Neuromuscular blocking agents (drugs that paralyze skeletal muscle) have been used in anesthesia since the 1930s. Their use in the intensive care unit, emergency department, and prehospital care (e.g., ground and air ambulances) is somewhat more recent, though still well-established. Despite the fact that these drugs are commonly administered to critically ill patients, many practitioners have only the most rudimentary knowledge regarding the proper selection of these agents, their broad classifications, their dosages, the monitoring technique necessary for their use, and other crucial aspects of this class of drugs. Indeed, a number of potentially lethal side effects from the acute or chronic administration of these agents may occur, resulting in significantly increased morbidity and mortality among patients receiving them.
- INTRODUCTION
- A BRIEF HISTORY OF NEUROMUSCULAR BLOCKERS
- THE NEUROMUSCULAR JUNCTION
- NEUROMUSCULAR BLOCKING AGENTS
- MONITORING NEUROMUSCULAR BLOCKADE
- REVERSAL OF NEUROMUSCULAR BLOCKADE
- COMMON USES OF NEURO-MUSCULAR BLOCKING AGENTS AND AREAS OF CONCERN FOR THE PRACTITIONER
- CONCLUSION
- Works Cited
- Evidence-Based Practice Recommendations Citations
This course is designed for nurses, nurse practitioners, and other allied health professionals in a variety of settings, including the intensive care unit, emergency department, acute care, prehospital settings, critical care, and post-anesthesia care.
The purpose of this course is to provide intensive care, emergency, and prehospital providers with the clinical knowledge to administer neuromuscular blocking agents in a safe and effective fashion, as well as to know how such agents can be effectively monitored and, ultimately, safely and efficiently reversed.
Upon completion of this course, you should be able to:
- Review the pertinent history surrounding the discovery and early administration of neuromuscular blocking agents.
- Outline the anatomy and physiology of the neuromuscular junction.
- Identify commonly used neuromuscular blockers.
- Discuss the use and effects of benzylisoquinolinium nondepolarizing neuromuscular blocking agents.
- Describe the use and effects of amino steroid nondepolarizing neuromuscular blockers.
- Identify the crucial effects and side effects of succinylcholine, listing both relative and absolute contraindications to its use.
- Analyze approaches to monitoring neuromuscular blockade.
- Evaluate the effects and use of traditional agents used to reverse neuromuscular blockade.
- Discuss the reversal agent sugammadex.
- Analyze the role of neuromuscular blockers in various patient populations.
Richard E. Haas, BSN, MSN, EdM, PhD, CRNA, LTC US Army Nurse Corps (Retired), is a retired nurse anesthetist and prehospital registered nurse (instructor) who has published extensively in various areas of healthcare research while providing clinical care in arenas ranging from academic medical centers to austere environments in the third world during both wartime and peacetime. He has a bachelor’s degree in nursing from Georgetown University, Master’s degrees in education (Boston University) and nursing specializing in anesthesia (State University of New York in Buffalo and U.S. Army), and a PhD from the University of South Carolina. He is a retired lieutenant colonel in the U.S. Army Nurse Corps. He has taught nursing anesthesia, pharmacology, and physiology; mentored students in doctoral programs; and used advanced patient simulation to train students. Dr. Haas has worked in clinical, administrative, education, and research roles. He continues to work as an independent consultant, while taking more time to enjoy life with his wife of nearly 50 years and their children and grandchildren.
Contributing faculty, Richard E. Haas, BSN, MSN, EdM, PhD, CRNA, LTC US Army Nurse Corps (Retired), has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Margo A. Halm, RN, PhD, NEA-BC, FAAN
The division planner has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Sarah Campbell
The Director of Development and Academic Affairs has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
The purpose of NetCE is to provide challenging curricula to assist healthcare professionals to raise their levels of expertise while fulfilling their continuing education requirements, thereby improving the quality of healthcare.
Our contributing faculty members have taken care to ensure that the information and recommendations are accurate and compatible with the standards generally accepted at the time of publication. The publisher disclaims any liability, loss or damage incurred as a consequence, directly or indirectly, of the use and application of any of the contents. Participants are cautioned about the potential risk of using limited knowledge when integrating new techniques into practice.
It is the policy of NetCE not to accept commercial support. Furthermore, commercial interests are prohibited from distributing or providing access to this activity to learners.
Supported browsers for Windows include Microsoft Internet Explorer 9.0 and up, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Supported browsers for Macintosh include Safari, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Other operating systems and browsers that include complete implementations of ECMAScript edition 3 and CSS 2.0 may work, but are not supported. Supported browsers must utilize the TLS encryption protocol v1.1 or v1.2 in order to connect to pages that require a secured HTTPS connection. TLS v1.0 is not supported.
The role of implicit biases on healthcare outcomes has become a concern, as there is some evidence that implicit biases contribute to health disparities, professionals' attitudes toward and interactions with patients, quality of care, diagnoses, and treatment decisions. This may produce differences in help-seeking, diagnoses, and ultimately treatments and interventions. Implicit biases may also unwittingly produce professional behaviors, attitudes, and interactions that reduce patients' trust and comfort with their provider, leading to earlier termination of visits and/or reduced adherence and follow-up. Disadvantaged groups are marginalized in the healthcare system and vulnerable on multiple levels; health professionals' implicit biases can further exacerbate these existing disadvantages.
Interventions or strategies designed to reduce implicit bias may be categorized as change-based or control-based. Change-based interventions focus on reducing or changing cognitive associations underlying implicit biases. These interventions might include challenging stereotypes. Conversely, control-based interventions involve reducing the effects of the implicit bias on the individual's behaviors. These strategies include increasing awareness of biased thoughts and responses. The two types of interventions are not mutually exclusive and may be used synergistically.
#35111: Clinical Use of Neuromuscular Blocking Agents
For those who administer drugs, the five "rights" (i.e., right dose, right drug, right patient, right time, and right route) are emphasized beginning in the earliest days of instruction. There is, however, one class of drugs—neuromuscular blocking agents—that has the potential to end a patient's life each time they are administered, no matter how scrupulously the "five rights" are followed. The body's ability to move in order to flee adverse stimuli; to seek food, warmth, and shelter; and even to breathe has many redundant motor pathways, emphasizing the importance of motor function in the maintenance of homeostasis. The interruption of motor function by neuromuscular blocking agents places the patient's life in the hands of the practitioner administering the drug. With this in mind, the following continuing education course is designed to make the skilled practitioner aware of the problems that can occur with the administration of these agents, guide selection of the best agent for a particular intervention, and provide guidelines for monitoring a relaxed (i.e., paralyzed) patient and reversing the effects of these drugs.
The history of neuromuscular blockers, and how they came into day-to-day clinical use, is a fascinating one. Curare (Chondrodendron tomentosum) is the archetypal neuromuscular blocking agent, becoming popular in the 1930s, though it is no longer commonly used in practice in the United States [1]. Several authors describe the first "discoverer" of curare as Sir Walter Raleigh, though at least one expert disputes this idea [2,3,4]. Raleigh witnessed the natives in Guyana making a poison and applying it to the tips of their arrows when hunting monkeys. The slightest wound resulted in death to the monkey. In 1804, Charles Waterton (1782–1865) left England for his family's sugar estates in Guyana, and there he observed native Guyanese men hunting with curare. Encouraged by English scientists to find the secret of these poison arrows, Waterton brought curare back to England in 1812 for further study, thinking it might be especially useful in the treatment of rabies (then called hydrophobia) [5]. In the course of his time in Guyana, he witnessed an event that affected him profoundly: the death of a native man who was accidentally hit by his own arrow after missing the monkey at which it was aimed. His eyewitness description is [2]:
"Never," he (the Indian) said to his companion in a broken voice, and having looked at his bow while he spoke, "will I bend this bow again." Having spoken these words, he removed from his shoulder the small bamboo box that contained the poison, and having placed his bow and arrows on the ground, he lay down, said goodbye to his companion, and never spoke again. "It will be a consolation for tender souls," remarked Waterton, "to know that the victim did not suffer, because 'wourali' take life gently."
Waterton continued his research in curare, as did others, most notably the physiologist Claude Bernard (1813–1878), who in 1840 discovered that the cause of death of the drug was neuromuscular paralysis [4]. One of Bernard's early experiments involved the application of a piece of dried curare beneath the skin of a frog [2]. Bernard reported the following [6]:
On opening the poisoned frog, I saw that its heart continued to beat. Its blood became red on exposure to the air and appeared physiologically normal. I then used electrical stimuli as the most convenient method of provoking a reaction in the nerves and muscles. Stimulating the muscle directly produced violent contractions in every part of the body, but on stimulating the nerves there was no reaction. The nerves, that is, the bundles of nervous tissue, were completely dead, while the other bodily components, the muscles, the blood, the mucous membranes, retained their physiological properties for a number of hours, as one sees in cold-blooded animals.
Bernard then linked the effects of curare to its ability to paralyze skeletal muscle while allowing smooth muscle and cardiac muscle to continue to function. Though Bernard died in 1878, the clinical utility of his findings and their use in the care of patients did not begin to occur until the early 20th century. In 1932, West described numerous proposed uses of curare, though most of his uses differ diametrically from the reasons that neuromuscular blocking agents are administered today [7]. Indeed, West's efforts focused on the degree to which the side effects of curare could be best used in patient care. He explained making the drug itself from the raw material, which he describes as "a resinous mass of the consistency of hard toffee…incompletely soluble in water" [7]. The mass was sterilized and placed in glass ampoules of 2–20 mg designed for clinical administration. He injected the drug "hypodermically" into 30 patients and observed patients had no effects at 10 minutes, at which point a feeling of "giddiness" and "feeling stupid or fuddled" along with nystagmus began to occur. Blood pressure fell 20 to 30 mm Hg, and the hypotension was accompanied by both bradycardia and by a headache that could be "entirely relieved by administering 5 or 10 minims of adrenalin with or shortly after the curare injection" [7]. In retrospect, the "giddiness" and "fuddled" feelings could be the result of hypoxia, though ventilatory responses to the drug were not measured. His subsequent attempts to treat hypertension with curare resulted in elevations in blood pressure of 10 to 16 mm Hg, most likely associated with a sympathetic discharge associated with hypoxia, although again, no records of ventilatory response were obtained [7]. It is easy now, after 80 years of research, to be critical of West's findings. However, he helped advanced the research of the use of neuromuscular blockers when he stated, "There is the possibility of the myoneural junction being a more selective apparatus than it is usually considered to be. I have in mind a structure upon which curare could actually act selectively, removing discharges of certain electrical patterns, while allowing others to pass" [7]. In fact, he was describing the nicotinic acetylcholine receptor on the posterior neuromuscular junction far before it was discovered.
The researchers who best directed use of neuromuscular blockers into what they have become today are Griffith and Johnson who, in 1942, reported on the use of a new tool for general anesthesia: curare [8]. They reported that the use of curare in doses of "10–20 mg per 20 lbs. of body weight" improved skeletal muscle relaxation in their first cohort of 25 patients to whom it was administered. They described its effect in many of their patients, but it is important to note that in all of these cases, patients breathed spontaneously and required no "artificial respiration" [8]. In a particularly descriptive part of the paper, the authors described the surgeon's difficulty in closing an abdominal wound after an appendectomy. The administration of 5 mL of Incostrin (the trade name for curare) resulted in the patient's abdomen being "soft as dough" within one minute [8].
While curare began to take its place in the armamentarium of anesthesia through the 1940s, some disturbing data, most of it anecdotal, began to appear. Practitioners were finding that individuals receiving curare during surgery were dying at higher rates than those who did not. This led to the groundbreaking 1954 study of Beecher and Todd in which they reviewed 599,548 patients administered anesthetics between 1948 and 1952 [9]. The study placed a special emphasis on the curares, as described by the authors when they state, "The muscle relaxants have been singled out from the other agents and technics [sic] for particular discussion because of their newness, because of their greatly increasing use, and because their employment appears to be associated with certain anesthetic hazards not yet entirely clear, nor completely appreciated" [9]. Could it be that these new agents, in clinical use for less than two decades, would be removed from practice? Indeed, they found a six-fold increase in death rate when "curares" were used, as compared to when they were withheld [9]. The researchers, however, placed the responsibilities for these deaths not on the drug itself, but rather stated [9]:
The data presented strongly suggest that great caution in the use of the muscle relaxants is indicated, that the agents available at present be considered as on trial, and that they be employed only where there are clear advantages to be gained by their use, that they not be employed for trivial purposes, or as a corrective for generally inadequate anesthesia.
This is the best possible advice to all practicing clinicians when it comes to the use of neuromuscular blockade; it is only administered after careful risk/benefit considerations, and its use is not trivial. Though there are historical developments that occur after this, they are mainly in the creation of various types of agents.
The neuromuscular junction has been the subject of intense research over the past 50 years. The work of Griffith and Johnson in introducing curare into clinical practice prompted the work of two generations of bench scientists to uncover the secrets of this structure [8]. In order to understand why neuromuscular blocking agents work the way they do, it is vital to understand the structural anatomy and physiology related to the neuromuscular junction.
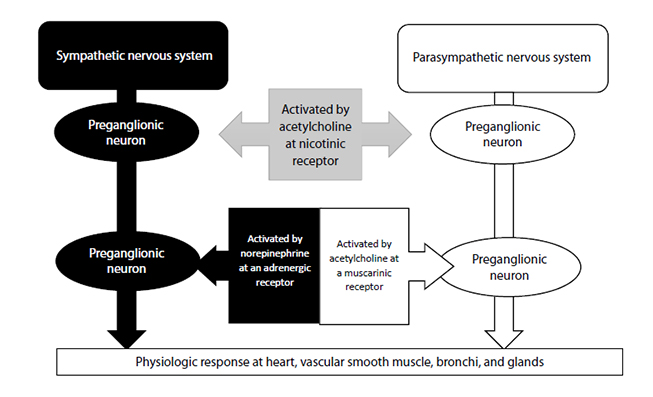
The cells in the brain communicate with those in the rest of the body by sending and receiving small electric impulses (called action potentials) along nerves from one area of the body to another. When one wishes to move, specialized areas in the brain send action potentials along specific motor neurons that descend through the spinal cord. Bundles of neurons, running together within an anatomic sheath, are called nerves. The neurons comprising the nerves are myelinated; that is, each neuron is wrapped in a series of Schwann cells, increasing the speed of transmission of a muscle action potential along the nerve through a process called saltatory conduction[10]. There are small spaces between the Schwann cells called the nodes of Ranvier, and the underlying nerve is exposed at these points. These exposed areas of the nerve allow the action potential to skip from space to space, significantly increasing the speed of transmission of the impulse[10]. The point at which two nerves meet is called a synapse.
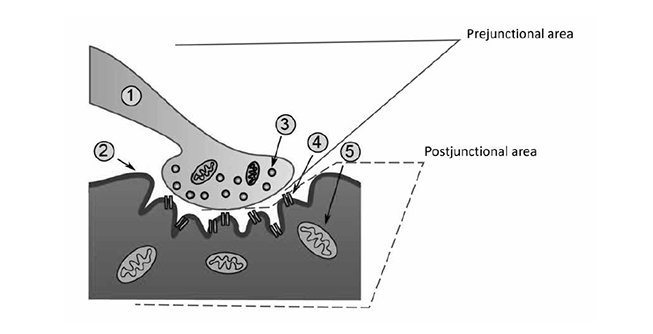
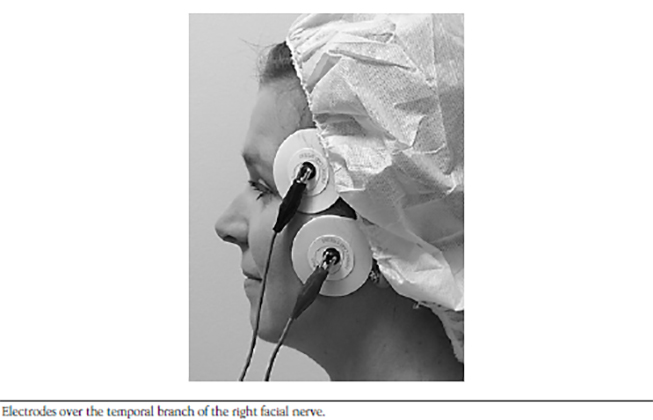
The neuron that sends the signal by releasing a neurotransmitter is called a first-order or, more commonly, a prejunctional neuron. The neuron or muscle cell that receives the action potential can be referred to as a second-order or postjunctional neuron. These second-order neurons, or the second nerve in a series of connecting nerves (bundles of which are called peripheral nerves), exit the ventral horn of the spinal cord and begin branching, creating 10 to 300 separate nerve fibers, traveling throughout the body, and finally ending in skeletal muscle fibers[11]. The point at which the nerve meets the muscle is referred to as the neuromuscular junction, and the muscle fiber is the postjunctional area (Figure 1). Though the nerve terminal (or endpoint of the motor nerve) approaches each individual muscle fiber, it does not contact it. Instead, the terminal ending of the nerve sits in a highly specialized invagination of the muscle fiber called the neuromuscular junction. Each muscle fiber has its own neuromuscular junction.
The neuromuscular junction is a complex of numerous anatomic structures and is itself a synapse. At any synapse, some ligand (an intracellular substance) is released from a prejunctional neuron, crosses a synaptic space, and binds with a receptor on a postjunctional neuron (or postjunctional muscle). As noted, the terminal nerve ending does not touch the muscle fiber itself. Rather, there is a small space between the two structures, referred to as the synaptic cleft or the synaptic space [10,11]. The terminal nerve ending consists of thousands of small synaptic vesicles that contain the neurotransmitter acetylcholine. When an action potential leaves the spinal cord via a lower motor neuron and travels to the terminal nerve ending, the amount of voltage within the nerve changes as the impulse passes. When the action potential (electric impulse) arrives at the prejunctional nerve terminal, the resting membrane potential (i.e., the electric "charge" inside the cell) suddenly increases from a resting level of -90 millivolts to a depolarizing level of +50 millivolts [11]. This change in voltage activates small receptors in the prejunctional neurons called voltage-gated calcium channels. These channels are specialized proteins that open and close, creating a tube through which calcium ions (Ca2+) can flow when open [10]. Because there is 10,000 times more calcium outside of the nerve cell than inside, Ca2+ rushes through these tubes into the prejunctional nerve cell [10]. The entry of calcium effects a change in the vesicles, causing them to move toward and fuse with the cell membrane. After the fusion takes place, the vesicles are opened to the extracellular space, resulting in thousands of molecules of acetylcholine being released from the prejunctional neuron and entering the synaptic space [10].
