnaire assessing pain, stiffness, and physical function, was used. Sub-analyses suggest that the participants with the lowest dietary intake of meat, as well as those with the greatest joint degeneration at baseline, may have been more likely to experience benefit [1].
In another study, taking collagen peptides (Colatech) 10 grams daily for three months reduced pain by about 2 points on a 100-point scale, compared with a 0.9-point reduction in those taking glucosamine sulfate 1,500 mg. There was no reduction in the use of ibuprofen in these patients, so it is unlikely that this improvement would be considered clinically relevant [1].
Also, one large clinical study found no benefit with the use of collagen peptides 10 grams daily for six months when compared with placebo. There was a trend towards reduced pain and improved function in patients with severe symptoms at baseline, but further research is needed to confirm this finding [1].
Although some research suggests the potential for modest benefit, it is not clear that collagen peptides can improve pain to a clinically meaningful degree. Patients should not rely on collagen peptides for the management of knee osteoarthritis, although it may be considered as an adjunct therapy in patients with more severe disease [1].
Collagen type II is the only form of collagen that has been evaluated for use in rheumatoid arthritis (RA). Although the available research is mixed, the highest quality evidence suggests a lack of benefit [2].
Clinical research in adults with RA, some of whom were taking disease-modifying antirheumatic drugs (DMARDs), shows that taking chicken collagen type II 0.1–2.5 mg daily, or bovine collagen type II 0.1–0.5 mg daily, for six months does not improve clinical response rates based on the American College of Rheumatology (ACR) criteria when compared with placebo. Also, one clinical study in patients stabilized on methotrexate shows that switching to chicken collagen type II 0.5 mg daily increases disease activity when compared to continued treatment with methotrexate [2].
Some small, low-quality studies have suggested possible benefit with the use of collagen type II, but these findings are limited by poor study methodology. Also, when response rates are evaluated by the ACR criteria, any identified benefits seem to disappear [2].
Some very small, low-quality studies in children with juvenile idiopathic arthritis have suggested that chicken collagen type II may improve some symptoms. However, these studies did not enroll a control group, limiting the validity of these findings [2].
Based on the available evidence, patients with RA should be advised to steer clear of collagen supplements. However, it should be noted that the doses used in the available research are much lower than those used in other studies. Further research is needed to determine if higher doses may be beneficial for RA [2].
Collagen Peptides
Research in otherwise healthy athletes with joint pain suggests that collagen peptides may provide modest relief. A study in student athletes with joint pain shows that taking a specific product (FORTIGEL) providing collagen peptides 10 grams daily for 24 weeks modestly improves pain during movement and at rest when compared with placebo [1].
Similarly, another study in athletes with knee pain shows that taking collagen peptides 5 grams daily for 12 weeks reduces pain intensity during activity by 38% to 41%, compared with a 26% to 28% reduction in those taking placebo. However, there was no improvement in pain at rest or after the activity ended [1].
Collagen Type II
A clinical study in adults with exercise-induced joint pain shows that taking a specific collagen type II product (UC-II) 40 mg daily for four months increases knee extension by 9% when compared with placebo. But it is unclear if this improvement is clinically relevant, or if collagen type II reduces pain [3].
Research on the use of collagen peptides in older adults with joint pain has yielded conflicting findings. A study in patients 50 years of age or older with general joint pain shows that taking a specific collagen peptides product (Genacol) 1.2 grams daily for six months does not reduce pain or improve function. In contrast, another study in otherwise healthy adults 40 to 65 years of age with joint discomfort shows that taking collagen peptides (AVC-H2) 1.25 grams twice daily for eight weeks improves scores on the Western Ontario and McMaster Universities (WOMAC) scale by 37% at four weeks, compared with an improvement of only 14% in the placebo group. However, this improvement was not maintained at eight weeks. Until more is known, patients should not rely on collagen peptides for the relief of non-osteoarthritis joint pain [1].
There is interest in the use of collagen for various skin conditions, including acne, cellulite, dry skin, eczema, scleroderma, and melasma, but there is limited and/or conflicting research available on its use for these purposes. For now, research has focused predominantly on the use of collagen for reducing wrinkles due to age and photodamage. Although there has been interest in using all forms of collagen for this purpose, the available research has evaluated collagen peptides, as opposed to intact collagens [1,5].
Oral collagen peptides, when taken in doses of 4–10 grams daily for 4 to 12 weeks, have been shown to improve skin hydration and elasticity in older adults. However, not all studies have evaluated the visual appearance of wrinkles, and it remains unclear whether collagen peptides can improve the appearance of wrinkles to a cosmetically significant degree [1].
