Food allergy affects approximately 5.8% to 8.0% of children and approximately 6.2% to 10.8% of adults in the United States, and the prevalence has been increasing. The number of deaths associated with food allergy remains relatively low, but some reactions can be life-threatening, making it necessary to ensure that individuals with food allergy and their families understand the potential severity of the allergy. This course provides an overview of food allergy, beginning with a definition of food allergy and a description of the two primary types of adverse food reactions. Brief discussions of the risk factors, epidemiology and natural history, and prevention of food allergies are followed by details on the cutaneous, gastrointestinal, and respiratory manifestations of food allergy. The focus of the course is a description of the diagnostic process involved in identifying food allergies, with an exploration of the benefits and risks of testing and comment on appropriate referrals. The management of food allergy is also discussed, highlighting the treatment of severe reactions after inadvertent ingestion of an allergen. The course closes by addressing the need for patient education and a brief look to the future of treatment.
This course is designed for dental professionals involved in the care of patients with food allergies who would benefit from a better understanding of the natural history, diagnosis, and treatment of food allergies.
The purpose of this course is to encourage dental professionals to raise the issue of reactions to food during patient encounters, especially with parents of young patients, and to educate patients about the importance of protecting themselves or their children from allergic reactions.
Upon completion of this course, you should be able to:
- Distinguish between the different types of adverse reactions to food.
- Discuss the prevalence of food allergy and the natural history of the disease, including risk factors.
- Analyze the data on strategies to prevent food allergy.
- Identify the cutaneous, gastrointestinal, and respiratory manifestations of food allergy.
- Summarize the recommended methods of diagnosing food allergy, including considerations for non-English-proficient patients.
- Describe the appropriate management of food allergies and food-induced anaphylaxis.
- Summarize the most important points of the emergency treatment of food-induced anaphylaxis.
Lori L. Alexander, MTPW, ELS, MWC, is President of Editorial Rx, Inc., which provides medical writing and editing services on a wide variety of clinical topics and in a range of media. A medical writer and editor for more than 30 years, Ms. Alexander has written for both professional and lay audiences, with a focus on continuing education materials, medical meeting coverage, and educational resources for patients. She is the Editor Emeritus of the American Medical Writers Association (AMWA) Journal, the peer-review journal representing the largest association of medical communicators in the United States. Ms. Alexander earned a Master’s degree in technical and professional writing, with a concentration in medical writing, at Northeastern University, Boston. She has also earned certification as a life sciences editor and as a medical writer.
Contributing faculty, Lori L. Alexander, MTPW, ELS, MWC, has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Mark J. Szarejko, DDS, FAGD
The division planner has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
Sarah Campbell
The Director of Development and Academic Affairs has disclosed no relevant financial relationship with any product manufacturer or service provider mentioned.
The purpose of NetCE is to provide challenging curricula to assist healthcare professionals to raise their levels of expertise while fulfilling their continuing education requirements, thereby improving the quality of healthcare.
Our contributing faculty members have taken care to ensure that the information and recommendations are accurate and compatible with the standards generally accepted at the time of publication. The publisher disclaims any liability, loss or damage incurred as a consequence, directly or indirectly, of the use and application of any of the contents. Participants are cautioned about the potential risk of using limited knowledge when integrating new techniques into practice.
It is the policy of NetCE not to accept commercial support. Furthermore, commercial interests are prohibited from distributing or providing access to this activity to learners.
Supported browsers for Windows include Microsoft Internet Explorer 9.0 and up, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Supported browsers for Macintosh include Safari, Mozilla Firefox 3.0 and up, Opera 9.0 and up, and Google Chrome. Other operating systems and browsers that include complete implementations of ECMAScript edition 3 and CSS 2.0 may work, but are not supported. Supported browsers must utilize the TLS encryption protocol v1.1 or v1.2 in order to connect to pages that require a secured HTTPS connection. TLS v1.0 is not supported.
The role of implicit biases on healthcare outcomes has become a concern, as there is some evidence that implicit biases contribute to health disparities, professionals' attitudes toward and interactions with patients, quality of care, diagnoses, and treatment decisions. This may produce differences in help-seeking, diagnoses, and ultimately treatments and interventions. Implicit biases may also unwittingly produce professional behaviors, attitudes, and interactions that reduce patients' trust and comfort with their provider, leading to earlier termination of visits and/or reduced adherence and follow-up. Disadvantaged groups are marginalized in the healthcare system and vulnerable on multiple levels; health professionals' implicit biases can further exacerbate these existing disadvantages.
Interventions or strategies designed to reduce implicit bias may be categorized as change-based or control-based. Change-based interventions focus on reducing or changing cognitive associations underlying implicit biases. These interventions might include challenging stereotypes. Conversely, control-based interventions involve reducing the effects of the implicit bias on the individual's behaviors. These strategies include increasing awareness of biased thoughts and responses. The two types of interventions are not mutually exclusive and may be used synergistically.
#58794: Food Allergies
Food allergy affects approximately 33 million individuals in the United States, including 5.8% to 8% of children and approximately 6.2% to 10.8% of all adults [1,3,4,5,17]. Despite an overall lower prevalence of food allergy in comparison to skin or respiratory allergy, there is cause for concern, as severe allergic food reactions can be life-threatening. It is estimated that 3.4 million patients annually require emergency medical care for food-induced allergic reactions, including medical procedures to treat anaphylaxis [1,4,17]. Food-induced anaphylaxis is the most frequent cause of anaphylactic reaction outside of the hospital setting, and more than 40% of children and 50% of adults with food allergy have experienced anaphylaxis or another severe allergic reaction [1,4,17]. In addition, food allergy is a serious public health and economic issue; the cost among families caring for children with food allergy in the United States was estimated to be $33 billion dollars in 2024 alone (adjusted from 2011–2012 figures) [6,17].
There is currently no cure for food allergy, and the cornerstones of management are strict avoidance of the causal food and swift response to allergic reactions. Most food allergies occur before the age of 2 years and are lost by late childhood [8]. Seafood (fish and/or shellfish) and peanut are the two primary persistent food allergens. Allergies that persist have a negative effect on the quality of life and can be especially challenging for teenagers and adolescents.
Guidelines for the diagnosis and management of food allergy are available. The American Academy of Allergy, Asthma and Immunology (AAAAI), the American College of Allergy, Asthma and Immunology (ACAAI), and the Joint Council of Allergy, Asthma and Immunology (JCAAI) jointly developed a practice parameter (first published in 2006, updated in 2014 by AAAAI/ACAAI and others), and comprehensive evidence-based guidelines were developed by an expert panel convened by the National Institute of Allergy and Infectious Disease (NIAID) in 2010, with peanut allergy diagnosis practice parameter update in 2020 [2,9,12,43]. All healthcare professionals should become familiar with these guidelines, as educational gaps regarding food allergy have been reported in nearly every specialty, including among primary care physicians, emergency departments, pediatricians, and school nurses. There are also differences noted in knowledge, beliefs, and attitudes between allergists and nonallergists [10,13,15,16].
Surveys have shown that food allergy education among healthcare professionals is lacking, and many professionals indicate they would benefit from further education. For example, in a assessment of charts from emergency departments, more than 50% of the primary care and emergency medicine physicians responding expressed the need for more education about food allergy, especially directed at referral guidelines (59%), diagnosis (52%), and patient education (50%) [15]. Additional need for food allergy education has been noted among students in food and nutrition, nursing, and pre-medicine [13]. Knowledge and increased awareness among individuals with food allergy and the general population is also needed, especially regarding the distinction between food allergy and food intolerance, the absence of a cure, and the current approach to treatment [16].
This course provides an overview of food allergy, beginning with a definition of food allergy and a description of the two primary types of adverse food reactions. Brief discussions of the epidemiology and natural history, risk factors, and prevention of food allergies are followed by details on the cutaneous, gastrointestinal, and respiratory manifestations of food allergy. The focus of the course is a description of the diagnostic process involved in identifying immunoglobulin E (IgE)-mediated food allergies, with details on diagnostic testing. The management of food allergy is also discussed, highlighting patient (and family) education about avoidance of risk, the accurate interpretation of food labels, the treatment of severe reactions after inadvertent ingestion of an allergen, supportive management, the future of immunotherapy, and the safety of routine vaccinations.
Food allergy is often misinterpreted by the general population to be any nontoxic adverse reaction to food [16]. However, food allergy represents a cluster of disorders that are characterized by an abnormal immunologic response to a substance in the food, usually a protein (sometimes a hapten) [2]. Food allergy is defined in the NIAID-sponsored guidelines on food allergy as an "adverse health effect arising from a specific immune response that occurs reproducibly on exposure to a given food" [2]. Adverse reactions to food are usually classified in two broad categories: IgE-mediated allergy or hypersensitivity (true food allergy) and non-IgE-mediated reactions; the latter group includes cell-mediated reactions and disorders that are a combination of IgE-mediated and cell-mediated reactions (Table 1) [2]. Non-IgE-mediated reactions include primarily gastrointestinal food allergies such as celiac disease, food protein-induced enteropathy and enterocolitis/proctocolitis, and eosinophilic disorders [2]. Allergic sensitization (presence of allergen-specific IgE) to a food can occur without clinical signs and symptoms on exposure to that food, but both sensitization and clinical symptoms are needed for a definition of food allergy [2].
ADVERSE REACTIONS TO FOOD
| Type of Reaction | Associated Condition | ||||
|---|---|---|---|---|---|
| Immunoglobulin E (IgE)-mediated |
| ||||
| Cell-mediated (non-IgE-mediated) |
| ||||
| Mixed (IgE-mediated and cell-mediated) |
| ||||
| Non-immune-mediated (primarily food intolerance) |
|
Food allergy is also distinct from adverse reactions that do not involve an immune response. These adverse reactions may result from a metabolic disorder (such as lactose or alcohol intolerance), a pharmacologic reaction (such as sensitivity to caffeine), a structural abnormality (such as hiatal hernia), or another, undefined response [2,18,19]. Headache, heartburn, vomiting, irritability or nervousness, and gas or bloating are symptoms related to food intolerance, whereas the hallmark symptoms of food allergy are rash or hives, itchy skin, cramping stomach pain, diarrhea, and in severe cases, shortness of breath, wheezing, and chest pain [2,18].
With an IgE-mediated response, food-specific IgE antibodies are produced after exposure to certain proteins that bind to tissue mast cells and basophils, leading to the release of mediators such as histamines and leukotrienes [19]. The resultant reaction typically manifests in symptoms or disorders related to the skin, gastrointestinal tract, and respiratory system [19]. Symptoms occur within minutes to 1 to 2 hours after the causal food has been ingested and vary from mild (oral or cutaneous symptoms only) to a life-threatening systemic reaction [2,19]. Sensitization without clinical symptoms is common; for example, approximately 1% of the population has a true allergy to peanut (sensitization plus symptoms), whereas approximately 8% will have sensitization to peanut (a positive test result) but no symptoms [21,22].
In general, nine allergens account for approximately 85% to 90% of IgE-mediated food allergies: cow's milk, hen's egg, peanut, tree nuts (e.g., walnuts, cashews.), fish (fin fish), shellfish, soy, wheat, and sesame. With shellfish, allergy to crustaceans (shrimp, crab, and lobster) is more common than allergy to mollusks (e.g., clams, oysters) [2,20].
Allergy to fresh fruits and vegetables is less common and is primarily attributed to oral allergy syndrome, a mild IgE-mediated reaction discussed later in this course [18,25]. Allergic reaction to fruits and vegetables not attributed to oral allergy syndrome can be more serious. In one study, researchers evaluated 346 allergic reactions to fruit and found that 52% consisted of only oral symptoms; 37% consisted of oral symptoms and a systemic reaction; and 11% consisted of a systemic reaction only [23]. Melon, kiwifruit, and avocado were the most frequent causes of isolated oral symptoms, whereas peach, banana, and kiwifruit were most often associated with a systemic reaction. Of the 38 solely systemic reactions, 13 were severe and five were life-threatening [23].
Fruits and vegetables are also implicated in cross-reactivity, or an allergy to foods that have proteins similar to those in other allergens (Table 2). For example, the Bet v1 and Bet v2 (profilin) proteins are found in birch pollen as well as several fruits, vegetables, and nuts, and approximately 70% of patients who are allergic to birch pollen may have symptoms after eating foods in this group [18,25]. Latex-fruit allergy is another example; an allergy to fruit will develop in 30% to 50% of individuals who are allergic to latex [25]. Common food allergens associated with latex allergy are avocado, banana, and kiwifruit [18]. Cross-reactivity also refers to an allergy to more than one food in a particular food group; it is estimated that 70% to 90% of individuals with seafood allergy have had reactions to multiple types of fish [4].
CROSS-REACTIVITY OF ALLERGENS
| Known Allergen | Cross-Reactivity |
|---|---|
| Natural rubber latex | Apple, avocado, banana, buckwheat, carrot, celery, chestnut, dill, kiwifruit, melon, oregano, papaya, potato, sage, tomato; possibly: apricot, cherry, grape, orange, passion fruit, peach, peanut, pear, pineapple, rye, soybean, strawberry, walnut |
| Bird feathers | Egg yolk |
| Pollens | |
| Alder | Almond, apple, celery, cherry, hazelnut, parsley, peach, pear |
| Birch | Almond, apple, apricot, buckwheat, carrot, celery, cherry, coriander, fennel, hazelnut, honey, kiwifruit, nectarine, parsley, parsnip, pear, peach, peanut, pepper, plum, potato, prune, spinach, tomato, walnut, wheat |
| Grass | Melon, orange, pear, Swiss chard, tomato, watermelon, wheat |
| Mugwort | Carrot, celery, coriander, fennel, melon, parsley, pepper, spices, sunflower seed, watermelon |
| Ragweed | Apple, banana, cantaloupe, chamomile tea, honey, honeydew melon, nuts, sunflower seed, watermelon |
One of the fastest growing new allergies is to sesame, with both IgE-mediated hypersensitivity and cell-mediated reactions occurring [3,5]. At the other end of the spectrum is a rare allergy that developed in an Inupiat boy; allergy was confirmed to bearded seal and bowhead whale, staples in the diet of residents of coastal Alaska [28]. This case is thought to be the first documentation of an IgE-mediated reaction to these species and is a reminder that all foods in a patient's diet should be considered as potential allergens.
The understanding of non-IgE-mediated reactions is not as clear as that of IgE-mediated reactions. Most adverse food reactions have no immunologic basis. However, for many adverse reactions that affect primarily the gastrointestinal tract, a cell-mediated response is involved. Several mechanisms have been suggested to play a role in these reactions, including an abnormal mucosal immune response and responses involving mast cells, eosinophils, macrophages, and T-cells. In contrast to IgE-mediated reactions, the symptoms associated with non-IgE-mediated reactions are delayed, often not occurring for hours or days after the suspected food was ingested [18,24,26].