Acetylcholine is one of the most important neurotransmitters in the human body. It is crucial for the function of both the autonomic and motor nervous systems. This course will focus on acetylcholine's role in ultimately making skeletal muscle contract.
Acetylcholine is synthesized in the neuron in both the soma and the terminal nerve ending[10]. It is the product of acetyl-coenzyme-A, produced by the mitochondria, and choline from dietary sources. These substances are combined in the presence of the enzyme choline acetyl-transferase and then placed in the vesicles.
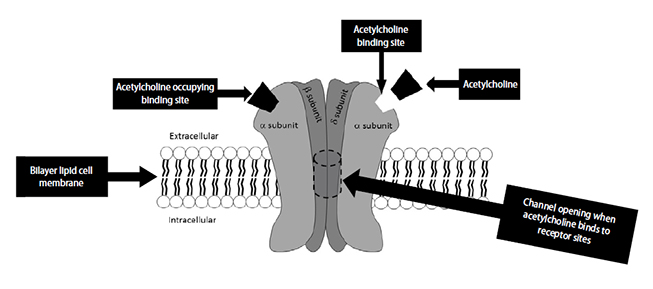
After being released from the vesicles, acetylcholine molecules travel a short distance across the synaptic cleft, where they bind to the nicotinic acetylcholine receptor (Figure 2)[11,12]. This receptor is crucially important to neuromuscular function and has been intensively studied. The receptor is a large protein that spans from the cytosol outside the muscle cell to the cytoplasm on the inside of the cell [13]. The protein has five major subunits: two alpha, two beta, and one delta. There are two acetylcholine binding sites on the receptor. Once bound, the receptor undergoes a change in shape and creates an opening through which sodium ions (Na+) can pass. As Na+ passes into the muscle cell, the electrical charge in the muscle rises, causing a muscular action potential, similar to the neuronal action potential.
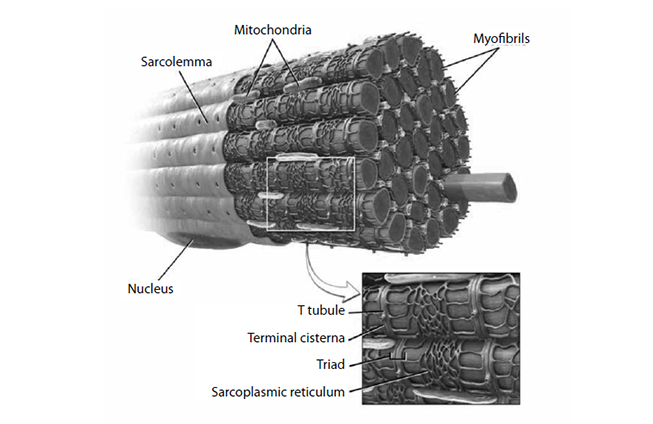
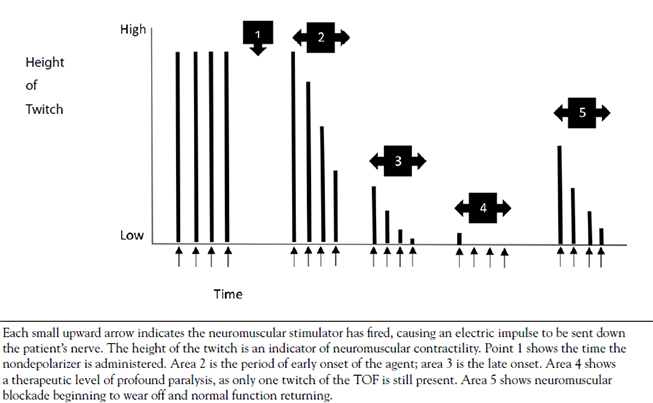
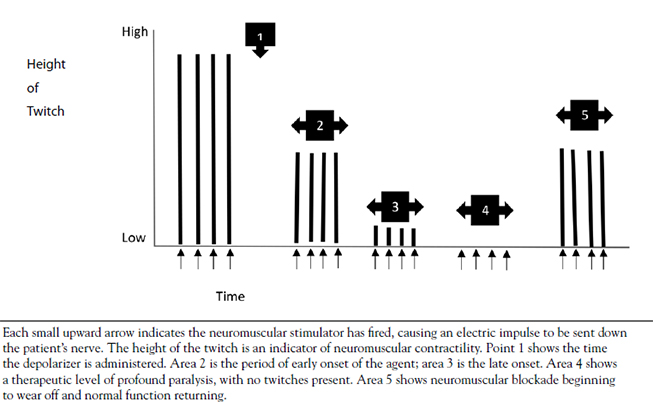
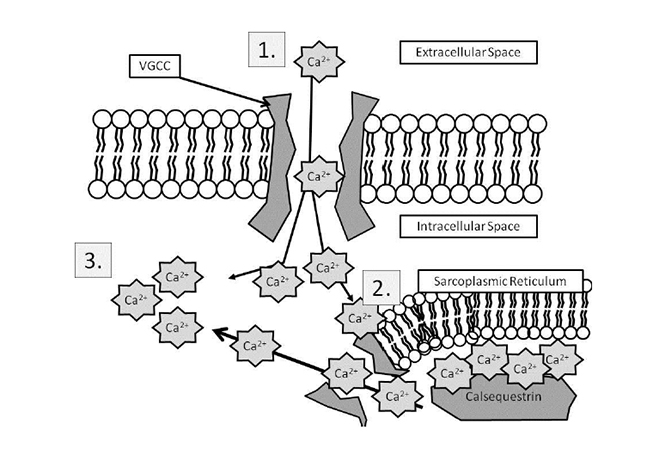
Muscle cells have their own electrical charge when at rest—in this case, approximately -90 millivolts [14]. When Na+ enters the muscle, this potential rises and causes the depolarization of the cell, sending an action potential into the muscle fiber. Muscles, however, are anatomically thicker than neurons, and the action potential must activate the fibers inside a mass of muscle (Figure 3) in the same manner it activates those closer to the surface. The t-tubules accomplish this, as they dive deep into the muscle and allow the action potential to spread. In the presence of this action potential, a series of vesicular structures called the sarcoplasmic reticula become permeable to Ca2+. This happens via specific channels on the sarcoplasmic reticulum called ryanodine receptors. Figure 4 illustrates the entry of Ca2+ into the skeletal muscle, which then binds with the ryanodine receptor, releasing more Ca2+ from the sarcoplasmic reticulum and allowing a buildup of Ca2+ in the cell [15]. Ca2+ is important in beginning and maintaining the contraction of skeletal muscle.
CALCIUM RELEASE IN SKELETAL MUSCLE
 | ||||
|
The operant part of the skeletal muscle is the interdigitation of actin and myosin fibers within the muscle cell. Actin has binding sites for the myosin heads of the myosin fibers, but at rest, these are covered by a structure called the troponin-tropomyosin complex. The tropomyosin portion of this complex is tightly wound around the actin fiber and covers the actin-binding sites [10]. Once covered, the myosin heads remain quiescent, unable to bind with the sites. In the presence of increased Ca2+ levels, such as those that occur after an excitatory muscle potential, the binding sites are uncovered. The Ca2+ binds with one part of the troponin-tropomyosin complex called troponin C. Once bound, the tropomyosin undergoes a conformational change and "rolls" off the actin binding sites. Myosin heads, in the presence of magnesium (Mg2+) and adenosine triphosphate (ATP), bind with the actin binding sites. As the ATP dissociates into adenosine diphosphate (ADP) and a free phosphate ion, the head of the myosin assumes an acute angle, and the myosin head binds with the now-exposed actin-binding site, releasing the ADP and phosphate ion. The myosin head has been compared to a "cocked spring" [10]. Once bound to actin, the spring releases, causing the head to change to a more acute angle, thus pulling the actin fibers closer together. In the presence of ATP, the myosin releases from the actin-binding site. Ca2+ is actively pumped back into the sarcoplasmic reticulum and pumped outside the muscle cell, and the process is free to begin again. Indeed, the muscle stiffness called rigor mortis seen in individuals who have died is the result of a lack of ATP allowing the myosin heads to release from the actin fibers.
Understanding the nature by which muscle contracts in the face of the stimulation of the neuromuscular junction is at the heart of understanding how to safely administer neuromuscular blocking agents. As described, there are two binding sites for acetylcholine on the alpha subunits of the neuromuscular junction.
Some neuromuscular blockers, called depolarizing agents, have structures similar to acetylcholine. These agents bind with the neuromuscular junction, causing it to discharge and initiating random depolarization of skeletal muscle, similar to acetylcholine. The difference is that these agents remain bound to the receptor and do not allow the muscle to repolarize. In short, the muscle shortens, then relaxes, and then remains relaxed for a longer period than it would if the depolarization was caused by acetylcholine [15].
Other agents, called nondepolarizing agents, bind with the receptor but do not cause it to be activated. These are considered true antagonists, because they interact with the receptor but the interaction results in no downstream activity. In the case of these agents, the muscles progressively weaken as impulses through the neuromuscular junction are halted. As drug molecules redistribute to other tissues and/or undergo degradation, their numbers decrease, which again allows acetylcholine to function normally and muscle to contract and relax [15,16].
Motor nerves themselves will still conduct motor action potentials; it is only at the point where the nerve meets the muscle that contractility of the skeletal muscle fibers is obtunded. Further, administration of reversal agents, which either increases the amount of acetylcholine or surrounds the neuromuscular blocking agent molecules to render them inert, will result in the accelerated return of normal neuromuscular function [16].
As noted, there are two major classes of neuromuscular blocking agents: depolarizing and nondepolarizing. These agents interfere with the normal transmission of messages from the prejunctional neuron to the postjunctional muscle fiber.
There is only one depolarizing agent currently available for use in the United States—succinylcholine[16]. As discussed, the nicotinic acetylcholine receptor has two binding sites, one on each alpha subunit. When bound to these sites, succinylcholine causes an uncontrolled depolarization of the muscle cell, resulting in random muscle movement (fasciculation). The binding of succinylcholine with these sites prevents the repolarization of the muscle, causing paralysis.
There are numerous nondepolarizing agents, which are subsequently broken down into two major subgroups: benzylisoquinoliniums and amino steroids[16,17]. However, both of these groups have the same mechanism of action when it comes to blocking neuromuscular transmission. Upon injection, they travel to the nicotinic receptor and bind with one of the two receptors. As can be recalled, in order for a neuromuscular action potential to be passed from the nerve to the muscle, both binding sites on the receptor must be occupied by acetylcholine[10]. As the binding of a nondepolarizer to one of the two binding sites will prevent this, neuromuscular excitation is precluded and no muscular contraction will occur.
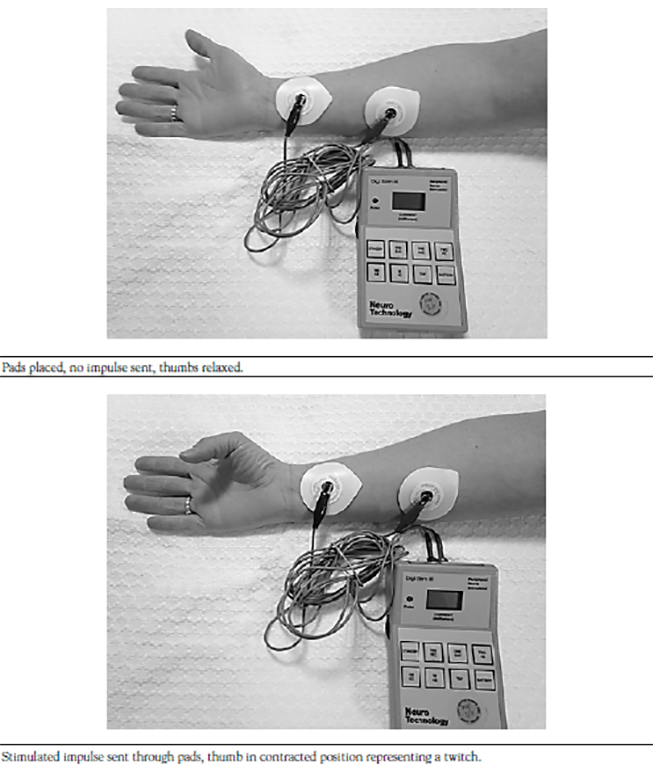
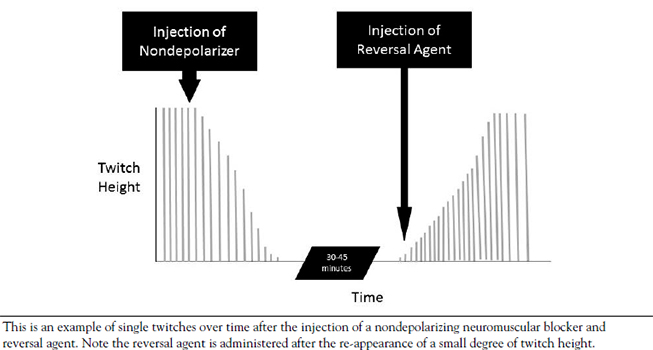
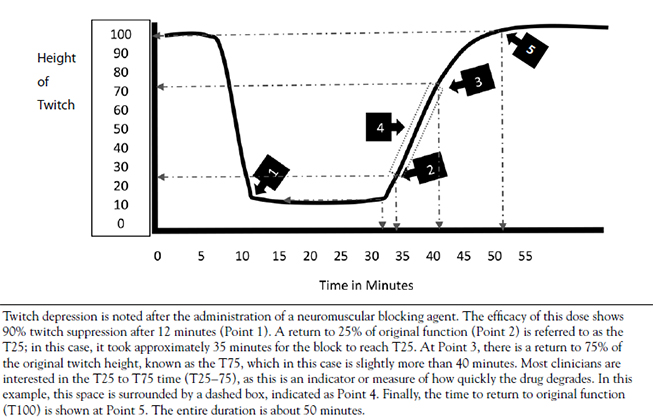
During the testing and development of neuromuscular blocking agents, a dose that results in some level of paralysis in a representative sample of patients is established. If the dose results in an effect in 95% of the patients tested, that dose is referred to as the ED95 or the estimated dose at which 95% of patients receive the desired effect (i.e., paralysis) [16]. Though an agent may have an ED95, there is often a significant time lapse between the time of administration and peak effect. During endotracheal intubation and airway management, time is of the essence. The prolonged onset of these agents in lower doses may be deleterious, so most agents used for endotracheal intubation are administered to patients at two to three times the ED95[14,17]. This increased dose speeds the onset of action, though it is important for the practitioner to remember that the increased dose also prolongs the effect of the injected agent. Table 1 shows the typical ED95 dose as well as the intubating dose of commonly used neuromuscular blocking agents [17].
NEUROMUSCULAR BLOCKING AGENT DOSAGES
| Agent | Approximate ED95 | Factor to Calculate Intubating Dose (× ED95) | Intubating Dose | Intubating Dose For A Healthy 80-Kg Male |
|---|---|---|---|---|
| Atracurium | 0.25 mg/kg | 2 | 0.5 mg/kg | 40 mg |
| Cisatracurium | 0.05 mg/kg | 5 | 0.3 mg/kg | 24 mg |
| Mivacurium | 0.07 mg/kg | 2 | 0.15 mg/kg | 12 mg |
| Pancuronium | 0.07 mg/kg | 1.5 | 0.1 mg/kg | 8 mg |
| Rocuronium | 0.3 mg/kg | 4 | 1.2 mg/kg | 100 mg |
| Succinylcholine | 1 mg/kg | 1.5 | 1.5 mg/kg | 120 mg |
| Vecuronium | 0.05 mg/kg | 4 | 0.2 mg/kg | 16 mg |
There are a number of amino-steroid agents on the market today, most of them routinely used. These agents all have a quaternary amine structure, which prevents them from crossing the blood-brain barrier and interacting with cholinergic receptors in the brain. They are also highly water-soluble, allowing them to move quickly from central circulation to the peripheral neuromuscular junctions on skeletal muscle. In nearly all cases, these agents are metabolized in the liver and excreted by the kidneys.
Pancuronium bromide is a long-acting, nondepolarizing neuromuscular blocking agent. It is an older agent (as are most neuromuscular blockers) first synthesized in 1966 [16]. The agent can be used for long procedures in the operating room, prolonged air or ground transport during which paralysis is required for patients who are mechanically ventilated, and in the intensive care unit (ICU) for patients requiring immobility for long periods of time [18]. Table 2 provides the dosing regimens for pancuronium.
Cardiovascular Effects
Pancuronium bromide is noted for its cardiovascular stability, with a slight propensity to cause minor tachycardia in higher doses. This mechanism is related to a combination of blockade of muscarinic receptors and blockade of the re-uptake of norepinephrine in the periphery [20,21]. In one study of patients undergoing open-heart surgery, pancuronium increased heart rate an average of 8 beats per minute (from 53 to 61) while changing neither arterial blood pressure nor cardiac index [22]. Indeed, the authors stated this mild increase in heart rate may be "beneficial" [22].
Hepatic Effects
As pancuronium undergoes 40% to 60% of its degradation in the liver, patients with cirrhosis may expect prolonged time to recovery [16]. The key to determining the duration of pancuronium's effects is a patient's hepatocyte status. Laboratory indications of destruction of hepatic parenchyma should lead to caution in the administration of pancuronium, as its action may be substantially lengthened [23]. In the presence of patients taking other drugs that undergo extensive degradation by the liver (e.g., phenytoin), the upregulation of microsomal enzymes may result in a substantial decrease in the duration of action [16,24].