The studies that have evaluated this endpoint were all conducted in women older than 40 years of age and have yielded conflicting findings. One small clinical study shows that taking a low molecular weight collagen peptide product (Evercollagen) 1 gram daily for 12 weeks modestly improves visual appearance of wrinkles around the eyes when compared with placebo [1].
Another small study evaluating collagen peptides made from porcine collagen type I (VERISOL) shows that taking 2.5 grams daily for eight weeks reduces eye wrinkle volume by 20% when compared with placebo [1]. Additionally, a small study evaluating fish scale-derived collagen peptides (Wellnex) shows that taking 5 grams orally daily for eight weeks modestly improves wrinkle depth and roughness, but not wrinkle volume, when compared with placebo. Conversely, one small study shows that taking a fish-derived collagen peptide product (Vinh Wellness Collagen) 10 grams daily for 12 weeks does not improve patient-reported measures of wrinkles when compared with placebo [1].
There has been interest in the use of collagen for improving muscle function and strength in both younger and older adults. Thus far, the available research is limited and has only evaluated collagen peptides, not intact collagens.
One small study in about 60 active female runners undergoing resistance and endurance training three times per week shows that taking collagen peptides 7.5 grams twice daily for 12 weeks increases running distance by approximately 1,034 meters, compared with an increase of 703 meters in those taking placebo. However, it did not improve speed, squat leg strength, or endurance. A similar small study that evaluated untrained women shows that taking collagen peptides (Bodybalance) 15 grams daily in addition to resistance training three times weekly for 12 weeks improves hand-grip strength, but not leg strength, when compared with resistance training plus placebo [1].
Research in untrained men 30 to 60 years of age also shows that taking collagen peptides (Bodybalance) 15 grams daily in addition to resistance training three times weekly for 12 weeks improves hand-grip strength, but not leg strength, when compared with resistance training plus placebo. These participants also experienced an increase in fat-free mass. However, a post-hoc exploratory analysis suggests that the effects seen in this study are comparable to those seen with whey protein supplementation [1].
Research in healthy, recreationally active adults suggests that taking collagen peptides (Peptan) 15–20 grams daily, starting seven days prior to strenuous exercise, does not reduce exercise-induced muscle soreness when compared with placebo. It is unclear if a longer duration of supplementation would be more beneficial [1].
A small clinical study in elderly men shows that taking collagen peptides made from collagen type I as 15 grams daily for 12 weeks along with resistance training three times per week modestly increases fat-free mass and power when compared with resistance training plus placebo. It is unclear how this compares to supplementation with other forms of protein, such as soy and whey, which have also been studied for this purpose [2].
It is unclear how collagen peptides may compare with the use of other sports supplements or protein products. Until more is known, patients should not rely on collagen peptides for improving muscle strength or reducing muscle soreness [1].
Overall, collagen appears to be safe for adults when taken by mouth at doses evaluated in clinical research. For collagen peptides, these doses range from 2–10 grams daily for up to five months [1]. Collagen type I appears to be safe at a dose of up to 8 mg daily for up to three months, although much smaller doses of 0.5 mg daily have been used for up to one year [2]. Collagen type II appears to be safe when used at a dose of up to 40 mg daily for up to six months [3]. Collagen type III has not been adequately evaluated in clinical research; safety is unknown.
There are very limited reports of adverse effects with collagen products. Some minor gastrointestinal upset has been reported rarely with the use of collagen peptides [1]. Similarly, adverse effects with intact collagen have been reported in very few patients per clinical study, so it is unclear if those events can be attributed to collagen [2,3].
Safety in children, or during pregnancy or lactation, is unknown due to a lack of research. However, there is no biological reason to expect safety concerns in these populations as long as collagen is not relied upon as a sole source of dietary protein [1,2,3].
To date, there have been no reports of interactions between intact collagen or collagen peptides and any drugs or supplements [1,2,3].
Protein powder products that provide only collagen protein are becoming increasingly available. These products may claim to provide skin and joint health benefits. They may also be marketed for use by athletes and for overall nutrition.
Although there are no specific safety concerns related to the consumption of collagen protein, it is important to understand the composition of collagen in relation to other proteins. Many other proteins that are provided in protein powder products, such as pea, whey, soy, and lupin, are considered complete proteins. This means that they contain all essential amino acids, which are amino acids that cannot be made by the body and must be obtained from the diet [6,7].