The true prevalence of food allergy has been difficult to determine for many reasons, including lack of uniform diagnostic criteria, misclassification of adverse reactions, the use of self-reports, and the potential for allergy resolution [27]. In general, the prevalence has been higher when food allergies are self-reported than when the food allergy has been documented after diagnostic testing. Studies have shown that parent- and self-reporting of food allergy are higher when compared with rates of food allergy determined by diagnosis or history of IgE-mediated food allergy. In one study, parent-reported food allergy accounted for 11.4% of candidates, but only 7.6% had diagnosed allergy [1]. Among adults, one study showed that 19% of individuals self-reported food allergy, but only 11% had documented diagnosis of food allergy [4].
As noted, studies have found that food allergy affects approximately 5.8% to 8% of children and 6.2% to 10.8% of all adults in the United States [1,3,4,5,17]. CDC data indicate a steadily rising prevalence rate, with a greater than 50% increase in food allergy among children from 1997 to 2011, and again a greater than 50% increase from 2007 to 2021 [4]. The CDC also noted that the prevalence of food allergy increases with age in children (from 4.4% in ages 0 to 5 years), peaks in adolescence (7.1% in ages 12 to 17 years), then decreases with age in adults (from 6.6% in ages 18 to 44 to 5.1% in ages 65 to 74, and 4.5% in adults ages 75 and older), although the trend is not linear when allergen types are taken into account [3,5]. The prevalence of food allergy to specific allergen types varies significantly between children (0 to 17 years of age) and adults (18 years of age and older). Among children in the United States with food allergy, the most common allergens, in order of prevalence, are peanut, milk, shellfish, and tree nut; the most common allergens among adults are shellfish, milk, peanut, and tree nut (Table 3) [1,4]. In addition, nearly 40% of children and 46% of adults with food allergies report allergy to more than one food [4,17].
ESTIMATED PREVALENCE OF FOOD ALLERGY IN THE UNITED STATES, BY TOP NINE RECOGNIZED ALLERGENS
| Allergen | Prevalence | |
|---|---|---|
| Children | Adults | |
| Peanut | 2.2% | 1.8% |
| Milk | 1.9% | 1.9% |
| Crustacean shellfish (e.g., shrimp, crab, lobster) | 1.3% | 2.9% |
| Tree nuts (e.g., almond, cashew, walnut, pecan, hazelnut) | 1.2% | 1.2% |
| Egg | 0.9% | 0.8% |
| Fish (fin fish) | 0.6% | 0.9% |
| Wheat | 0.5% | 0.8% |
| Soy | 0.5% | 0.6% |
| Sesame | 0.2% | 0.2% |
Data from the 2021 National Health Interview Survey (NHIS) provide information on children and adolescents with food allergy according to gender and race/ethnicity. In 2021, the prevalence was essentially the same in girls and boys (5.8% vs. 5.9%). The prevalence among children with food allergy was highest among Black children (7.6%), followed by Asian (6.6%), White (5.3%), and lowest among Hispanic children [3]. Income may also indicate prevalence, with 2018 NHIS data showing the highest prevalence in households with incomes greater than $100,000 (7.3%) compared with households with incomes $50,000–$74,999 (5.2%). Geographic area is also a factor in the prevalence of food allergy, with the highest prevalence in the West (7.4%), followed by the South (6.5%), Northeast (6.4%), and Midwest (5.4%) [7,41].
Among adults with food allergy, 7.5% of men and 13.8% of women have food allergy, and 3.0% and 7.2% were adult-onset, respectively. Prevalence of food allergy according to race and ethnicity among adults were different in some ways to those of children, with the lowest rate seen in non-Hispanic White adults (10.1%) and higher rates among non-Hispanic Black adults (11.2%), Asian adults (11.4%), and Hispanic adults (11.6%); this survey also included adults with multiple or other races, who had the highest rate (15.9%) [4]. Household income of adults with food allergy also differs from that of children; prevalence appears to be highest when household income is $50,000–$99,999, and there is a decreased prevalence for incomes greater than and less than that range. Geographic distribution of adult food allergy was also slightly different than that described for children, with the West accounting for the greatest prevalence (11.5%), followed by the Northeast (11.2%), South (10.4%), and Midwest (10.3%) [4,7].
There are few known risk factors for food allergy, but researchers continue to explore and identify possible genetic and environmental causes. Atopic diseases include inflammatory or allergic diseases such as atopic dermatitis (eczema), allergic rhinitis, asthma, and food allergy; atopy is the genetic predisposition of an exaggerated IgE-mediated response to allergens that cause atopic diseases [29]. It has long been thought that the strongest risk factors for atopic disease, including food allergy, were atopic dermatitis or family history of atopy [2,9]. However, ongoing research has identified atopic dermatitis or atopy of the individual, rather than family history, as a better indicator of risk for food allergy [30]. In addition, a family history of food allergy has shown little impact on predicting risk of a child developing peanut allergy in the absence of atopic dermatitis [31].
The prevalence of IgE-mediated food allergy increases with the severity of atopic dermatitis [32]. In fact, of all atopic diseases, the strongest association is between childhood eczema and IgE-mediated food allergy, with a 4.7 hazard ratio, almost twofold higher than that observed for the risk of asthma or allergic rhinitis. Additionally, children that develop atopic dermatitis by 3 months of age and with more severe atopic dermatitis have the greatest risk of food sensitization. Overall, approximately 30% of children with moderate-to-severe atopic dermatitis exhibit clinical evidence of food allergy [32].
Studies have shown that rates of asthma, eczema or skin allergy, and respiratory allergy are substantially higher among children and adults with food allergy than those individuals without food allergy. One study noted respiratory allergy in 31.5% of children with food allergy (vs. 8.7% without), asthma in 29.4% (vs. 12.4% without), and atopic dermatitis in 27.2% (vs. 8.1% without) [33]. Among adults, the most common comorbid conditions with food allergy include latex allergy (28.8%), urticaria or chronic hives (27.8%), insect sting allergy (22.9%), asthma (20.9%), atopic dermatitis (19.2%), medication allergy (18.5%), and environmental allergies (17.2%) [4].
Another risk factor appears to be food allergy itself. Among individuals who have an IgE-mediated reaction to one food allergen, the likelihood is high that reaction will occur to another food allergen, as well as to aeroallergens, such as pollens. As noted, 40% of children and 46% of adults with food allergy have multiple food allergies [4,17].
Food allergy is thought to be the result of immaturity of both the immune system and the mucosal barrier in the gastrointestinal tract. Early exposure to food proteins leads to allergic sensitization against a specific food. Thus, most food allergies develop before the age of 2 years, with the prevalence peaking by 2 years of age and then gradually decreasing through late childhood before peaking again in early adulthood (18 to 29 years of age). As noted, the rate of adult-onset food allergies are becoming more common; among the 10.8% of adults who indicated that they had a food allergy, adult-onset allergy occurred most commonly with wheat (52.6%), shellfish (48.2%), soy (45.4%), fin fish (39.9%), tree nuts (34.6%), eggs (29%), sesame (25.7%), milk (22.7%), and peanut (17.5%) [4].
The percentage of children who achieve desensitization or resolution of food allergy varies according to the allergen and increases with age (Table 4) [2,8,34]. Most children who have allergy to milk, egg, soy, or wheat lose the sensitivity over time, with the time varying according to food. In contrast, allergy to peanut, tree nuts, and shellfish usually persists into adulthood [2]. Allergy to peanut or tree nuts is lost in about 20% of children after the age of 5 years [8]. The level of allergen-specific IgE is often an indicator of persistence; high initial levels of allergen-specific IgE have been associated with lower rates of resolution, and decreases in IgE levels over time often indicate the onset of tolerance [2,8].
RESOLUTION OF COMMON FOOD ALLERGIES
| Allergen | Percentage of Resolution | ||||
|---|---|---|---|---|---|
| Cow's milk |
| ||||
| Hen's egg |
| ||||
| Soy |
| ||||
| Wheat |
| ||||
| Peanut | 20% after 5 years | ||||
| Tree nuts | 20% after 5 years | ||||
| Shellfish | Persistent | ||||
| Seafood | Persistent | ||||
| Sesame | 32% after 4 years |
Several strategies have been proposed as measures to prevent the development of food allergy, including maternal dietary restrictions, the use of soy-based formula, exclusive breastfeeding, and delayed or early introduction of solid foods and of allergenic foods. Maternal dietary restrictions and/or use of soy formula have not been shown to be effective in preventing food allergy and are not recommended in current guidelines [40,42].
Recommendations regarding the timing of the introduction of solid foods have evolved over the past three decades as more research data have become available. According to an updated 2022 Policy Statement, the American Academy of Pediatrics (AAP) recommends breastfeeding exclusively for the first six months, with appropriate solid foods being introduced at about 6 months of age while continuing to breastfeed up to 2 years of age or longer. Infants at high risk of peanut allergy because of the presence of severe eczema and/or egg allergy are the exception to early introduction of foods; an expert panel has advised peanut introduction as early as 4 to 6 months of age for infants at high risk, but not until 6 months for infants at moderate or low risk [11,40]. These recommendations are in contrast to those previously published by the AAP in 2008 and 2019, which indicated that there was little benefit of delaying the introduction of solid foods, including potential allergens such as peanuts, eggs, and fish, beyond 4 to 6 months of age to prevent food allergy or atopic disease in general [35,39].
In 2021, the AAAAI, ACAAI, and the Canadian Society for Allergy and Clinical Immunology (CSACI) released a joint consensus statement with recommendations for the prevention of food allergy through nutrition. Regarding breastfeeding, the AAAAI/ACAAI/CSACI states, "although exclusive breastfeeding is universally recommended for all mothers, there is no specific association between exclusive breastfeeding and the primary prevention of any specific food allergy" [42]. Recommendations on initiating solid foods for prevention of food allergy include introducing both peanut and egg around 6 months of life, but not before 4 months, in at-risk or high-risk individuals. Screening before introduction is not required but may be preferred by some families [42].
In addition, NIAID-sponsored guidelines were revised in 2017 to recommend introducing peanut-containing foods as early as 4 to 6 months of age as a strategy to prevent peanut allergy in high-risk infants [11].
While guidelines vary, it is accepted overall that breastfeeding exclusively for the first three to four months has been shown to have short- and long-term medical and neurodevelopmental advantages, and while there is no direct link to primary prevention of food allergy, breastfeeding in the first 3 to 4 months of life appears to be a protective factor [40]. Research in food allergies prevention is expanding and evolving rapidly [11; 40; 42].
Food-induced adverse reactions vary from mild to severe and life-threatening. As noted, an estimated 3.4 million patients annually require emergency medical care for food-induced allergic reactions, including medical procedures to treat anaphylaxis. Most reactions are mild to moderate, with the exception of reactions to peanut, which are often severe. However, it has been reported that 51% of adults and 42% of children with food allergy have experienced a severe reaction to food allergens [1,4,17] .
The severity of allergic reactions varies according to several factors, including the amount of food ingested, the form of the food (raw, cooked, or processed), the ingestion of other foods at the same time, the patient's age, the degree of sensitization, and the presence of comorbidities. The presence of asthma is the factor most commonly associated with the most severe reactions. The degree of severity of past reactions cannot be used to accurately predict future reactions [2,4].
Accidental ingestion of a food is the most common cause of an adverse reaction, and reactions may occur frequently, even though the food allergy is known. In a study of infants (3 to 15 months of age) with a documented or likely allergy, more than half of the children had more than one reaction over 36 months of evaluation. Reactions were significantly associated with a higher number of food allergies in a child and a higher food-specific IgE level [37]. Young children who frequently put their hands in their mouths are more prone to accidental ingestion and are therefore more likely to experience adverse effects to allergens such as peanut [17].
Allergic reactions can also be caused by exposure to food allergens through saliva—either through kissing or the sharing of utensils or drinking straws. Approximately 5% to 16% of people with food allergy have reported an allergic reaction caused by kissing [112].
Food allergy manifests itself primarily through the skin, gastrointestinal tract, and respiratory system, and symptoms are categorized as acute or delayed (Table 5) [2]. Cutaneous symptoms are typically the most common.
SYMPTOMS OF FOOD-INDUCED ALLERGIC REACTIONS
| Target Organ | Immediate Symptoms | Delayed Symptoms | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cutaneous |
|
| ||||||||||||
| Ocular |
|
| ||||||||||||
| Upper respiratory |
| — | ||||||||||||
| Lower respiratory |
|
| ||||||||||||
| Gastrointestinal (oral) |
| — | ||||||||||||
| Gastrointestinal (lower) |
|
| ||||||||||||
| Cardiovascular |
| — | ||||||||||||
| Miscellaneous |
| — |
The most common cutaneous conditions associated with food allergy are urticaria (hives) and angioedema. These skin conditions occur in approximately 20% of the general population with food allergy and are more common in younger patients and patients with atopy. Urticaria is characterized by transient erythematous raised, well-demarcated plaques that are often intensely pruritic. The plaques frequently have central pallor and blanch when pressure is applied; they are usually the result of an inflammatory reaction. Approximately 20% of cases of acute urticaria (duration of less than 6 weeks) are caused by IgE-mediated reactions. Clinicians should take care in interpreting the cause of urticaria, as only a small fraction of people who believe the skin condition is associated with food actually have this manifestation in placebo-controlled studies [45].
Angioedema is considered a more severe form of the same pathologic process as urticaria. Whereas urticaria is limited to the superficial dermis, angioedema affects vessels in the deep dermis and subcutaneous tissue. Angioedema is characterized by edema of distensible tissue, including the face, genitals, extremities, lips, tongue, and uvula. If angioedema occurs in the respiratory tract, it can result in dysphagia, respiratory distress, or complete airway obstruction [45].
The gastrointestinal tract is a common target organ for cell-mediated reactions to foods. Gastrointestinal disorders can be difficult to identify and diagnose, particularly because symptoms are not always easily associated with ingestion of causal foods. The American Gastroenterological Association (AGA) notes several elements that may suggest food allergy as a cause of gastrointestinal disease (Table 6) [46].
ELEMENTS SUGGESTING FOOD ALLERGY AS A CAUSE OF GASTROINTESTINAL DISEASE
|
Oral allergy syndrome, also known as pollen-associated food allergy syndrome, is most common among individuals with pollen allergy. This syndrome is primarily a localized IgE-mediated reaction, with mild symptoms that include itching, irritation, or swelling occurring around the mouth after eating raw fresh fruits and vegetables, and other symptoms, such as rash, hives, watering of the eyes, nasal congestion, or tingling of the lips or tongue, may also develop. Symptoms usually resolve within a few minutes after ingestion and rarely progress to a systemic reaction. Often, no allergic reaction occurs after ingestion of fruits and vegetables that have been cooked, as heating destroys the foods' proteins. Due to cross-reactivity, allergic reactions can be more common when levels of ragweed pollen are high [2,25].