Renal Effects
Pancuronium is 85% eliminated by the kidneys [25]. In patients with renal failure, the substantial need for renal degradation of the agent combined with decreased glomerular filtration rate can result in prolonged duration of action [17]. In the elderly patient with decreased cardiac output, glomerular filtration rate, and hepatic blood flow, one may expect to see these comorbid conditions combine to result in a prolongation of the drug's effects [16]. Pancuronium clearance time in the patient with renal disease is doubled, resulting in prolonged action [26]. At least two case studies involving three patients reported prolonged paralysis after the administration of pancuronium to patients with renal disorders, in one case for nine days [27]. In the other two cases, patients with comorbid kidney disease who received pancuronium as part of an anesthesia plan for open-heart coronary artery bypass surgery. In these two patients, one remained paralyzed for two days after the administration of pancuronium, the other for six days [28].
Ophthalmologic Effects
During ophthalmologic cases, pancuronium does not change intra-ocular pressure and may be considered for prolonged surgical cases involving the eye [29].
Effects of Pancuronium in Special Populations
Pediatric Patients. Pancuronium may be safely administered to pediatric patients (including neonates), though one should expect a somewhat shorter duration of action. Its duration in pediatric patients is approximately 60 minutes [16]. Pancuronium is especially useful in the pediatric population, as heart rate rather than stroke volume is a key indicator of cardiac output. Cote describes pancuronium as "an appropriate choice" in pediatric patients in whom maintaining an elevated heart rate is particularly important [30]. Pancuronium may also be used in the pediatric intensive care unit (PICU). In one study of 25 pediatric patients receiving pancuronium for three to five days in the PICU, no signs of residual weakness were reported after discontinuation of the of the drug [19].
Elderly Patients. Initial research on pancuronium revealed that despite having both decreased clearance and a prolonged elimination half-life, pancuronium administered to healthy elderly patients has a profile that does not differ markedly from that of young adults [31]. More recent reports recommend caution with the use of pancuronium in the elderly, especially in those with known age-related decrements in hepatic or renal function [32].
Obese Patients. Dosing of any drug is always a challenge in the obese patient. Research has varied, with some clinicians using total body weight and others using ideal body weight or the patient's body surface area to calculate dose [14,16,17,33,34]. The best clinical method of dosing the extremely obese patient with pancuronium is to begin with the ideal-body-weight dose and then modify the dose upward, if needed, based on neuromuscular blocking results [17]. The induction dose may need to be increased if speed of onset is crucial for patient safety. As obese patients frequently present with complex airway problems, a long-acting agent such as pancuronium may be best replaced with one of the shorter-acting agents. The presence of prolonged neuromuscular blockade, combined with a "can't intubate/can't ventilate" scenario with the obese patient, may result in severe comorbidity or death.
Rocuronium is the most recently developed neuromuscular blocking agent, introduced in 1992 and developed as a short- to intermediate-acting nondepolarizing agent with an extremely rapid, dose-based onset (Table 3) [14,16,17]. This agent is primarily used as an induction and maintenance agent in anesthesia or when neuromuscular relaxation is needed for a comparatively short period in the non-surgical venue. Rocuronium is typically used in the prehospital environment to facilitate endotracheal intubation by paramedics in the field[35]. Its rapid onset has placed it as a nondepolarizing alternative to succinylcholine; however, doses sufficient to speed onset to this degree come with long durations of action. In the patient whose airway is difficult and in whom the chance of failure to rapidly intubate may lead to a comorbid or mortal event, succinylcholine remains the criterion standard. This circumstance, however, has changed with the introduction of sugammadex to clinical practice in the United States (as will be discussed in more detail later in this course). This novel reversal agent works by surrounding the molecules of rocuronium, precluding it from binding to the nicotinic acetylcholine receptor[36]. Following an intubating dose of rocuronium, administration of sugammadex allows the complete recovery of neuromuscular function in a shorter time than an equipotent dose of succinylcholine. This innovation has dramatically increased the use of rocuronium, making it ideal for the prehospital environment.
Cardiovascular Effects
Rocuronium is a very cardiovascularly stable neuromuscular blocking agent [16,37]. In one study in which it was compared with atracurium, rocuronium was significantly more cardiovascularly stable, with no change in heart rate and significantly less decrease in mean arterial pressure [38]. In another study of 80 healthy adults, rocuronium and cisatracurium were found to have equal cardiovascular stability when the parameters of heart rate, mean arterial pressure, cardiac index, and stroke index were measured [39]. The study showed that during a 90-minute monitoring period, mean arterial pressure and heart rate were essentially unchanged throughout the surgical procedures. Additionally, cardiac index varied less than 0.4 L/min/m2, from a high of 3.4 L/min/m2 to a low of 3.0 L/min/m2[39]. Finally, its use in managing the airways of prehospital trauma patients also speaks to its stability in the injured patient [35].
Hepatic Effects
Degradation of the liver in any fashion prolongs the effects of rocuronium [40]. In one study of 50 patients, patients with cirrhosis showed a slower and more variable return to neuromuscular function and impaired elimination of the drug [41]. In another study of 38 patients, 17 of whom had mild or moderate cirrhosis, researchers found a definite prolongation of duration of rocuronium in patients with liver impairment, with a mean increase in total duration of more than 30 minutes compared with healthy controls [42].
Renal Effects
Rocuronium is eliminated 20% to 35% unchanged in the urine and 55% to 80% unchanged in the bile [15,37]. It is, therefore, dependent on renal clearance for its subsequent elimination from the body. In one study comparing patients with renal disease to healthy controls, equipotent doses of rocuronium had clinical durations approximately 18 minutes longer in those patients with kidney failure, and the recovery time for patients with renal impairment was 30 minutes longer than for those with normal kidneys [43].
Respiratory Effects
Rocuronium does not have a significant histamine release and may be safely administered to patients with reactive airway disease [37].
Effects of Rocuronium on Special Populations
Pediatrics Patients. Rocuronium has been safely administered to pediatric patients both perioperatively and in the ICU. In its usual dose of 0.6 mg/kg, children will experience 90% degradation of the first twitch (T1) in a train of four (TOF) series within 90 seconds [44]. While rocuronium may be used as a rapid sequence induction agent for children as well, it is important to remember that as dose speeds onset, it also increases the duration of the block. Bock and colleagues successfully used priming doses of rocuronium, administered to children premedicated with oral midazolam in subclinical doses of 0.045–0.06 mcg/kg prior to a subsequent intubating dose of 0.405–0.504 mg/kg [44]. They found they were able to decrease the time required for maximal suppression of T1 from a maximum of 90 seconds in the control group to 40 seconds while increasing the maximum duration of the block less than 10 minutes [44]. Rocuronium has not been reported to change heart rate or blood pressure in children in doses of two times the ED95 (0.6 mg/kg) [45]. It is important to note that in the operating room environment, the concomitant administration of the volatile anesthetic sevoflurane may prolong the effect of rocuronium in children [46].
Elderly Patients. Rocuronium is well tolerated by the elderly population, and there have been no reports of significant change in hemodynamic variables. Elderly patients have inherent decreases in cardiac output, hepatic blood flow, and both renal blood flow and glomerular filtration rate. One might expect a longer time until onset with any agent, but the literature reports different findings. One study reveals rocuronium's onset was the same in the elderly as in younger patients, but the duration of action was markedly increased, from an average of 82 minutes in younger patients to 98 minutes in the elderly after a standard double ED95 dose of the agent [25]. A second study showed that the onset of rocuronium administered at a dose of 1 mg/kg (slightly more than three times the ED95) was, on average, 18 seconds longer compared with younger adult patients [47]. This 18-second difference may or may not be clinically significant to practitioners, depending on the circumstance of use. During times when gaining control of the airway in an elderly patient is crucial, this relatively short prolongation of onset may have dramatic effects on clinical outcomes. As rocuronium undergoes both hepatic and renal degradation and excretion, this prolonged effect is expected [48]. At least one source suggests that recovery from rocuronium after the administration of sugammadex as a reversal agent may also be prolonged when compared with a younger adult patient [48].
Obese Patients. Rocuronium dosage modification in obese patients is similar to that necessary for other neuromuscular blockers. Specifically, it is important to consider whether to dose by ideal body weight, actual body weight, or some value between the two. In their review of dosing morbidly obese patients, Ingrande and Lemmons recommend using ideal body weight in order to preclude prolongation of the effects [49]. Their recommendation is confirmed by other sources [37,50]. In one reported case study of bariatric surgery on a super-obese patient (77 kg, body mass index [BMI] 66), rocuronium was successfully administered based on ideal body weight [51].
Trauma Patients. The cardiovascular stability and rapid onset offered by rocuronium provide superior advantages in its use in emergency rapid sequence induction and intubation in the traumatically injured patient. It is crucial, however, that these advantages be balanced against the disadvantage of a prolonged neuromuscular blockade coupled with failed airway management. The latter results in the necessity for rapid surgical management of the airway (quite difficult in the prehospital environment and never routine outside of the operating room). If the airway can be safely obtained in the prehospital setting, the duration of rocuronium works to the advantage of care providers during transport, as the patient will not be able to move and inadvertently remove the endotracheal tube [52].
Introduced in 1980, vecuronium is an intermediate-acting nondepolarizing neuromuscular blocker that is quite potent; this can be expected, as it is a monoquaternary analogue of pancuronium. As a result, in larger doses one would expect to see prolonged duration, though in normal doses (Table 4) it is classed as intermediate-acting [15]. Further, vecuronium is unstable as an aqueous solution, so the agent is produced as a dry white powder that must be reconstituted prior to its administration [15,16,37]. Vecuronium undergoes elimination in the liver and is excreted in the bile and urine [14]. One of vecuronium's metabolites, 3-desacetyl vecuronium, is active and cleared more slowly than vecuronium, which may result in prolongation of action in patients with liver and kidney disorders.
Cardiovascular Effects
Vecuronium's hemodynamically stable profile has contributed to its popularity. In one study, vecuronium administered in a dose of 0.1 mg/kg showed no significant changes in heart rate or blood pressure throughout the surgical case, during which both the narcotic remifentanil and volatile inhaled agent sevoflurane were also administered [53]. In another study of 60 adult patients with limited comorbidity undergoing laparotomy, vecuronium administered by infusion was noted to result in no significant changes in hemodynamic status. In this study, the average change in heart rate throughout the case was three beats per minute [54]. In a study of patients undergoing coronary artery bypass graft, Shah and colleagues found vecuronium somewhat less stable than rocuronium [55]. There was no difference in postoperative outcomes, despite vecuronium resulting in greater changes in heart rate and mean arterial pressure as well as higher pulmonary vascular resistance and decreased stroke volume [55].
Hepatic Effects
Vecuronium's primary route of excretion is via the biliary tract, with 40% to 80% of the drug being eliminated in the bile and the rest excreted in the urine [16,26]. In patients with hepatic cirrhosis, onset is slower at lower doses (0.1 mg/kg) compared with healthy controls [26]. When the dose is increased to 0.2 mg/kg, the opposite effect occurs; onset becomes closer to normal controls, but duration is markedly lengthened [26]. In all patients with significant hepatic disorder, the elimination half-life and the duration of any dose of vecuronium is markedly increased [17]. Additionally, doses of vecuronium tend to accumulate, making the administration of multiple doses even more likely to lead to prolonged neuromuscular blockade in these patients [56].
Renal Effects
Though the primary route of excretion for vecuronium is the biliary route, a significant portion of the agent is also excreted renally. Renal clearance is the primary mechanism of excretion of the drug from the blood. A prolonged effect of vecuronium should be expected in patients with renal disease. In one study, duration of the agent increased approximately 35% in patients with chronic renal failure versus those with normal renal function [57]. The extent to which this prolongation can be seen is described in a case study of a woman (61 years of age) who, after losing consciousness in her home, was transported to the hospital, placed in the ICU, and paralyzed with vecuronium for mechanical ventilation and sedation for 15 hours [58]. The patient did not recover central nervous system function and was diagnosed brain dead, allowing her to serve as an organ donor. The paralyzing effects of vecuronium lasted for 64 hours after its cessation, an extreme duration of action attributed to polycystic kidney disease resulting in renal insufficiency and decreased clearance [58]. Naturally, this is an extreme case, but the prolonged duration of vecuronium in patients with renal disease, including renal failure, is confirmed by other studies [37,59].
Effects of Vecuronium on Special Populations
Pediatric Patients. Vecuronium may be safely administered to infants and children. Its cardiovascular stability results in no change in heart rate. However, the concomitant administration of narcotics or high doses of volatile agents may result in a decreased heart rate and resultant hypotension [16,37]. Although these effects are not caused by vecuronium, they could be ameliorated with an agent that increases heart rate (e.g., pancuronium). The United Kingdom Paediatric Intensive Care Society Sedation, Analgesia, and Neuromuscular Blockade Working Group has recommended vecuronium as one of a small group of agents acceptable for administration to children in the ICU [60]. As with patients in renal failure, neonates and infants may have reduced renal clearance of vecuronium, resulting in a prolonged action [30].
Elderly Patients. Elderly patients have less circulating body water than young adults. As monoquaternary agents are hydrophilic in nature, elderly patients will require a lower dose of vecuronium (generally decreased by about 35%) to obtain the same paralyzing effect as in younger adults [37,48,61]. Additionally, the decreased reserve manifested by these elderly patients results in the need for special care to be taken in ensuring that vecuronium has either been adequately reversed or allowed to completely wear off before a patient is considered stable and capable of maintaining his or her airway without assistance [16,37,48].
Obese Patients. Obese patients may safely receive vecuronium at an ideal-body-weight dosage, assuming there are no significant liver or kidney comorbidities [50]. In one study, researchers found that 0.015 mg/kg of vecuronium administered as an intravenous bolus, based on actual body weight, resulted in a faster onset in women than in men [62]. They attributed this difference to the greater body fat percentage in female patients compared with male patients. In a subsequent observation, they compared the onset times of the agent in obese and non-obese women, finding a faster onset in the obese group [62]. Other authors, however, have recommended using ideal body weight in the dosing of neuromuscular blocking drugs [34,49].
Trauma Patients. Vecuronium is an excellent agent for muscle relaxation in trauma patients. Its cardiovascular stability coupled with its ability to be administered for longer periods as an infusion make it ideal for patients requiring mechanical ventilation in the ICU. In the prehospital literature, at least one source suggests the use of longer-acting agents such as vecuronium to prevent excess movement during transport, particularly air transport [63].
As opposed to the amino-steroid neuromuscular blockers, there are primarily two benzylisoquinolinium-based agents on the market in the United States, and one of these (cisatracurium) is used far more than the other (atracurium). These agents have a bis-quaternary amine structure, which prevents them from crossing the blood-brain barrier and interacting with cholinergic receptors in the brain; the same properties are present in monoquaternary amino-steroids [37]. However, benzylisoquinoliniums were developed for a specific purpose, and their structure is closely related to that purpose. In the 1980s, Stenlake and colleagues developed a neuromuscular blocking agent that could be administered to patients with hepatic and renal comorbidities and still undergo predictable degradation [16,64]. The discovery of the application of the Hofmann elimination process in 1956, by which the drug "splits" into inactive parts in the presence of normal blood temperature and pH, and subsequent 25 years of testing and research resulted in the development of atracurium, followed by its cis isomer cisatracurium [15,16,65]. These agents are both classed as "intermediate-duration" nondepolarizing neuromuscular blocking agents [14,17].
A third benzylisoquinolinium-based agent—mivacurium—was developed in the 1980s but removed from the market in 2006 [149]. Mivacurium (name brand and generic) is not being marketed in the United States and is not currently available.
As noted, atracurium besylate was the first of its class, introduced into clinical practice in the United States in the early 1980s. It rapidly gained popularity due to its reliability of degradation even in patients with severe hepatic disease, renal disease, or a combination of both. The dosing details for atracurium can be seen in Table 5.
ATRACURIUM DOSING AND CHARACTERISTICS
| ED95 | Intubating Dose | Supplemental Doses | Onset | Return to Normal after Intubating Dose | Infusion |
|---|---|---|---|---|---|
| 0.25 mg/kg | 0.5–0.6a mg/kg | 0.1 mg/kg | 3 minutes | 45 minutes | 4–12 mcg/kg/min |
| aIn patients who have atopic tendencies, the release of histamine at this dosage level may result in a hypersensitivity response that, in its most severe form, may progress to anaphylaxis. | |||||
Cardiovascular Effects
Atracurium has been characterized as being hemodynamically stable, except when administered in doses resulting in histamine release. In other words, the drug itself provides good stability in the absence of an atopic response on the part of the patient at doses of 0.4 mg/kg and lower [16,64]. In one study of healthy adult patients (American Society of Anesthesiology [ASA] Class I or II), the administration of atracurium in a dose of twice the ED95 (0.5 mg/kg) resulted in an average of a 10-mm Hg drop in mean arterial pressure and an average increase of heart rate of approximately 10 beats per minute [38]. These changes were correlated with a peak plasma histamine increase 700 pg/mL higher than in an equivalent group receiving rocuronium. This represented a doubling of the average plasma histamine level before administration of the drug [38]. In another study comparing atracurium at a dose of 0.5 mg/kg with vecuronium at 0.1 mg/kg and a combination of both agents (atracurium 0.25 mg/kg and vecuronium 0.05 mg/kg), there was no significant difference in heart rate or blood pressure measured perioperatively between the atracurium group and the vecuronium group [66]. Of interest, however, is that the group that combined both agents showed greater hemodynamic stability than either one given alone [66]. In another study comparing benzylisoquinolines to amino-steroid blockers, a dose of 0.6 mg/kg of atracurium resulted again in a doubling of plasma histamine levels within two minutes after a five-second bolus injection of the agent [67]. These levels were accompanied by significant hemodynamic changes, including pulse elevation and decreased blood pressure [67]. Contrary to this, one study describes the administration of atracurium to ASA class III and IV patients (those with significant and life-threatening comorbidities) diagnosed with sepsis and inherent cardiovascular instability in which no mention was made of drug-induced instability [68]. One phenomenon that seems to be receiving greater scrutiny is long QT syndrome, in which a triggering agent or condition results in the polymorphic ventricular tachycardia torsade des pointes [69]. Atracurium is not a triggering agent for this phenomenon and may be safely administered [69].