Collagen, however, is not a complete protein. It lacks tryptophan, an essential amino acid that acts as a precursor to niacin (vitamin B3) in the body. Thus, collagen protein should not be relied upon as the primary source of protein in the diet. Mixed protein products, which contain protein from various sources, including collagen, can provide a healthy mix of essential and nonessential amino acids. Similarly, a diet that incorporates collagen protein powder as only one source of dietary protein can also provide a well-balanced array of amino acids [6,7,8].
Let patients know that a healthy diet includes proteins from various sources, with an emphasis on whole foods that naturally contain protein, so as to ensure adequate intake of a wide range of nutrients and amino acids.
As noted, all collagen products, whether provided intact or as peptides, are derived from animal sources. This may be a cause for concern for some patients who follow a vegetarian or vegan lifestyle, as well as those who observe certain religious or cultural practices [1,2,3].
Some manufacturers prepare collagen products in accordance with specific practices, such as Kosher or Halal, and will indicate this on the product label. Products containing solely chicken-derived collagen can be considered for patients who abstain from consuming red meat. Similarly, some products provide collagen obtained solely from marine sources; this may be an alternative for patients who do not have restrictions related to the consumption of fish. Marine collagen is obtained from the skin and scales of finned fish. It is unclear if people with an allergy to finned fish might be at risk for an allergic reaction to marine collagen; use with caution [1,2,3].
As various forms of collagen can be used to make collagen peptides, the actual composition of the peptides found in these products can vary significantly. Factors associated with this variation include the animal source, the age of the animal, and the extraction method used. Analyses of products available on the market suggest that different collagen products may not be interchangeable. For now, it may be best to recommend products that have demonstrated benefit in clinical research.
To ensure the selection of a high-quality product, look for third-party quality certification stamps. The National Sanitation Foundation (NSF) certifies collagen products through its NSF Certified for Sport program. For a product to obtain this stamp, the manufacturer must pass an inspection for Good Manufacturing Practices (GMP) every six months. Additionally, the product that carries this stamp is subject to random off-the-shelf testing that confirms the quality of the product. These products can be identified by the stamp on the product label [1,2,3].
Although verification by the United States Pharmacopeia (USP) is often recommended as the criterion standard for identifying a quality supplement, USP does not currently provide verification for collagen products [1,2,3].
For patients who are not proficient in English, it is important that information regarding the benefits and risks associated with the use of dietary supplements be provided in their native language, if possible. When there is an obvious disconnect in the communication process between the practitioner and patient due to the patient's lack of proficiency in the English language, an interpreter is required. Interpreters can be a valuable resource to help bridge the communication and cultural gap between patients and practitioners. Interpreters are more than passive agents who translate and transmit information back and forth from party to party. When they are enlisted and treated as part of the interdisciplinary clinical team, they serve as cultural brokers who ultimately enhance the clinical encounter.
Collagen products, including intact collagens and collagen peptides, are generally safe when used in studied doses. However, collagen protein does not provide all essential amino acids and should not be relied upon as a primary source of dietary protein. Additionally, as collagen is derived from animal sources, collagen supplements may not be an option for all patients.
Most research and interest in collagen are related to use for joint and skin health. The available clinical research suggests that certain forms of collagen may provide modest benefit for knee osteoarthritis and skin wrinkles, but the jury is still out on its use for other skin and joint conditions.
1. TRC Healthcare Natural Medicines Database. Collagen Peptides. Available at https://naturalmedicines.therapeuticresearch.com/Data/ProMonographs/Collagen-Peptides. Last accessed October 23, 2025.
2. TRC Healthcare Natural Medicines Database. Collagen Type I (Native). Available at https://naturalmedicines.therapeuticresearch.com/Data/ProMonographs/Collagen-Type-I-native. Last accessed October 23, 2025.
3. TRC Healthcare Natural Medicines Database. Collagen Type II (Native). Available at https://naturalmedicines.therapeuticresearch.com/Data/ProMonographs/Collagen-Type-II-native. Last accessed October 23, 2025.
4. Kuivaniemi H, Tromp G. Type III collagen (COL3A1): Gene and protein structure, tissue distribution, and associated diseases. Gene. 2019;707:151-171.
5. Aguirre-Cruz G, León-López A, Cruz-Gómez V, Jiménez-Alvarado R, Aguirre-Álvarez G. Collagen hydrolysates for skin protection: oral administration and topical formulation. Antioxidants (Basel). 2020;9(2):181.
6. U.S. Department of Agriculture. Dietary Guidelines for Americans (DGA) 2020–2025. Available at https://www.dietaryguidelines.gov/sites/default/files/2021-03/Dietary_Guidelines_for_Americans-2020-2025.pdf. Last accessed October 23, 2025.
Mention of commercial products does not indicate endorsement.