Celiac disease is a cell-mediated reaction to gluten that occurs in approximately 1% to 4% of the global population [47]. It is usually characterized by diarrhea, borborygmus (rumbling of the stomach or gut), steatorrhea, abdominal pain, bloating, and weight loss attributed to maldigestion and malabsorption associated with intestinal villous atrophy [.However, some individuals present with constipation and/or recurrent vomiting or heartburn, often mistaken for a functional disorder or irritable bowel syndrome. Fatigue is common in individuals with celiac disease, with an incidence as high as 37% at diagnosis [48]. The standard criterion for diagnosis is detection of celiac-specific antibodies with serologic testing, confirmation with biopsy of the jejunal mucosa (which shows flattening of villi), and positive clinical and serologic response to a gluten-free diet. Symptoms are alleviated by avoidance of gluten, which must be maintained over the individual's lifetime [49].
Non-IgE-mediated gastrointestinal food allergic disorders include food protein-induced enterocolitis syndrome (FPIES), allergic proctocolitis (FPIAP), allergic enteropathy (FPE), and dysmotility disorders (GORD and constipation). These diseases affect infants, and the cell-mediated reactions are usually in response to cow's milk or soy. However, other foods may also be implicated. Symptoms include protracted diarrhea and profuse vomiting, which can lead to malabsorption, dehydration, and lethargy. Biopsy specimens show increased intraepithelial lymphocytes and eosinophils and flattened villi, as in celiac disease. Elimination diets can help identify the food allergen. The diseases usually resolve within one to two years, making it helpful to monitor the child with follow-up diagnostic testing [50].
The incidence of eosinophilic esophagitis is rising rapidly, increasing more than fivefold since 2009. Incidence rates are estimated to be between 50 to 100 per 100,000 individuals in the United States [51,52]. Approximately 60% to 80% of individuals with the disease will have a concomitant allergy such as eczema, allergic rhinitis, asthma, or another chronic respiratory disease. Symptoms of eosinophilic esophagitis include heartburn, chest pain, dysphagia, vomiting, abdominal pain, and food impaction. Eosinophilic esophagitis is diagnosed with three criteria: symptoms of esophageal dysfunction; dense infiltrate of eosinophils (at least 15 per high-power field) on esophageal biopsy; and an evaluation for non-eosinophilic esophagitis disorders that cause or potentially contribute to esophageal eosinophilia Findings on endoscopy are subtle granularity with linear furrows or rings, adherent white plaques, or friable mucosa. In addition to avoidance of food allergens, treatment may include topical or systemic corticosteroids, proton pump inhibitors, or leukotriene inhibitors [50,51,53]. A 2025 guideline for the diagnosis and management of eosinophilic esophagitis is available from the American College of Gastroenterology [50].
As with eosinophilic esophagitis, this disorder is characterized by eosinophilic inflammation and is caused by a combination of IgE-mediated and cell-mediated responses. Two-thirds of individuals with the disorder will also have peripheral eosinophilia. Approximately 50% to 70% of individuals have food allergy, atopic disease, or a family history of allergies. The most common symptoms are postprandial abdominal pain, diarrhea, vomiting, and early satiety. Biopsy specimens obtained through endoscopy show prominent tissue eosinophilia with mild mastocytosis. The approach to treatment is elimination of food allergens and corticosteroids for symptom control [53].
The respiratory system is not as commonly affected as the skin and gastrointestinal tract; its involvement usually indicates a systemic effect. The food allergens most commonly associated with respiratory system manifestations are egg, milk, peanut, fish, shellfish, and tree nuts. Manifestations range from mild (rhinitis) to severe (asthma and anaphylaxis). Anaphylaxis is discussed in detail later in this course, but a rare entity—food-associated, exercise-induced anaphylaxis—is discussed in this section.
Allergic rhinitis affects up to 60 million people in the United States annually and includes symptoms such as nasal congestion, rhinorrhea, sneezing, and pruritus. Isolated rhinitis is not a common manifestation of IgE-mediated food allergy; rather, it occurs along with cutaneous and/or gastrointestinal manifestations [54].
Like food allergy, asthma is an atopic disease, and, as noted previously, there is a strong association between the two conditions. Food-induced wheezing and bronchospasms occur in up to 50% of children during acute allergic reactions to food. In addition, asthma has been identified as a risk factor for anaphylaxis and is associated with poorer outcomes in children with food allergy. One study found that children with an allergy to cow's milk had a 10 times greater chance of severe reaction if they also had asthma. It has been recommended that any child with asthma be evaluated for food allergy, especially when acute episodes are unexplained or when asthmatic symptoms are accompanied by other manifestations of food allergy. Similarly, children with food allergy, especially those who have allergy to more than one food or who have severe allergy, should be evaluated for asthma [55].
Food-associated, exercise-induced anaphylaxis is a rare entity that occurs when ingestion of a food allergen is followed by exercise within several hours. The unique factor is that neither the food allergen nor the exercise alone induces anaphylaxis. The pathophysiology is not clearly defined, but it is thought to be related to degranulation of mast cells after the metabolic changes brought on by exercise. The condition occurs primarily in individuals with atopy, more often in women than men, and usually in young adults (adolescence through the thirties). People with the condition have reported several episodes per year. A variety of food allergens have been associated with the condition, including shellfish, fish, celery, tomato, wheat, grapes, chicken, dairy products, and matsutake mushrooms. The most common symptoms are pruritus, urticaria, angioedema, flushing, and shortness of breath. Treatment is aimed at preventing recurrence, and once the food allergen has been identified through diagnostic testing, the individual should refrain from exercising within 4 to 6 hours after eating the causal food [9,56].
Many individuals seek medical attention for evaluation of reactions to food, interpreting the reactions as food allergy. Several studies have indicated that 50% to 90% of food-related adverse reactions are not true food allergies [2]. Even when medical attention is sought, diagnostic testing is not always done. The NIAID guidelines recommend a detailed history or physical examination as an essential first step in the diagnosis of food allergy but note that they alone cannot provide a definitive diagnosis of food allergy, and an objective evaluation should be carried out to confirm or disprove a suspected food allergy. The history will suggest whether the reaction was IgE-mediated or non-IgE-mediated and can guide the selection of the most appropriate diagnostic testing. Diagnostic testing for non-IgE-mediated food allergies is complex; the focus here is on testing for IgE-mediated allergy [2,11,27].
Given the increasing rate of food allergies over time, practitioners should ask all parents of infants and young children specific questions about reactions after eating or drinking. According to the NIAID guidelines, a food allergy should be considered for the following [2,27]:
Infants, young children, and selected older children with a diagnosis of moderate-to-severe atopic dermatitis, eosinophilic esophagitis, enterocolitis, enteropathy, or allergic proctocolitis
Adults with eosinophilic esophagitis
Any individual with anaphylaxis or any combination of typical symptoms that occur within minutes to hours after ingesting food, especially young children and/or if symptoms have occurred after ingestion of a specific food on more than one occasion
In obtaining a detailed history, several questions are crucial, and healthcare professionals should ask the following [2,27]:
What food(s) do you suspect as the cause of the reaction?
How much time elapsed between eating the suspected food and the reaction?
How much of the suspected food did the patient eat before having the reaction?
Was the suspected food raw or cooked?
What specifically happened during the reaction? What symptoms did the patient have? How long did the symptoms last?
Has the patient had a similar reaction to the same food in the past? If so, how often has it occurred?
Is it possible that there was cross contamination of the suspected food?
Has this reaction ever occurred before at a time other than after exposure to the suspected food?
Was any treatment given?
Where did the reaction occur?
It may be helpful to request emergency department records or information from another physician who has evaluated the patient; details about the most recent reaction are of the most benefit. If the history includes an anaphylactic episode, the physician should gain as much information as possible about the reaction to help predict future reactions and develop an appropriate emergency plan. In addition, the history should elicit information about personal or family history of atopy or other allergies. A history of asthma or sensitivity to latex, for example, should prompt further diagnostic testing. When the patient and/or parents cannot suggest a causal food, they should be asked to keep a food diary and note any symptoms that correlate with dietary intake [2,27].
Even the most detailed history can lack the details sufficient for an accurate diagnosis. For example, it is difficult to isolate a single food that caused a reaction after a meal, especially when it may not be known how the suspected food was manufactured or prepared or if there was cross contamination. Symptoms that are thought to be related to a food allergy (such as urticaria or symptoms of anaphylaxis) may be associated with another cause. Also, symptoms of non-IgE-mediated reactions are difficult to relate to a food due to the long interval of time between ingestion and symptoms.
Unless the patient is being examined within a short time after an adverse reaction to food, the findings on physical examination may be unremarkable. Symptoms related to the skin, gastrointestinal tract, and respiratory system should be evaluated for their potential association with a food allergy, as previously discussed.
Although such symptoms may suggest the likelihood of food allergy, chronic conditions are rarely indicators. Urticaria, diarrhea, rhinitis, and cough are related to food allergy only if they occur within minutes to hours after ingestion of the offending food and last only a few hours. The presence of severe atopic dermatitis should raise suspicion of food allergy [2,27].
When food allergy is suspected on the basis of the history and/or physical examination, diagnostic testing should be done to confirm the identity of the causal food. Allergy testing can provide information on the likelihood of a reaction but it cannot predict the severity of clinical reaction. The NIAID-sponsored guidelines include recommendations for tests that should and should not be used to diagnose food allergy (Table 7) [2]. The three primary methods used to diagnose food allergy are skin prick testing, determination of allergen-specific IgE levels, and oral food challenges. A systematic review showed that each of these tests has advantages and drawbacks, and no one test is superior in terms of sensitivity and specificity. These recommendations were emphasized as part of the Choosing Wisely campaign, a successful initiative of the American Board of Internal Medicine Foundation that was in place from 2012–2023. In that campaign, the AAAAI noted that "unproven diagnostic tests, such as immunoglobulin G (IgG) testing or an indiscriminate battery of immunoglobulin E (IgE) tests" should not be used to evaluate allergy [57]. Instead, the appropriate diagnosis (and treatment) of allergies requires specific IgE testing on either skin or blood. The 2020 joint practice parameter on the diagnosis of peanut allergy recommends skin prick testing or whole peanut serum-specific IgE (sIgE) (or component-specific peanut sIgE) testing for individuals with physician-judged high pretest probability of peanut allergy or a moderate pretest probability before an oral food challenge [12].
EVIDENCE-BASED RECOMMENDATIONS FOR DIAGNOSTIC TESTING FOR FOOD ALLERGY
| Recommended | Not Recommended | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| aMay be useful in specific cases. | |||||||||
The oldest method for determining sensitization to food allergens is the skin prick test. The test is simple, provides rapid results, has high sensitivity, is inexpensive, and can be carried out in the primary care setting. Taken together with the history and physical examination, the findings of the skin prick test will help identify the food or foods that may be causing an allergic reaction. Intradermal allergy skin tests are associated with a high rate of false-positive results and are more painful than skin prick testing [2,27]. The NIAID-sponsored guidelines recommend that intradermal testing not be used to diagnose food allergy [2].
The skin prick test is designed to elicit a histamine reaction to a small amount of extract of a suspected allergen. The patient's history dictates the allergen extracts to be used, and the number of extracts should be kept to a minimum to avoid confusion in interpreting the results. When evaluating an individual for oral allergy syndrome, testing may be more sensitive when the prick technique is used with fresh foods, especially fruits and vegetables. This is also true in cases in which the findings with commercial extracts do not correlate with the clinical history [2,27].
The skin prick test is performed with a lancet containing a 1 mm point. A drop of the selected allergen is introduced into the skin, usually on the volar or inner aspect of the forearm. A pen is commonly used to mark a grid on the arm, and the allergens are instilled at intervals of at least 2 cm. The reaction is usually obvious after 10 to 15 minutes. In general, a wheal with a diameter of 3 mm or more is considered positive, and the larger the wheal, the more likely an allergy is present. However, the size of the wheal does not predict the severity of a reaction, and there are no standards for interpreting the results of skin prick tests [2,22,27].
Negative findings on a skin prick test are of the most value, as the test has an excellent negative predictive value (95% or more), especially when testing for allergy to egg, milk, wheat, peanut, tree nuts, fish, and shellfish. Negative skin prick test results rarely occur in an individual who has an IgE-mediated reaction to one of these foods; nevertheless, if the history is strong, a food allergy should not be ruled out on the basis of negative results on a skin prick test alone. The combination of a positive test result and an inconclusive history should prompt an oral food challenge [2,22,27].
Some issues to consider with skin prick testing include [2,27]:
A physician and emergency equipment must be readily available.
Particular care must be taken when testing is done on a child who has had a previous anaphylactic reaction.
Eczematous areas should be avoided.
The reaction site may be smaller when the test is performed where the skin is loose (as in the wrist).
Bleeding may lead to false-positive results.
Antihistamines and corticosteroids may affect the result. They should not be given for 48 to 72 hours before testing.
Test results may vary according to the time of day.
Standardization is lacking for the development of some natural extracts.
The NIAID-sponsored guidelines recommend that measurement of the amount of food allergen-specific IgE antibodies in the serum can help identify foods that have the potential for provoking an IgE-mediated reaction in an individual. The sensitivity of early assays was only slightly better than that of skin testing, but advances have led to more definitive results. Fluorescent enzyme immunoassay has become the preferred testing method. The results of different commercially available assays are not comparable, and although diagnostic decision points have been established, they vary across research groups because of differences in patient populations, especially with regard to age. For most food allergens, the likelihood of a definitive food allergy (rather than sensitization only) increases with higher levels of allergen-specific IgE; the exceptions are soy and wheat [2,27].
As with the skin prick test, the negative-predictive values for food allergen-specific IgE testing are better than its positive-predictive values. Thus, the results should be considered within the context of the history, physical examination, and the findings of other studies. If there is a history of previous allergic reactions and the results of testing are above threshold levels, an oral food challenge does not need to be done. In contrast, if there is no history of a reaction, a high level should be interpreted carefully, and an oral food challenge may be helpful in verifying the diagnosis. An oral food challenge may also be useful when the history provides unclear information and the results of food allergen-specific IgE testing are slightly below the threshold levels [2,12,27].
Serum food allergen-specific IgE levels do not always correlate with clinical symptoms, and as mentioned previously, sensitization without clinical allergy is common. In addition, testing may identify a cross-reactive food that has not caused an allergic reaction. The following are some issues to consider when interpreting the results of food allergen-specific IgE levels [2,22,24,25]:
Threshold levels of milk and egg are lower for younger children.
Laboratories vary in their ability to perform testing, and quality control is essential.
Threshold levels are not conclusive, as they have been determined only in children who had positive reactions to the allergen.
Higher IgE levels reflect a greater likelihood of a reaction but cannot predict the severity of a reaction.