Hepatic Effects
A significant part of atracurium'sraison d'êtreis to better treat patients with hepatic disease[14,15,16]. It has been shown to reliably metabolize in patients with severe liver disease at the same rate as in healthy controls[23]. In one study of six patients with acute hepatic and renal failure, a dose of atracurium 0.7 mg/kg resulted in a mean plasma elimination half-life of 22 minutes, compared with 21 minutes in healthy controls[70]. Another case study described the administration of multiple doses of atracurium to a patient with severe hepatic disease[71]. The agent degraded reliably, and the patient's remaining neuromuscular block was reversed at the end of the case without difficulty[71]. In one particularly interesting case, a woman (37 years of age, primigravida) presented for emergency cesarean delivery with the diagnoses of twin pregnancy, pre-eclampsia, and acute fatty liver of pregnancy syndrome with elevated liver function tests, as well as fetal decelerations[72]. General anesthesia was administered using atracurium for muscle relaxation, and the resulting extubation and recovery postoperatively was described as "uneventful"[72]. Other authors have also recommended the perioperative use of atracurium during surgery for pregnant patients with liver disease[73].
Renal Effects
Atracurium was also developed to assist in the effective treatment of patients with renal dysfunction. In a manner analogous to that of hepatic function, the Hofmann elimination aspects of the drug make it very attractive for use in the patient with kidney disease, removing renal excretion from the degradation pathway [15,16,59]. Atracurium is valued for its use in the critically ill patient, as it does not possess the accumulative properties leading to prolonged blockade, and is preferred over the amino-steroids for this class of patients [74]. One concern is the partial production of laudanosine, one of the metabolites of Hofmann elimination. This metabolite, in large doses, has rarely been associated with central nervous system disorders in the form of seizure activity [16,74]. In a study comparing the effects of atracurium in patients with renal failure against those of healthy controls, Della Rocca and colleagues found that there was no difference in onset, duration, or recovery from 25% blockade recovery to 80% blockade recovery [57]. Atracurium is also indicated in renal transplant surgery [59,75].
Effects of Atracurium on Special Populations
Pediatric Patients. Atracurium may be administered to pediatric patients, but it is important to bear in mind the significance of histamine release if administered quickly or in high doses. Neonates and infants have decreased renal clearance of anesthetic drugs, so classes of agents that do not rely on renal excretion are helpful in the care of these patients [30]. The onset of atracurium in neonates is more rapid than in children [45]. A standard induction dose of 0.5 mg/kg administered to children establishes sufficient muscle relaxation for intubation in 1.2 minutes; in neonates, these same conditions occur at 0.9 minutes [45]. In children, compared with neonates and infants, atracurium has a more rapid clearance rate [60].
Elderly Patients. In general, muscle relaxants, like other drugs, have longer onsets (usually associated with decreased cardiac output) and longer durations (associated with degraded performance of the hepatic and renal systems) when administered to elderly patients. The duration of atracurium follows this model, in spite of its lack of reliance on hepatic and renal systems [61]. The ED50 needed for neuromuscular blockade was slightly higher in the elderly when compared with younger controls (137 mcg/kg vs. 126 mcg/kg), and the difference in dose to reach ED95 was higher still (249 mcg/kg vs. 226 mcg/kg) [25]. Some research, however, has found no difference in recovery from atracurium [48].
Obese Patients. As discussed, the decision of dosing the obese patient by actual body weight, ideal body weight, or some scaled body weight between the two can be difficult. Van Kralingen and colleagues compared two different atracurium dosing protocols on morbidly obese patients in their study [76]. A group of 20 patients was randomized to receive either atracurium 0.5 mg/kg based on ideal body weight or the same dose based on actual body weight. The researchers measured the onset in terms of time to 100% blockade of a TOF and return to 5%, 25%, 75%, and 95% of the control value before atracurium administration [76]. They found that dosing on ideal body weight resulted in a predictable return to function identical to that of a non-obese patient of the same weight, while the total-body-weight-dosing group had longer and unpredictable durations [76]. Their findings coincide with those of other researchers, advising the practitioner to dose on an ideal body weight basis, monitor results carefully, and be prepared to provide supplemental doses as needed [33,49].
Trauma Patients. While the traumatically injured patient may receive atracurium acutely, those requiring prolonged mechanical ventilation in the ICU should not. The buildup of the metabolite laudanosine, which is not easily cleared by those with renal impairment or decreased glomerular filtration, may result in seizure activity [37]. In the prehospital setting, atracurium can easily be replaced by other agents that will provide a more rapid onset of muscle relaxation. Further, at the dose needed for a rapid onset, atracurium may cause histamine release accompanied by hemodynamic instability and reactive airway response that is especially deleterious in the trauma patient. In one study of 566 patients at two tertiary care centers, 287 of whom received neuromuscular blockers, atracurium was not administered to any of the patients requiring airway management, while rocuronium and succinylcholine were administered 70.4% and 29.6% of the time, respectively [35].
Cisatracurium was developed and approved in the mid-1990s as an alternative to atracurium and is one of atracurium's 10 stereoisomers [16,17]. After numerous reports of problems with atracurium's propensity to result in release of histamine, cisatracurium was designed to result in less likelihood of histamine release and to increase potency. The increased potency allows prolonged administration of the agent via infusion, with less buildup of the toxic metabolite laudanosine [37]. Its use of Hofmann elimination as the primary pathway of degradation has contributed to its popularity in clinical use. The dosing characteristics of cisatracurium are outlined in Table 6.
Cardiovascular Effects
Cisatracurium has demonstrated hemodynamic stability in both the operating room and in the ICU [16]. In a study of 70 patients undergoing myocardial revascularization, patients received a dose of cisatracurium of 0.1 mg/kg (approximately two times the cited ED95) [77]. Cisatracurium was administered over 60 seconds in one group, over 30 seconds in a second group, and over 5 to 10 seconds in a third group. None of the groups had hemodynamic changes greater than or equal to 20% of baseline [77]. These results were replicated in a similar study in which 79 patients diagnosed with coronary artery disease undergoing elective bypass received a cisatracurium dose of either two or four times the ED95[78]. No hemodynamic instability was noted in any of the patients, regardless of dose. In another study of 60 patients who received an intubating dose of 0.25 mg/kg of cisatracurium (roughly five times the ED95), there were no significant changes in hemodynamic status noted [79]. A study of 80 patients receiving either rocuronium or cisatracurium for general anesthesia, combined with either intravenous propofol or inhaled sevoflurane, showed no significant changes in heart rate, mean arterial blood pressure, or cardiac index [80]. Finally, a group of researchers compared two doses of cisatracurium administered to patients in respiratory failure in the ICU for periods ranging from 1.6 to 650 hours [81]. Dose was maintained via infusion pump and monitoring TOF at the orbicularis oculi to obtain either 0 or 2 out of 4 twitches. The researchers reported that, even after long durations of the infusion, no patients had evidence of hemodynamic instability [81].
Hepatic Effects
As with atracurium, cisatracurium was designed for patients with either hepatic or renal comorbidities. The Hofmann elimination process is applicable to cisatracurium [16]. In patients with cirrhosis, cisatracurium is a muscle relaxant of choice, although the fluid retention found in patients with cirrhosis necessitates an increased dose to obtain a similar effect as in non-cirrhotic patients [82]. However, a study assessing the effects of cisatracurium on patients with a Child-Pugh Score of A or B (the lowest and intermediate indicators of hepatic disease) found the dose response curve to be "clinically insignificant" when compared to normal controls [83]. Based on the evidence at hand, cisatracurium is also favored for hepatic resection secondary to tumor excision [84].
Renal Effects
In a study comparing various neuromuscular blockers in patients with renal disorders, there were no significant differences in onset, duration, or supplemental doses, but offset from 25% recovery to 75% recovery was roughly doubled (from 9 minutes in healthy controls to 18 minutes in the renal disease group), and offset from 25% recovery to 80% recovery was slightly longer in patients with renal disease (20 minutes vs. 16 minutes in healthy patients) [57].
Effects of Cisatracurium on Special Populations
Pediatric Patients. Cisatracurium may be safely administered to pediatric patients [16]. In a comparison of all neuromuscular blockers, cisatracurium was noted to have no cardiovascular effects and no histamine-releasing effects in children [85]. In a study of 75 children, cisatracurium doses of 0.1 mg/kg resulted in significantly longer onset; larger doses (0.15 mg/kg and 0.2 mg/kg) did not [86]. No significant difference in recovery time was found at the highest two doses, and no evidence of cardiovascular instability was noted at any dose. In a study of intubating conditions in 181 infants and children 1 month to 12 years of age, a dose of 0.15 mg/kg produced "acceptable intubating conditions" in two minutes [87].
Elderly Patients. Cisatracurium's pharmacodynamics are similar in both elderly and younger adult populations [37,61]. This assumes that the elderly have only the normal renal changes brought on by aging rather than renal comorbidities [25].
Obese Patients. Cisatracurium may be safely administered to patients who are obese. In a study of 20 obese women with BMIs greater than 40, cisatracurium was administered based either on actual or ideal body weight [88]. There was no difference in onset time between the actual-body-weight group and non-obese controls, though the ideal-body-weight group had an onset delayed by 50 seconds on average. The duration of the block to 25% recovery was approximately 30 minutes longer in the actual-body-weight patients when compared with ideal-body-weight patients [88]. One report of anesthesia for a super-obese patient (BMI: 70.7) used cisatracurium at a dose of 0.2 mg/kg using an ideal body weight of 80 kg (compared with an actual body weight of 219 kg) [89]. The authors reported the patient recovered uneventfully after reversal of the neuromuscular blockade [89].
Trauma Patients. There is no reference to the use of cisatracurium in the prehospital role in the professional literature. In the traumatized patient in the ICU, it is crucial that the demands made upon the patient's liver and kidneys be minimized to the extent possible. Not infrequently, patients with severe cardiovascular stressors decrease both hepatic and renal blood flow [74]. As this is the case, agents not relying on these routes for degradation would seem preferable to those that do require hepatic or renal elimination. Further, the absence of inherent cardiovascular instability makes cisatracurium desirable in this setting [90].
Despite the plethora of nondepolarizing neuromuscular blocking agents currently in use in modern healthcare practice, there remains one depolarizing agent—succinylcholine chloride. As new drugs are brought to the market, succinylcholine still has a place in modern practice, despite its invention in the 1950s[16]. The drug has numerous effects and side effects that stand among its principal purpose, which is to rapidly induce neuromuscular paralysis and nearly as rapidly have it wear off again. Succinylcholine differs in its mechanism of action significantly from the nondepolarizing agents.
Succinylcholine, like its nondepolarizing counterparts, works at the postsynaptic neuromuscular junction. Upon injection, it rapidly distributes throughout the body, binding to the acetylcholine receptors on the postsynaptic muscle tissue. Instead of preventing muscular contraction, it causes a random and uncoordinated firing of these receptors, resulting in the physical manifestation of anything from minor twitching to tonic contraction of major muscle groups. These fasciculations are an indicator that the drug is working. After the muscle groups tighten, they relax, but the presence of the drug on the receptors does not allow the muscle tissue to immediately repolarize[16]. The subsequent relaxation of the skeletal muscle results in a transient paralysis that quickly dissipates. The pharmacodynamic characteristics of succinylcholine are described in Table 7.
SUCCINYLCHOLINE DOSING AND CHARACTERISTICS
| ED95 | Intubating Dose | Supplemental Doses | Onset | Return to Normal after Intubating Dose | Infusion |
|---|---|---|---|---|---|
| 0.5–0.6 mg/kg | 1.0–1.5 mg/kg | 0.5–0.6 mg/kga | 1 minute | 9 to 13 minutes | 0.5–10 mg/kg/mina |
| aAt doses exceeding 5 mg/kg, patients may transition into a phase II block, which unpredictably prolongs the action of succinylcholine. | |||||
Succinylcholine has been intensively studied since its introduction into clinical practice in the 1960s. A small molecule that is the functional analog of two acetylcholine molecules joined together, succinylcholine is degraded in the blood by plasma cholinesterase (also referred to as butyrylcholinesterase or pseudocholinesterase) [14,17]. In patients with normal physiology, the abundance of this enzyme allows rapid breakdown of succinylcholine into succinylmonocholine and choline, with approximately 10% being excreted in the urine [91]. This agent has been successfully used in all areas of clinical care, including prehospital, intensive care, anesthesia/surgery, and surgical obstetric practice, and on all age groups. Perhaps due to this longevity, it has been subjected to many clinical trials and research studies. In light of this research, succinylcholine has been found to have numerous side effects and post-administration sequelae. Before addressing these, however, it is important to stress that succinylcholine still has a role in clinical practice. Succinylcholine is the criterion standard for rapid relaxation of skeletal muscle and for its ability to degrade allowing the rapid return of muscular function [15,16,17,37]. When skeletal muscle relaxation is needed quickly, such as for rapid-sequence induction for intubation, succinylcholine remains an excellent choice. Its ability to wear off quickly also ensures that failed intubation does not result in serious comorbidity or, in the presence of a "can't intubate/can't ventilate" scenario, possible patient death due to hypoxia. These facts being accepted, there are many times when succinylcholine may cause complications, some of which are quite serious.
Succinylcholine Side Effects
Post-Administration Myalgias. Patients receiving succinylcholine may complain of a diffuse musculoskeletal pain after recovering from its use. These muscle pains are believed to be the result of generalized inflammation following uncoordinated muscular fasciculations [56]. Numerous interventions have been developed to decrease this pain, which may be severe. The foremost technique is the use of a defasciculating dose of nondepolarizing agents two to three minutes before the administration of succinylcholine, resulting in visibly decreased fasciculations [16]. A meta-analysis assessed a broad compendium of possible treatments to decrease post-administration myalgias, including decreasing the succinylcholine dose and the co-administration of vitamin C, calcium gluconate, lidocaine, and aspirin and nonsteroidal analgesics [92]. Most of the treatments decreased reported pain, but none proved to be ideal. In one study of 393 patients, authors administered lidocaine, d-tubocurarine in a defasciculating dose, a combination of the two, or a placebo prior to the administration of succinylcholine [93]. The authors found that the combination of lidocaine and d-tubocurarine resulted in 8% of the patients having postoperative myalgias, compared with 41.3% reporting myalgias in the placebo group [93].
Hyperkalemia. When muscle and nerve cells depolarize, sodium ions enter the cell, followed by a release of potassium ions during repolarization [10]. The action of the sodium potassium pump then moves these ions against their concentration gradient to ensure the re-equilibration of the ions in their correct concentrations both inside and outside of the cell. However, the administration of succinylcholine, with its accompanying massive depolarization of skeletal muscle, may cause the elevation of serum potassium levels, usually about 0.5–1.0 mmol/L [16,56]. This is especially important in patients with prolonged immobility, neuromuscular weakness as a result of stroke, and/or paralysis secondary to injury [16]. These patients undergo a physiologic change in which they upregulate the number of receptors at their neuromuscular junctions [94]. A 2012 study sought to determine the degree to which hyperkalemia becomes problematic in the critically ill. In a study of 131 critically ill patients who were intubated 158 times (some requiring second intubation in the course of their care), using succinylcholine as the muscle relaxant of choice increased potassium levels an average of 0.4 mmol/L [94]. The primary impact on the elevation in potassium in this study was the length of stay in the ICU. However, in some patients, succinylcholine can cause potassium to rise precipitously. In a case study of a male adolescent (16 years of age) with Klebsiella pneumoniae-associated sepsis, the administration of succinylcholine to facilitate intubation resulted in an increase in his potassium level from 3.19 mmol/L to 8.64 mmol/L [95]. The patient developed cardiac arrest and was aggressively resuscitated. The patient's illness before intubation resulted in him being on bed rest for 15 days prior to the episode, illustrating the impact of immobility on the upregulation of the receptors [95]. Practitioners should make a careful risk/benefit analysis involving any use of succinylcholine. In each case of its use, the question is whether the short onset and, perhaps more importantly, the short duration of this agent overcome the possibility of creating a critical hyperkalemia in an immobile patient.
Masseter Muscle Spasm. One of the primary uses of succinylcholine is to temporarily paralyze the patient to ease the performance of laryngoscopy and endotracheal intubation. Occasionally, the administration of succinylcholine will result in the spasm of the masseter muscle in the jaw, resulting in a patient with a tightly clenched mouth and jaw [16,37]. This condition results in extraordinarily difficult airway manipulation. In one case study, a man became unconscious after an overdose of oral clonidine and the decision was made to intubate him [96]. The patient received 30 mg of etomidate to obtund consciousness, followed by 1.5 mg/kg of succinylcholine. With the jaw rigidly closed, two subsequent attempts at intubation failed, relieved only by the administration of the nondepolarizer vecuronium 10 mg, resulting in sufficient relaxation to allow successful intubation [96]. In a more extreme case, a man presented via ambulance to the emergency room after significant hypovolemia secondary to upper and lower gastrointestinal bleeds [97]. In the face of impending respiratory failure, rapid sequence intubation was selected, and the patient received etomidate 20 mg and succinylcholine 100 mg, after which he rapidly developed a masseter spasm so tight the mouth could not be opened nor could the mandible be moved. Following these findings, a repeat dose of succinylcholine 100 mg was administered without effect [97]. The neck was subsequently prepped and, after a failed nasal fiber optic attempt at intubation, a cricothyrotomy performed with the successful placement of a 5.0 endotracheal tube. It is vital to anticipate the possibility of masseter muscle spasm in patients receiving succinylcholine. Deepening sedation or the administration of a nondepolarizing agent will ameliorate this condition [56].
Cardiovascular Effects. The acetylcholine-based structure of succinylcholine may activate the muscarinic receptors in the parasympathetic nervous system, resulting in bradycardia and bradyarrhythmias. This is most common in patients with high vagal tone and could be of concern in patients requiring an elevated pulse rate to maintain cardiac output[16,17,99]. It is not uncommon to see these effects after large initial doses of succinylcholine or supplemental doses after an initial intubating dose (e.g., in the case of an initial failure of intubation). The slowing of heart rate may result in the generation of ventricular dysrhythmias, which are further aggravated by elevations in potassium[37]. The administration of an anticholinergic such as atropine or glycopyrrolate will ameliorate both the bradycardia and excessive secretions in the mouth and upper airway (another problem of cholinergic stimulation)[15].
Hepatic Effects. As succinylcholine is primarily metabolized by circulating plasma cholinesterases, one would not imagine variations in liver function to be of much concern. However, plasma cholinesterase is produced in the liver, and its levels can be used as proxy indicators of hepatic function[91]. Hepatic abnormalities, ranging from hepatitis to cirrhosis, and normal changes of pregnancy may result in decreased production of plasma cholinesterase[16,37,56].
Genetic Issues. Certain genetic disorders result in abnormal cholinesterases that will degrade succinylcholine far more slowly. This genetic predisposition can be assessed using the dibucaine test, which provides a dibucaine number based on the total activity of cholinesterase and its modified activity in the presence of the local anesthetic dibucaine. Table 8 shows the relationship between dibucaine activity and the ability of cholinesterase to degrade succinylcholine. Dibucaine numbers less than 70 indicate genetic variation that may result in prolonged activity of succinylcholine[37,91].
CHANGES IN SUCCINYLCHOLINE ACTION BASED ON DIBUCAINE TEST RESULTS
| Type of Cholinesterase | Prevalence in Population | Genetic Identifier | Dibucaine Number | Succinylcholine Duration |
|---|---|---|---|---|
| Normal | 96% | Homozygous U (typical) | 70–80 | Normal |
| Atypical | 3% | Heterozygous atypical | 50–69 | Lengthened 50% to 100% |
| Fluoride-resistant, silent, and other variants | 1% | Homozygous atypical | 16–30 | Markedly prolonged (4 to 8 hours) |
Unfortunately, for some patients, this genetic predisposition only becomes evident after the first administration of succinylcholine. A prolonged response is usually followed by genetic testing, and the patient can then have the information placed in their medical records. In extreme cases (such as the "silent" variant), patients may obtain an alert bracelet to warn emergency medical service providers of their altered response to succinylcholine.
Renal Effects. Hyperkalemic effects of succinylcholine can be even more severe in the patient with acute or chronic renal failure. Ordinarily, ingested potassium exceeds requirements, so the kidney excretes excess potassium in the urine. The principal cells in the kidney secrete potassium into the filtrate via specialized channels [100]. Because this loss of potassium is dependent upon glomerular filtration in the kidney, decreased or absent blood flow in the kidney will result in increased potassium levels. One study indicated that while succinylcholine can be safely administered to patients in renal failure, patients with comorbidities or with a predisposition toward hyperkalemia should not receive the drug without a careful risk/benefit analysis [101].
Malignant Hyperthermia. Malignant hyperthermia is a rare genetic disorder occurring in both adults and children, characterized by an uncontrolled release of cellular calcium creating a hypermetabolic state [102,103]. In extreme cases, the patient's temperature can rise as high as 110 degrees Fahrenheit [102]. The syndrome is triggered by the administration of volatile anesthesia inhalation agents or succinylcholine. A prior history of masseter muscle spasm has been noted in 20% to 30% of those with malignant hyperthermia and decreases the onset time of symptoms [103]. Though symptoms can occur immediately after the administration of a triggering agent, there can also be a significant delay. One 2014 study reviewing 477 reported cases of malignant hyperthermia showed its onset from 10 minutes to 75 minutes after the administration of succinylcholine [103]. The initial onset is characterized by an unexplained tachycardia, elevation of end tidal carbon dioxide, and elevation of body temperature. A history of malignant hyperthermia is an absolute contraindication to the use of succinylcholine.
Effects of Succinylcholine on Special Populations
Pediatric Patients. The use of succinylcholine in pediatric patients has generated a great deal of controversy over the past 20 years. In a healthy child with no comorbidities, succinylcholine works well, with the added advantage of being able to be administered intramuscularly. In the event of laryngospasm during the inhalation induction of general anesthesia, succinylcholine may be used in small intramuscular doses to allow muscular relaxation and reopening of the glottis. Infants require somewhat larger doses of succinylcholine; 2 mg/kg is the dose most efficacious for quick blockade [30]. Because both infants and children are dependent on heart rate for cardiac output and tend to have higher vagal tone than adults, an anticholinergic agent should be administered to offset succinylcholine's cholinergic action (and resultant bradycardia) [16,30].
There have been a significant number of case reports of hyperkalemia leading to death in children receiving succinylcholine. One review cites 13 patients with no previous report of neuromuscular disorders, almost all of whom were 8 years of age or younger, who experienced hyperkalemic cardiac arrest after exposure to succinylcholine [104]. All of these patients had underlying Duchenne muscular dystrophy or Becker muscular dystrophy, leading to upregulation of nicotinic receptors. This problem reached sufficient severity to prompt the U.S. Food and Drug Administration to place a "black-box" warning on the succinylcholine product insert. Part of this warning reads as follows [105]:
When a healthy-appearing infant or child develops cardiac arrest soon after administration of succinylcholine, not felt to be due to inadequate ventilation, oxygenation, or anesthetic overdose, immediate treatment for hyperkalemia should be instituted. This should include administration of intravenous calcium, bicarbonate, and glucose with insulin, with hyperventilation. Due to the abrupt onset of this syndrome, routine resuscitative measures are likely to be unsuccessful. However, extraordinary and prolonged resuscitative efforts have resulted in successful resuscitation in some reported cases. In addition, in the presence of signs of malignant hyperthermia, appropriate treatment should be instituted concurrently.
Based on these findings, a nondepolarizing agent should be strongly considered. Bearing this in mind, succinylcholine has been successfully administered to children in the prehospital environment with success, with no reports of malignant hyperthermia-like symptoms [106]. Succinylcholine should be reserved for cases in which rapid onset and short action are crucial to the success of the larger plan of care.
Elderly Patients. The normal degradation of organ systems that accompany aging should be considered when administering any neuromuscular blocker, and succinylcholine is not an exception to this rule. While no difference in duration of action has been found, the elderly may experience longer onsets when their decreased cardiac output is considered [25,61].
Obese Patients. Obese patients frequently have airways that are difficult to manage, whether in the inpatient environment, in the operating room, or in the field during prehospital rescue or resuscitation. Succinylcholine has been used to help facilitate endotracheal intubation in these patients primarily because of its ability to wear off quickly in the event of failed airway management, allowing the patient to breathe spontaneously [16,49]. Succinylcholine should be administered to adult patients using total body weight, rather than ideal body weight, at a dose of 1 mg/kg [33,49]. This use of total body weight to calculate dose is also appropriate for obese adolescents [107]. It is important to remember that these doses result in both rapid onset and somewhat prolonged activity, with duration increasing to approximately 12 minutes [49].
Trauma Patients. In both the inpatient emergency setting and the prehospital setting, succinylcholine has been successfully administered to facilitate intubation of the critically ill or injured patient. In the Eastern Association for the Surgery of Trauma practice guideline, succinylcholine is described as the neuromuscular blocking agent of choice for use in securing the airway via emergency intubation in the prehospital setting [108]. However, European literature has recommended rocuronium, which, with the advent sugammadex, can be readily reversed in a period as short as (or shorter than) the degradation period of succinylcholine [109].
Burn Patients. The burn patient, like the immobilized patient, presents with special concerns regarding the administration of succinylcholine. Patients who have been burned within the last 24 hours usually have had insufficient time to upregulate nicotinic receptors and will not demonstrate the hyperkalemia associated with patients extensively burned more than 24 to 48 hours prior [16,98]. The quick onset and offset of succinylcholine may be of benefit for patients whose emergent burn injuries involve or are near the head and face, so the airway may be rapidly and reliably secured despite any anatomic anomalies. Indeed, inhalation of smoke and superheated air affects the delicate oropharyngeal and nasopharyngeal mucosa, and intubation should be attempted at the earliest practical moment. Patients with burns will frequently return for various grafts and wound maintenance. For these cases, and for airway management in the burn unit, nondepolarizing agents represent the safest choice for neuromuscular blockade [16,17,98].
Neuromuscular blocking agents are different from other classes of agents in many ways, but perhaps most exceptional is the ability to accurately and precisely track levels and degrees of neuromuscular blockade[15]. With most other agents, the patient's description is relied upon to determine efficacy (e.g., "My pain is less" or "My nausea is better"). Using a simple nerve stimulator (or "twitch monitor"), an anesthetized surgical patient or sedated intensive care patient can be precisely evaluated. Note that the sedation aspect in the description of these patients is important—as the frequency of the impulse rises, so does the degree of pain. An impulse of 50 Hz administered to produce tetany is quite painful, so patients being tested in such a fashion should be sedated or deemed insensate to pain secondary to severe injury or illness.
Peripheral nerve stimulators (PNSs) have been used for more than 40 years to monitor neuromuscular blockade, although its routine use is not as ubiquitous as might be expected[11,16]. In a 2015 editorial, Rodney and associates reported that a PNS was routinely used by only 18% of those in anesthesia practice in Europe and 34% of those in the United States[110]. This low use rate is despite the fact that the monitor is small and easy to use and that the patient who is inadequately recovered from neuromuscular blockade is at risk for severe complications, including death[111,112,113]. The average PNS (Image 1) is roughly 2–3 inches wide, 1 inch deep, and 4–6 inches long. It has two wires, a positive and negative, which, when attached to gel-filled pads (e.g., electrocardiogram pads), provides a mechanism by which to apply small electrical impulses down a nerve, simulating the electrical synapses that would ordinarily cause the contraction of skeletal muscle. They range in complexity from extremely simple (a twitch switch, a tetany switch, and little else) to very complex (multiple settings, ability to select the exact frequency needed, automatic settings for various techniques of monitoring). Most are less than $300 in price.
The PNS has several settings that are helpful to the clinician and can be activated by pressing the correct button. In this example, there are several modes available by which the degree of neuromuscular blockade can be monitored. In the upper left side of the control panel is the "standby" switch, which stops all electrical impulse generation while maintaining the PNS in the "on" state. Next is the "TEN SEC" button, which will generate one impulse every ten seconds. To the right is the "ONE SEC" button, which will generate one twitch per second. The "TWO HZ" button increases the frequency of the twitch from 1 Hz to 2 Hz. In the second row on the left is the "100 HZ" button, which delivers a high-intensity tetanic pulse for as long as the button is pressed. To the right is the "50 HZ" button, which will also deliver a tetanic stimulation, albeit at half the frequency, for as long as it is pressed. The next button to the right is the "TOF" (train of four) button, which will activate a single series of four impulses. Next is the "TET" button, meaning tetany. Finally, there is the "BATTERY" button. If pressed, how much battery power is left in the device will be displayed. Other, more advanced PNSs may have other controls. For example, some have a control marked "DBS" for double-burst stimulation. The blank screen would show exactly how many Hz are being administered during the production of electric impulses. It is important to remember that the PNS shown in Image 1 is just one example of a PNS; they vary a great deal in complexity, utility, and cost. At a minimum, all should provide single twitches and tetany.
Monitoring of neuromuscular blockade could conceivably occur anywhere that a motor nerve is close enough to the skin that it may be stimulated [16]. Most practitioners, however, use either the adductor pollicis muscle in the patient's forearm and wrist (Image 2) or the orbicularis oculi muscles surrounding the eye in the face (Image 3). Both Image 2 and Image 3 show the correct placement of the electrodes on the forearm (in the case of the adductor pollicis) or over the temporal branch of the facial nerve (cranial nerve VII), stimulation of which results in twitches around the eye. In addition, Image 2 shows a comparison of the thumb in the relaxed mode and the contracted mode. The orbicularis oculi may be used if the practitioner cannot gain access to the patient's arms (e.g., due to injury or positioning for surgery). The use of the orbicularis oculi has been shown to be as effective as the adductor pollicis in determining adequate muscle relaxation for endotracheal intubation[114].
The most commonly used method of neuromuscular blockade monitoring is the TOF, which will fire four 2-Hz impulses every half second for two seconds [115]. If there is no neuromuscular blocking agent present, one should see four muscular contractions of equal intensity[16,115]. The impulses should be a monophasic, square type, and last no longer than three milliseconds[115].
When activating the PNS to produce a 1-Hz impulse each second, a tracing similar to the one seen in Figure 5 could be produced. Using this figure as an example, consider a patient who has received a dose of neuromuscular blocking agent at the time indicated inFigure 5. As the agent reaches the nicotinic receptors, the degree of muscular contraction, represented by the height of the lines, will diminish. As the muscle relaxant is degraded, the height of the twitch returns to its original pre-block height. Figure 6 shows the effect of the neuromuscular blocker on the twitch height, and the specific measures showing how efficacious the agent is (after a normal dose, to what extent paralysis occurs), its onset (how long it took to reach the maximum point of twitch suppression), and its duration (how long it took to fully degrade and allow twitch height to return to normal). These types of diagrams are ordinarily used in research studies and occasionally by clinicians fortunate enough to have a complex accelerometer available for monitoring time.
The average neuromuscular stimulator, however, does not have this level of complexity. Instead, the clinician uses either a series of four single twitches, observed visually and/or using tactile senses, repeatedly administered one second apart (the TOF). The TOF tells the clinician the approximate number of receptors occupied by the neuromuscular blocker at the point in time when the clinician activates the stimulator. As previously mentioned, the stimulator administers four 2-Hz impulses to the patient. By simply counting the number of muscular contractions produced by the electrical impulses, the practitioner can estimate the degree to which neuromuscular blockade is in force. The TOF looks different in patients receiving succinylcholine than it does in all of the nondepolarizing agents.
Figure 7 shows the height of TOF muscular twitches, represented by lines reaching a certain height in the patient receiving a nondepolarizing neuromuscular blocker. In clinical practice, one would equate the height of these lines with the degree of muscular contraction. Therefore, a tall line is indicative of a strong muscular contraction, a short line is a weak contraction, and no line indicates no response to the stimulus. A patient is tested before receiving an initial dose of neuromuscular blocking agent (after receiving sedation in the ICU or an induction dose of intravenous or inhalation anesthesia). Following the establishment of this baseline, the nondepolarizing agent is administered, a short amount of time (i.e., 30 to 60 seconds) is permitted to pass, and the patient is again retested. Note that all of the twitches are reduced, with the first twitch being strongest and the fourth being weakest. Subsequent tests show further diminution of these twitch heights, until finally only the first twitch is manifested. As the block wears off, the twitches return in ascending height from first to fourth, until, after reversal or significant time for degradation, all four twitches are equal in height and equal to the test conducted before block administration. The interpretation of these twitches is outlined in Table 9 [13,16,116].
INTERPRETATION OF TWITCHES DURING TRAIN OF FOUR STIMULATION AFTER THE ADMINISTRATION OF A NONDEPOLARIZING NEUROMUSCULAR BLOCKING DRUG
| Number of Twitches Seen | Clinical Interpretation |
|---|---|
| 0 | 100% of the neuromuscular junctions are blocked. It is possible that the concentration of drug is so high that the amount available exceeds the amount needed for a maximal response. |
| 1 | 90% of the neuromuscular junctions are blocked. This degree of relaxation is ordinarily suitable for most surgical or intensive care patients. |
| 2 | 80% of the neuromuscular junctions are blocked. The patient may be readily reversed with the administration of reversal agents. |
| 3 | 75% of the neuromuscular junctions are blocked. This patient may have inadequate blockade for some types of surgery and/or may attempt to breathe over the ventilator. |
| 4 | Less than 70% of the neuromuscular junctions are blocked. The patient may breathe adequately but may still be in a weakened state. |
Figure 8 shows the TOF response one sees when succinylcholine is administered. The stair-step appearance of the height of the twitches seen with a nondepolarizing agent is no longer present. The depolarizing action of this blocker suppresses muscle response equally, resulting in all twitch heights diminishing to the same degree until the relaxant sets up, at which point they are completely abolished. The twitches return in the same manner in which they were ablated, with four twitches of the same height increasing in contractility until a return to normal function.
The degree to which a patient is relaxed may also be determined by administering a tetanic impulse of 50–100 Hz for a given period of time, usually five seconds. For most practitioners, the presence of five seconds of sustained tetany is used as a proxy measure for adequate muscle contraction needed for spontaneous and effective ventilation. However, the transmission of these impulses may provide a clouded picture of the patient's blocked status. Consider the patient who has received a nondepolarizing neuromuscular blocking agent. The practitioner administers TOF stimulation, but notes zero twitches out of four. After waiting a short period (i.e., one minute), the practitioner administers five seconds of tetany, followed almost immediately by a TOF stimulation, after which two of four twitches can be seen. This latter reading is inaccurate, as the tetanic impulse caused the temporary release of large amounts of acetylcholine from the presynaptic neuron. This transient efflux results in a brief period during which it appears the neuromuscular blockade has dissipated. If one re-tests the TOF after four to five minutes, during which the transiently increased release of acetylcholine is redistributed and/or broken down, the TOF will return to zero of four twitches.
Double-burst stimulation is also useful, though it should be noted that the higher impulses used by this technique increase its painfulness and patients who are sedated but not anesthetized may react to this testing method. The double-burst stimulation technique involves firing either two or three 50-Hz impulse bursts, followed by a delay of 750 milliseconds[117]. A second two- or three-burst series follows. The goal is to feel the degree of muscular contraction of the first burst compared to the second burst in terms of both strength of the contraction and lack of fade (or sustained contractility throughout the electric impulse). If the contraction on the second set of bursts indicates fade, the patient patient's TOF ratio (current values compared to baseline values) are 40% to 60%, indicating the patient has residual weakness[117].