The purpose of an elimination diet is to exclude suspected food allergens from the diet to alleviate symptoms and determine the causal food. An elimination diet is recommended especially for identifying foods causing non-IgE-mediated allergic disorders and some mixed IgE- and non-IgE-mediated food induced allergic disorders [2]. There are three types of elimination diets. With the most commonly used elimination diet, one or more specific foods are excluded from the diet on the basis of the history and the findings of skin prick testing. After one to two weeks, the food items are reintroduced into the diet, one at a time, to determine the allergen. This type is most helpful for patients who have IgE-mediated reactions [46].
The second type of elimination diet is the oligoantigenic diet, in which the only foods the patient is allowed to eat are those considered to have an overall low risk of being allergenic. The third type of elimination diet is an elemental diet, in which only a hypoallergenic formula is allowed; in some instances, a few so-called safe foods are allowed. The elemental diet is best for infants who have been eating few or no solid foods or for patients who are thought to have reactions to many foods [46].
Compliance with elimination diets, especially the elemental diet, is a tremendous challenge as a result of many contributing factors [2,27]:
Nutrition may be compromised.
More time is needed to plan and prepare meals.
Patient is restricted about where he or she can eat (cannot eat foods prepared outside of the home).
Cross-reactivity may occur.
The oral food challenge is the most effective diagnostic allergy test and can be carried out as an open challenge, a single-blind challenge, or a double-blind, placebo-controlled challenge. Despite this, a survey of AAAAI members identified many barriers to open food challenges, including lack of time, poor reimbursement, lack of staff, and lack of office space [58]. The double-blind, placebo-controlled challenge is the criterion standard for diagnosing food allergy and is used in research for comparing the results of other tests [2]. The open and single-blinded challenges can be carried out in the pediatric or primary care setting, but the double-blind, placebo-controlled challenge is usually performed by an allergist.
The open challenge is the easiest to perform. This type of challenge is effective for ruling out a food allergen and is generally used for people at low risk for reaction [59]. For example, an open food challenge is reasonable if a child has had a reaction to a food in its natural form but has not had a reaction to eating other foods in which the suspected food is an ingredient.
With a single-blind food challenge, the suspected food allergen is hidden (in another food or a capsule) and the identity of the food allergen and the placebo are known only to the examiner. This type of challenge is commonly used when the patient and/or parents have concerns about ingestion of the suspected food. The vehicle used to hide the food being challenged should be selected according to the patient's age. It is important to use a vehicle that provides complete masking of the food item [2,58].
In the double-blind placebo-controlled challenge, neither the examiner nor the patient and/or parent know the identity of the food allergen and the placebo. The test is valuable when it is important to eliminate the perspective of bias for everyone involved and can be helpful in getting patients and/or parents with firmly held beliefs to accept the results. The drawbacks of this type of challenge are its need for specialized personnel, the length of time needed to perform the test, the risk of anaphylaxis, and a lack of criteria for positive results [2,58].
The selection of patients for oral food challenges is important, as the test can be time-consuming and labor-intensive for the healthcare professional and can pose risks to the patient. In general, the decision to perform a food challenge is influenced by several factors, including the patient's age, past adverse food reactions, results of other diagnostic testing, the importance of the food to the patient (either because of nutrition or presence in an ethnic diet), and the patient's and/or caregiver's preferences. According to the practice parameter developed by the AAAAI, ACAAI, and JCAAI, the decision to perform an oral food challenge should be based on the results of serum food allergen-specific IgE testing within the context of the clinical history and not on the basis of the test results alone [9]. The optimum candidate for a food challenge is a young patient with a 50% or less likelihood of reacting to a food. Patients with either a very high or very low pretest probability of reaction are unlikely to benefit from an oral food challenge [2,9].
Information from the history should be used to design an appropriate oral food challenge for an individual patient. Details on the timing of symptoms in previous reactions can help determine how long the patient should be observed after ingesting the food. The quantity of food used in the challenge should also be based on the patient's history. The initial amount given is usually lower than that expected to prompt a reaction, and increasing amounts are given at intervals of 15 to 30 minutes. A description of the most recent reaction can be helpful for monitoring symptoms during the challenge. Some anxiety-related symptoms are similar to allergic reactions, and close observation and careful interpretation are needed to accurately identify a true allergic reaction [2,27].
Careful supervision by a physician or nurse is needed, and emergency personnel and equipment must be readily available, especially when testing children who have had severe reactions in the past. Children with a history of anaphylaxis should be tested in a hospital setting rather than a practice setting. Overall, however, food challenges are safe, with severe reactions occurring in approximately 1% of individuals tested [60]. Only one death during an oral food challenge has been reported in the literature since 1976 (a 3-year-old boy in Alabama in 2017) [61]. In a retrospective review of 1,273 oral food challenges, 436 reactions occurred, with epinephrine administered in 50 challenges (11% of the positive challenges, 3.9% of the total). The authors found that older age and peanut allergy were significant risk factors for anaphylaxis during oral food challenges [62]. Another survey of 6,377 oral food challenges showed a 14% rate of reaction and a 2% rate of anaphylaxis [63].
Due to the potential risks of a food challenge, it is important to explain the procedure carefully to the patient and/or parents and to describe why it is being carried out. This should be done in a language that is familiar to the patient and his or her parents. Parents should be assured of the safety of the test and reminded that the test setting is safer than an inadvertent ingestion of the causal food in school or elsewhere away from home. For children who take antihistamines or asthma medication, the drug(s) should be discontinued before the test, if possible. Asthma must be stable in order for the test to be carried out, however, so a child should continue taking a maintenance medication if necessary. Some children have had a positive result on an oral challenge even when they have continued to take antihistamines or asthma medication [2,27].
Although referrals from primary care physicians to board-certified allergists are common, primary care physicians have expressed a desire for referral guidelines [15]. Referrals are not discussed in the NIAID-sponsored guidelines but are addressed in the AAAAI guidelines. The AAAAI recommends referrals for the following [64]:
Persons who have limited their diet based on perceived adverse reactions to foods or additives
Persons with a diagnosed food allergy
Atopic families with, or expecting, a newborn who are interested in identifying risks for, and preventing allergy
Persons who have experienced allergic symptoms (urticaria, angioedema, itch, wheezing, gastrointestinal responses) in association with food exposure
Persons who experience an itchy mouth from raw fruits and vegetables
The care of patients with food allergy requires a partnership, involving not only the primary care physician and an allergist/immunologist but also a gastroenterologist, a dermatologist, and a nutritionist, as appropriate. When the results of allergen-specific IgE testing are positive, an allergist/immunologist can provide special expertise in the following areas [15,26,27]:
Determining whether an oral food challenge is needed
Providing education about eliminating food allergens
Managing allergic reactions
Carrying out follow-up testing
There is currently no cure for food allergy, and the mainstay of management is avoidance of the offending food. The NIAID-sponsored guidelines recommend the following [2,27]:
Individuals with IgE-mediated and non-IgE-mediated food allergies should avoid ingesting their specific allergen or allergens.
Individuals with food allergy and their caregivers should be given information on avoiding their food allergen and emergency management that is age- and culture-appropriate.
Individuals with food allergy and their caregivers should receive education and training on how to interpret ingredient lists on food labels and how to recognize labeling of the food allergens used as ingredients in foods.
All children with food allergy should have nutritional counseling and regular growth monitoring.
Follow-up diagnostic testing should be done to monitor a child's allergy status, especially for those food allergies that are most likely to be lost during later childhood (milk, egg, soy, and wheat).
Parents of children with food allergy have expressed a desire for comprehensive information on the management of food allergy and have noted the following specific topics: early signs and symptoms, cross contamination, reading of food labels, self-injectable epinephrine, and becoming a teacher and advocate. Studies and surveys of children with food allergy and their families have also shown that improved education is needed in these areas [37]. Every healthcare professional involved in an individual's care (pediatrician, family physician, allergist, nutritionist, nurse, etc.) should collaborate to ensure that patients with food allergy and their families understand these topics. The NIAID expert panel coined the SAFE mnemonic for patient education [2]:
Seek support
Allergen identification and avoidance
Follow-up with specialty care
Epinephrine for emergencies
Many educational resources are available for individuals with food allergy and their families. Healthcare professionals should supplement their discussions with patients and families by encouraging them to access reliable, credible information on the websites of professional associations, government agencies, and specialty organizations.
Given the importance of educating patients and their caregivers about how to manage food allergy, it is crucial to ensure understanding, especially when the patient and/or caregiver has low health literacy, lacks proficiency in the English language, or speaks a language other than that of the healthcare professional. Health literacy, the ability to understand health information and make informed health decisions, is integral to good health outcomes. Yet, the National Assessment of Adult Literacy estimated that only 12% of adults have "proficient" health literacy and 14% have "below basic" health literacy [65]. Rates of health literacy are especially low among ethnic minority populations. Compounding the issue of health literacy is the high rate of individuals with limited English proficiency. According to U.S. Census Bureau data from 2023, almost 69 million Americans speak a language other than English in the home, with approximately 26.3 million of them (8.4% of the population) speaking English less than "very well" [66]. Healthcare professionals should assess their patients' literacy level and understanding and implement interventions as appropriate. Translated and/or low-literacy resources may be beneficial for some patients and families.
The American Medication Association offers several health literacy resources for healthcare professionals on its website (https://www.ama-assn.org), and the U.S. Department of Health and Human Resources offers valuable information on cultural competency from the Office of Disease Prevention and Health Promotion (https://odphp.health.gov/our-work/national-health-initiatives/health-literacy) and the Office of Minority Health (https://minorityhealth.hhs.gov).
The results of studies have shown that both children and adults may underestimate the severity of a food allergy, which means that education on the consequences of risk-taking behaviors is essential. Many individuals with food allergy or their parents fear that a severe reaction will occur in a setting where immediate help will not be available. However, studies have shown that most severe reactions occur in a setting that is considered to be "safe," such as home, work, or school [4,16]. Although this fact should be reassuring, it does suggest that better education is needed to help individuals with food allergy and/or their parents be better able to avoid causal foods.
Patients and their families need help in identifying so-called hidden sources of food allergens to avoid inadvertent ingestion of a food allergen by cross contamination. For example, some deli meats may have trace amounts of dairy product if the meat was cut on a slicer also used to cut cheese. Particular emphasis should be placed on nonfood items as potential sources of allergens; for example, many cosmetics may contain milk, tree nut oils, wheat, or soy; modeling dough may contain wheat; and beanbag stuffing often includes nut shells (Table 8) [112].
HIDDEN SOURCES OF FOOD ALLERGENS
| Food Allergen | Potential Sources |
|---|---|
| Milk/dairy products | Gravies and gravy mixes, butter, casein, cheese, ghee, lactose, whey, nondairy products, packaged soup, luncheon meat (from deli slicer), hot dogs, sausages, artificial butter flavor, breakfast foods, chocolate, some prepared fish/shellfish (dipped in milk to maintain freshness/odor control), some medications, cosmetics |
| Egg | Creamy fillings, cake decorations, malted cocoa drinks, creamy salad dressing, egg substitute products, albumin, mayonnaise, processed pasta, chips, crackers, marshmallows, tortillas, finger paints (egg white) |
| Peanuts | Candy, nut butters, sunflower seeds, arachis oil (another name for peanut oil), baked goods, ice cream, cultural foods (African, Chinese, Indonesian, Mexican, Thai, and Vietnamese), chili, glazes and marinades, granola, vegetarian meat substitutes, pet food, compost/lawn fertilizer (peanut shells are sometimes added) |
| Tree nuts | Cereals, crackers, cookies, candy, chocolates, confections, baked goods, energy bars, flavored coffee, frozen desserts, marinades, barbeque sauces, some cold cuts (e.g., mortadella), cosmetics, "natural" sponges or brushes |
| Shellfish | Caesar salad or dressing, steak sauce, Worcestershire sauce, bouillabaisse, glucosamine, seafood flavoring (e.g., crab or clam extract), fish sauce |
| Fish (fin fish) | Fish flavoring, fish gelatin, fish oil, fish sticks, barbecue sauce, bouillabaisse, Caesar dressing, imitation fish or shellfish, Worcestershire sauce, cultural foods (African, Chinese, Indonesian, Mexican, Thai, and Vietnamese), kimchi |
| Soy | Peanut butter, soy sauce, Worcestershire sauce, tofu, cereals, infant formulas, baked goods, canned tuna, crackers, hot dogs, processed meats, vegetable gum, vegetable starch, vegetable broth, adhesives, printing inks, soaps, cosmetics, pet food |
| Wheat | Beer/ale, sausage, hot dogs, luncheon meats, ice cream, candy, pasta, glucose syrup, soy sauce, starches, plant-based meat alternatives, marinara sauce, potato chips, rice cakes, salad dressings, spices, turkey patties, decorative wreaths, modeling dough |
| Sesame | Spice blends or flavoring, Asian cuisine (sesame oil/seed), baked goods, bread crumbs, cereals, dipping sauces, dressings, margarine, processed meats and sauces, protein and energy bars, pretzels, sushi, vegetarian burgers, cosmetics, medications, nutritional supplements, perfumes, pet food |
The rising prevalence of food allergy and the associated public concern has heightened awareness of the problem in restaurants, schools, day care settings, camps, airplanes, and other community-based institutions. Still, vigilance and precaution are required. In a study of food-induced allergic reactions among infants (3 to 15 months of age), half of the reactions were caused by food given to them by someone other than a parent [37]. Precaution is needed with older children and teenagers, as well, whose behaviors are often guided by a need to be accepted by peers. Practitioners should emphasize the importance of asking about ingredients when eating at a restaurant or away from home and of accurate interpretation of food labels. Issues with eating at restaurants include cross contamination (the most common cause of allergic reactions related to meals in a restaurant), knowledge gaps among restaurant staff, and nondisclosure of an allergy to restaurant staff [67].
Parents and children should also be cautioned about the risks of exposure through means other than eating. For example, they should understand the risks of kissing and sharing utensils with people who have ingested an individual's food allergen. After eating peanut, preventive measures, such as brushing the teeth, rinsing the mouth and chewing gum, can reduce salivary Ara h1 (a peanut protein marker), but the allergen has remained detectable in about 40% of instances after such measures. People who have ingested peanut should wait several hours before kissing a person with peanut allergy [112].
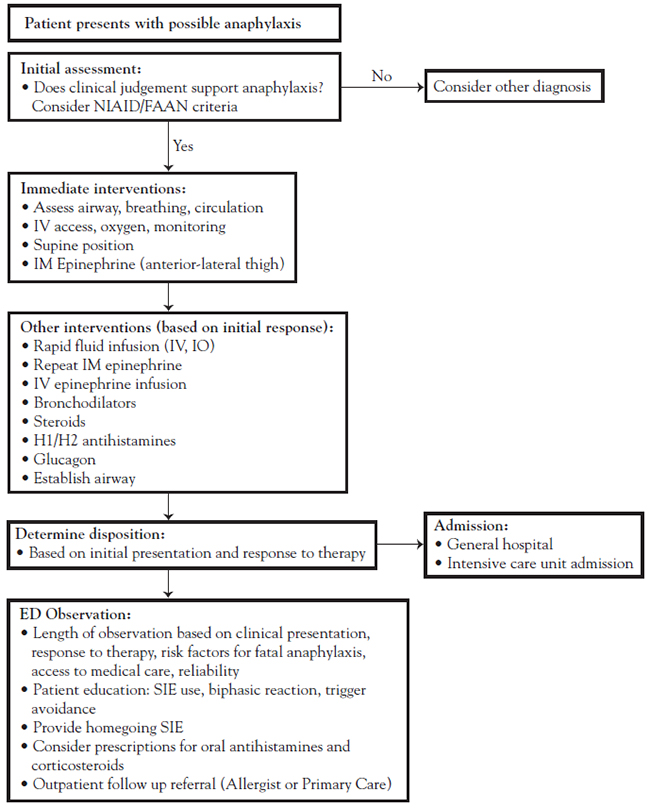
Individuals who are at risk for food-induced anaphylaxis should have medication on hand in case of inadvertent ingestion of a food allergen. Self-injectable epinephrine should be provided in a preloaded syringe to facilitate its use in an emergency situation, and the patient, as well as caregivers and all members of the family, should be instructed in how to administer the injection.
The most commonly used self-injectable epinephrine in the United States is EpiPen, although other brands are available, including Adrenaclick, Auvi-Q, Twinject, Adrenalin, and Symjepi. The EpiPen disposable drug-delivery system comes in two doses: 0.3 mg in 0.3 mL (EpiPen) and 0.15 mg in 0.3 mL (EpiPen Jr) autoinjectors, designed to be given intramuscularly. The manufacturer's labeling recommends one initial 0.15-mg dose for children weighing 15 to <30 kg or one 0.3-mg initial dose for children and adults who weigh ≥30 kg (the standard for all epinephrine-containing autoinjectors) [68]. Another brand available in the United States, Auvi-Q, is a 0.1-mg autoinjector approved for use in children who weigh 7.5 to <15 kg [68].
Most allergists prescribe EpiPen Jr for children who weigh 22 to 44 pounds and the EpiPen for children who weigh 62 pounds or more [68]. The dose for children who weigh 44 to 61 pounds primarily depends on the physician's assessment of the child's risk for a severe anaphylactic reaction. Expert consensus and a pharmacokinetic study recommend switching children to the 0.3-mg dose when a child weighs 55 to 66 pounds [69]. The conventional approach, that children who weigh less than 22 pounds should receive a 0.01-mg/kg dose of epinephrine drawn up in a syringe from an ampule, has been challenged because several factors (e.g., caregivers' inability to draw and administer a correct dose in a reasonable amount of time) that ultimately leads to a delay in dosing, incorrect dosing, or no dose at all [69]. The 2017 AAP clinical report for management of anaphylaxis and the 2020 AAAAI anaphylaxis practice parameter, among other sources, recommend using the 0.15-mg (EpiPen Jr) or 0.1-mg (Auvi-Q) autoinjector for infants and children weighing less than 22 pounds, stating that the benefit-to-risk ratio is favorable [12,69]. Patients and caregivers should be instructed that the autoinjector should be administered at the middle thigh in average BMI individuals; for obese or severely obese individuals, the dose should be administered in the lower thigh or the calf muscle, respectively. The autoinjector will deliver the full dose in less than three seconds, and it should be removed promptly. If additional doses are required, they should be administered at an alternate site (e.g., the other thigh) [68].
Barriers to using self-injectable epinephrine have increased, with the rising cost of the self-injectable epinephrine pens, an underutilization of the pens when needed, and a supply shortage of injectable medications [68]. Between 2007 and 2016, there was a more than 500% increase in the price of an EpiPen two-pack, from an average of $100 to more than $600. This presented a major barrier to the initial filling and refilling of prescriptions and left many without emergency life-saving treatment [70]. In 2016, generic epinephrine autoinjectors became available; however, the generic formulation was originally listed for approximately $400/two-pack. Criticism of the high cost led to an attempt at lower-cost treatment, with some drug stores and pharmacies beginning to stock generic epinephrine autoinjectors for approximately $100/2-pack. In addition, state legislation has been enacted in some places, with New York signing a law to cap the price of EpiPens to $100 starting January 1, 2026. Manufacturers have worked to make generic injectable pens more affordable, but cost and insurance/prescription coverage remain barriers to many [71,72].
Researchers also found that there is a need for more physicians to prescribe self-injectable epinephrine for their patients and to emphasize the importance of immediate treatment for food-induced anaphylaxis. Surveys of primary care physicians and of individuals with nut allergies have shown that approximately 50% to 75% of individuals with nut allergies have not been prescribed self-injectable epinephrine [15,73].
Surveys of parents and physicians have indicated a need for improved education on emergency preparedness and the use of epinephrine. Studies vary with regard to individuals who use epinephrine for anaphylactic reaction before receiving emergency care, with rates varying between 65% and 85% of children presenting to the emergency department with anaphylaxis [15,63]. Among the reasons given for not administering epinephrine are lack of recognition of the severity of the reaction, unavailability of epinephrine, and fear of administering the drug [2,37,63]. A majority of parents, emergency medical personnel, and even physicians are reluctant to administer epinephrine due to a lack of knowledge of the necessity in anaphylaxis and of the drug's inherent safety (i.e., it is a chemical already present in the body, potentially in high levels, such as during exercise); other reasons for failing to administer epinephrine include lack of training and fear of worsening the patient's condition [74,75]. These findings suggest that practitioners should evaluate all patients carefully, use and follow treatment guidelines, talk to parents about identifying reactions and the need to act, prescribe self-injectable epinephrine, and instruct families in its use and safety. Studies have shown that up to 25% of children with previously unknown food allergy will experience their first allergic reaction while at school. As such, at least 46 states have created legislation to require or allow kindergarten–12th grade schools to stock undesignated epinephrine auto-injectors for use on students who do not have a prescription, due to unknown allergy or unprescribed allergy, who cannot afford the device, or who are not currently carrying an epinephrine pen. Law regarding schools carrying epinephrine varies by state and can vary by ability to implement at the school level; parents and healthcare providers should check the status of their local legislation and guidelines [76].
As the child grows older, parents and physicians should decide when it is appropriate for the child to assume increasing responsibility for management of his or her food allergy. Many parents have anxiety about their child assuming this increasing responsibility, especially with regard to selecting foods, but 85% agreed that their child should carry injectable epinephrine with them to school [10]. Most pediatric allergists believe that children should recognize the signs and symptoms of anaphylaxis and be able to use epinephrine by the time they are 12 to 14 years of age, with consideration given to developmental level [77]. Teenagers especially need reminders about the importance of having epinephrine available. In addition, cultural and ethnic differences may influence the types of behaviors in which teenagers will engage. Any possible cultural barriers to the proper use of the medication should be addressed. Older children and teenagers should also be cautioned that the availability of epinephrine does not mean that they can relax avoidance of the food allergen [78].
Practitioners should encourage the patient and parents to make the allergy known to all family and friends and to ensure that family and close friends know what to do in case of accidental ingestion and when to seek emergency medical care. An emergency call should be made as soon as an anaphylactic reaction begins, and the patient should be taken to an emergency department in an ambulance. Other points of discussion include documenting the allergy with a medical emergency tag or bracelet and providing an emergency care plan for day care providers, schools, and camps and when traveling. Parents of children who have been prescribed self-injectable epinephrine should inform school personnel about the allergy and the availability of the medication.
The Federal Food, Drug and Cosmetic Act (FD&C Act) was enacted in 1938 to protect the public health by ensuring safety of food, drugs, medical devices, and cosmetics that are manufactured and sold in the United States. The Act authorizes the FDA to oversee these products, including approval of new drugs and devices, inspecting of manufacturing facilities, and enforcing labeling requirements [81]. The FD&C Act is amended as evidence supports, with The Food Allergen Labeling and Consumer Protection Act of 2004 (FALCPA) being the first change, which went into effect January 1, 2006, and requires that labels clearly indicate ingredients and specifically note the presence any of the eight major food allergens. The FD&C was again amended in 2021, when the Food Allergy Safety, Treatment, Education, and Research Act (FASTER Act) was passed. The FASTER Act added sesame as the ninth major food allergen recognized in the United States and began food allergen labeling and manufacturing requirements (established by FALCPA) as of January 1, 2023 [82]. In January 2025, the FDA published the Food Allergen Labeling Requirements of the Federal Food, Drug, and Cosmetic Act (Edition 5), to include the following changes to existing food labeling to be implemented by January 1, 2028 [79,113]:
Tree nuts to continue requiring labeling have been reduced to 12 types: almond, black walnut, Brazil nut, cashew, filbert (hazelnut), heartnut (Japanese walnut), macadamia nut (Bush nut), pecan, pine nut (pinon nut), pistachio, and English and Persian walnut.
Tree nuts to be excluded from required labeling include coconut, cola (kola) nut, beech nut, butternut, chestnut, chinquapin, ginkgo nut, hickory nut, palm nut, pili nut, and shea or shea nut.
The definition of "egg" has been expanded from "hen's egg" to include domesticated chickens, ducks, geese, quail, and other birds.
The definition of "milk" has been expanded from "cow's milk" to cow, goat, sheep, or other ruminants.
The FDA enforces the provisions of these laws in most packaged food products containing the nine major food allergens, including dietary supplements, but does not include meat, poultry, and egg products (which are regulated by the U.S. Department of Agriculture); alcoholic beverages subject to Alcohol and Tobacco Tax and Trade Bureau labeling regulations; raw agricultural commodities; highly refined oils; drugs; cosmetics; and most foods sold at retail or food service establishments that are not pre-packaged with a label [82].
Product labels are required to identify the presence of food allergens in the ingredient list or immediately after next the ingredient list in a "contains" statement (e.g., "Contains Wheat, Milk, and Soy"). Allergen source ingredients must be listed in English and may either be listed in the ingredient statement, for example, "wheat flour" instead of "flour," or in parentheses following the source ingredient, as in "whey (milk)" or "natural flavor (peanut)" [82]. Patient education on how to read food labels for those with food allergy is available on the AAAAI website at https://www.aaaai.org/tools-for-the-public/conditions-library/allergies/food-labels [79,85].
Another barrier to identifying food allergens is using precautionary allergen labels as marketing language. Phrases that are used to indicate possible cross-contact with allergens include variations of the following [82,85]:
May contain
May contain traces of
Processed in a facility with
Produced in a factory with
Manufactured on shared equipment with
These phrases (among others) are not regulated by the FDA and do not accurately indicate different levels of risk. The distinction between varying precautionary phrases is unclear, and this type of marketing has been shown to lead to poor understanding of allergen labeling and varying degrees of risk perception among food allergic and non-allergic consumers alike [82,84].
The interpretation of food labels is a complex process, involving general food knowledge, literacy, and other factors. When reading labels, people with food allergy and caregivers draw on factors in addition to precautionary labels, such as trust of a particular brand or manufacturer, previous experience with a product, and images and product names (not intended to denote risk) [82,85]. The NIAID expert panel suggests that healthcare professionals provide education and training to patients with food allergies and their caregivers about how to best interpret ingredient lists on food labels and how to recognize incomplete labeling of ingredients. The panel also suggests that individuals with food allergy avoid products with precautionary labeling [2].
Individuals with food allergy, especially those with allergy to multiple foods, are at risk for nutrient deficiency during either diagnostic elimination diets or long-term allergen avoidance. A nutritionist or dietician plays a key role by helping parents to [83]:
Avoid all forms of the allergen
Select alternate foods to maintain an adequate diet with the appropriate vitamins and minerals (Table 9)
Prepare foods using substitutes for food allergens
ALTERNATIVE SOURCES FOR VITAMINS AND MINERALS FOUND IN COMMON FOOD ALLERGENS
| Food Allergen | Vitamins/Minerals | Other Sources |
|---|---|---|
| Milk | Vitamin A | Liver, spinach, dark leafy greens, broccoli, deep orange fruits and vegetables (apricots, cantaloupe, squash, carrots, sweet potato, pumpkin) |
| Vitamin D | Liver, sunlight | |
| Riboflavin (B2) | Meat, leafy green vegetables, whole or enriched grains or cereals | |
| Pantothenic acid (B5) | Meat, cereals, legumes, fruits, vegetables | |
| Vitamin B12 | Meat, fish, poultry, shellfish, eggs | |
| Calcium | Greens, legumes, calcium-fortified products (juices, rice, etc.) | |
| Egg | Vitamin B12 | Meat, fish, poultry, shellfish, milk, cheese |
| Riboflavin (B2) | Meat, leafy green vegetables, whole or enriched grains or cereals | |
| Pyridoxine (B6) | Grains, seeds, liver, meat, milk, vegetables | |
| Biotin | Liver, soy flour, cereals, tomatoes, yeast | |
| Selenium | Seafood, grains, meats | |
| Peanuts, tree nuts | Vitamin E | Leafy green vegetables, wheat germ, whole-grain products, seeds |
| Niacin | Meat, legumes, whole or enriched grains | |
| Magnesium | Fruits, vegetables, cereals | |
| Manganese | Whole grains, leafy green vegetables, wheat germ | |
| Chromium | Molasses, whole grains, seafood | |
| Wheat | Thiamin | Pork, beef, liver, legumes, nuts |
| Riboflavin | Milk, yogurt, cottage cheese, meat, leafy green vegetables | |
| Niacin | Meat, peanuts, legumes | |
| Iron | Red meat, fish, poultry, shellfish, legumes, dried fruits | |
| Folate (fortified wheat) | Liver, leafy green vegetables, lentils, oranges | |
| Soy | Thiamin | Pork, beef, liver, whole or enriched grains, legumes, nuts |
| Riboflavin | Milk, yogurt, cottage cheese, meat, leafy green vegetables, whole or enriched grains or cereals | |
| Pyridoxine (B6) | Grains, seeds, liver, meat, milk, eggs, vegetables | |
| Folate | Liver, leafy green vegetables, lentils, oranges | |
| Calcium | Milk, greens, legumes, calcium-fortified products | |
| Phosphorus | Milk, poultry, fish, meat, carbonated beverages | |
| Magnesium | Nuts, fruits, vegetables, cereals | |
| Iron | Red meat, fish, poultry, shellfish, legumes, dried fruits | |
| Zinc | Red meat, seafood (especially oysters), beans | |
| Fish, shellfish | Vitamin B12 | Meat, poultry, milk, cheese, eggs |
| Chromium | Molasses, whole grains | |
| Iron | Red meat, poultry, legumes, dried fruits | |
| Phosphorus | Milk, poultry, fish, meat, carbonated beverages | |
| Selenium | Grains, organ and muscle meats | |
| Zinc | Red meat, beans |
In addition, a nutritionist or dietician is the best equipped to conduct an annual nutrition assessment to monitor overall health effects of patients and prevent growth and nutritional issues in children.