A final way of measuring neuromuscular function is the use of post-tetanic count. As with the double-burst stimulation, this is a painful technique and patients who are not anesthetized may view this as a noxious stimulus. It should be used with great discretion in the ICU and prehospital arenas. In this technique, 50 Hz is applied for five seconds, followed after three seconds of no impulse with a 1-Hz twitch. The presence of a twitch indicates the patient is not completely (100%) blocked[117]. It also gives an indication of the time to the return of the first twitch in TOF stimulation; after post-tetanic count, the presence of a twitch during vecuronium or atracurium relaxation indicates a return of T1 in a TOF within seven to eight minutes[117].
For most patients receiving nondepolarizing agents as part of anesthesia for surgical procedures, as well as for most patients in the ICU requiring paralysis, the return of the first twitch only in a series provided by the TOF indicates the presence of a therapeutic degree of neuromuscular blockade. It is possible (and a common, though not desirable, occurrence) that a dose of neuromuscular blocking drug can completely extinguish all twitches in a TOF. Though indicative of a completely paralyzed patient, this level of relaxation is, in most cases, both unneeded and undesirable. In such cases, one must attempt to anticipate how long it will take the block to wear off in an environment of supersaturation. In other words, the amount of neuromuscular blocking agent exceeds the amount needed for a therapeutic effect; there is excessive drug present even as the body starts to break down the initial dose. This is analogous to a "standing-room only" crowd. When one patron leaves his/her seat, another person is waiting to take it; no longer can the number of patrons be estimated by counting the seats.
There are rare cases in the ICU, post-anesthesia care unit (PACU), operating theatre, and prehospital arena in which the benefits gained by the patient being utterly immobile exceed the benefits of the knowledge gained by seeing one twitch and knowing when offset will occur. However, these occasions should be carefully planned and should not happen as a default administration of neuromuscular blocking drugs. No patient should receive a relative overdose of any agent unless there is benefit to be gained by the use of that larger dose[113].
Note that this applies only to nondepolarizing agents. Complete ablation of the TOF is common with the depolarizing agent succinylcholine and is a good indicator of adequate blockade. This reflects the fact that succinylcholine, when administered to a patient with normal plasma cholinesterase levels and function, has a short duration, resulting in the patient's rapid return to normal neuromuscular function [118]. The absence of any twitches only becomes problematic in patients with pathologic responses to the drug (e.g., atypical plasma cholinesterases) or in those developing a phase 2 block. For the most part, such occasions are quite rare, and succinylcholine reliably degrades very quickly, obviating the need for one-twitch maintenance [118].
One of the properties that makes nondepolarizing muscle relaxants so useful is their ability to be reversed. In comparison, depolarizing blockers cannot be reversed; the agent must run its natural course and degrade spontaneously. As discussed, it is vital to dose the agent correctly. In nearly all cases, this means leaving a minimum of one twitch in the TOF (for nondepolarizing agents). The biggest concern with reversing neuromuscular blocking drugs is the fact that reversal agents work at both the nicotinic and muscarinic receptor sites. As the nicotinic receptor and its activity have been discussed in detail in a previous section, this section will provide a brief review of the muscarinic receptor.
Muscarinic receptors are found ubiquitously throughout the body, though its main areas of concern when reversing neuromuscular blockade are its effects in the heart, vasculature, upper airway, conducting airways, and nervous system. The autonomic nervous system (Figure 9) is composed of two major divisions: the sympathetic (or adrenergic) nervous system and the parasympathetic (or cholinergic) nervous systems. The sympathetic nervous system is associated with the "fight-or-flight" phenomenon, while the parasympathetic system is associated with the "rest-and-digest" functions. Both of these nervous systems are further divided into the preganglionic neurons and the postganglionic neurons.
In the reversal of neuromuscular blockade, concerns revolve around the inadvertent and excessive activation of the parasympathetic nervous system and its attendant muscarinic receptor activation. This is because classic neuromuscular blockade reversal agents work by deactivating acetylcholinesterase, the enzyme primarily responsible for inactivating acetylcholine. Lack of acetylcholinesterase causes increased levels of acetylcholine to build up. These acetylcholine molecules compete with nondepolarizing drug molecules for the acetylcholine receptor site at the nicotinic receptor on the postsynaptic neuromuscular junction membrane. This is a therapeutic action, resulting in the return to normal functioning of skeletal muscle.
Unfortunately, elevated levels of acetylcholine will also bind with muscarinic receptors. Muscarinic receptors are a family of receptor subtypes that are primarily responsible for carrying out the physiologic effects associated with the parasympathetic nervous system [16]. When activated by acetylcholine, muscarinic receptors have a wide range of physiologic responses that can be deleterious to the patient, including bradycardia, bronchospasm, increased nasal and oral secretions, and hypotension [37,116]. In patients with reactive airway disease, cholinergic responses can result in full-blown asthma attacks. The solution is to administer a concomitant dose of an anticholinergic agent, which will work at the muscarinic receptor to block the attachment of acetylcholine while not affecting the nicotinic receptors. It is crucial that an adequate dose of anticholinergic drug, either glycopyrrolate or atropine, is administered with the anti-acetylcholinesterase agent. Failure to administer an anticholinergic may result in severe cardiovascular collapse, pulmonary failure, and, in extreme cases, death [119]. Fortunately, these drugs are easy to administer.
Upon release from the prejunctional neuron at the neuromuscular junction, acetylcholine rapidly crosses the synaptic space and binds with the nicotinic acetylcholine receptor on the muscle membrane. The synaptic cleft, however, is also lined with acetylcholinesterase that has the function of breaking down acetylcholine. This breakdown happens very quickly, usually within 80 to 100 microseconds [116]. In order to overcome this rapid breakdown and to allow the neurotransmitter sufficient time to build up to a level at which it can compete with the nondepolarizing drug, the acetylcholinesterase must be temporarily degraded. There are three commonly used anti-acetylcholinesterase agents that will accomplish this task: edrophonium, neostigmine, and pyridostigmine. Of these three, neostigmine is the one used most frequently for the reversal of neuromuscular blockade induced by a nondepolarizing agent.
Injecting neostigmine intravenously results in the drug quickly binding with acetylcholinesterase. This binding process accomplishes two actions. First, the acetylcholinesterase is inactivated, and then the neostigmine itself is hydrolyzed, deactivating the action of the drug as well[116]. These actions raise the concentration of acetylcholine in the synapse of the neuromuscular junction, resulting in a return to normal function of the skeletal muscle[15]. The kidney excretes approximately 50% of neostigmine, so its effects may be prolonged in the patient with renal disease[116]. Of the remaining drug, 30% undergoes degradation in the plasma via cholinesterases, and the rest in the liver[120]. Neostigmine has a quick onset (i.e., 1 minute) but a slower peak activity (approximately 10 minutes) after injection[119,121]. A dose of 0.07 mg/kg (maximum: 5 mg) has been deemed sufficient to provide reversal for patients who manifest at least one twitch in a TOF; however, more recent research has called this into question[15,16]. A standard dose of neostigmine does not reflect the pharmacologic standard of varying drug doses with patient needs. The ability to monitor patients receiving neuromuscular blockers allows one to determine the extent of the blockade and thus modify the dose of reversal agent required. In Table 10, the works of several researchers have been combined to provide dosage guidance for the administration of neostigmine in the face of neuromuscular block monitoring[11,17,113,119,121].
This agent is a short-acting anti-acetylcholinesterase with a more rapid onset than neostigmine. While the administration of neostigmine results in the degradation of the acetylcholinesterase enzyme, edrophonium merely binds and then releases, leaving the enzyme intact [116]. The dose of edrophonium is 0.5–1.0 mg/kg, and, like neostigmine, it must be administered with an anticholinergic agent [16,116].
Pyridostigmine has a somewhat longer duration of action than neostigmine and, like neostigmine, degrades the acetylcholinesterase enzyme. Pyridostigmine is not as effective as either neostigmine or edrophonium at doses of 0.38 mg/kg [123]. A review of the literature reveals the nearly total absence of use of this agent as a reversal drug, and it is included here solely in the interest of being complete.
As with every other medication, the patient's underlying comorbidities, pathology, and circumstances regarding its administration should be considered. The most common way of administering neuromuscular reversal agents is to mix them together in a syringe of adequate size, usually 10 mL in capacity. To prepare the medication for injection, the clinician first aseptically draws up 5 mL of either atropine (in a concentration of 400 mcg/mL; total dose 2 mg) or glycopyrrolate (in a concentration of 200 mcg/mL; total dose 1 mg). Having filled the syringe halfway with anticholinergic, the practitioner fills the remaining 5 mL of the syringe with 5 mg of neostigmine, resulting in a 10-mL syringe filled with 5 mg of neostigmine in a concentration of 0.5 mg/mL, and either 2 mg of atropine at a concentration of 200 mcg/mL or 1 mg of glycopyrrolate at a concentration of 100 mcg/mL. The drugs are now ready for administration. Referring to Table 9, consider a 60-kg patient with two twitches on a TOF being reversed with glycopyrrolate and neostigmine. The correct dose for reversal is 0.05–0.07 mg of neostigmine per kilogram of body weight. This calculates to a dose of 3.5–4.2 mg of neostigmine. The dose of 3.5 mg is selected. As the syringe contains 0.5 mg neostigmine per mL, 7 mL of the mixed agents is required; this will give a concomitant dose of 700 mcg of glycopyrrolate. Glycopyrrolate will ameliorate the adverse cardiovascular and pulmonary side effects of neostigmine, while the therapeutic effects of increasing acetylcholine at the neuromuscular junction will continue, resulting in the reversal of neuromuscular blockade. This technique also works with atropine, though atropine's ability to cross the blood-brain barrier (which is not present in glycopyrrolate) has resulted in its use as a part of the reversal mixture decreasing over time.
Occasionally, patients may experience a brief episode of bradycardia if neostigmine binds with muscarinic receptors before the anticholinergic can occupy and block those same muscarinic receptors. If it is important that the patient not become bradycardic (e.g., cardiac valvular disease with regurgitant flow), the practitioner can mix the neostigmine and anticholinergic in two different syringes. A small dose of the anticholinergic is then administered first, and, upon seeing an increase in heart rate, the practitioner can then inject the anti-acetylcholinesterase agent and the remaining anticholinergic agent.
Sugammadex is the newest reversal drug to come on the market, and it has been described as "revolutionary" [36]. It was approved for use in the United States by the FDA in 2015 [36]. Prior to this, it was used successfully in Europe for nearly a decade. Sugammadex is described as a cyclodextrin with a hydrophobic interior and hydrophilic exterior [124]. Its structure allows other hydrophobic agents to enter the center of the molecule and prevent the agent from binding with the receptor. The drug was designed to have a specific affinity for the nondepolarizing agents rocuronium and vecuronium [125]. It differs from other neuromuscular block reversal agents in that it inactivates the nondepolarizing drug while leaving acetylcholinesterase unaffected. As sugammadex combines with the amino-steroid nondepolarizers, there are fewer free nondepolarizing agent molecules available to bind with the nicotinic receptor. This in turn allows the increased binding of acetylcholine and a return to normal muscle function [36]. One of the shortcomings of this drug is that it does not work to reverse blocks from cisatracurium, atracurium, or succinylcholine [36].
The most crucial impact of sugammadex is that, when combined with the nondepolarizing agent rocuronium, it offers an alternative to succinylcholine in a patient who requires both a rapid onset and a rapid offset of muscle relaxation. This is even more important when coupled with the fact that the duration of time the block has been in effect, the presence or absence of twitches, and the dose of the block are immaterial to its reversal [124]. As an example of the efficacy of this reversal agent, consider the effects of a dose of 0.6 mg/kg of rocuronium, which should last approximately 40 minutes. After the administration of a dose of 8 mg/kg of sugammadex, neuromuscular function returns to normal in less than two minutes [124]. A dose of conventional neuromuscular block reversal agent, such as neostigmine, would have been ineffective until two twitches of the TOF had recurred (approximately 20 minutes), and then the reversal agent itself would have taken another 10 minutes to peak. The recovery profile of sugammadex coupled with rocuronium exceeds that of succinylcholine in terms of onset and offset [113].
The dose of sugammadex varies depending on the time delay between the administration of the nondepolarizing agent and the subsequent reversal dose of the drug. The doses of sugammadex for shallow, moderate, and deep levels of neuromuscular blockade are 2 mg/kg, 4 mg/kg, and 16 mg/kg, respectively [113,119]. The latter dose of 16 mg/kg is referred to as a rescue dose and can reverse doses of rocuronium as high as 1.2 mg/kg in as little as three to five minutes [113].
Sugammadex is eliminated via the kidney, with 95% of the drug being eliminated unchanged in the urine [126]. The drug has not been associated with any pulmonary or cardiovascular side effects, despite initial concerns that its administration could aggravate long QT syndrome [126].
Neuromuscular blockers are commonly administered in the operating room, and nurses in the PACU will frequently receive patients who either still have neuromuscular blocking drugs on board or who have just undergone reversal. It is important for the PACU nurse to rapidly and accurately determine the patient's neuromuscular blockade status, as the inadequate reversal of neuromuscular blockade combined with an extubated patient may result in hypoxemia, the need for emergent reintubation, cardiac dysrhythmias, and, in extreme cases, death [112,113]. While using the PNS to determine the degree of relaxant remaining in the patient would be of greatest use, this is somewhat difficult unless the patient arrives in the PACU sedated and intubated, as the PNS tests may be painful. Fortunately, other tests can be done to determine the degree to which the patient's neuromuscular function has returned [111,113,121]. These tests include:
A sustained head lift for longer than five seconds: This tests the core muscles of the patient and mimics the "five seconds of tetany" produced by the PNS. The drawback of this test is it may be impossible for certain patients (e.g., those who have undergone abdominal surgery).
Protrusion of the tongue for longer than five seconds: The tongue is the most likely anatomic structure to occlude the upper airway. Patients who can stick their tongue out and hold it for five seconds reassure the practitioner that they can clear their own airway. Additionally, the five-second period shows prolonged muscular activity is possible.
Firm handgrip for longer than five seconds: This test offers the added benefit of determining the patient's level of mentation and his or her ability to respond appropriately to verbal stimulus. As with the first two tests, the five-second period shows the ability of the patient for prolonged muscular contraction.
An inspiratory force exceeding -25 cm H2O: The ability of the patient to breathe deeply is the prime reason all of the other tests are performed. The ability to generate this level of negative pressure (as measured by spirometry) indicates the return of muscular function of the diaphragm.
Tidal volume of 5 mL/kg and a vital capacity of greater than 15 mL/kg: These tests are ordinarily performed by respiratory therapy during a pulmonary function test but may be performed at the bedside if the patient is intubated. The ability to generate these values on command is indicative of sufficient respiratory function to allow normal breathing. It is important to note these values may be modified in patients with significant pulmonary comorbidities.
The prehospital arena presents numerous problems for practitioners. There is a consistent dynamic tension between the option of staying on scene longer to stabilize the patient prior to transport and loading the patient for rapid transport to a hospital for definitive care. The area in which these two schools of thought converge is that of time management. The "golden hour" applies not just to traumatically injured patients, but to those suffering from cardiovascular issues (e.g., myocardial infarction) or cerebrovascular issues (e.g., stroke). In all of these cases, perfusion of the damaged tissue with oxygenated blood is among the highest priorities. Providing supplemental oxygen via facemask, nasal cannula, or even continuous positive airway pressure may not be sufficient to meet the patients' needs. In these cases, the airway must be secured, either with a supraglottic device blindly inserted or via endotracheal intubation.
Administration of neuromuscular blockers can relax the musculature of the jaw and oropharynx and speed endotracheal intubation. The same relaxation that makes endotracheal intubation easier comes with the downside of increasing the likelihood of vomiting and aspiration. As early as 1998, researchers have sought to determine the extent to which succinylcholine would ease intubations in the emergency department [127]. Though not in the prehospital venue as such, most prehospital policies emanate from emergency departments. In this study, researchers found that the administration of succinylcholine decreased the number of intubations defined as "difficult" and advocated for the use of the drug [127]. This study was followed by a second confirming the utility of neuromuscular blockers in the hands of non-anesthesia personnel in the emergency room, again using succinylcholine [128]. The rates of complications such as esophageal intubation, aspiration, and airway trauma were reduced after the administration of succinylcholine for airway management.
Other studies conducted in the late 1990s began to branch into the use of neuromuscular blocking agents in the field by non-physician providers. One such study advocated the use of succinylcholine, along with lidocaine, oxygen, and midazolam, for patients receiving aeromedical evacuation [129]. The researchers found an increased rate of successful intubation, moving from 69.6% success in non-medicated patients to 97.5% in those receiving paralysis [129]. A particularly interesting study dealt with the paramedic administration of succinylcholine to critically ill and injured children. As discussed, succinylcholine may lead to significant problems in children, such as bradycardia and hyperkalemia, the latter complication in those with undiagnosed muscle disorders. Despite these possible concerns, the study found that paramedics, under close medical direction, could use succinylcholine to successfully intubate pediatric patients, with a reduced prevalence of airway complications and no episodes of bradycardia [130]. This theme was echoed by researchers determining the utility of neuromuscular blockade assisted intubation in patients with severe head injuries [131]. They used succinylcholine followed by rocuronium after endotracheal tube placement to provide ease in ventilation during transport. Severely hypoxic patients had statistically higher mortality rates than those without hypoxia, and the success rate for oral tracheal intubation was 84.5% [131]. Another study in which paramedics intubated patients with severe traumatic brain injury using succinylcholine showed increased rates of favorable neurologic outcome six months after the injury [132].
As prehospital care continued to develop, the use of other types of medications for neuromuscular blockade were researched. As with succinylcholine, early research seeking to use rocuronium as a substitute was performed in the emergency department. In a study comparing the use of succinylcholine and rocuronium for airway management in a tertiary care facility, both agents were found to be equally efficacious, though succinylcholine was preferred by the clinicians, presumably due to its fast offset [133]. It is important to note that during the time of this study (2009) sugammadex was not yet approved for use in the United States. In a later review article, the author suggested rocuronium be substituted for succinylcholine for the prehospital provider [63]. The availability of sugammadex takes the problem of prolonged paralysis out of the equation while also eliminating the plethora of side effects associated with succinylcholine. In short, neuromuscular blocking agents used by well-trained field personnel decrease the likelihood of airway complication, facilitate airway management in the field, and, when accompanied by appropriate sedation protocols, prevent the patient from fighting restraints and mechanical ventilation during transport.