The interval for follow-up diagnostic testing depends on the food in question, the age of the child, and the intervening medical history. Decreases in food-specific IgE levels over time may indicate that the child will become tolerant of the food; thus, lower IgE levels may prompt repeat oral food challenges to determine resolution. For example, an oral challenge can be offered to patients who have a low peanut-specific IgE and have not had an allergic reaction within the past year. IgE levels may also remain elevated for some time, and an oral food challenge can determine if clinical reactivity still exists. The resolution of atopic dermatitis may also be associated with the onset of tolerance to food allergens. A skin prick test is not helpful in determining resolution of allergy, as the results can remain positive even after tolerance has developed; however, a reduction in wheal size may indicate tolerance [2,27].
Food allergy can have a tremendous impact on quality of life, and attention should be given to the psychosocial needs of individuals with food allergy, as well as caregivers of those with food allergy. Studies have shown that adults and children with food allergy report lower general quality of life; physical health-related quality of life; quality of life within school; and emotional, psychologic, and social quality of life than their counterparts without allergy [86,88,89]. Approximately 45% of children with food allergy have said they were bullied or harassed (for any reason), and 31% said the bullying was specifically related to the food allergy. This bullying is also associated with lower quality of life and increased distress, for both the child and the parents [87,90].
Social limitations are primary concerns of parents. In one survey, about half of caregivers said the food allergy affected the family's social activities, and 10% opted to home-school the child because of the food allergy [90]. More than half of parents have reported that some family relatives do not accommodate the child's food allergy, and 40% report hostility from other parents [10].
Food allergy affects anxiety and stress levels, and parents, especially mothers, have reported high scores for stress and anxiety. Parents have also noted that the food allergy causes a strain on their marriage/relationship and that their career has suffered because of the allergy [10]. In one survey, 70% of caregivers said that mental health support would have been helpful, but only 23% sought such help, even when it was available [91].
These negative effects of food allergy call for practitioners' heightened awareness of the psychosocial health of people with food allergy and their families, and greater encouragement of the use of mental health support. Practitioners should also encourage parents to discuss issues such as bullying with school administrators and suggest ways to better empower children. Education has been shown to enhance coping, and prescription of injectable epinephrine has decreased anxiety among mothers and children with food allergy [90,91].
Oral antihistamines and corticosteroids are commonly used to provide relief of symptoms associated with mild-to-moderate allergies, and bronchodilators should be prescribed for patients with asthma [25]. Physicians must emphasize that antihistamines or bronchodilators should not be used as a preventive measure before ingesting a possible food allergen, as oral food challenges have elicited positive results even in children who have taken such medication before the test [27].
Oral immunotherapy is now recognized as an alternative treatment to food avoidance in some patients with IgE-mediated food allergy. Several types of immunotherapy have been evaluated, including subcutaneous, epicutaneous, heated food, sublingual, and oral immunotherapy. Subcutaneous immunotherapy is no longer used because of severe systemic reactions, and although epicutaneous immunotherapy uses the lowest maintenance dose of the immunotherapies and also has an improved safety profile, it is less efficacious [73,92,96].
Immunotherapy with heated food proteins has been evaluated in children with generally transient allergies, such as to egg or milk. Heating egg and milk proteins at high temperature denatures allergenic proteins, making them less allergenic. Approximately 70% to 75% of children with egg or milk allergy have tolerated baked egg or milk, and introducing baked egg into the diet of children with egg allergy has accelerated the development of tolerance to regular egg, compared with strict avoidance of the food. In a 2021 study, researchers found that after 18 months of boiled egg white immunotherapy, 88% of children were regularly consuming egg and 72% were desensitized to the target dose [94]. A study published in 2024 found that, among children with milk allergy, immunotherapy using baked milk showed desensitization after 12 and 24 months of treatment, with the longer duration of treatment (24 months) increasing efficacy [93]. This treatment approach may not be effective for children with severe food allergy or for those with a high milk-specific IgE.
In 2020, the FDA approved the first oral immunotherapy peanut allergen powder (Palforzia) for the mitigation of allergic reaction, including anaphylaxis, with accidental exposure to peanuts in individuals 1 to 17 years of age with a confirmed peanut diagnosis. The powder is packaged in pull-apart capsules, allowing the allergen to be added to a small amount of semisolid food and consumed. Efficacy studies showed 67.2% of recipients tolerated a 600-mg dose, compared with 4.0% of placebo participants [97,112]. Peanut allergen powder is to be used in conjunction with a peanut-avoidant diet and epinephrine should be available during use [112].
Sublingual immunotherapy has been evaluated in children with allergies to nuts or milk, and this strategy has led to an increase in the amount of food that can be tolerated on an oral food challenge and generally mild reactions. However, the maximum amount of food that can be tolerated is limited by the amount of food that can be given sublingually [73]. In 2013, sublingual immunotherapy for peanut allergy was evaluated in 40 individuals, 12 to 37 years old, in a randomized, double-blind, placebo-controlled multicenter trial, one of the first of its kind [95]. After 44 weeks of daily therapy, 70% of the individuals were able to consume at least 10 times more peanut powder than they could at the beginning of the study (compared with 15% of individuals given placebo). Longer therapy (65 weeks) led to the ability to consume significantly more peanut powder without an allergic reaction. The treatment appeared safe, with side effects being minor (itching in the mouth) [95]. A three-year follow-up to this trial showed that 50% of patients discontinued therapy, and 10.8% of patients were desensitized to 10 g of peanut powder and achieved sustained unresponsiveness eight weeks after therapy concluded [73]. While sublingual immunotherapy has an improved safety profile and promising results, it appears to be less efficacious than oral immunotherapy.
Most adverse events in oral immunotherapy studies have occurred during the initial phase of increasing the dose of allergen, and new studies are focusing on ways to address this problem by pretreatment with an anti-IgE monoclonal antibody (such as omalizumab) or initial use of sublingual doses, with gradual increases in oral doses. Omalizumab is currently U.S. Food and Drug Administration (FDA)-approved for allergic asthma, and its use as an adjunct to oral immunotherapy in trials has allowed more rapid and higher doses of immunotherapy [68,98]. In one study, 79% of participants in the omalizumab group (who were started on 250 mg of peanut protein, versus 22.5 mg for the placebo group) were able to tolerate 2,000 mg of peanut protein six weeks after stopping omalizumab, compared with only 12% in the placebo group [98]. Additionally, a 2025 study found that omalizumab was superior to oral immunotherapy for multi-food allergy, with 36% of study participants who received an extended course of treatment able to tolerate 2 grams or more of peanut protein and two other food allergens, compared with 19% of participants who received multi-food oral immunotherapy [99,101].
The cost of oral immunotherapy protocols is expensive because of the time and monitoring needed to complete them; FDA approval and health insurance coverage are needed.
Questions have arisen about the safety of some vaccinations for individuals with food allergy, specifically the measles-mumps-rubella (MMR) vaccine and certain types of influenza vaccine, both of which are cultured in egg embryos. Studies have demonstrated that the MMR vaccine is safe for children with egg allergy, and the Advisory Committee on Immunization Practices (ACIP), the American Academy of Pediatrics (AAP), and the NIAID-sponsored guidelines all support MMR vaccination for children with egg allergy, even children who have a history of severe reactions [2].
The 2010 NIAID-sponsored guidelines note that there is insufficient evidence to recommend administering either trivalent inactivated or live-attenuated influenza vaccines to children with egg allergy who have a history of hives, angioedema, allergic asthma, or systemic anaphylaxis to egg proteins [2]. However, since that time, the results of several studies have shown that the influenza vaccine is safe for most people with a history of egg allergy, without the need to divide and administer the vaccine by a two-step approach or for skin testing with vaccine [102,103]. Based on these findings, the ACIP recommends that mild (hives only) or more severe symptoms (angioedema, respiratory distress, lightheadedness, recurrent emesis, administration of epinephrine or another emergency medical intervention) after exposure to egg are no longer contradictions for any influenza vaccine in adults or children. These individuals should receive any licensed, recommended, age-appropriate influenza vaccine [103,104]. The vaccine should be administered by a healthcare provider who is familiar with identifying and managing the potential manifestations of egg allergy if any symptoms are previously known. A previous severe allergic reaction to influenza vaccine, regardless of the component suspected of causing the reaction, is a contraindication to future receipt of the vaccine [103]. The AAP and a joint AAAAI and ACAAI task force support these recommendations, noting that the risks of not vaccinating outweigh the risks of vaccinating [102].
Food-induced anaphylaxis is under-recognized, underdiagnosed, and undertreated [2,73]. The rate of food-induced anaphylaxis appears to be rising, with one study showing an increase of 380% in the number of medical procedures to treat anaphylaxis due to food allergy between 2007 and 2016 [17]. Experts have noted, however, that most estimates of anaphylaxis do not reflect the substantial variation in several factors, such as patient age, geographic location, criteria used for diagnosis of anaphylaxis, and study methods [2]. Food-induced allergic reactions and anaphylaxis cost an estimated half billion dollars per year, with ambulatory visits accounting for more than half of that cost [105].
Food-induced anaphylaxis is rarely fatal, with about 150 to 250 deaths per year; this rate is even lower among adults. Among the factors associated with a greater risk for fatal anaphylaxis have been asthma; adolescence or young adulthood; peanut, tree nut, and seafood allergy; not carrying epinephrine; restaurant food; time in schools and childcare settings; and lack of information from healthcare providers [106].
Among children who have food-induced anaphylaxis, the causal food has varied according to age. The most common food triggers have been reported to be milk products and peanut among infants (younger than 2 years of age), peanut and tree nuts among preschool and school-aged children (2 to 11 years of age), and shellfish and tree nuts among adolescents [1,3].
Until 2006, there was no universal agreement on the definition of anaphylaxis or the criteria for its diagnosis. The NIAID and the Food Allergy and Anaphylaxis Network (FAAN) held two symposia to collaborate with representatives from 16 organizations and government bodies to develop a universally accepted definition of anaphylaxis as well as criteria for its diagnosis. They defined anaphylaxis as, "a serious allergic reaction that is rapid in onset and may cause death" [108]. The collaborative effort also led to the establishment of clinical criteria for diagnosis (Table 10) [107,108]. In a validation study of 86 emergency department patients who met these criteria, the criteria demonstrated a sensitivity of 96.7%, a specificity of 82.4%, a positive predictive value of 68.6%, and a negative predictive value of 98.4%, indicating that the criteria are likely to be useful in an emergency department setting [109].
CLINICAL CRITERIA FOR DIAGNOSING ANAPHYLAXIS
|
Anaphylaxis is highly likely if any one of the following three criteria is fulfilled:
|
| aLow systolic blood pressure for children is defined as less than 70 mm Hg from 1 month to 1 year, less than 70 mm Hg +(2 x age) from 1 to 10 years, and less than 90 mm Hg from 11 to 17 years. |
Early recognition of the clinical signs and symptoms of anaphylaxis is necessary to ensure immediate, appropriate treatment. In most cases, these signs and symptoms will occur within one hour after the accidental ingestion (ranging from within less than one minute to a few hours) and will vary in terms of presence, sequence, and severity. In 1% to 20% of anaphylaxis cases, there will be a biphasic response, with recurrence of symptoms 8 to 12 hours later, after the individual had seemed to recover [100]. The interval between the initial reaction and the recurrence has ranged from 1 to 72 hours. A biphasic reaction occurs in approximately 6% to 11% of children; such reactions typically occur within 8 hours after the first reaction but may occur as long as 72 hours later [12,108] .
As with less severe food-induced allergic reactions, cutaneous manifestations are the most common, followed by respiratory and gastrointestinal symptoms [12,110]. In one study of more than 600 children, cutaneous manifestations were documented in 87% to 98% of children; respiratory manifestations, in 59% to 81%; and gastrointestinal manifestations, in 50% to 59% [110]. The cardiovascular system is less frequently involved and is more often involved in adolescents [110]. Still, cutaneous manifestations may be absent in about 10% to 20% of cases of anaphylaxis, which may contribute to under-recognition [2].
Appropriate treatment of anaphylaxis must be immediate, as death can occur within 30 to 60 minutes [12,107]. Guidelines for the treatment of anaphylaxis have been developed jointly by the AAAAI and the ACAAI as well as by the NIAID and by the World Allergy Organization [2,12,107,108]. The drug of choice for the treatment of anaphylaxis is epinephrine, and several studies have shown that the lack of early epinephrine is associated with an increase in biphasic reactions as well as greater morbidity and mortality. Antihistamines (H1 and H2 blockers) and corticosteroids have been used to treat anaphylaxis, and the use of antihistamines is the most common reason given for not administering epinephrine. However, it has been demonstrated that there are few or no data to support the effectiveness of antihistamines or corticosteroids and that epinephrine is the only first-line treatment for anaphylaxis [2,12,69]. The recommended dose of epinephrine is 0.01 mg/kg (maximum dose: 0.3 mg for children and 0.5 mg for adults), given intramuscularly every 5 to 15 minutes as necessary to control symptoms and maintain blood pressure [2,12,108]. Peak plasma concentrations are highest and achieved fastest when epinephrine is administered intramuscularly in the thigh (versus the arm) [12,112].
Treatment of anaphylaxis must begin before the individual is transported to an emergency care facility. The NIAID expert panel recommends that the following steps be carried out concurrently as soon as an anaphylactic reaction has started [2]:
Eliminate additional exposure to the allergen
Call for help (emergency response team or 911)
Inject epinephrine intramuscularly
When the patient arrives at the emergency care facility, the first action is to assess the airway, breathing, and circulation. In the emergency care setting, additional injections of epinephrine may be necessary. There are multiple other components to treatment that vary according to many factors, most notably the individual's symptoms, the type of reactions that have occurred in the past, and the response to epinephrine (Figure 1) [100,108,111]. According to evidence-based guidelines for the treatment of anaphylaxis, adjunctive treatment may include [2,12,108,111]:
Intravenous crystalloid solutions or colloid volume expanders to reverse hypotension
Vasopressor for persistent hypotension
Antihistamine (H1 antagonists) for symptomatic relief of cutaneous reactions
Corticosteroids for anti-inflammatory protection and to help prevent protracted and biphasic reactions
Supplemental oxygen for respiratory symptoms
Inhaled bronchodilator (such as albuterol); nebulized therapy is preferred over metered-dose inhaler
Due to the potential for a biphasic response, the individual should remain in the emergency department for observation for four to six hours. In some instances, such as a history of severe reaction, it may be reasonable to admit the individual for longer observation. When discharged, the individual should be given self-injectable epinephrine, as well as a prescription for at least two doses, provided with educational resources on the symptoms of anaphylaxis and the use of epinephrine, and advised to make a prompt appointment with an allergist and to notify his or her primary care clinician [2,111].
Despite the availability of guidelines, anaphylaxis is often not appropriately treated. A review of randomly selected charts of individuals treated for food-induced anaphylaxis at 21 emergency departments in North America indicated the following [14,72]:
72% of patients received antihistamines
48% of patients received systemic corticosteroids
33% of patients received respiratory treatment (aerosolized beta-adrenergic agent)
16% of patients received epinephrine (24% with severe reactions received epinephrine)
16% of patients were prescribed self-injectable epinephrine at discharge
12% of patients were referred to an allergist at discharge
The establishment of treatment protocols and the availability of necessary supplies and equipment have been shown to significantly improve the management of anaphylaxis, with higher rates of epinephrine use and prescription, greater rates of admission to an observation area in the emergency department and a longer time in such an area, and lower rates of corticosteroid use (as monotherapy) and discharges without follow-up instructions [14,72]. In its guidelines on anaphylaxis, the World Allergy Organization provides examples of protocols and supply lists [107].
Patient J is a man, 35 years of age, who presents to the emergency room with acute dysphagia, vomiting, and abdominal pain. The symptoms began when he was having lunch at work, and they have persisted for a long period of time. The patient has a history of indigestion, asthma, and atopic dermatitis. The symptoms appear to be indicative of gastroesophageal reflux disease, and lansoprazole, 30 mg daily is prescribed. However, because the patient has a history of atopy and the onset of symptoms was acute, he is referred to a gastroenterologist for additional work-up.
The gastroenterologist performs an upper endoscopy with biopsy to determine if the symptoms are the result of an allergic response. Endoscopic examination shows the esophagus to be pink with linear furrows. Analysis of the biopsy samples demonstrates increased levels of eosinophils, approximately 26 per high-power field, in samples taken from the esophagus but not in those taken from the stomach or duodenum. Given the history of atopy, the results of the endoscopy and analysis indicate eosinophilic esophagitis.
The patient is referred to an allergist for testing to determine the allergen responsible for the response. Results of the skin prick tests demonstrate a response to bananas and honey. Patient J has eliminated these food items from his diet, and symptoms have resolved without recurrence.
A woman brings her son, Patient M, 1 year of age, to the pediatrician because of a persistent rash on the child's face, arms, and legs. The history regarding dietary intake indicates that cow's milk was newly introduced into Patient M's diet. Furthermore, the child's mother has a history of asthma and remembers that she drank soy milk as a child because she was allergic to cow's milk. On examination, Patient M's rash is limited to his cheeks and the extensor surface of his arms and legs. The rash is raised and intensely pruritic, with some small erythematous patches. The child has no respiratory or gastrointestinal symptoms and is afebrile. It is suspected that the patient is having an IgE-mediated response to cow's milk manifesting as atopic dermatitis. An immediate referral to an allergist is made, and the mother is advised to remove milk from the child's diet.
After reviewing Patient M's history and to confirm the initial diagnosis of IgE-mediated atopic dermatitis, the allergist performs a skin prick test. There is a positive response to cow's milk, with a wheal 6.5 mm in diameter. No reactions to other substances are noted. The positive reaction on the skin test and the family history of milk allergy negate the need for additional tests to confirm the diagnosis. Management includes removal of milk from Patient M's diet and treatment of pruritus with children's strength diphenhydramine (Benadryl) and a topical steroid cream. A follow-up appointment is made to monitor Patient M's allergy status.
Patient A is a woman, 21 years of age, with a known allergy to peanuts. She was having dinner at a Chinese restaurant with friends when she began to experience trouble breathing, which progressed to wheezing within a few minutes. She also showed signs of confusion and had slurred speech. Emergency response personnel were summoned to the scene.
On arrival, emergency personnel note diffuse and severe urticaria on Patient A's arms, legs, and face, particularly around the eyes and mouth. The patient also appears to have angioedema of the throat and/or tongue. Examination reveals pulmonary edema and a pulse of 140 beats per minute. The woman's friends tell the emergency response personnel that she has a peanut allergy. During transportation of the patient to the local hospital, an endotracheal tube is placed to create a patent airway, and 0.3 mg of epinephrine is administered intramuscularly in the thigh. After the epinephrine is administered, Patient A's symptoms begin to clear, and it is determined that no additional epinephrine is required.
When the patient arrives at the hospital, she has a pulse of 100 beats per minute and her breathing is substantially improved. Intravenous corticosteroid is given in order to minimize lingering allergic response. It is determined that, although the patient did not intentionally consume peanuts, there had been some cross contamination at the restaurant, which does serve several dishes containing peanuts or peanut butter. Because Patient A has a known allergy, she has a prescription for self-injectable epinephrine. However, she states that she usually leaves the medication at home because she avoids peanuts and, therefore, has had no need for it. The patient is advised to always keep the self-injectable epinephrine with her and to tell friends and companions where the medication is and when to use it. It is also recommended that she wear a necklace or bracelet identifying her severe peanut allergy in order to assist emergency personnel in the future.
Many types of adverse reactions to food occur, and true food allergy, an IgE-mediated reaction, must be distinguished from cell-mediated reactions as well as reactions that have no immunologic basis. True food allergy affects a small percentage of children and adults, but its increasing prevalence and the potential severity of allergic reactions are cause for public health concern. It is estimated that 3.4 million individuals require emergency medical care for food-induced allergic reactions and anaphylaxis each year [17]. Nine allergens account for up to 90% of all allergies: cow's milk, hen's egg, soy, wheat, peanut, tree nuts, shellfish, fish, and sesame [20]. There are few risk factors for food allergy, but family history of atopy or food allergy is common. Most food allergies are lost later in childhood, with allergies to nuts and seafood most commonly persisting into adulthood. Although guidelines once recommended late introduction of solid foods and common food allergens as a way to help prevent food allergy, more recent data contradict those recommendations.
Food allergy and other adverse food reactions manifest primarily in the skin, gastrointestinal tract, and respiratory system, and the related symptoms can help determine whether the reaction is a true food allergy. Guidelines have been developed for the diagnosis and management of food allergy, and recommendations include performing diagnostic testing within the context of a carefully taken history and physical examination. Skin prick testing and measurement of serum food allergen-specific IgE levels can help identify IgE-mediated reactions, and elimination diets and oral food challenges may be done on the basis of the results of these tests.
Preliminary research indicates that immunotherapy may hold the future key to the treatment of food allergy, but there is currently no cure. Strict avoidance of the causal food and response to allergic reactions are the cornerstones of management. A multidisciplinary approach is optimum for the management of food allergy, with participation of the pediatrician or primary care physician to coordinate early diagnostic testing, an allergist/immunologist to conduct food challenges and monitor follow-up diagnostic testing, and a nutritionist to help the patient maintain proper nutrition.
Food allergies affect the quality of life for children and their parents and can be especially challenging for teenagers. Patient education about the consequences of risky behavior and the need for emergency preparedness is essential. Psychosocial support is needed to help both children and parents cope. Several educational resources are available, and healthcare professionals should recommend these resources to help patients and families cope with all aspects of food allergy.
In treating allergic reactions, antihistamines and corticosteroids are helpful for mild reactions, and epinephrine is the preferred treatment for severe reactions and anaphylaxis. Evidence-based guidelines for the treatment of anaphylaxis have been developed. Their availability should help to provide more consistency in the treatment provided in emergency facilities, thus enhancing the quality of care.
1. Gupta RS, Warren CM, Smith BM, et al. The public health impact of parent-reported childhood food allergies in the United States. Pediatrics. 2018;142(6):e20181235.
2. Boyce JA, Assa'ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6):S1-S58.
3. Zablotsky B, Black LI, Akinbami LJ. Diagnosed allergic conditions in children 0–17 years: United States, 2021. NCHS Data Brief. 2023;459:1-8.
4. Gupta RS, Warren CM, Smith BM, et al. Prevalence and severity of food allergies among U.S. adults. JAMA Netw Open. 2019;2(1):e185630.
5. Ng AE, Boersma P. Diagnosed allergic conditions in adults: United States, 2021. NCHS Data Brief. 2023;460:1-8.
6. Gupta R, Holdford D, Bilaver L, et al. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167(11):1026–1031.
7. Jiang J, Warren CM, Brewer A, Soffer G, Gupta RS. Racial, ethnic, and socioeconomic differences in food allergies in the US. JAMA Netw Open. 2023;6(6):e2318162.
8. Sicherer SH, Warren CM, Dant C, Gupta RS, Nadeau KC. Food allergy from infancy through adulthood. J Allergy Clin Immunol Pract. 2020;8(6):1854-1864.
9. Sampson HA, Aceves S, Bock SA, et al. Food allergy: a practice parameter update, 2014. J Allergy Clin Immunol. 2014;134(5): 1016-1025.
10. Gupta RS, Springston EE, Kim JS, et al. Food allergy knowledge, attitudes, and beliefs of primary care physicians. Pediatrics. 2010;125(1):126-132.
11. Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J Allergy Clin Immunol. 2017;139(1):29-44.
12. Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis: a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145(4):1082-1123.
13. Shen W, Shih CH, Lim TJ. Food allergy knowledge among college students majoring in food and nutrition, nursing, and pre-medicine. Ann Allergy Asthma Immunol. 2023;130(2):256-257.
14. Clark S, Bock SA, Gaeta TJ, Brenner BE, Cydulka RK, Camargo CA. Multicenter study of emergency department visits for food allergies. J Allergy Clin Immunol. 2004;113(2):347-352.
15. Kumar A, Bruhn C, Teuber SS, Sicherer SH. Food allergy education for non-allergist physicians: a needs assessment survey. J Allergy Clin Immunol. 2006;117(2 Suppl 1):S43.
16. Sansweet S, Rolling C, Ebisawa M. Reaching communities through food allergy advocacy, research, and education: a comprehensive analysis. J Allergy Clin Immunol. 2024;12(2):310-315.
17. Food Allergy Research and Education. Facts and Statistics. Available at https://www.foodallergy.org/resources/facts-and-statistics. Last accessed April 1, 2025.
18. Bischoff S, Crowe SE. Gastrointestinal food allergy: new insights into pathophysiology and clinical perspectives. Gastroenterology. 2005;128(4):1089-1113.
19. Burks AW, Tang M, Sicherer S, et al. ICON: food allergy. J Allergy Clin Immunol. 2012;129(4):906-920.
20. United States Department of Agriculture. Food Allergies: The "Big 9." Available at https://www.fsis.usda.gov/food-safety/safe-food-handling-and-preparation/food-safety-basics/food-allergies-big-9. Last accessed April 1, 2025.
21. Liu AH, Jaramillo R, Sicherer SH, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2010;126(4):798-806.
22. Sicherer SH, Wood RA, Section on Allergy and Immunology. Allergy testing in childhood: using allergen-specific IgE tests. Pediatrics. 2012;129(1):193-197.
23. Rodriguez J, Mielgo R, Gonzalez A, Crespo JF. Allergic reactions to fresh fruits: beyond oral symptoms. J Allergy Clin Immunol. 2006;117(2 Suppl 1):S301.
24. Parrish CP. A review of food allergy panels and their consequences. Ann Allergy Asthma Immunol. 2023;131(4):421-426.
25. Francis OL, Wang KY, Kim EH, Moran TP. Common food allergens and cross-reactivity. J Food Allergy. 2020;2(1):17-21.
26. Sur LM, Armat I, Duca E, et al. Food allergy a constant concern to the medical world and healthcare providers: practical aspects.Life (Basel). 2021;11(11):1204.
27. Sicherer SH, Sampson HA. Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol. 2018;141(1):41-58.
28. Moore L, Demain JG, Sanner CJ, Whisman BA, Rathkopf MM. Seal and whale meat: a newly recognized food allergy. Ann Allergy Asthma Immunol. 2007;98(1):92-96.
29. Asthma and Allergy Foundation of America. Allergies: The Allergic or Atopic March. Available at https://aafa.org/allergies/prevent-allergies/allergic-march. Last accessed April 1, 2025.
30. Baloh CH, Mathias RA. Recent progress in the genetic and epigenetic underpinnings of atopy. J Allergy Clin Immunol. 2023;151(1):60-69.
31. Park J, Jang H, Kim M, et al. Predicting allergic diseases in children using genome-wide association study (GWAS) data and family history. World Allergy Organ J. 2021;14(5):100539.
32. Kenney HM, Battaglia J, Herman K, Beck LA. Atopic dermatitis and IgE-mediated food allergy. Ann Allergy Asthma Immunol. 2024;133(3):262-277.
33. Gupta R, Warren C, Blumenstock J, et al. The prevalence of childhood food allergy in the United States: an update. Ann Allergy Asthma Immunol. 2017;119(5):S11.
34. Mahlab-Guri K, Guri A, Kadar L, et al. Characteristics of patients with spontaneous resolution of sesame allergy. Ann Allergy Asthma Immunol. 2022;128(2):206-212.
35. Greer FR, Sicherer SH, Burks AW, Committee on Nutrition, Section on Allergy and Immunology. The effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, hydrolyzed formulas, and timing of introduction of allergenic complementary foods. Pediatrics. 2019;e20190281.
36. Maslova E, Granström C, Hansen S, et al. Peanut and tree nut consumption during pregnancy and allergic disease in children-should mothers decrease their intake? Longitudinal evidence from the Danish National Birth Cohort. J Allergy Clin Immunol. 2012;130(3):724-732.
37. Fleischer DM, Perry TT, Atkins D, et al. Allergic reactions to foods in preschool-aged children in a prospective observational food allergy study. Pediatrics. 2012;130(1):e25-e32.
38. Koukou Z, Papadopoulou E, Panteris E, et al. The effect of breastfeeding on food allergies in newborns and infants. Children (Basel). 2023;10(6):1046.
39. Greer FR, Sicherer SH, Burks AW. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008;121(1):183-191.
40. Meek JY, Noble L. American Academy of Pediatrics policy statement: breastfeeding and the use of human milk. Pediatrics. 2022;150(1):e2022057988.
41. Centers for Disease Control and Prevention. Summary Health Statistics: National Health Interview Survey, 2018. Available at https://archive.cdc.gov/#/details?url=https://www.cdc.gov/nchs/nhis/shs/tables.htm. Last accessed April 1, 2025.
42. Fleischer DM, Chan ES, Venter C, et al. A consensus approach to the primary prevention of food allergy through nutrition: guidance from the American Academy of Allergy, Asthma, and Immunology; American College of Allergy, Asthma, and Immunology; and the Canadian Society for Allergy and Clinical Immunology. J Allergy Clin Immunol Pract. 2021;9(1):22-43.
43. Chapman JA, Bernstein IL, Lee RE, et al. Food allergy: a practice parameter. Ann Allergy Asthma Immunol. 2006;96(3 Suppl):S1-S68.
44. Jackson KD, Howie LD, Akinbami LJ. Trends in allergic conditions among children: United States, 1997–2011. NCHS Data Brief. 2013;(121):1-8.