The most common use of neuromuscular blocking agents is during surgical procedures. Neuromuscular blocking agents allow the anesthesia provider the muscular relaxation needed to facilitate manipulation of the patient's airway, direct or indirect exposure of the glottic opening, and placement of an endotracheal tube to facilitate perianesthesia ventilation and maximally protect the patient's airway during the procedure. Additionally, in the majority of surgical cases, relaxation of skeletal muscle aids the surgeon's visualization of the organs and results in the administration of lower doses of other anesthetics. As discussed, neuromuscular blocking agents work peripherally at the neuromuscular junction rather than centrally in the motor cortex of the brain. It is, however, possible to decrease patient movement and decrease the tension in skeletal muscle by deepening the level of other anesthetic agents, particularly the inhaled (volatile) anesthetic agents. Years of experience have revealed that the dose of inhalation agent required to accomplish this is quite high and affects not only the brain but also mechanisms at the spinal cord [134]. It is dramatically higher than the amount of inhaled agents needed to obtund consciousness and obliterate the patient's memory during the surgical procedure [134]. As this is the case, neuromuscular blocking agents provide a quiet surgical field, increase the patient's tolerance of mechanical ventilation, and quickly reverse the patient at the end of the case, shortening the perianesthesia period.
The anesthetist is well placed to measure the degree of neuromuscular blockade continuously throughout the anesthetic and surgical procedure. With rare exceptions, the anesthetist has access to either the patient's forearm (to stimulate adductor pollicis muscles) or head (to stimulate orbicularis oculi muscles). As this continuous monitoring is both quick and easy to quantify, the guidelines recommend that only intermediate-acting neuromuscular blockers be administered and that the long-acting neuromuscular blockers (e.g., pancuronium) be removed from anesthesia practice [113]. Further, the anesthetist should endeavor, whenever clinically possible, to maintain a minimum of one twitch in a TOF series. These two practices, both of which are easily accomplished, have the potential to decrease the problems with prolonged or inadequately reversed neuromuscular blockade. Long-acting muscle relaxants may still have a place in anesthesia practice for patients who require a long recovery, intensive care hospitalization, and prolonged post-procedure ventilation. As surgical practice moves more and more toward "fast-tracking" the patient's recovery, these cases will probably be less common.
Note: There are many types of ICUs, some generalized in nature and some only accepting specific patients. In this course, the term ICU includes any unit in which critically ill or injured patients require extensive monitoring and in which mechanical ventilation is used.
Critically ill patients in the ICU may be unable to breathe for themselves secondary to severe injury, extensive surgery, or respiratory failure secondary to cardiac or pulmonary comorbidities. In cases such as this, intubated patients (or those with tracheotomy) may require mechanical ventilation. While such ventilation is possible with the co-administration of neuromuscular blocking drugs, some forms of mechanical ventilation will be difficult for the patient to tolerate. For example, as the mechanical ventilator inflates the lungs, a volume will be reached that triggers the Hering-Breuer response [135]. This reflex ordinarily stops negative pressure inspiration in the normally breathing adult; however, in the mechanically ventilated patient, it may cause the patient to violently attempt to cough, more commonly known as "bucking" on the ventilator. This phenomenon may be attenuated by either sedation or narcotic analgesia, but the doses of such medications may result in prolonged inhibition of normal respiration and delay extubation. Further, in patients with either acute lung injury or adult respiratory distress syndrome (sometimes referred to as noncardiogenic pulmonary edema), the administration of neuromuscular blockade can decrease oxygen consumption [74]. In one study of 340 patients requiring mechanical ventilation secondary to adult respiratory distress syndrome, a group that received a 15-mg bolus dose of cisatracurium followed by an infusion of 37.5 mg/hour for 48 hours resulted in a 90-day mortality rate of 31.6%, compared with a mortality rate of 40.7% in those not receiving the protocol [136]. While the importance of the judicious use of neuromuscular blockers in the critically ill cannot be understated, one study provides clear and convincing evidence of the importance of a sufficient level of sedation that must also be given to these patients. In their study, patients were more likely to develop delirium in the absence of or inadequacy of sedation [137]. In their research, 64.4% of mechanically ventilated patients experienced delirium and had a 30-day mortality rate of 30.3%, compared with a rate of 11.9% in those not experiencing delirium [137]. Clinicians should always keep in mind the concept that neuromuscular blockers offer no diminution of central nervous system function whatsoever, and there can be few things as terrifying as being paralyzed and wide awake.
With the use of a PNS and the co-administration of sedation, the ICU staff can closely monitor a patient's degree of neuromuscular blockade. Indeed, one author has referred to the use of the PNS as the "criterion standard" in musculoskeletal monitoring [138]. Patients requiring prolonged neuromuscular blockade in the ICU are best treated with intravenous infusions of intermediate-acting agents. As with all hospitalized patients, the goal is to treat the underlying disease or injury as quickly as possible, and then wean the patient from the ventilator in a quick and efficient manner. While intermittent boluses of neuromuscular blockers will prevent patient movement, they may impede the reversal and weaning process. A large loading dose of neuromuscular blocking drug, given to ensure quick onset, has the disadvantage of exceeding the therapeutic dose levels and extinguishing the TOF response (no twitches). Until the dose begins to degrade, the clinician is "flying blind," as there is no way to determine the extent of neuromuscular blockade. Indeed, there may be a supramaximal dose, resulting in prolonged blockade. The infusion dose, though taking a bit longer to set up, stops at the desired point. The lack of peaks and nadirs ensures the correct dose throughout the administration of the drug, easing recovery from neuromuscular blockade. Finally, the administration of large bolus doses of neuromuscular blocking agents in the ICU has been associated with prolonged blockade [138]. This is attributed primarily to those agents degraded by the liver and eliminated by the kidney. During the peak stages of the patient's illness or injury, hepatic and/or renal function may decrease. Agents such as pancuronium and vecuronium, with significant hepatic breakdown, active metabolites, and diminished renal excretion, have been associated with prolonged neuromuscular blockade lasting days or weeks after the cessation of administration [138]. This phenomenon appears especially linked to those patients presenting with sepsis. One group of researchers speculated the aggravating effects of neuromuscular blockers in these patients may also be due to degraded renal function [139].
Another significant problem that may occur in ICU patients receiving muscle relaxants is the phenomenon of ICU-acquired weakness. This weakness may be nerve-centered (critical illness polyneuropathy) or muscle-centered (critical illness myopathy) [140]. One study reports an incidence of between 24% and 77% in patients with stays in the ICU as short as one week, and men appear to be twice as likely to experience this as women [140,141]. The nature and role of neuromuscular blocking in aggravating or preventing this weakness is still under consideration. One study, for example, found that cisatracurium had no effect on the development or exacerbation of ICU-acquired weakness [140]. A contrary view states that the risk of myopathy increases with the concomitant administration of corticosteroids and neuromuscular blockers [138]. From this perspective, the institution of "drug-free holidays" during which neuromuscular blockers are allowed to completely wear off, as verified by the PNS, may be warranted. Their findings were echoed by other researchers, who identified prolonged neuromuscular blockade as a risk factor for prolonged weakness [141]. These researchers recommended the same drug-free holidays, as well as the use of an infusion to keep the dose at a sufficient level to allow a TOF of two twitches, with regular PNS monitoring.
Neuromuscular blockers have a specific and carefully defined place in health care. The primary warning that comes with each of the agents is that an individual who is an expert in airway management should always be immediately available prior to their administration. The practitioner should always consider carefully the comorbidities of the patient receiving these agents. Further, every patient who receives these agents should receive some form of sedation, preferably deep sedation, to prevent him or her from consciously experiencing the terrifying phenomenon of being awake and paralyzed. Finally, patients who have received neuromuscular blocking agents should always be carefully monitored and, with rare exceptions, the minimum dose to achieve the therapeutic goal administered. Following these guidelines will result in patient care that is safe and comfortable.
1. Raintree. Tropical Plant Database: Curare. Available at http://www.rain-tree.com/curare.htm. Last accessed March 1, 2023.
9. Beecher HK, Todd DP. A study of deaths associated with anesthesia and surgery. Ann Surg. 1954;140(1):2-34.
10. Hall JE. Excitation of skeletal muscle: neuromuscular transmission and excitation-contraction coupling. In: Hall JE, Guyton AC (eds). Guyton and Hall's Textbook of Medical Physiology. 13th ed. Philadelphia, PA: Elsevier, Inc.; 2016: 89-95.
11. Brull SJ. Physiology of neuromuscular transmission. In: Murray MJ, Rose SH, Wedel DJ, et al. (eds). Faust's Anesthesiology Review. 4th ed. Philadelphia, PA: Elsevier; 2015: 98-100.
12. Rossman AC. The physiology of the nicotinic acetylcholine receptor and its importance in the administration of anesthesia. AANA J. 2011;79(5):443-440.
14. Martyn JA. Neuromuscular physiology and pharmacology. In: Miller RD (ed). Miller's Anesthesia. 8th ed. Philadelphia, PA: Elsevier; 2015: 423-443.
15. Calvey TN, Williams NE. Drugs that act on the neuromuscular junction. In: Calvey TN, Williams NE (eds). Principles and Practice of Pharmacology for Anesthetists. 5th ed. Malden, MA: Blackwell Publishing; 2008: 171-194.
16. Haas RE, Darsey DA, Powell D. Neuromuscular blocking agents, reversal agents, and their monitoring. In: Nurse Anesthesia. 6th ed. St. Louis, MO: Elsevier; 2018: 162-195.
17. Lien C, Eikermann M. Neuromuscular blockers and reversal drugs. In: Hemmings HC, Egan TD (eds.) Physiology and Pharmacology for Anesthesia: Foundations and Clinical Application. 2nd ed. Philadelphia, PA: Elsevier; 2019: 325-348.
18. Chambers SL, Ferry TG. Patients requiring mechanical ventilation. In: Hurd WW, Jernigan JG (eds). Aeromedical Evacuation: Management of Acute and Stabilized Patients. New York, NY: Springer-Verlag; 2003: 256-264.
19. Tobias JD, Lynch A, McDuffie A, Garrett JS. Pancuronium infusion for neuromuscular block in children in the pediatric intensive care unit. Anesth Analg. 1995;81(1):13-16.
20. Kobayashi O, Nagashima H, Duncalf D, et al. Direct evidence that pancuronium and gallamine enhance the release of norepinephrine from the atrial sympathetic nerve by inhibiting prejunctional muscarinic receptors. J Auton Nerv Syst. 1987;18(1):55-61.
21. Dalton DW, Tyers MB. A comparison of the muscarinic antagonist actions of pancuronium and alcuronium. J Auton Pharmacol. 1982;2(4):261-266.
22. Brinkmann M, Güenniker M, Freund U, Schieffer M, Peters J. Histamine plasma concentration and cardiovascular effects of non-depolarizing muscle relaxants: comparison of atracurium, vecuronium, pancuronium and pipecuronium in coronary surgical patients at risk. Anasthesiol Intensivmed Notfallmed Schmerzther. 1998;33(6):362-366.
23. Rahimzadeh P, Safari S, Faiz SHR, Alavian SM. Anesthesia for patients with liver disease. Hepat Mon. 2014;14(7):1-7.
24. Haas RE, Masters JE. Comparative recovery from three nondepolarizing neuromuscular blockers in a patient before and after chronic anticonvulsant therapy: a case report. AANA Journal. 1997;65(5):475-478.
25. Cope TM, Hunter JM. Selecting neuromuscular-blocking drugs for elderly patients. Drugs Aging. 2003;20(2):125-140.
26. Craig RG, Hunter JM. Neuromuscular blocking drugs and their antagonists in patients with organ disease. Anaesthesia. 2009;64(suppl 1):55-65.
28. Oliveri L, Plourdes G. Prolonged (more than ten hours) neuromuscular blockade after cardiac surgery. Can J Anaesth. 2005;52(1):88-93.
29. Nagaraja AS, Nagesha KA. A clinical study of intraocular pressure changes with vecuronium bromide and pancuronium bromide. Journal of Evolution of Medical and Dental Sciences. 2014;3(15):3887-3894.
30. Cote C. Pediatric anesthesia. In: Miller RD (ed). Miller's Anesthesia. 8th ed. Philadelphia, PA: Elsevier Saunders; 2015: 2757-2798.
31. Rupp SM, Castagnoli KP, Fisher DM, Miller RD. Pancuronium and vecuronium pharmacokinetics and pharmacodynamics in younger and elderly adults. Anesthesiology. 1987;67(1):45-49.
32. Barnett SR. Eldrely patients. In: Pardo M Jr, Miller RD (eds). Basics of Anesthesia. 7th ed. Philadelphia, PA: Elsevier Saunders; 2011: 568-579.
33. Ingrande J, Hendrikus JML. Anesthetic pharmacology and the morbidly obese patient. Curr Anesthesiol Rep. 2013;3,(1):10-17.
34. Casati A, Putzu M. Anesthesia in the obese patient: pharmacokinetic considerations. J Clin Anesth. 2005;17(2):134-145.
35. Wilcox SR, Bittner EA, Elmer J, et al. Neuromuscular blocking agent administration for emergent tracheal intubation is associated with decreased prevalence of procedure-related complications. Crit Care Med. 2012;40(6):1808-1813.
36. Naguib M. Sugammadex: another milestone in clinical neuromuscular pharmacology. Anesth Analg. 2007;104(3):575-581.
37. Naguib M, Lien C, Meistelman C. Pharmacology of neuromuscular blocking drugs. In: Miller RD (ed). Miller's Anesthesia. 8th ed. Philadelphia, PA: Elsevier Saunders; 2015: 958-994.
38. El-Baradie S. Neuromuscular efficacy and histamine-release hemodynamic changes produced by rocuronium versus atracurium: a comparitive study. J Egypt Natl Canc Inst. 2004;16(2):1007-1013.
39. Amin AM, Mohammad MY, Ibrahim MF. Comparative study of neuromuscular blocking and hemodynamic effects of rocuronium and cisatracurium under sevoflurane or total intravenous anesthesia. Middle East J Anesthesiol. 2009;20(1):39-51.
40. Rothenberg DM, O'Connor CJ, Tuman KJ. Anesthesia and the hepatobiliary system. In: Miller RD (ed). Miller's Anesthesia. 8th ed. Philadelphia, PA: Elsevier Saunders; 2015: 2244-2261.
41. Servin FS, Lavaut E, Kleef U, Desmonts JM. Repeated doses of rocuronium bromide administered to cirrhotic and control patients receiving isoflurane: a clinical and pharmacokinetic study. Anesthesiology. 1996;84(5):1092-1100.
42. van Miert MM, Eastwood NB, Boyd AH, Parker CJ, Hunter JM. The pharmacokinetics and pharmacodynamics of rocuronium in patients with hepatic cirrhosis. Br J Clin Pharmacol. 1997;44(2):139-144.
43. Robertson EN, DriessenJJ, Booij LH. Pharmacokinetics and pharmacodynamics of rocuronium in patients with and without renal failure. Eur J Anaesthesiol. 2005;22(1):4-10.
44. Bock M, Haselmann L, Böettiger BW, Motsch J. Priming with rocuronium accelerates neuromuscular block in children: a prospective randomized study. Can J Anesth. 2007;54(7):538-543.
45. Meakin GH. Role of muscle relaxants in pediatric anesthesia. Cur Opin Anaesthesiol. 2007;20(3):227-231.
46. Woloszczuk-Gebicka B, Wyska E, Grabowski T. Sevoflurane increases fade of neuromuscular response to TOF stimulation following rocuronium administration in children: a PK/PD analysis. Paediatr Anesth. 2007;17(7):637-646.
47. Baykara N, Solak M, Toker K. Predicting recovery from deep neuromuscular block by rocuronium in the elderly. J Clin Anesth. 2003;15(5):328-333.
48. Sieber F, Pauldine R. Geriatric anesthesia. In: Miller RD (ed). Miller's Anesthesia. 8th ed. Philadelphia, PA: Elsevier Saunders; 2015: 2407-2422.
49. Ingrande J, Lemmons HJ. Dose adjustments of anaesthetics in the morbidly obese. Br J Anaesth. 2010;105(Suppl 1):i16-i23.
50. Eckmann D. Anesthesia for bariatric surgery. In: Miller RD (ed). Miller's Anesthesia. 8th ed. Philadelphia, PA: Elsevier Saunders; 2015: 2200-2216.
51. Pellis T, Leykin Y, Albano G, et al. Perioperative management and monitoring of a super-obese patient. Obes Surg. 2004;14(10):1423-1428.
52. Sherren PB, Tricklebank S, Glover G. Development of a standard operating procedure and checklist for rapid sequence induction in the critically ill. Scand J Trauma, Resusc Emerg Med. 2014;14(21):1-10.
53. Keles GT, Yentür, Cavuş Z, Zakarya M. Assessment of neuromuscular and haemodynamic effects of cisatracurium and vecuronium under sevoflurane-remifentanil anaesthesia in elderly patients. Eur J Anaesthesiol. 2004;21(11):877-881.
54. Basu R, Sinha I. Comparison of intra operative muscle relaxation and neuromuscular recovery from continuous infusion of vecuronium and atracurium in ASA grade I & II patients undergoing midline and paramedian laparotomies. Al Ameen J Med Sci. 2015;8(1):55-63.
55. Shah H, Bhavsar M, Upadhyaya R, Rathod H. Rocuronium vs vecuronium: a comparison of intubating condition, hemodynamic parameters and post-operative outcome in patients of coronary artery bypass graft surgery. International Journal of Biomedical and Advance Research. 2013;04(12):857-864.
56. Aronson JK. Neuromuscular blocking drugs and relaxants. In: Aronson JK (ed). Meyler's Side Effects of Drugs Used in Anesthesia. Amsterdam: Elsevier; 2009: 179-264.
57. Della Rocca G, Pompei L, Coccia MG, et al. Atracurium, cisatracurium, vecuronium and rocuronium in patients with renal failure. Minerva Anesthesiol. 2003;69(7-8):605-615.
58. Kainuma M, Miyake T, Kanno T. Extremely prolonged vecuronium clearance in a brain death case. Anesthesiology. 2001;95(4):1023-1024.
59. Holt NF. Renal disease. In: Hines RL, Marschall KE (eds). Stoelting's Anesthesia and Co-Existing Disease. 7th ed. Philadelphia, PA: Elsevier; 2012: 334-356.
60. Playfor S, Jenkins I, Boyles C, et al. Consensus guidelines for sustained neuromuscular blockade in critically ill children. Pediatr Anesth. 2007;17(9):881-887.
61. Rivera R, Antognini JF. Perioperative drug therapy in elderly patients. Anesthesiology. 2009;110(5):1176-1181.
62. Takaya T, Takeyama K, Miura M, Takiguchi M. The influence of body fat on the onset of vecuronium induced neuromuscular blockade. Tokai J Exp Clin Med. 2001;26(3):107-111.
63. Birks J. Conducting a safe rapid sequence induction in pre-hospital care. Trauma. 2016;18(2):1-5.
64. Miller Rd, Rupp SM, Fisher DM, Cronnelly R, Fahey MR, Sonh YJ. Clinical pharmacology of vecuronium and atracurium. Anesthesiology. 1984;61(4):444-453.
65. Raghavendra T. Neuromuscular blocking drugs: discovery and development. J R Soc Med. 2002;95(7):363-367.
66. Karnalkar A. Neuromuscular blockade of injectable atracurium and injectable vecuronium. Journal of Evidence-Based Medical Care. 2015;2(59):8976-8980.
67. Naguib M, Samarkandi AH, Bakhamees HS, Magboul MA, el-Bakry AK. Histamine release hemodynamic changes produced by rocuronium, vecuronium, mivacurium, atracurium and tubocurarine. Br J Anesth. 1995;75(5):588-592.
68. Singh A, Rao WRP, Ramakanth P. Volume of distribution of atracurium in different cohorts: a comparative study. Journal of Evidence-Based Medicine and Health Care. 2015;2(38):6001-6009.
69. Kies SJ, Pabelick CM, Hurley HA, White RD, Ackerman MJ. Anesthesia for patients with congenital long QT syndrome. Anesthesiology. 2005;102(1):204-210.
70. Ward S, Neill EA. Pharmacokinetics of atracurium in acute hepatic failure (with acute renal failure). Br J Anaesth. 1983;55(12):1169-1172.
71. Gyasi HK, Naguib M. Atracurium and severe hepatic disease: a case report. Can Anaesth Soc J. 1985;32(2):161-164.
72. Philip S, George S, Sunil G. Acute fatty liver of pregnancy complicating a twin pregnancy. Indian J Anaesth. 2014;58(1):73-75.
73. Bajwa SJS, Bajwa SK, Ghuman G. Pregnancy with co-morbidities: anesthetic aspects during operative intervention. Anesth Essays Res. 2013;7(3):294-301.
74. Warr J, Thiboutot Z, Rose L, Mehta S, Burry LD. Current therapeutic uses, pharmacology, and clinical considerations of neuromuscular blocking agents for critically ill adults. Ann Pharmacother. 2011;45(9):1116-1126.
75. SarinKapoor H, Kaur R, Kaur H. Anaesthesia for renal transplant surgery. Acta Anesthesiol Scand. 2007;51(10):1354-1367.
76. van Kralingen S, van de Garde EM, Knibbe C, et al. Comparative evaluation of atracurium dosed on ideal body weight vs. total body weight in morbidly obese patients. Br J Clin Pharmacol. 2011;71(1):34-40.
77. Konstadt SN, Reich DL, Stanley DE, et al. A two-center comparison of the cardiovascular effects of cisatracurium (Nimbex) and vecuronium in patients with coronary artery disease. Anesth Analg. 1995;81(5):1010-1014.
78. Searle NR, Thomson I, DuPont C, et al. A two-center study evaluating the hemodynamic and pharmacodynamic effects of cisatracurium and vecuronium in patients undergoing coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 1999;13(1):20-25.
79. Doenicke AW, Czeslick E, Moss J, Hoernecke R. Onset time, endotracheal intubating conditions, and plasma histamine after cisatracurium and vecuronium administration. Anesth Analg. 1998;87(2):434-438.
80. Amin AM, Mohammad MY, Ibriham MF. Comparative study of neuromuscular blocking and hemodynamic effects of rocuronium and cisatracurium under sevoflurane of total intravenous anesthesia. Middle East J Anesthesiol. 2009;20(1):39-51.
81. Lagneau F, D'honneur G, Plaud B, et al. A comparison of two depths of prolonged neuromuscular blockade induced by cisatracurium in mechanically ventilated critically ill patients. Intensive Care Med. 2002;28(12):1735-1741.
82. Eagar MA. The patient with cirrhosis who presents for non-hepatic surgery. Southern African Journal of Anaesthesia and Analgesia. 2011;17(1):120-123.
83. Ali MA, Ebied RS, Atallah MA, et al. Cisatracurium dose-response relationship in patients with chronic liver disease. Egyptian Journal of Anesthesia. 2014;30(2):197-202.
84. Lentschener C, Ozier Y. Anaesthesia for elective liver resection. Eur J Anesthesiol. 2002;19(11):780-788.
85. Fisher DM. Neuromuscular blocking agents in pediatric anaesthesia. Br J Anesth. 1999;83(1):58-64.
86. ShangGuan W, Lian Q, Li J, Gao F. Clinical pharmacology of cisatracurium during nitrous oxide-propofol anesthesia in children.J Clin Anesth. 2008;20(6):411-414.
87. Meakin GH, Meretoja OA, Perkins RJ, et al. Tracheal intubating conditions and pharmacodynamics following cisatracurium in infants and children undergoing halothane and thiopental-fentanyl anesthesia. Pediatr Anesth. 2007;17(2):113-120.
88. Leykin Y, Pellis T, Lucca M, Lomangina G, Marzano B, Gullo A. The effects of cisatracurium on morbidly obese women. Anesth Analg. 2004;99(4):1090-1094.
89. Gaszyński T, Gaszyński W, Strzelcyk J. General anaesthesia with remifentanil and cisatracurium for a superobese patient. Eur J Anesthesiol. 2003;20(1):77-78.
90. Claudius C, Garvey LH, Viby-Morgenson J. The undesirable effects of neuromuscular blocking drugs. Anaesthesia. 2009;64(Suppl 1):10-21.
91. Keegan MT. Prolongation of succinylcholine effect. In: Murray MJ, Rose SH, Wedel DJ, et al. (eds). Faust's Anesthesiology Review. 4th ed. Philadelphia, PA: Elsevier Saunders; 2015: 180-182.
92. Wong SF, Chung F. Succinylcholine associated postoperative myalgias. Anaesthesia. 2000;55(2):144-152.
93. Melnick B, Chalasani J, Uy NT, Phitayakorn P, Mallett SV, Rudy TE. Decreasing post-succinylcholine myalgias in outpatients. Can J Anaesth. 1987;34(3Pt1):238-241.
94. Blanié, Ract C, LeBlanc PE, et al. The limits of succinylcholine for critically ill patients. Anesth Analg. 2012;115(4):873-879.
95. Piotrowski AJ, Fendler WM. Hyperkalemia and cardiac arrest following succinycholine administration in a 16-year-old boy with acute nonlymphoblastic leukemia and sepsis. Pediatr Crit Care Med. 2007;8(2):183-185.
96. Roman CS, Rosin A. Succinylcholine-induced masseter muscle rigidity associated with rapid sequence intubation. Am J Emerg Med. 2007;25(1):102-104.
97. Bauer SJ, Orio K, Adams BD. Succinylcholine induced masseter spasm during rapid sequence intubation may require a surgical airway: a case report. Emerg Med J. 2005;22(6):456-458.
99. Hunter JM. Muscle function and neuromuscular blockade. In: Aitkenhead AR, Moppett IK, Thompson JP (eds). Smith and Aitkenhead's Textbook of Anaesthesia. 6th ed. London: Churchill Livingstone Elsevier; 2013: 87-104.
100. Hall JE. Renal regulation of potassium, calcium, phosphate, and magnesium; integration of renal mechanisms for control of blood volume and extracellular fluid volume. In: Hall JE, Guyton AC (eds). Guyton and Hall Textbook of Medical Physiology. 13th ed. Philadelphia, PA: Elsevier; 2016: 389-407.
101. Thapa S, Brull SJ. Succinylcholine-induced hyperkalemia in patients with renal failure: an old question revisited. Anesth Analg. 2000;91(1):237-241.
102. Stolworthy C, Haas RE. Malignant hyperthermia: a potentially fatal complication of anesthesia. Semin Perioper Nurs. 1998;7(1):58-66.
103. Visiou M, Young MC, Wieland K, Brandom BW. Anesthetic drugs and onset of malignant hyperthermia. Anesth Analg. 2014;118(2):388-396.
104. Hayes J, Veyckemans F, Bissonette B. Duchenne muscular dystrophy: an old anesthesia problem revisited. Pediatr Anesth. 2008;18(2):100-106.
105. U.S. Food and Drug Administration. Succinylcholine Package Insert. Lake Forest, IL: Hospira; 2010.
106. Brownstein D, Shugerman R, Cummings P, Rivera F, Copass M. Prehospital endotracheal intubation of children by paramedics.Ann Emerg Med. 1996;28(1):34-39.
107. Rose JB, Theroux MC, Katz MS. The potency of succinylcholine in obese adolescents. Anesth Analg. 2000;90(3):576-578.
108. Mayglothling J, Duane TM, Gibbs M, et al. Emergency tracheal intubation immediately following traumatic injury: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg. 2012;73(5 Suppl 4):S333-S340.
109. Sherren PB, Tricklebank S, Glover G. Development of a standard operating procedure and checklist for rapid sequence induction in the critically ill. Scand J Trauma Resusc Emerg Med. 2014;22:41.
110. Rodney G, Raju PK, Ball DR. Not just monitoring; a strategy for managing neuromuscular blockade. Anaesthesia. 2015;70(10):1105-1118.
111. Kervin MW. Residual neuromuscular blockade in the immediate postoperative period. J Perianesth Nurs. 2002;17(3):152-158.
112. Murphy GS, Brull SJ. Residual neuromuscular block: lessons unlearned. Part I: definitions, incidence, and adverse physiologic effects of residual neuromuscular block. Anesth Analg. 2010;111(1):120-128.
113. Brull SJ, Murphy GS. Residual neuromuscular block: lessons unlearned. Part II: methods to reduce the risk of residual weakness. Anesth Analg. 2010;111(1):129-140.
114. Dabaene B, Beaussier M, Meistleman C, Donati F, Lienhart A. Monitoring the onset of neuromuscular block at the orbicularis oculi can predict good intubating conditions during atracurium-induced neuromuscular block. Anesth Analg. 1995;80(2):360-363.
116. Murphy GS, de Boer HD, Eriksson LI, Miller RD. Reversal (antagonism) of neuromuscular blockade. In: Miller RD, Eriksson LI, Fleisher LA, Wiener-Kronish JP, Cohen NH, Young WL (eds). Miller's Anesthesia. 8th ed. Philadelphia, PA: Elsevier Saunders; 2015: 995-1027.
117. Ghai B, Makkar JK, Wig J. Neuromuscular monitoring: a review. J Anaesth Clin Pharmacol. 2006;22(4):347-356.
118. Kopman AF, Zhaku B, Lai KS. The "intubating dose" of succinylcholine. Anesthesiology. 2003;99(5):1050-1054.
120. Farooq K, Hunter JM. Neuromuscular blocking agents and reversal agents. Anesth Intensive Care Med. 2011;12(6):266-270.
122. Kirkegaard-Nielsen H, Helbo-Hansen HS, Lindholm P, Severinsen IK, Pedersen HS, Jensen EW. Optimum time for neostigmine reversal of atracurium-induced neuromuscular blockade. Can J Anaesth. 1996;43(9):932-938.
123. Donati F, Lahoud J, McReady D, Bevan DR. Neostigmine, pyridostigmine, and edrophonium as antagonists of deep pancuronium blockade. Can J Anesth. 1987;34(6):589-593.
124. Kovac AL. Sugammadex: the first selective binding reversal agent for neuromuscular block. J Clin Anesth. 2009;21(6):444-453.
125. Nag K, Singh DR, Shetti AN, Kumar H, Sivashanmugam T, Parthasarathy S. Sugammadex: a revolutionary drug in neuromuscular pharmacology. Anesth Essays Res. 2013;7(3):302-306.
126. Lobaz S, Clymer M, Sammut M. Safety and efficacy of sugammadex for neuromuscular blockade reversal. Clin Med Insights Ther. 2014;6:1-14.
127. Vijayakumar E, Bosscher H, Renzi FP, Baker S, Heard SO. The use of neuromuscular blocking agents in the emergency department to facilitate tracheal intubation in the trauma patient: help or hindrance? J Crit Care. 1998;13(1):1-6.
128. Li J, Murphy-Lavoie H, Bugas C, Martinez J, Preston C. Complications of emergency intubation with and without paralysis. Am J Emerg Med. 1999;17(2):141-144.
129. Ma OJ, Atchley RB, Hatley T, Green M, Young J, Brady W. Intubation success rates improve for air medical program after implementing the use of neuromuscular blockers. Am J Emerg Med. 1998;16(2):125-127.
130. Brownstein D, Shugerman R, Cummings P, Rivera F, Copass M. Prehospital endotracheal intubation of children by paramedics.Ann Emerg Med. 1996;28(1):34-39.
131. Davis DP, Dunford JV, Poste JC, et al. The impact of hypoxia and hyperventilation on outcome after paramedic rapid sequence intubation of severely head-injured patients. J Trauma. 2004;57(1):1-8.
132. Bernard SA, Nguyen V, Cameron P, et al. Prehospital rapid sequence intubation improves functional outcome for patients with severe traumatic brain injury. Ann Surg. 2010;22(6):959-965.
133. Mallon WK, Keim SM, Shoenberger JM, Walls RM. Rocuronium vs. succinylcholine in the emergency department: a critical appraisal. J Emerg Med. 2009;37(2):183-188.
134. Perouansky M, Pearce RA, Hemmings HC Jr. Inhaled anesthetics. In: Miller RD, Eriksson LI, Fleisher LA, Wiener-Kronish JP, Cohen NH, Young WL (eds). Miller's Anesthesia. 8th ed. Philadelphia, PA: Elsevier Saunders: 2015; 614-637.
135. Hall J. Regulation of respiration. In: Hall JE, Guyton AC (eds). Guyton and Hall's Textbook of Medical Physiology. 13th ed. Philadelphia, PA: Elsevier, Inc.; 2016; 539-549.
136. Yegneswaran B, Murugan R. Neuromuscular blockers and ARDS: thou shalt not breathe, move, or die! Crit Care. 2011;15(5):311.
137. Shehabi Y, Riker RR, Bokesch PM, et al. Delirium duration and mortality in lightly sedated, mechnically ventilated intensive care patients. Crit Care Med. 2010;38(12):2311-2318.
138. Pandit L, Agrawal A. Neuromuscular disorders in critical illness. Clin Neurol Neurosurg. 2006;108(7):621-627.
139. Schefold JC, Bierbrauer J, Weber-Carstens S. Intensive care unit-acquired weakness (ICUAW) and muscle wasting in critically ill patients with severe sepsis and septic shock. J Cachexia Sarcopenia Muscle. 2010;1(2):147-157.
140. Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol. 2011;10(10):931-941.
141. Linos K, Foot C, Ziegenfuss M, Freeman WD, Tan KM. Critical illness weakness: common questions. Curr Anaesth Crit Care. 2007;18(5-6):252-260.
142. Kim TK, Obara S, Johnson KB. Basic principles of pharmacology. In: Miller RD (ed). Miller's Anesthesia. 8th ed. Philadelphia, PA: Elsevier/Saunders; 2015: 590-613.
143. Ortiz-Gómez JR, Carrascosa F, Pérez-Cajaraville JJ, Percaz-Baldos JA, Añez C. Comparative study of intubating conditions at the first minute with suxamethonium, rocuronium and different priming. Eur J Anesthesiol. 2005;22(4):263-268.
144. Ebert TJ. Autonomic nervous system pharmacology. In: Hemmings HC, Egan TD (eds). Pharmacology and Physiology for Anesthesia: Foundations and Clinical Application. Philadelphia, PA: Elsevier/Saunders; 2013: 282-299.
145. Roman J. FDA Panel Recommends Approval of Merck Drug Sugammadex To Reverse Effect of Muscle Relaxant. Available at https://www.techtimes.com/articles/104217/20151109/fda-panel-recommends-approval-of-merck-drug-sugammadex-to-reverse-effect-of-muscle-relaxant.htm. Last accessed March 1, 2023.
146. Naguib M. Sugammadex: another milestone in clinical neuromuscular pharmacology. Anesth Analg. 2007;104(3):575-581.
147. Dake. Neuromuscular Junction (Closer View). Available at https://commons.wikimedia.org/wiki/File:Synapse_diag4.png. Last accessed March 1, 2023.
148. Blaus B. Skeletal Muscle Fiber. Available at https://commons.wikimedia.org/wiki/File:Blausen_0801_SkeletalMuscle.png. Last accessed March 1, 2023.
1. Murray MJ, DeBlock H, Erstad B, et al. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med. 2016;44(11):2079-2103. Available at https://journals.lww.com/ccmjournal/Fulltext/2016/11000/Clinical_Practice_Guidelines_for_Sustained.16.aspx. Last accessed March 21, 2023.
Mention of commercial products does not indicate endorsement.