45. Tam JS. Cutaneous manifestation of food allergy. Immunol Allergy Clin North Am. 2017;37(1):217-231.
46. American Gastroenterological Association. American Gastroenterological Association medical position statement: guidelines for the evaluation of food allergies. Gastroenterology. 2001;120(4):1023-1025.
47. Wessels M, Auricchio R, Dolinsek J, et al. Review on pediatric coeliac disease from a clinical perspective. Eur J Pediatr. 2022;181(5):1785-1795.
48. Therrien A, Kelly CP, Silvester JA. Celiac disease: extraintestinal manifestations and associated conditions. J Clin Gastroenterol. 2020;54(1):8-21.
49. Rubio-Tapia A, Hill ID, Semrad C, et al. American College of Gastroenterology guidelines update: diagnosis and management of celiac disease. Am J Gastroenterol. 2023;118(1):59-76.
50. Al-Iede M, Sarhan L, Alshrouf MA, Said Y. Perspectives on non-IgE-mediated gastrointestinal food allergy in pediatrics: a review of current evidence and guidelines. J Asthma Allergy. 2023;16:279-291.
51. Dellon ES, Muir AB, Katzka DA, et al. ACG clinical guideline: diagnosis and management of eosinophilic esophagitis. Am J Gastroenterol. 2025;120(1):31-59.
52. Hahn JW, Lee K, Shin JI, et al. Global incidence and prevalence of eosinophilic esophagitis, 1976–2022: a systematic review and metanalysis. Clin Gastroenterol Hepatol. 2023;21(13):3270-3284.
53. Marasco G, Visaggi P, Vassallo M, et al. Current and novel therapies for eosinophilic gastrointestinal diseases. International Journal of Molecular Sciences. 2023;24(20):15165.
54. Dykewicz MS, Dinakar C, Dykewicz MS, et al. Rhinitis 2020: a practice parameter update. J Allergy Clin Immunol. 2020;146(4):721-767.
55. Foong RX, du Toit G, Fox AT. Asthma, food allergy, and how they relate to aach other. Front Pediatr. 2017;5:89.
56. Srisuwatchari W, Kanchanaphoomi K, Nawiboonwong J, et al. Food-dependent exercise-induced anaphylaxis: a distinct form of food allergy-an updated review of diagnostic approaches and treatments. Foods. 2023;12(20):3768.
57. Choosing Wisely. Choosing Wisely: A Watershed Moment in Health Care. Available at https://choosingwisely.org. Last accessed April 1, 2025.
58. Pongracic JA, Bock SA, Sicherer SH. Oral food challenge practices among allergists in the United States. J Allergy Clin Immunol. 2012;129(2):564-566.
59. Lieberman JA, Cox AL, Vitale M, Sampson HA. Outcomes of office-based, open food challenges in the management of food allergy [letter]. J Allergy Clin Immunol. 2011;128(5):1120-1122.
60. Kennedy K, Alfaro MKC, Spergel Z, et al. Differences in oral food challenge reaction severity based on increasing age in a pediatric population. Ann Allergy Asthma Immunol. 2021;127(5):562-567.
61. Frellick M. Allergists Respond to Death of Boy, 3, in Food Challenge. Available at https://www.medscape.com/viewarticle/883820. Last accessed April 1, 2025.
62. Järvinen KM, Amalanayagam S, Shreffler WG, et al. Epinephrine treatment is infrequent and biphasic reactions are rare in food-induced reactions during oral food challenges in children. J Allergy Clin Immunol. 2008;124(6):1267-1272.
63. Akuete K, Guffey D, Israelsen RB, et al. Multicenter prevalence of anaphylaxis in clinic-based oral food challenges. Ann Allergy Asthma Immunol. 2017;119(4):339-348.
64. American Academy of Allergy, Asthma and Immunology. Consultation and Referral Guidelines Citing the Evidence: How the Allergist/Immunologist Can Help: Food Allergy. Available at http://www.aaaai.org/Aaaai/media/MediaLibrary/PDF%20Documents/Referral%20Guidelines/Table-8-Food-allergy.pdf. Last accessed April 1, 2025.
65. Kutner M, Greenberg E, Jin Y, Paulsen C. The Health Literacy of America's Adults: Results from the 2003 National Assessment of Adult Literacy. Washington, DC: National Center for Education Statistics, U.S. Department of Education; 2006.
66. U.S. Census Bureau. Selected Social Characteristics in the United States: 2023 American Community Survey 5-Year Estimates. Available at https://data.census.gov/table/ACSDP5Y2023.DP02?d=ACS 5-Year Estimates Data Profiles. Last accessed April 1, 2025.
67. Centers for Disease Control and Prevention. Food Allergy Reactions. Available at https://www.cdc.gov/restaurant-food-safety/php/practices/food-allergy-reactions.html. Last accessed April 1, 2025.
68. LexiDrug. Available at https://online.lexi.com. Last accessed April 1, 2025.
69. Sicherer SH, Simons FER, Section on Allergy and Immunology. Epinephrine for first-aid management of anaphylaxis. Pediatrics. 2017;139(3):e20164006.
70. Siegel R. How Smart Marketing Transformed EpiPen Into A Billion-Dollar Product. Available at https://www.npr.org/2015/09/30/444790880/how-smart-marketing-transformed-epipen-into-a-billion-dollar-product. Last accessed April 1, 2025.
71. Food Allergy Research and Education. EpiPen Price Cap Bill Passes New York Legislature: EpiPens to Cost $100. Available at https://www.foodallergy.org/media-room/epipen-price-cap-bill-passes-new-york-legislature-epipens-cost-100. Last accessed April 1, 2025.
72. Prince BT, Mikhail I, Stukus DR. Underuse of epinephrine for the treatment of anaphylaxis: missed opportunities. J Asthma Allergy. 2018;11:143-151.
73. Chen M, Land M. The current state of food allergy therapeutics. Hum Vaccin Immunother. 2017;13(10):2434-2442.
74. Altman A, Wood RA. A majority of parents of children with peanut allergy fear using the epinephrine auto-injector. Pediatrics. 2014;134(Suppl 3):S148.
75. Russell WS, Farrar JR, Nowak R, et al. Evaluating the management of anaphylaxis in US emergency departments: guidelines vs. practice. World J Emerg Med. 2013;4(2):98-106.
76. Allergy and Asthma Network. State Laws. Available at https://advocacy.allergyasthmanetwork.org/laws-to-protect-those-with-allergies-and-asthma/state-laws. Last accessed April 1, 2025.
77. Simons E, Sicherer SH, Simons FE. Timing the transfer of responsibilities for anaphylaxis recognition and use of an epinephrine auto-injector from adults to children and teenagers: pediatric allergists' perspective. Ann Allergy Asthma Immunol. 2012;108(5):321-325.
78. Marrs T, Lack G. Why do few food-allergic adolescents treat anaphylaxis with adrenaline? Reviewing a pressing issue. Pediatr Allergy Immunol. 2013;24(3):222-229.
79. U.S. Food and Drug Administration. Guidance for Industry: Questions and Answers Regarding Food Allergen Labeling (Edition 5). Available at https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-questions-and-answers-regarding-food-allergen-labeling-edition-5. Last accessed April 1, 2025.
80. U.S. Department of Health and Human Services, U.S. Food and Drug Administration. Food Allergen Labeling and Consumer Protection Act of 2004 (FALCPA). Available at https://www.fda.gov/food/food-allergensgluten-free-guidance-documents-regulatory-information/food-allergen-labeling-and-consumer-protection-act-2004-falcpa. Last accessed April 1, 2025.
81. U.S. Food and Drug Administration. Federal Food, Drug, and Cosmetic Act (FD&C Act). Available at https://www.fda.gov/regulatory-information/laws-enforced-fda/federal-food-drug-and-cosmetic-act-fdc-act. Last accessed April 1, 2025.
82. U.S. Food and Drug Administration. Food Allergies. Available at https://www.fda.gov/food/nutrition-food-labeling-and-critical-foods/food-allergies. Last accessed April 1, 2025.
83. American Partnership for Eosinophilic Disorders. Alternate Sources of Nutrients. Available at https://apfed.org/resources/for-patients/for-adults/nutrition-and-recipes/alternate-sources-of-nutrients/. Last accessed April 1, 2025.
84. Holleman BC, van Os-Medendorp H, van den Bergh H, et al. Poor understanding of allergen labelling by allergic and non-allergic consumers. Clin Exp Allergy. 2021;51(10):1374-1382.
85. American Academy of Allergy, Asthma and Immunology. Food Labels: Read It Before You Eat It! Available at https://www.aaaai.org/tools-for-the-public/conditions-library/allergies/food-labels. Last accessed April 1, 2025.
86. Warren CM, Otto AK, Walkner MM, Gupta RS. Quality of life among food allergic patients and their caregivers. Curr Allergy Asthma Rep. 2016;16(5):38.
87. Danovich T. Parents, Schools Step up Efforts to Combat Food-Allergy Bullying Available at https://www.npr.org/sections/thesalt/2018/06/05/613933607/parents-schools-step-up-efforts-to-combat-food-allergy-bullying. Last accessed April 1, 2025.
88. Nguyen DTI, Pitts K, Staggers KA, et al. Quality of life is lower in food allergic adolescents compared to young children at a community educational symposium. Allergy Asthma Clin Immunol. 2023;19(99).
89. Protudjer J, Abrams EM. Enhancing health-related quality of life among those living with food allergy. J Allergy Clin Immunol. 2021;9(10):3715-3716.
90. Kachru R. Psychosocial issues and quality of life associated with food allergy. J Food Allergy. 2020;2(1):95-98.
91. Annunziato RA, Shemesh E, Weiss CC, Izzo GN, D'Urso C, Sicherer S. An assessment of the mental health needs and utilization by families of children with a food allergy. J Health Psychol. 2013;18(11):1456-1464.
92. Senti G, von Moos S, Tay F, Graf N, Johansen P, Kündig TM. Determinants of efficacy and safety in epicutaneous allergen immunotherapy: summary of three clinical trials. Allergy. 2015;70(6):707-710.
93. American Academy of Allergy, Asthma and Immunology. Baked Milk Oral Immunotherapy Found to be Effective Treatment for Cow's Milk Allergy. Available at https://www.aaaai.org/about/news/news/2024/baked. Last accessed April 1, 2025.
94. American Academy of Allergy, Asthma and Immunology. Egg Oral Immunotherapy Desensitizes Most Children After Prolonged Treatment. Available at https://www.aaaai.org/tools-for-the-public/latest-research-summaries/the-journal-of-allergy-and-clinical-immunology-in/2021/egg. Last accessed April 1, 2025.
95. Fleischer DM, Burks AW, Vickery BP, et al. Sublingual immunotherapy for peanut allergy: a randomized, double-blind, placebo-controlled multicenter trial. J Allergy Clin Immunol. 2013;131(1):119-127.
96. Pouessel G, Lezmi G. Oral immunotherapy for food allergy: translation from studies to clinical practice? World Allergy Organ J. 2023;16(2):100747.
97. U.S. Food and Drug Administration. FDA Approves First Drug for Treatment of Peanut Allergy for Children. Available at https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-treatment-peanut-allergy-children. Last accessed April 1, 2025.
98. Patrawala M, Shih J, Lee G, Vickery B. Peanut oral immunotherapy: a current perspective. Curr Allergy Asthma Rep. 2020;20(5):14.
99. American Academy of Allergy, Asthma and Immunology. Omalizumab Is Superior to Oral Immunotherapy in Multi-Food Allergy Treatment Study. Available at https://www.aaaai.org/about/news/news/2025/omalizumab. Last accessed April 1, 2025.
101. Wood R, Jones S, Dantzer J, et al. Treatment of multi-food allergy with omalizumab compared to omalizumab-facilitated multi-allergen OIT. J Allergy Clin Immunol. 2025;155(2):AB444.
102. Committee on Infectious Diseases, American Academy of Pediatrics. Recommendations for prevention and control of influenza in children, 2024–2025. Pediatrics. 2024;154(4):e2024068507.
103. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2024–25 influenza season. MMWR. 2024;73(5):1-25.
104. Kim DK, Bridges CB, Harriman KH; Advisory Committee on Immunization Practices (ACIP), ACIP Adult Immunization Work Group. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older—United States, 2019. MMWR. 2019;68(5):115-118.
105. Bilaver LA, Cadha AS, Doshi P, et al. Economic Burden of Food Allergy. Ann Allergy Asthma Immunol. 2019;122(4):373-380.
106. Turner PJ, Jerschow E, Umasunthar T, et al. Fatal anaphylaxis: mortality rate and risk factors. J Allergy Clin Immunol Pract. 2017;5(5):1169-1178.
107. Cardona V, Ansotegui IJ, Ebisawa M, et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organ J. 2020;13(10):100472.
108. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report-Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network Symposium. Ann Emerg Med. 2006;47(4):373-380.
109. Campbell RL, Hagan JB, Manivannan V, et al. Evaluation of National Institute of Allergy and Infectious Diseases/Food Allergy and Anaphylaxis Network criteria for the diagnosis of anaphylaxis in emergency department patients. J Allergy Clin Immunol. 2012;129(3):748-752.
110. Rudders SA, Banerji A, Clark S, Camargo CA Jr. Age-related differences in the clinical presentation of food-induced anaphylaxis.J Pediatr. 2011;158:326-328.
111. Gold DBK, Wang J, Waserman S, et al. Anaphylaxis: a 2023 practice parameter update. Ann Allergy Asthma Immunol. 2024;132(2):124-176.
112. Food Allergy Research and Education. Food Allergy Essentials: Common Allergens. Available at https://www.foodallergy.org/living-food-allergies/food-allergy-essentials/common-allergens. Last accessed April 1, 2025.
113. Food Allergy Research and Education. Update to FDA Guidance for Food Labeling. Available at https://www.foodallergy.org/fare-blog/update-fda-guidance-food-allergen-labeling. Last accessed April 1, 2025.
1. Muraro A, Roberts G (eds). European Academy of Allergy and Clinical Immunology Food Allergy and Anaphylaxis Guidelines: Translating Knowledge into Clinical Practice. Available at https://onlinelibrary.wiley.com/doi/full/10.1111/all.12429. Last accessed April 28, 2025.
2. Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(1 Suppl):S1-S55. Available at https://www.aaaai.org/Aaaai/media/Media-Library-PDFs/Allergist%20Resources/Statements%20and%20Practice%20Parameters/Allergen-immunotherapy-Jan-2011.pdf. Last accessed April 28, 2025.
3. Golden DBK, Wang J, Waserman S, et al. Anaphylaxis: a 2023 practice parameter update. Ann Allergy Asthma Immunol. 2024;132(2):124-176. Available at https://www.aaaai.org/Aaaai/media/Media-Library-PDFs/Allergist%20Resources/Statements%20and%20Practice%20Parameters/Anaphylaxis-Practice-Paramaters-2023.pdf. Last accessed April 28, 2025.
Mention of commercial products does not indicate endorsement.